Revalorization of Yerba Mate Residues: Biopolymers-Based Films of Dual Wettability as Potential Mulching Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nanocellulose Preparation and Characterization
2.2.2. Bilayer Films Preparation and Characterization
3. Results
3.1. Nanocellulose Fibers Isolation and Characterization
3.2. Bilayer Films
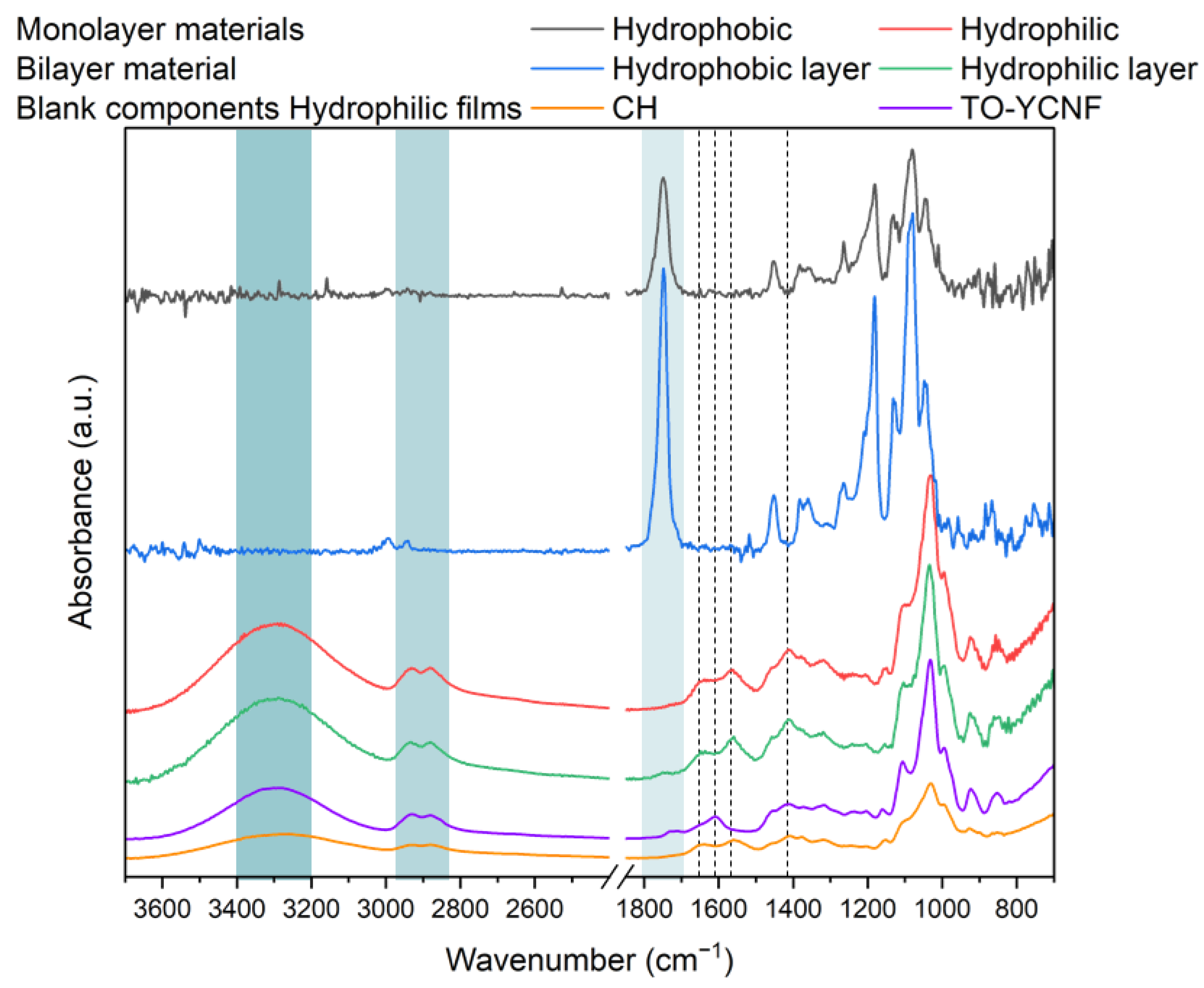
3.2.1. Textural and Chemical Analysis
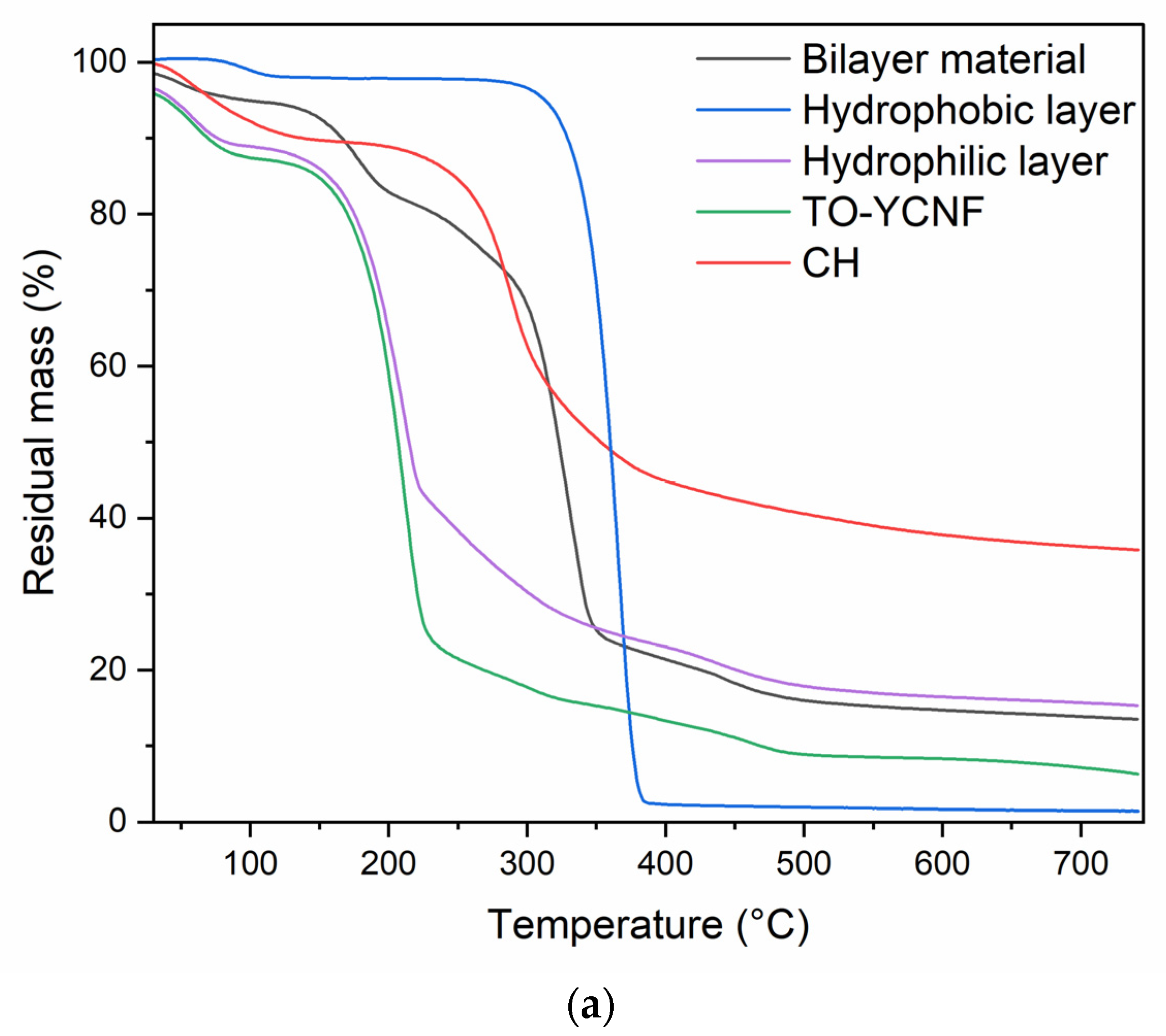
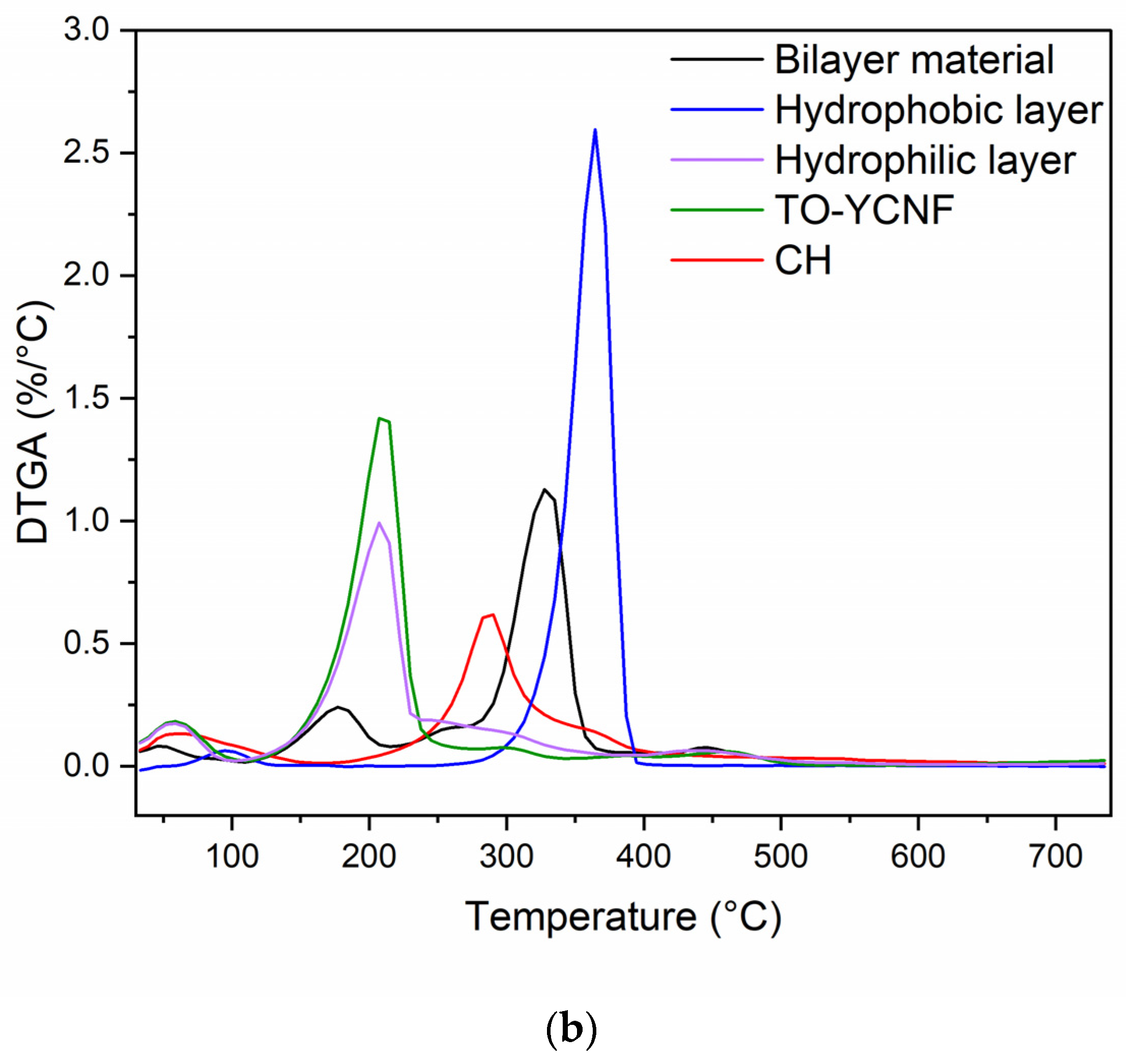
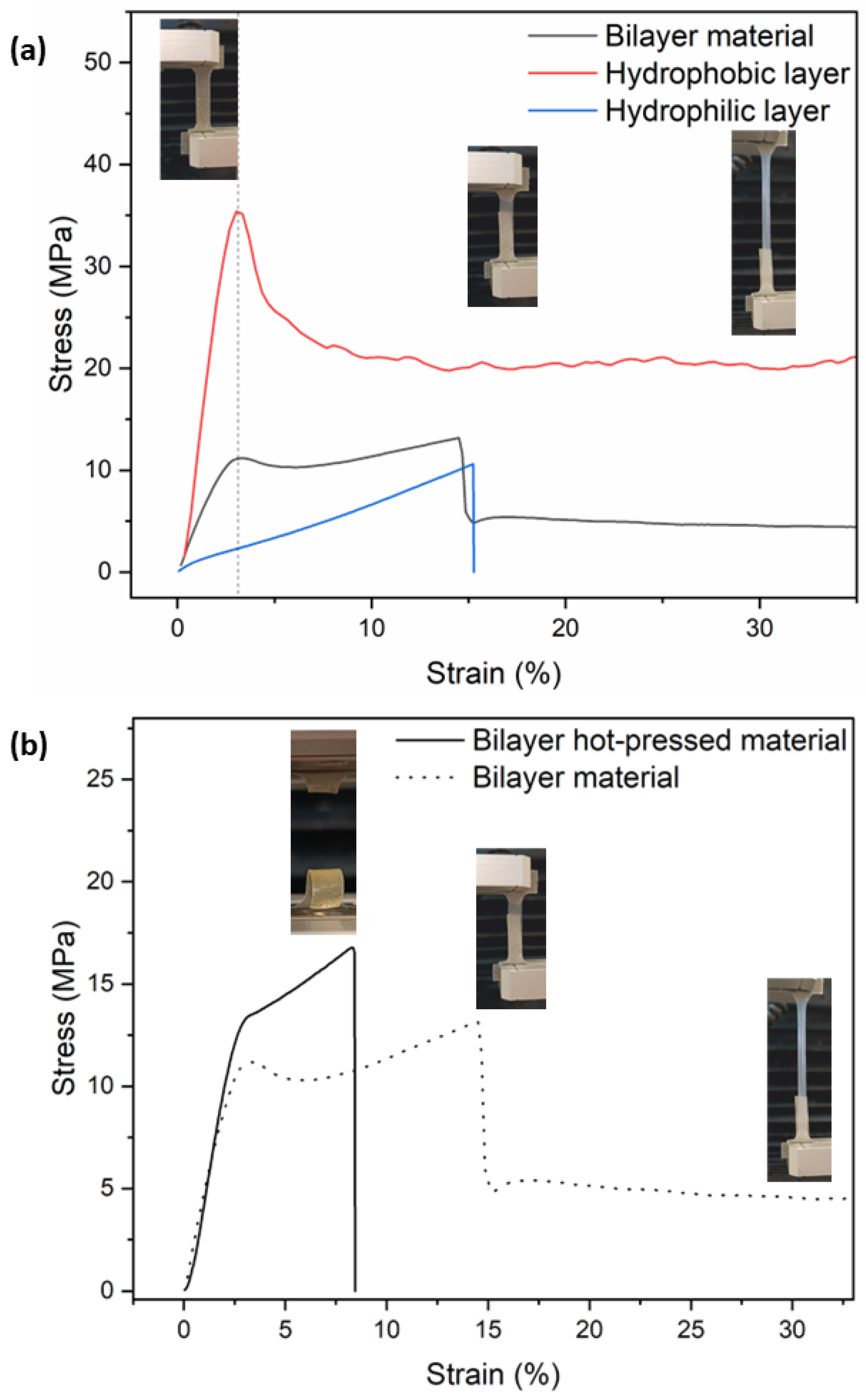
3.2.2. Mechanical and Thermal Characterization of Films
3.2.3. Film Interaction with Water and Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerrini, S.; Yan, C.; Malinconico, M.; Mormile, P. Agronomical Overview of Mulch Film Systems. In Polymers for Agri-Food Applications; Springer Nature: Cham, Switzerland, 2019; pp. 241–264. ISBN 9783030194161. [Google Scholar]
- Guo, H.; Li, S.; Kang, S.; Du, T.; Tong, L.; Hao, X.; Ding, R. Crop Coefficient for Spring Maize under Plastic Mulch Based on 12-Year Eddy Covariance Observation in the Arid Region of Northwest China. J. Hydrol. 2020, 588, 125108. [Google Scholar] [CrossRef]
- Braunack, M.V.; Zaja, A.; Tam, K.; Filipović, L.; Filipović, V.; Wang, Y.; Bristow, K.L. A Sprayable Biodegradable Polymer Membrane (SBPM) Technology: Effect of Band Width and Application Rate on Water Conservation and Seedling Emergence. Agric. Water Manag. 2020, 230, 105900. [Google Scholar] [CrossRef]
- Somanathan, H.; Sathasivam, R.; Sivaram, S.; Mariappan Kumaresan, S.; Muthuraman, M.S.; Park, S.U. An Update on Polyethylene and Biodegradable Plastic Mulch Films and Their Impact on the Environment. Chemosphere 2022, 307, 135839. [Google Scholar] [CrossRef] [PubMed]
- Martín-Closas, L.; Costa, J.; Pelacho, A.M. Agronomic Effects of Biodegradable Films on Crop and Field Environment. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 67–104. ISBN 9783662541302. [Google Scholar]
- Ajitha, A.R.; Aswathi, M.K.; Maria, H.J.; Izdebska, J.; Thomas, S. Multilayer Polymer Films. In Multicomponent Polymeric Materials; Springer: Dordrecht, The Netherlands, 2016; pp. 200–217. ISBN 9789401773249. [Google Scholar]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation Behavior of Three-Layer Sheets Based on Gelatin and Poly (Lactic Acid) Buried under Indoor Soil Conditions. Polym. Degrad. Stab. 2015, 116, 36–44. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, R.; Wang, B.; Chen, K. Development and Characterization of Bilayer Films Based on Pea Starch/Polylactic Acid and Use in the Cherry Tomatoes Packaging. Carbohydr. Polym. 2019, 222, 114912. [Google Scholar] [CrossRef] [PubMed]
- Góes, M.M.; Merci, A.; Andrello, A.C.; Yamashita, F.; de Carvalho, G.M. Design and Application of Multi-Layer Starch-Latex Blends as Phosphorous Delivery System. J. Polym. Environ. 2021, 29, 2000–2012. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Espinosa, E.; Mendoza Zélis, P.; Morcillo Martín, R.; de Haro Niza, J.; Rodríguez, A. Cellulose Nanofibers/PVA Blend Polymeric Beads Containing in-Situ Prepared Magnetic Nanorods as Dye Pollutants Adsorbents. Int. J. Biol. Macromol. 2022, 209, 1211–1221. [Google Scholar] [CrossRef]
- Liu, Z. A Review on the Emerging Conversion Technology of Cellulose, Starch, Lignin, Protein and Other Organics from Vegetable-Fruit-Based Waste. Int. J. Biol. Macromol. 2023, 242, 124804. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, P.; Narula, A.K.; Deswal, D. Polysaccharides and Lipoproteins as Reactants for the Synthesis of Pharmaceutically Important Scaffolds: A Review. Int. J. Biol. Macromol. 2023, 242, 124884. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Kajla, P.; Tavassoli, M. Value Addition of Rice Straw Cellulose Fibers as a Reinforcer in Packaging Applications. Int. J. Biol. Macromol. 2023, 243, 125320. [Google Scholar] [CrossRef]
- Gopan, G.; Jose, J.; Khot, K.B.; Bandiwadekar, A. The Use of Cellulose, Chitosan and Hyaluronic Acid in Transdermal Therapeutic Management of Obesity: A Review. Int. J. Biol. Macromol. 2023, 244, 125374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tarahomi, M.; Sheibani, R.; Xia, C.; Wang, W. Progresses in Lignin, Cellulose, Starch, Chitosan, Chitin, Alginate, and Gum/Carbon Nanotube (Nano)Composites for Environmental Applications: A Review. Int. J. Biol. Macromol. 2023, 241, 124472. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de la Yerba Mate (INYM). Informe Del Sector Yerbatero; Instituto Nacional de la Yerba Mate (INYM): Posadas, Argentina, 2023. [Google Scholar]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi Agricultural Waste and Industry By-Products by Recovering Bioactive Compounds and Applications as Food Additives: A Circular Economy Model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, L. Circular Economy and Waste Valorisation: Theory and Practice from and International Perspective; Springer Nature: Cham, Switzerland, 2017; ISBN 9780262337991. [Google Scholar]
- de Oliveira, J.P.; Bruni, G.P.; Fonseca, L.M.; da Silva, F.T.; da Rocha, J.C.; da Rosa Zavareze, E. Characterization of Aerogels as Bioactive Delivery Vehicles Produced through the Valorization of Yerba-Mate (Illex paraguariensis). Food Hydrocoll. 2020, 107, 105931. [Google Scholar] [CrossRef]
- Dahlem, M.A.; Borsoi, C.; Hansen, B.; Catto, A.L. Evaluation of Different Methods for Extraction of Nanocellulose from Yerba Mate Residues. Carbohydr. Polym. 2019, 218, 78–86. [Google Scholar] [CrossRef]
- Aliabadi, M.; Chee, B.S.; Matos, M.; Cortese, Y.J.; Nugent, M.J.D.; de Lima, T.A.M.; Magalhães, W.L.E.; de Lima, G.G. Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage. Polymers 2020, 12, 2807. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Herrera, R.; Labidi, J.; Gullón, P. Yerba Mate Waste: A Sustainable Resource of Antioxidant Compounds. Ind. Crops Prod. 2018, 113, 398–405. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Beltran, F.; de las Muelas, S.S.A.; Gaspar, G.; Hernandez, R.S.; de la Orden, M.U.; Urreaga, J.M. Development of Tri-Layer Antioxidant Packaging Systems Based on Recycled PLA/Sodium Caseinate/Recycled PLA Reinforced with Lignocellulosic Nanoparticles Extracted from Yerba Mate Waste. Express Polym. Lett. 2022, 16, 881–900. [Google Scholar] [CrossRef]
- Llive, L.; Bruno, E.; Molina-García, A.D.; Schneider-Teixeira, A.; Deladino, L. Biodegradation of Yerba Mate Waste Based Fertilizer Capsules. Effect of Temperature. J. Polym. Environ. 2019, 27, 1302–1316. [Google Scholar] [CrossRef]
- Schneider Teixeira, A.; Deladino, L.; Zaritzky, N. Yerba Mate (Ilex paraguariensis) Waste and Alginate as a Matrix for the Encapsulation of N Fertilizer. ACS Sustain. Chem. Eng. 2016, 4, 2449–2458. [Google Scholar] [CrossRef]
- Brombilla, V.D.L.; Lazarotto, J.S.; Silvestri, S.; Anschau, K.F.; Dotto, G.L.; Foletto, E.L. Biochar Derived from Yerba-Mate (Ilex Paraguariensis) as an Alternative TiO2 Support for Enhancement of Photocatalytic Activity toward Rhodamine-B Degradation in Water. Chem. Eng. Commun. 2022, 209, 1334–1347. [Google Scholar] [CrossRef]
- Tesio, A.Y.; de Haro Niza, J.; Sanchez, L.M.; Rodríguez, A.; Caballero, A. Turning Yerba Mate Waste into High-Performance Lithium–Sulfur Battery Cathodes. J. Energy Storage 2023, 67, 107627. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active Natural-Based Films for Food Packaging Applications: The Combined Effect of Chitosan and Nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, Y.; Li, X.; Wu, Y.; Tang, K.; Liu, J.; Zheng, X.; Wan, G. Injectable Antibacterial Cellulose Nanofiber/Chitosan Aerogel with Rapid Shape Recovery for Noncompressible Hemorrhage. Int. J. Biol. Macromol. 2020, 154, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Maldonado, D.; Filpponen, I.; Erramuspe, I.B.V.; Johansson, L.-S.; Mori, M.F.; Babu, R.J.; Waters, M.N.; Peresin, M.S. Development of a β-Cyclodextrin-Chitosan Polymer as Active Coating for Cellulosic Surfaces and Capturing of Microcystin-LR. Surf. Interfaces 2022, 33, 102192. [Google Scholar] [CrossRef]
- Gomez-Maldonado, D.; Reynolds, A.M.; Burnett, D.J.; Babu, R.J.; Waters, M.N.; Peresin, M.S. Delignified Wood Aerogels as Scaffolds Coated with an Oriented Chitosan-Cyclodextrin Co-Polymer for Removal of Microcystin-LR. RSC Adv. 2022, 12, 20330–20339. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Hisamatsu, M.; York, W.S.; Teranishi, K.; Yamada, T. Adhesion of β-D-Glucans to Cellulose. Carbohydr. Res. 1998, 308, 389–395. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.S.; Laine, J.; Rojas, O.J. Modification of Cellulose Films by Adsorption of Cmc and Chitosan for Controlled Attachment of Biomolecules. Biomacromolecules 2011, 12, 4311–4318. [Google Scholar] [CrossRef]
- Menossi, M.; Salcedo, F.; Capiel, J.; Adler, M.; Alvarez, V.A.; Ludueña, L.N. Effect of Starch Initial Moisture on Thermoplastic Starch Film Properties and Its Performance as Agricultural Mulch Film. J. Polym. Res. 2022, 29, 285. [Google Scholar] [CrossRef]
- Uzamurera, A.G.; Zhao, Z.Y.; Wang, P.Y.; Wei, Y.X.; Mo, F.; Zhou, R.; Wang, W.L.; Ullah, F.; Khan, A.; Xiong, X.B.; et al. Thickness Effects of Polyethylene and Biodegradable Film Residuals on Soil Properties and Dryland Maize Productivity. Chemosphere 2023, 329, 138602. [Google Scholar] [CrossRef]
- Qin, J.; Liang, B.; Peng, Z.; Lin, C. Generation of Microplastic Particles during Degradation of Polycarbonate Films in Various Aqueous Media and Their Characterization. J. Hazard. Mater. 2021, 415, 125640. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zhou, C.; Almatrafi, E.; Hu, T.; Zeng, G.; Zhang, Y. Recent Advances in Impacts of Microplastics on Nitrogen Cycling in the Environment: A Review. Sci. Total Environ. 2022, 815, 152740. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(Ligno)Nanocellulose Biocomposite Films. Effect of Residual Lignin Content on Structural, Mechanical, Barrier and Antioxidant Properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef]
- TAPPI. Hollocellulose in Wood; TAPPI: Atlanta, GA, USA, 1940. [Google Scholar]
- TAPPI. Alpha-Cellulose in Paper, Test Method T 429 Cm-10; TAPPI: Atlanta, GA, USA, 2023. [Google Scholar]
- TAPPI. Acid-Insoluble Lignin in Wood and Pulp (Reaffirmation of T 222 Om-02; TAPPI: Atlanta, GA, USA, 2006. [Google Scholar]
- TAPPI. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C (Five-Year Review of T 211 Om-02); TAPPI: Atlanta, GA, USA, 2007. [Google Scholar]
- TAPPI. Solvent Extractives of Wood and Pulp (Proposed Revision of T 204 Cm-97); TAPPI: Atlanta, GA, USA, 2007. [Google Scholar]
- Besbes, I.; Vilar, M.R.; Boufi, S. Nanofibrillated Cellulose from Alfa, Eucalyptus and Pine Fibres: Preparation, Characteristics and Reinforcing Potential. Carbohydr. Polym. 2011, 86, 1198–1206. [Google Scholar] [CrossRef]
- Carrasco, F.; Mutjé, P.; Pelach, M.A. Control of Retention in Paper-Making by Colloid Titration and Zeta Potential Techniques. Wood Sci. Technol. 1998, 32, 145–155. [Google Scholar] [CrossRef]
- ISO 5351:2010; Pulps: Determination of Limiting Viscosity Number in Cupri-Ethylenediamine (CED) Solution. ISO: Geneva, Switzerland, 2010.
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Tarrés, Q.; Delgado-Aguilar, M.; González, I.; Mutjé, P.; Rodríguez, A. Suitability of Wheat Straw Semichemical Pulp for the Fabrication of Lignocellulosic Nanofibres and Their Application to Papermaking Slurries. Cellulose 2016, 23, 837–852. [Google Scholar] [CrossRef]
- Espinosa, E.; Arrebola, R.I.; Bascón-Villegas, I.; Sánchez-Gutiérrez, M.; Domínguez-Robles, J.; Rodríguez, A. Industrial Application of Orange Tree Nanocellulose as Papermaking Reinforcement Agent. Cellulose 2020, 27, 10781–10797. [Google Scholar] [CrossRef]
- Detsi, E. Specific Surface Area of Nanoporous Materials. In Metallic Muscles; University of Groningen: Groningen, The Netherlands, 2012. [Google Scholar]
- Sanchez, L.M.; Rincón, E.; de Haro Niza, J.; Martín, R.M.; Espinosa, E.; Rodríguez, A. Vegetable Lignocellulosic Residues and Chitosan as Valuable Resources in the Superabsorbent Bio-Aerogel Development for Food Conservation. Food Bioprocess Technol. 2023, 1–16. [Google Scholar] [CrossRef]
- Abd Al-Ghani, M.M.; Azzam, R.A.; Madkour, T.M. Design and Development of Enhanced Antimicrobial Breathable Biodegradable Polymeric Films for Food Packaging Applications. Polymers 2021, 13, 3527. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Espinosa, E.; Rabasco-Vílchez, L.; Sanchez, L.M.; de Haro, J.; Rodríguez, A. Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-robles, J.; Rodríguez, A. A Comparative Study of the Suitability of Different Cereal Straws for Lignocellulose Nanofibers Isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef]
- Sun, Z.; Ning, R.; Qin, M.; Liang, J.; Jiang, J.; Sun, W.; Liu, X.; Zi, M. Sustainable and Hydrophobic Polysaccharide-Based Mulch Film with Thermally Stable and Ultraviolet Resistance Performance. Carbohydr. Polym. 2022, 295, 119865. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Czubenko, J.; Gierszewska-Druzyńska, M. Effect of Ionic Crosslinking on the Water State in Hydrogel Chitosan Membranes. Carbohydr. Polym. 2009, 77, 590–598. [Google Scholar] [CrossRef]
- Yang, S.L.; Wu, Z.H.; Yang, W.; Yang, M.B. Thermal and Mechanical Properties of Chemical Crosslinked Polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Cao, X.; Mohamed, A.; Gordon, S.H.; Willett, J.L.; Sessa, D.J. DSC Study of Biodegradable Poly(Lactic Acid) and Poly(Hydroxy Ester Ether) Blends. Thermochim. Acta 2003, 406, 115–127. [Google Scholar] [CrossRef]
- Najafi, M.; Zahid, M.; Ceseracciu, L.; Safarpour, M.; Athanassiou, A.; Bayer, I.S. Polylactic Acid-Graphene Emulsion Ink Based Conductive Cotton Fabrics. J. Mater. Res. Technol. 2022, 18, 5197–5211. [Google Scholar] [CrossRef]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Melt Free Radical Grafting of Glycidyl Methacrylate (GMA) onto Fully Biodegradable Poly(Lactic) Acid Films: Effect of Cellulose Nanocrystals and a Masterbatch Process. RSC Adv. 2015, 5, 32350–32357. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Soy Protein—Poly (Lactic Acid) Bilayer Films as Biodegradable Material for Active Food Packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical Isolation and Characterization of Different Cellulose Nanofibers from Cotton Stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y.; Akdoǧan, N. Wetting of Superhydrophobic Polylactic Acid Micropillared Patterns. Langmuir 2022, 38, 10052–10064. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of Biopolymer Coatings: Contact Angle Kinetics and Image Analysis Investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, E.Y.; Yoo, Y.T.; Im, S.S. Effect of Hydrophilicity on the Biodegradability of Polyesteramides. J. Appl. Polym. Sci. 2003, 90, 2708–2714. [Google Scholar] [CrossRef]
- Song, J.; Zhang, R.; Li, S.; Wei, Z.; Li, X. Properties of Phosphorus-Containing Polybutylene Succinate/Polylactic Acid Composite Film Material and Degradation Process Effects on Physiological Indexes of Lettuce Cultivation. Polym. Test. 2023, 119, 107921. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the Functionality of Bio-Based Plastic Mulching Films. Polym. Test. 2018, 67, 99–109. [Google Scholar] [CrossRef]
- Johnson, A.B. Studies: Study of the Effect of Lignin to a Nanocellulose Film; UPC: Barcelona, Spain, 2019. [Google Scholar]
- Feng, X.; Zhao, Y.; Jiang, Y.; Miao, M.; Cao, S.; Fang, J. Use of Carbon Dots to Enhance UV-Blocking of Transparent Nanocellulose Films. Carbohydr. Polym. 2017, 161, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Titone, V.; Teresi, R.; Scarlata, M.C.; Lo Re, G.; La Mantia, F.P.; Lopresti, F. Biocomposite PBAT/Lignin Blown Films with Enhanced Photo-Stability. Int. J. Biol. Macromol. 2022, 217, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Juraić, K.; Dubček, P.; Bohač, M.; Gajović, A.; Bernstorff, S.; Čeh, M.; Hodzic, A.; Gracin, D. Surface Morphology of Textured Transparent Conductive Oxide Thin Film Seen by Various Probes: Visible Light, X-rays, Electron Scattering and Contact Probe. Materials 2022, 15, 4814. [Google Scholar] [CrossRef]

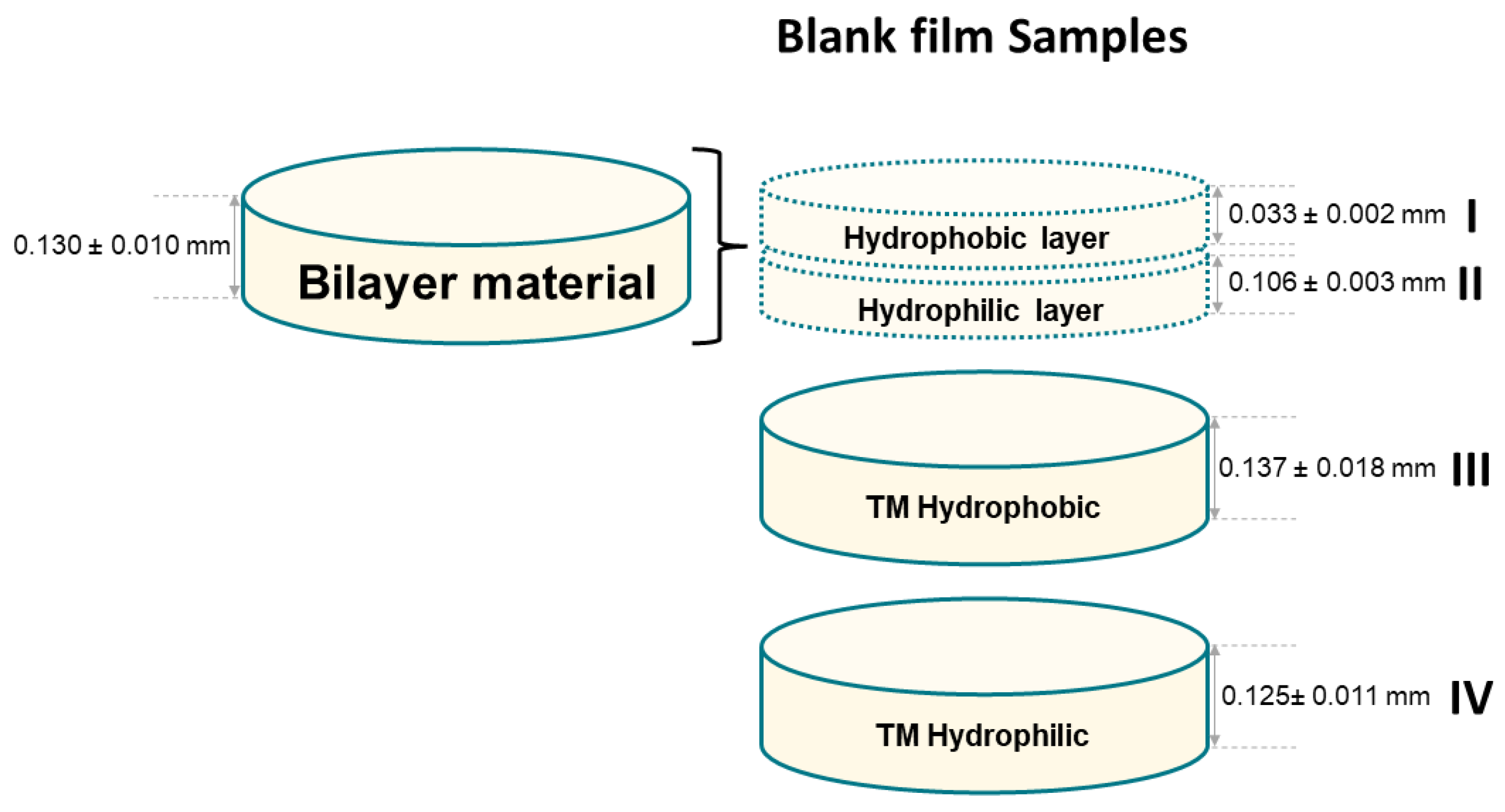
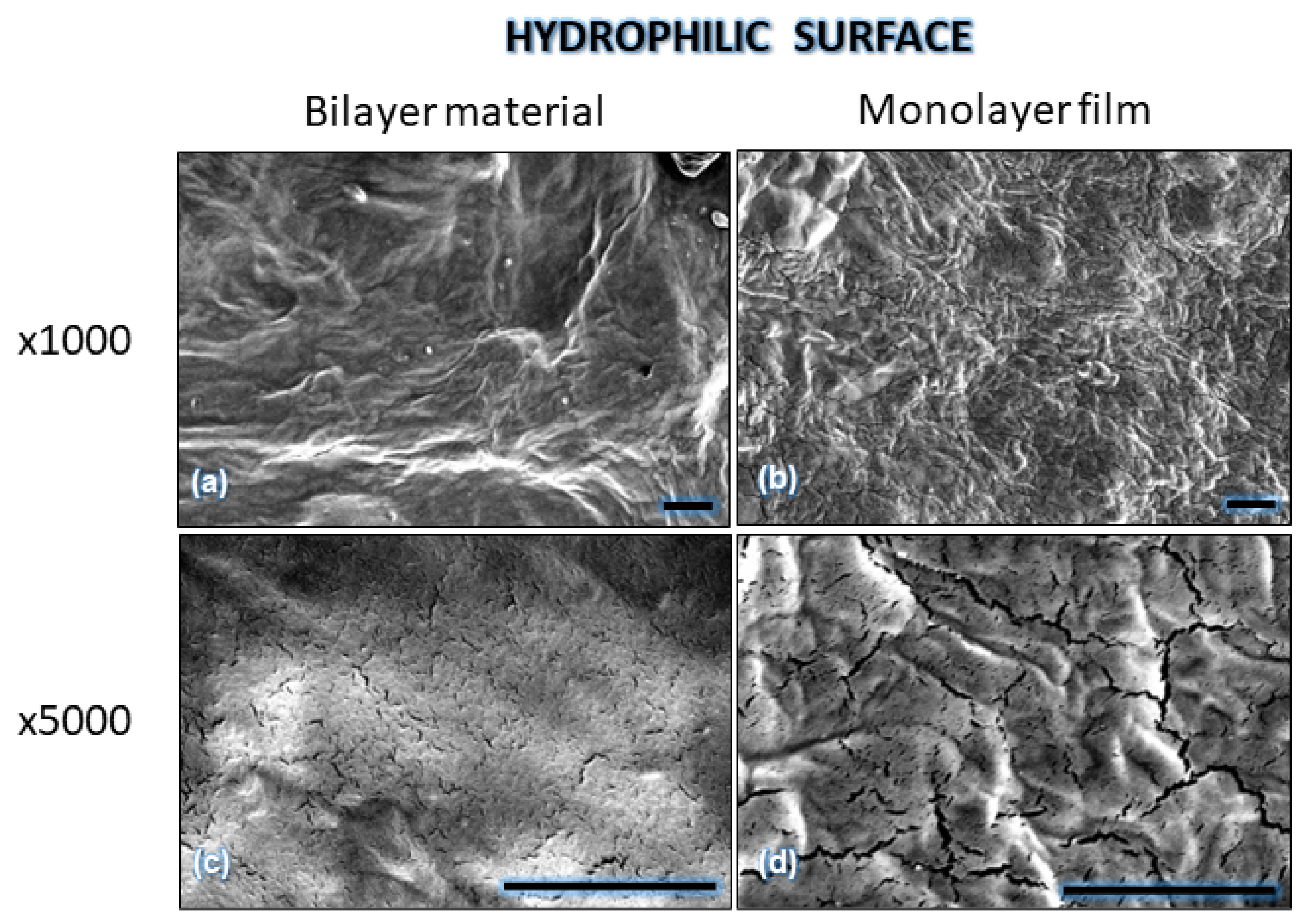
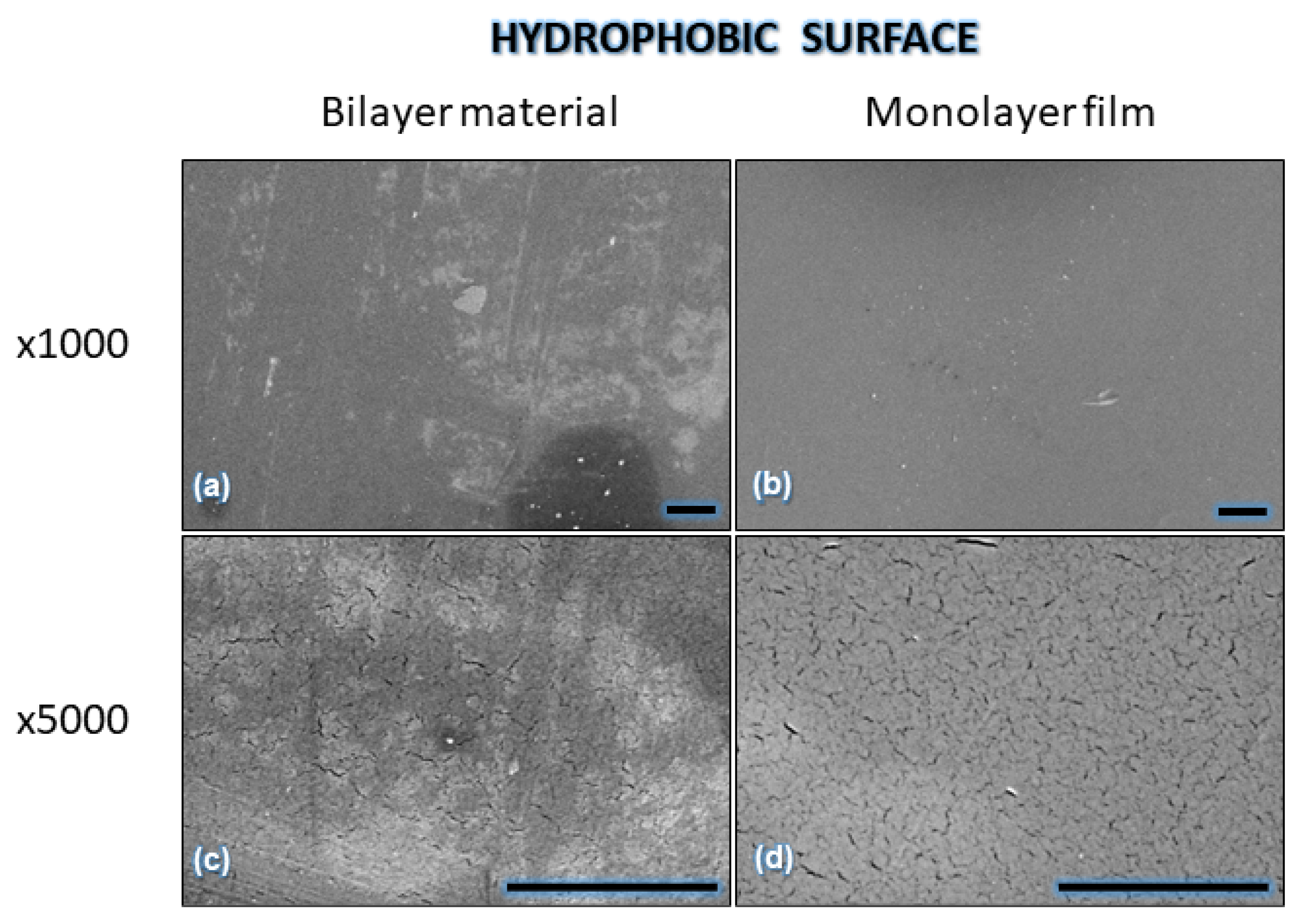
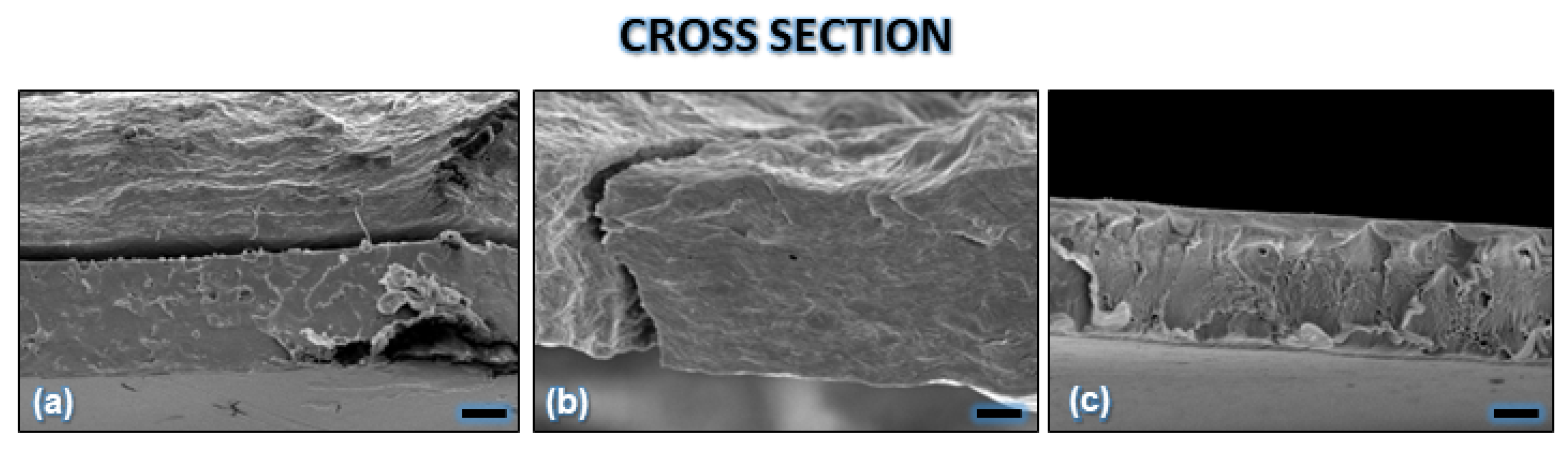

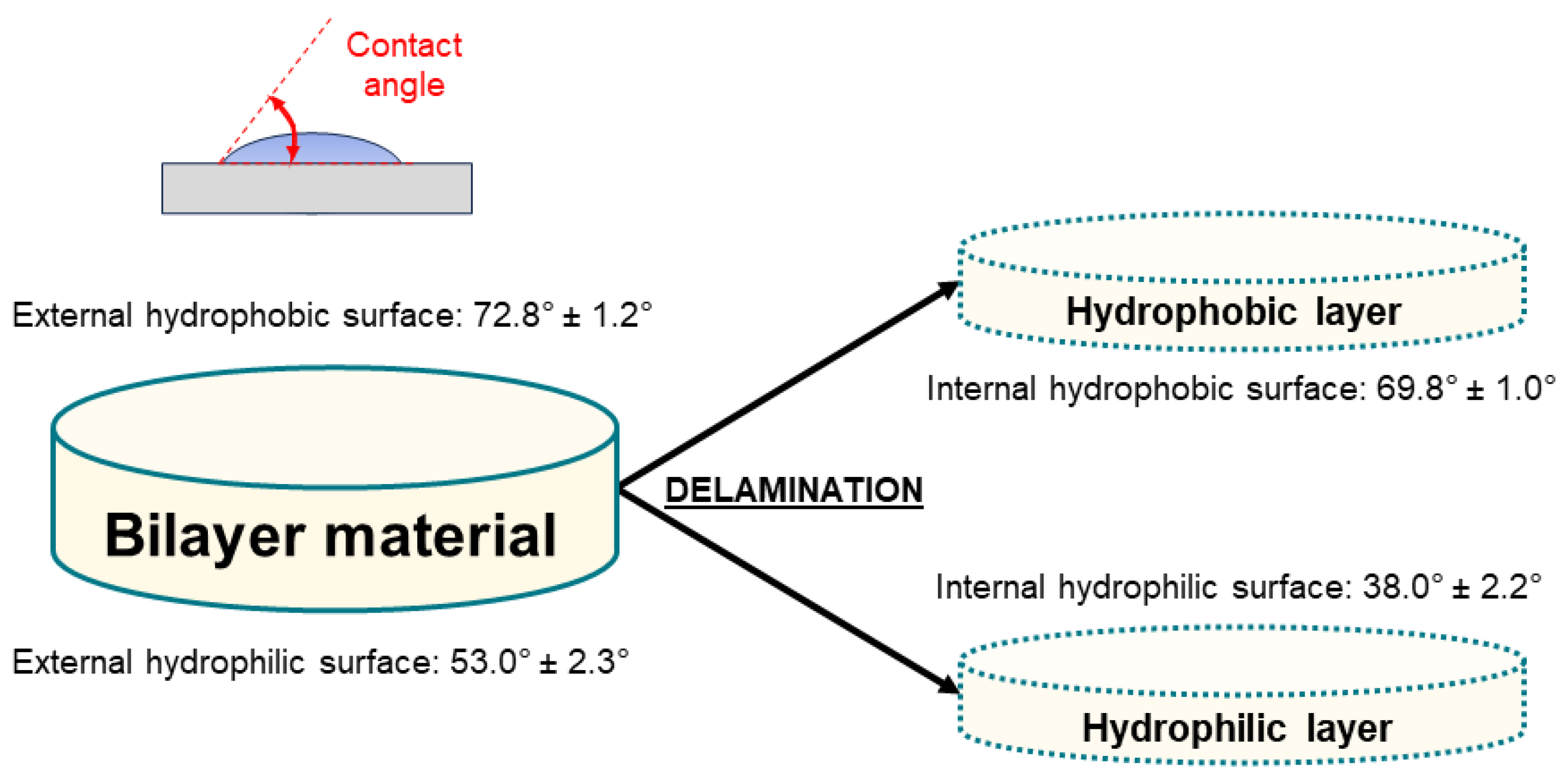
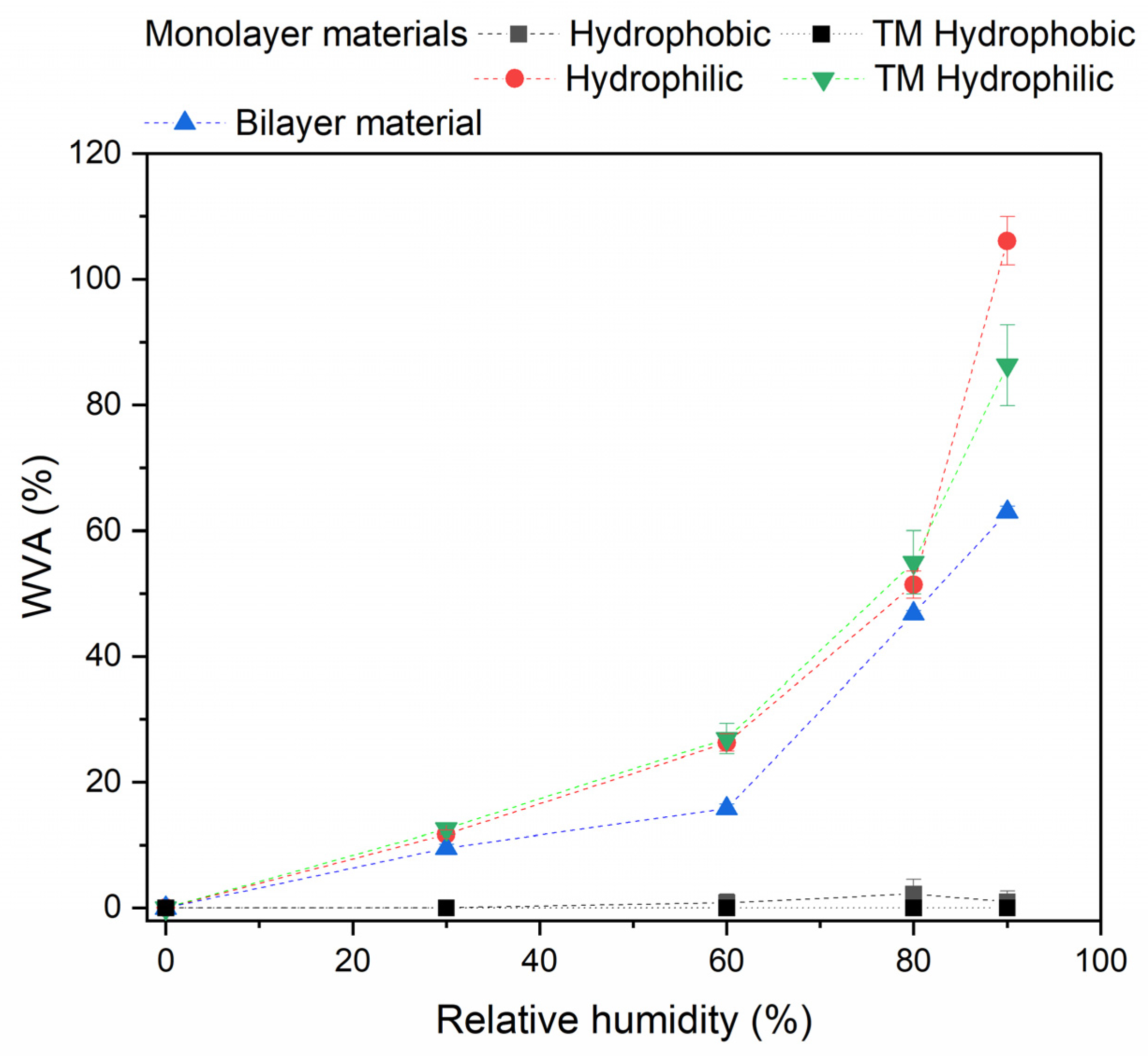

| Component | Infused Yerba Mate Residues (%) | Unbleached Pulp (%) | Bleached Pulp (%) |
|---|---|---|---|
| Ashes | 3.76 ± 0.13 | 2.45 ± 0.21 | 1.89 ± 0.19 |
| Extractables | 27.33 ± 1.34 | 21.26 ± 1.11 | 17.23 ± 1.63 |
| Lignin | 4.05 ± 0.41 | 7.41 ± 0.45 | 3.22 ± 0.3 |
| Hemicellulose | 29.23 ± 3.31 | 25.35 ± 3.41 | 20.12 ± 2.21 |
| α-Cellulose | 35.63 ± 3.25 | 43.53 ± 4.92 | 57.54 ± 4.39 |
| Sample | Yield (%) | T800 * (%) | CD (µeq/g) | CC (µeq/g) | Length (nm) | σspecific (m2/g) | Diameter (nm) | DP |
|---|---|---|---|---|---|---|---|---|
| TO-YCNF | 61.67 ± 3.5 | 52.3 | 1492.4 ± 41.43 | 190.41 ± 18.76 | 798 | 634 | 3.94 | 362.81 |
| TO-CNF [51] | 63.44 ± 4.52 | 74.7 | 1043.54 ± 18.2 | 148.12 ± 5.26 | 614.26 | 436 | 6 | 319.94 |
| TO-CNF [53] | 96.4 ± 0.4 | 1440.1 ± 20.1 | 369.5 ± 2.9 | 1033 | 521 | 5 | ||
| TO-CNF [54] | 98.7 | 90 | 1116.5 ± 43.1 | 367.0 ± 8.72 | 367.01 | 6.81 | 502 |
| Sample | 1st Heating | Cooling | 2nd Heating | ||
|---|---|---|---|---|---|
| Tg (°C) | Endothermic Peak | Tg (°C) | Tg (°C) | Endothermic Peak | |
| Bilayer | 39.6 | - | 47.5 | 53.2 | 125.5 °C * 0.444 J/g |
| Hydrophobic monolayer | 35.6 | - | 42.8 | 47.9 | - |
| Hydrophillic monolayer | - | - | - | 36.8 | 125.2 °C * 0.508 J/g |
| TM Hydrophobic | 24.1 *, (61.2) | 93.9 °C * 2.48 J/g | 41.6 | 45.6 | - |
| TM Hydrophilic | - | - | - | 35.1 | 125.6 °C * 0.683 J/g |
| Sample | Young’s Modulus (MPa) | Elongation at Break (%) | Tensile Strength at Break (MPa) | Tenacity (Nmm/mm3) (**) | Elongation Yield (%) | Tensile Yield (MPa) |
|---|---|---|---|---|---|---|
| Bilayer | 578 ± 121 (*) | 13.9 ± 1.9 (*) | 16.7 ± 3.2 (*) | 1.74 ± 0.19 | 3.8 ± 0.6 | 12.9 ± 2.6 |
| Hydrophobic monolayer | 1702 ± 130 | 65 ± 83 | 20.6 ± 3.7 | 13 ± 16 | 3.2 ± 0.4 | 34.4 ± 3.7 |
| Hydrophilic monolayer | 104 ± 20 | 16.8 ± 1.4 | 10.9 ± 1.7 | 0.88 ± 0.16 | - | - |
| TM Hydrophobic | 169 ± 26 | 490 ± 47 | 13.8 ± 1.8 | 43.3 ± 7.1 | - | - |
| TM Hydrophilic | 159 ± 24 | 16.2 ± 0.5 | 12.7 ± 1.0 | 1.05 ± 0.1 | - | - |
| Sample | Permeance (g/m2 d Pa) | Permeability (g m/m2 d Pa) × 106 |
|---|---|---|
| Bilayer | 0.0518 | 6.9 |
| Hydrophobic monolayer | 0.0666 | 2.2 |
| Hydrophilic monolayer | 0.7987 | 85 |
| TM Hydrophobic | 0.0074 | 1.0 |
| TM Hydrophilic | 0.7914 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, L.M.; de Haro, J.; Domínguez, E.; Rodríguez, A.; Heredia, A.; Benítez, J.J. Revalorization of Yerba Mate Residues: Biopolymers-Based Films of Dual Wettability as Potential Mulching Materials. Polymers 2024, 16, 815. https://doi.org/10.3390/polym16060815
Sanchez LM, de Haro J, Domínguez E, Rodríguez A, Heredia A, Benítez JJ. Revalorization of Yerba Mate Residues: Biopolymers-Based Films of Dual Wettability as Potential Mulching Materials. Polymers. 2024; 16(6):815. https://doi.org/10.3390/polym16060815
Chicago/Turabian StyleSanchez, Laura M., Jorge de Haro, Eva Domínguez, Alejandro Rodríguez, Antonio Heredia, and José J. Benítez. 2024. "Revalorization of Yerba Mate Residues: Biopolymers-Based Films of Dual Wettability as Potential Mulching Materials" Polymers 16, no. 6: 815. https://doi.org/10.3390/polym16060815
APA StyleSanchez, L. M., de Haro, J., Domínguez, E., Rodríguez, A., Heredia, A., & Benítez, J. J. (2024). Revalorization of Yerba Mate Residues: Biopolymers-Based Films of Dual Wettability as Potential Mulching Materials. Polymers, 16(6), 815. https://doi.org/10.3390/polym16060815








