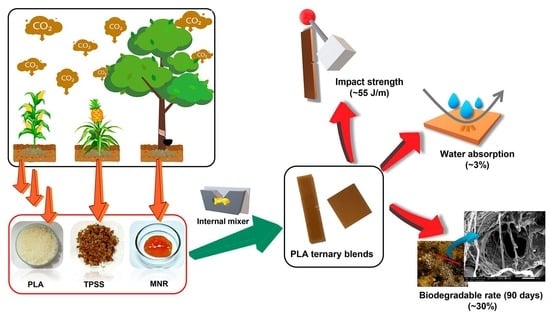
Sustainable Materials with Improved Biodegradability and Toughness from Blends of Poly(Lactic Acid), Pineapple Stem Starch and Modified Natural Rubber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
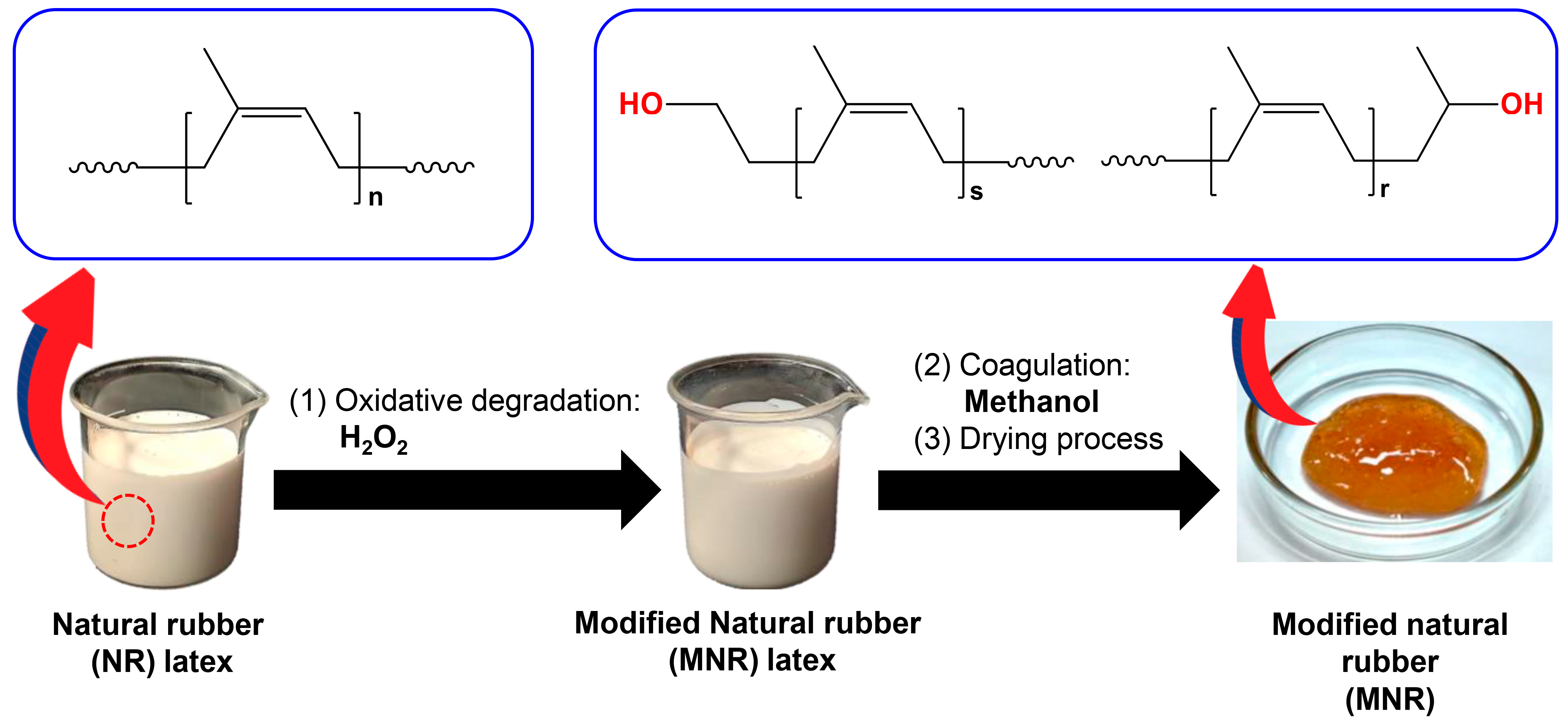
2.2. Preparation of Modified Natural Rubber (MNR)
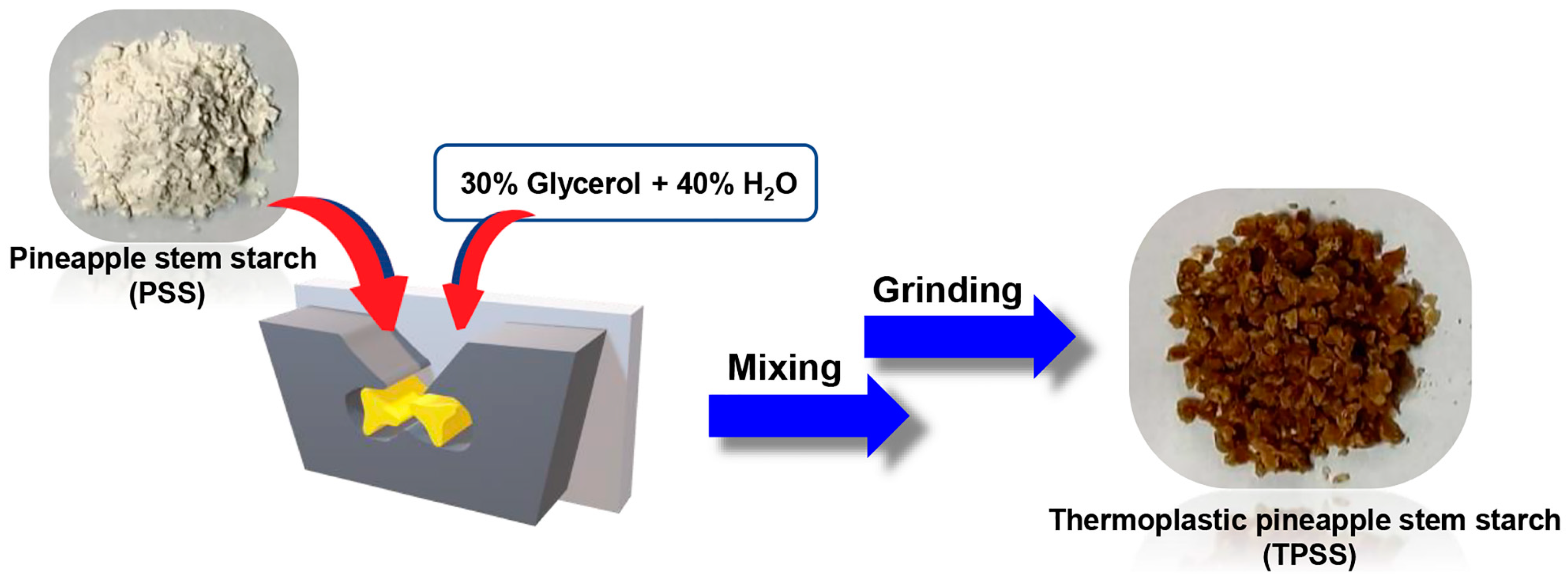
2.3. Preparation of Thermoplastic Pineapple Stem Starch (TPSS)
2.4. Preparation of Thermoplastic Pineapple Stem Starch (TPSS)
2.5. Material Characterizations
2.5.1. Determination of Hydroxyl Value of MNR
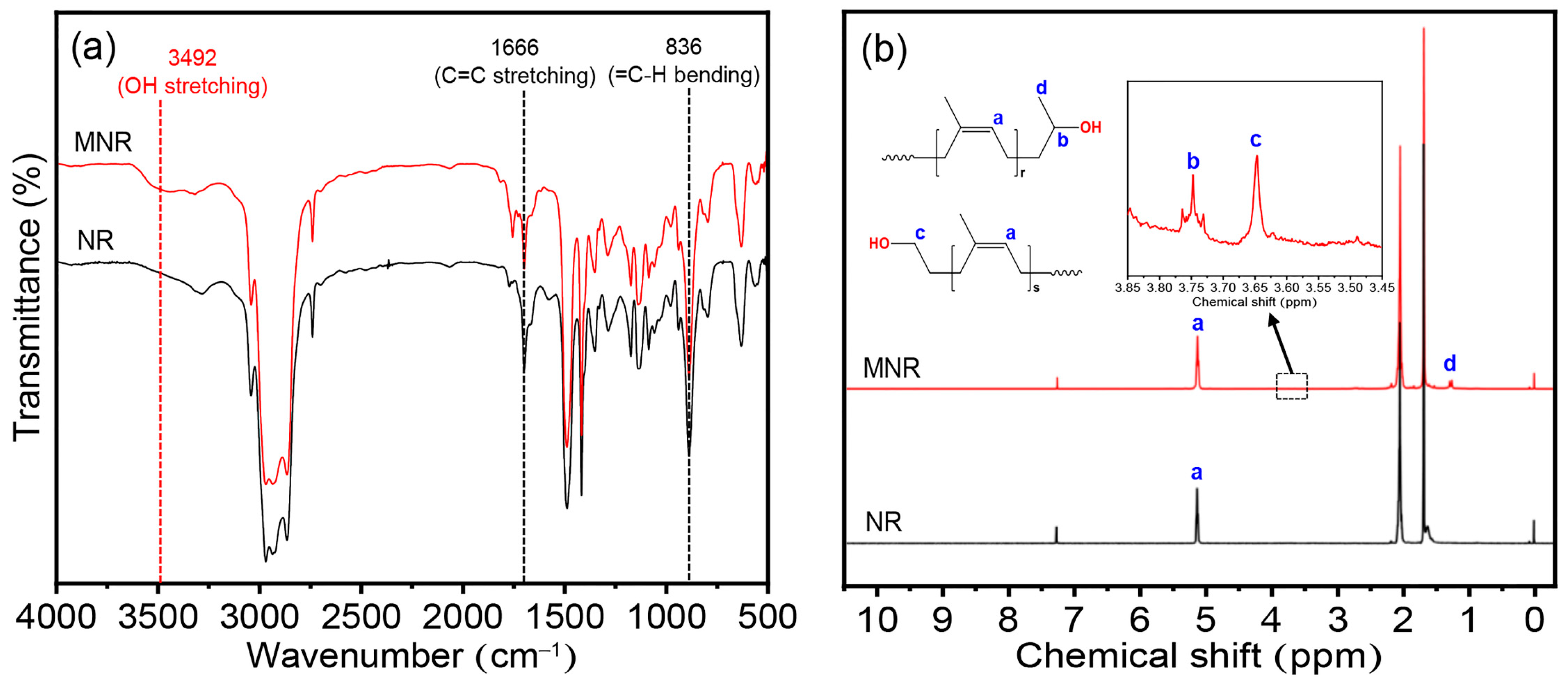
2.5.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.3. Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
2.5.4. Gel Permeation Chromatography (GPC)
2.5.5. Mechanical Properties
2.5.6. Dynamic Mechanical Analysis
2.5.7. Morphological Properties
2.5.8. Thermal Properties
2.5.9. Water Absorption
2.5.10. Soil Burial Degradation Test
3. Results and Discussion
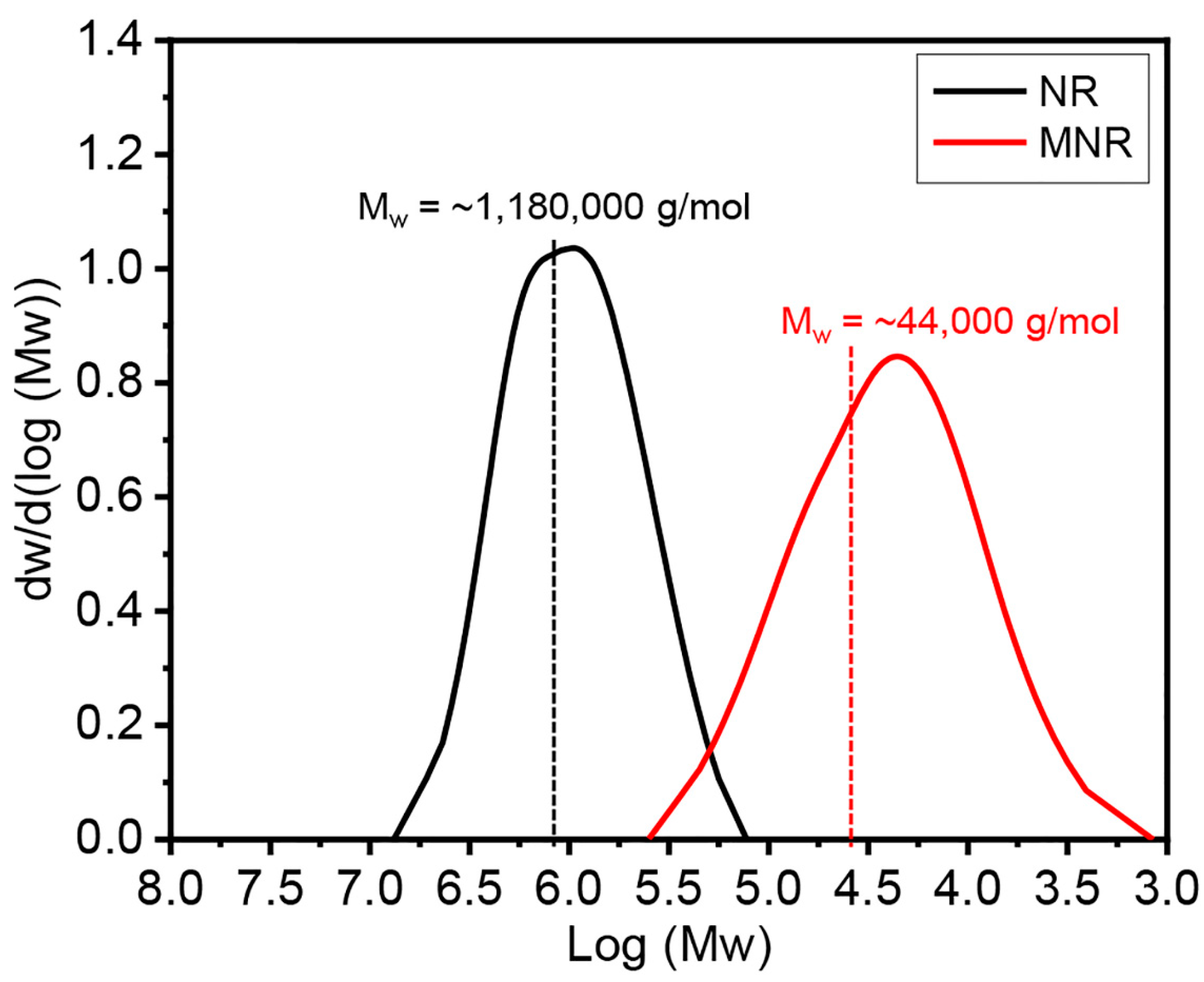
3.1. Chemical Structure, Hydroxyl Content, and Molecular Weight of Modified Natural Rubber
3.2. Properties of the Blends
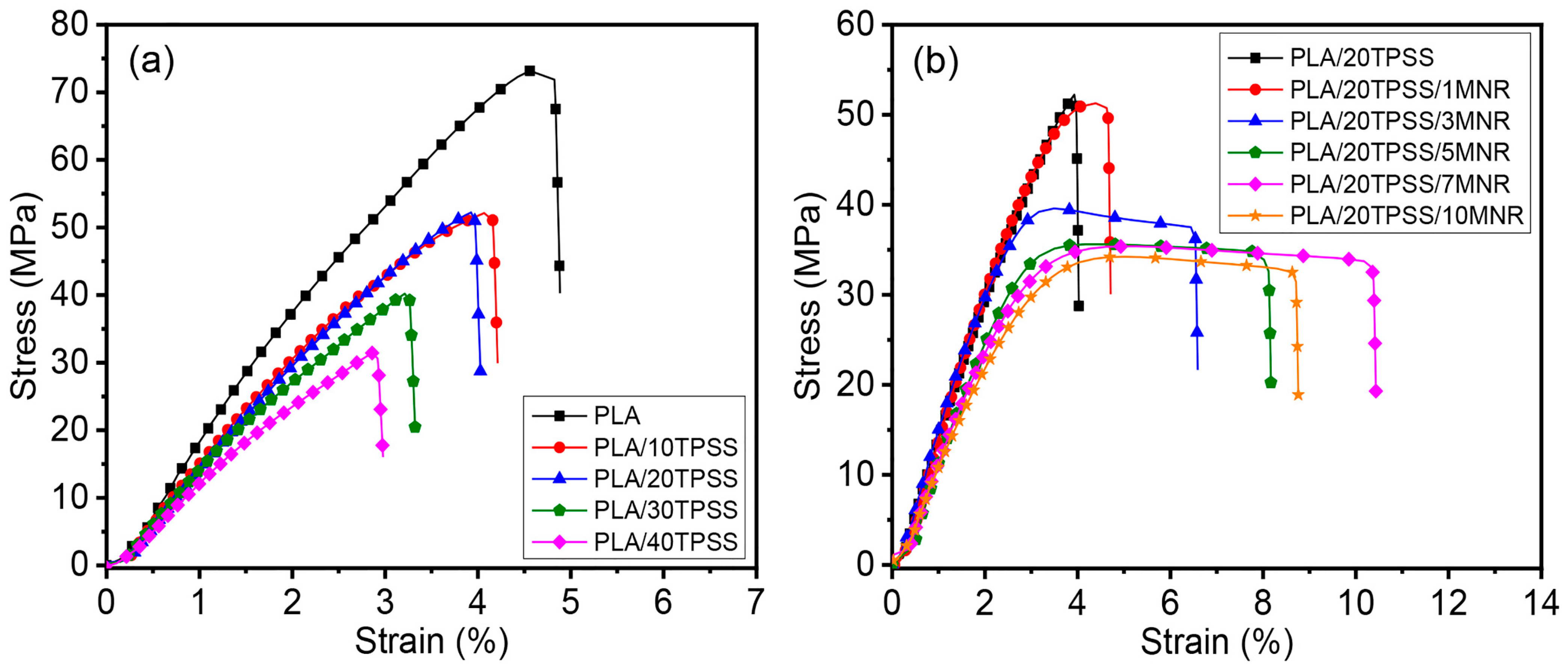
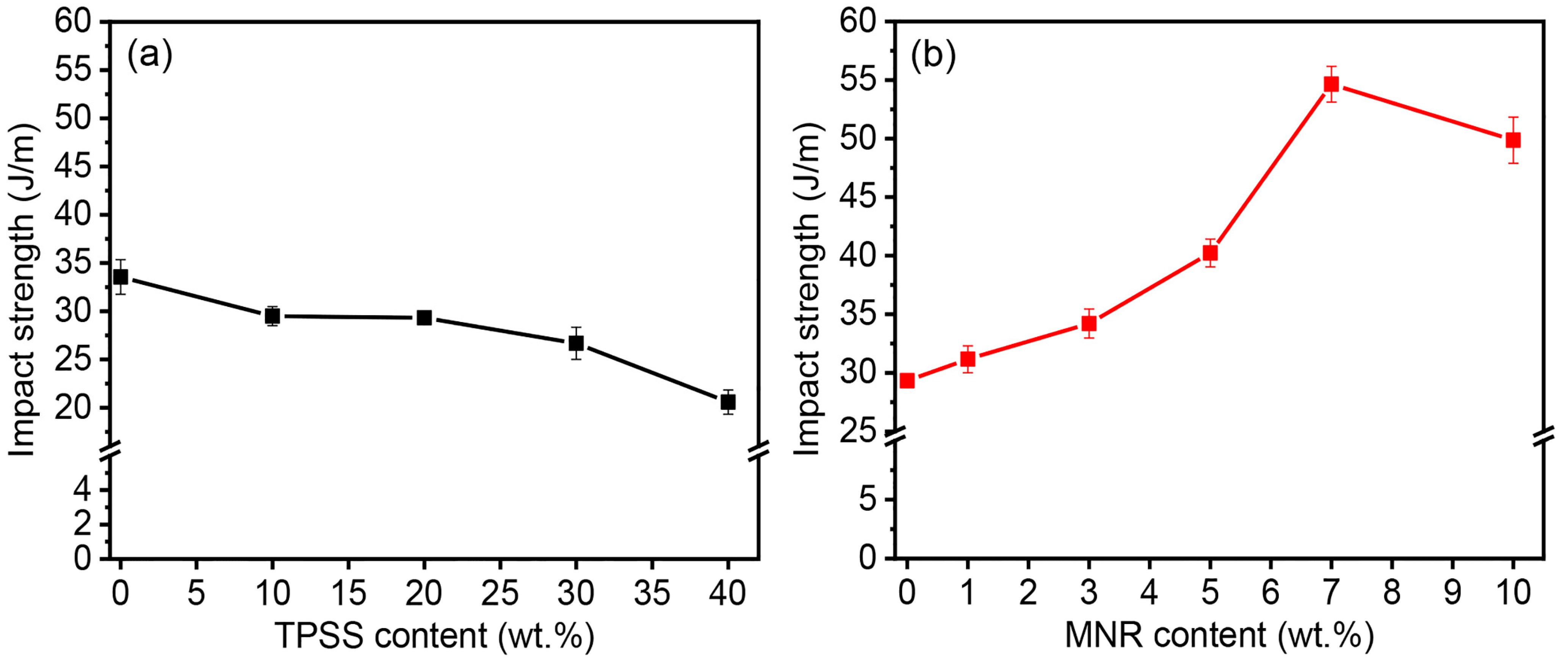
3.2.1. Mechanical Properties
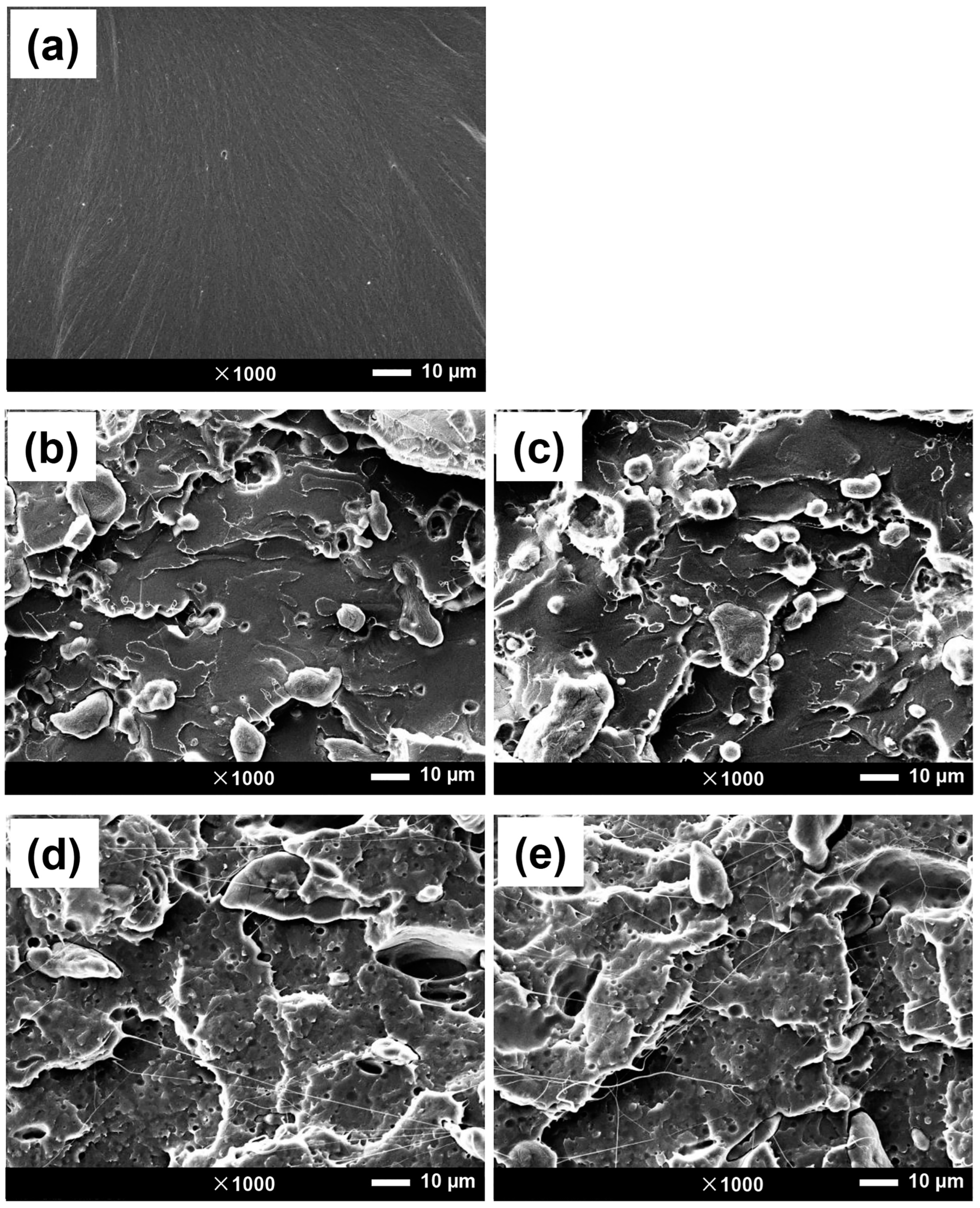
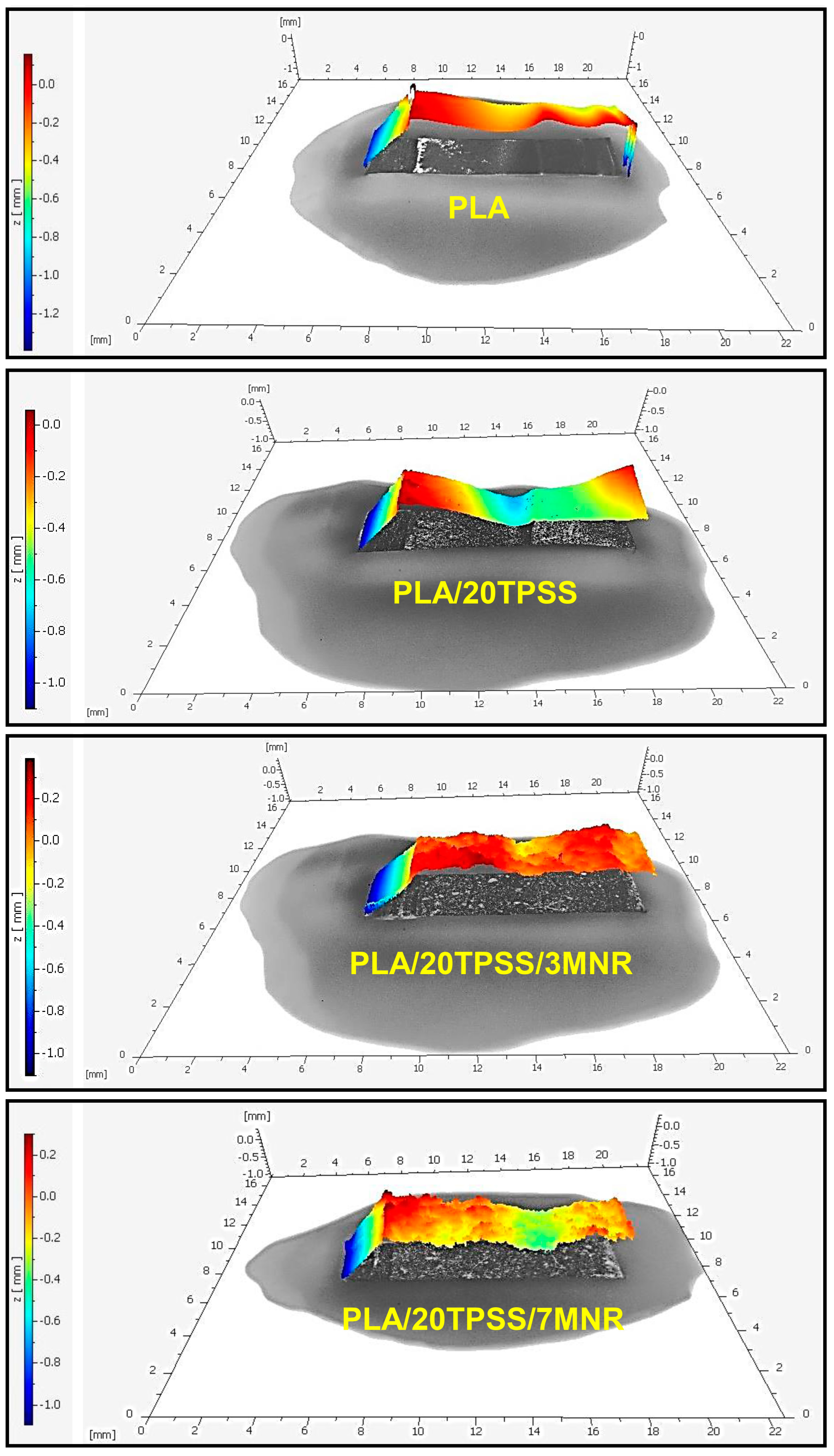
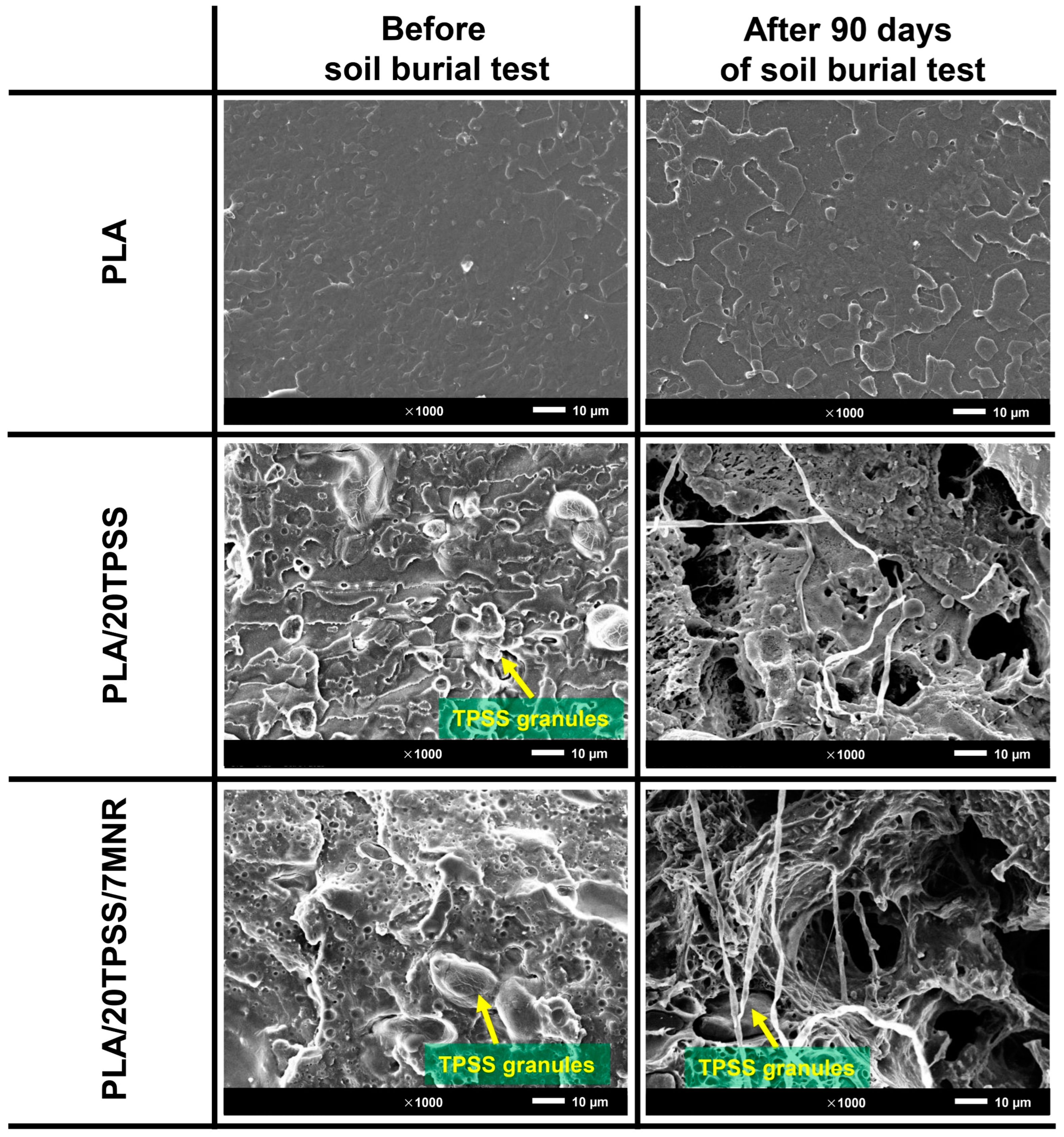
3.2.2. Morphological Aspects
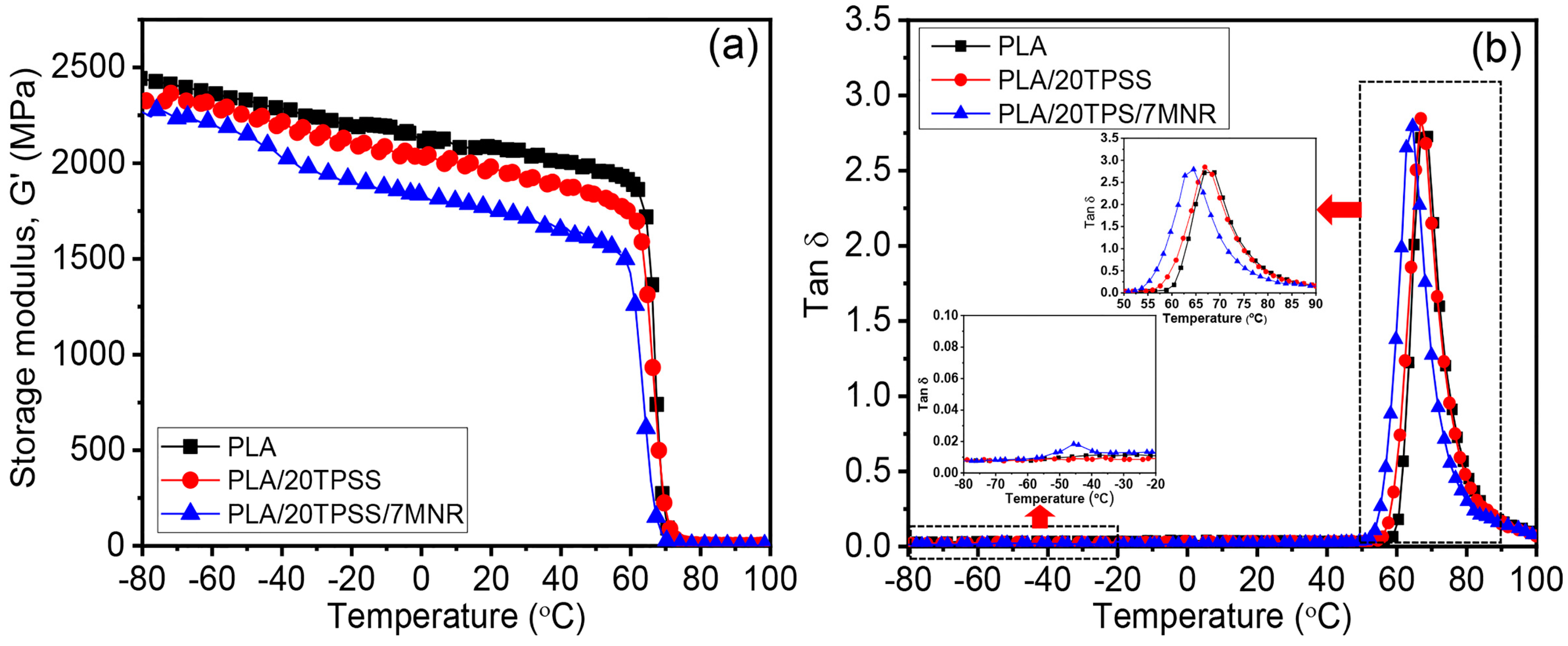
3.2.3. Dynamic Mechanical Properties
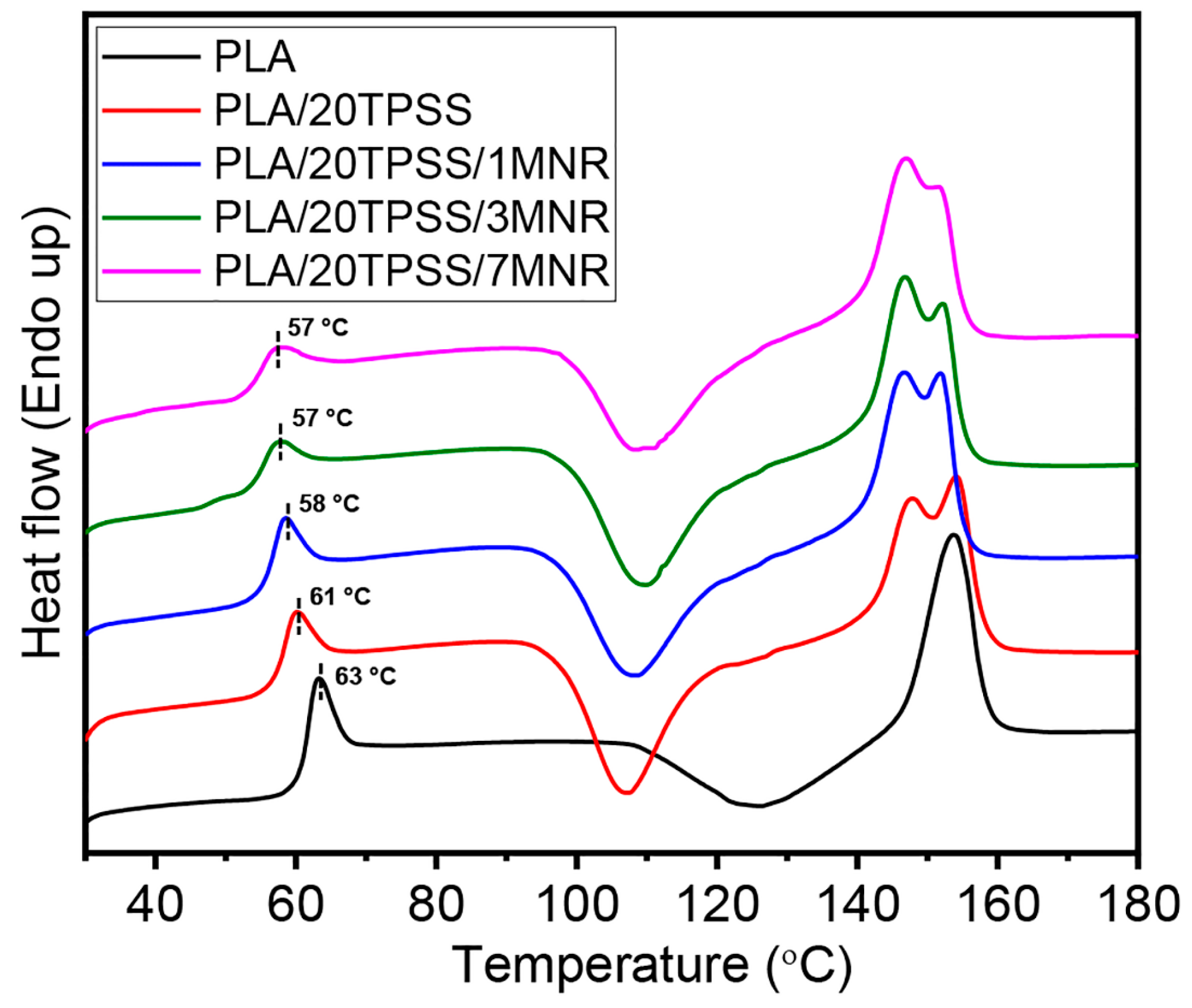
3.2.4. Thermal Properties
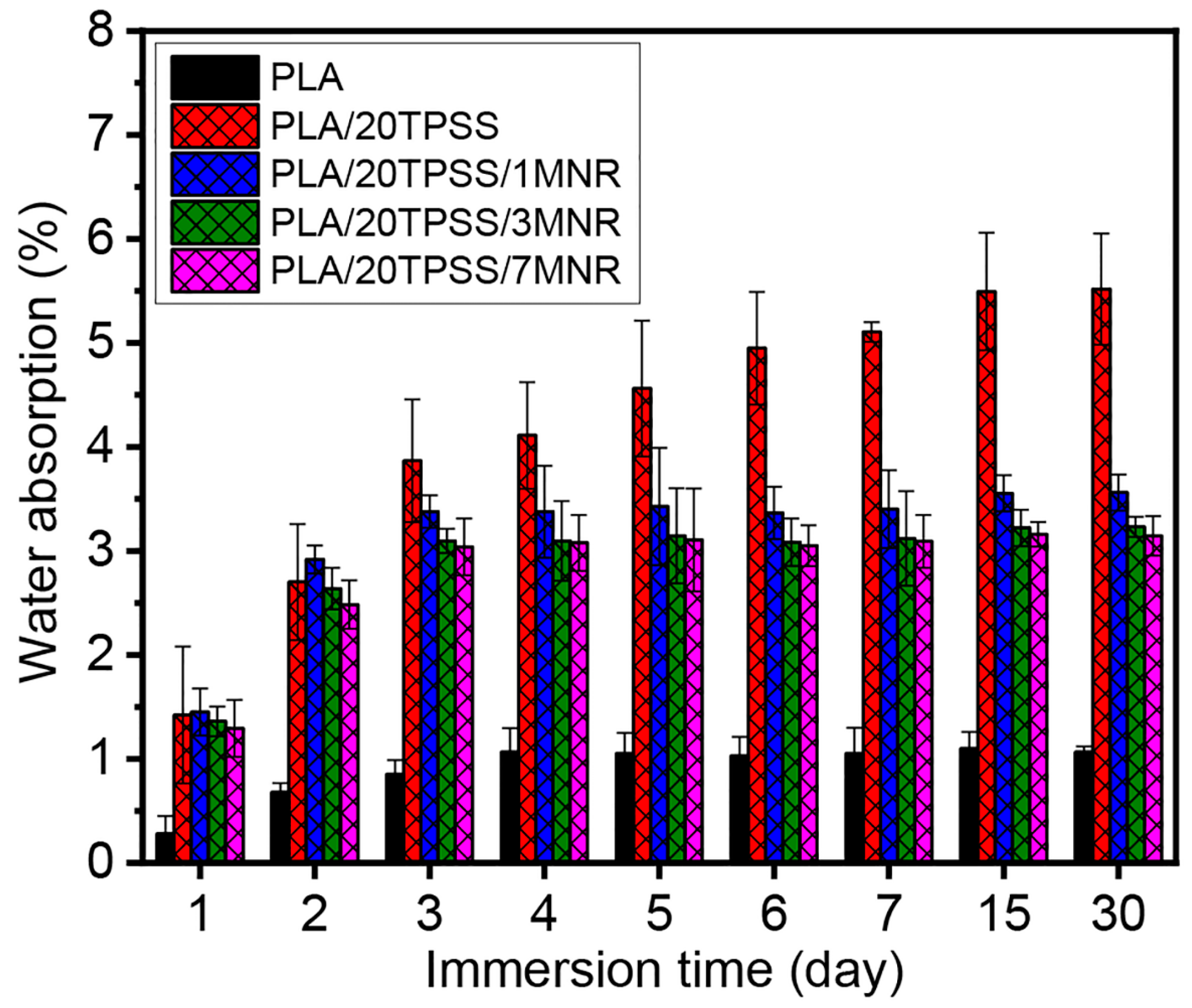
3.2.5. Water Absorption
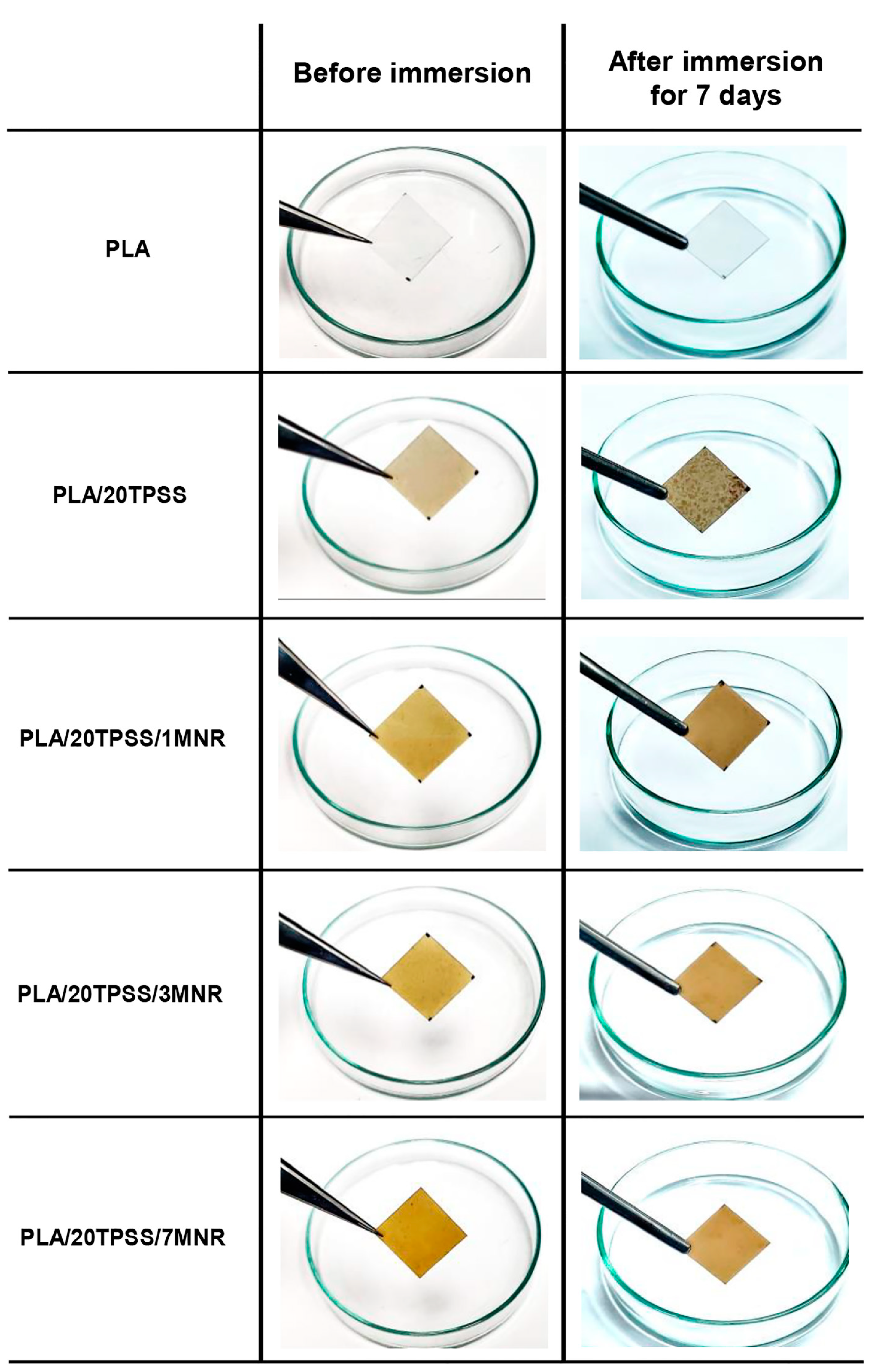
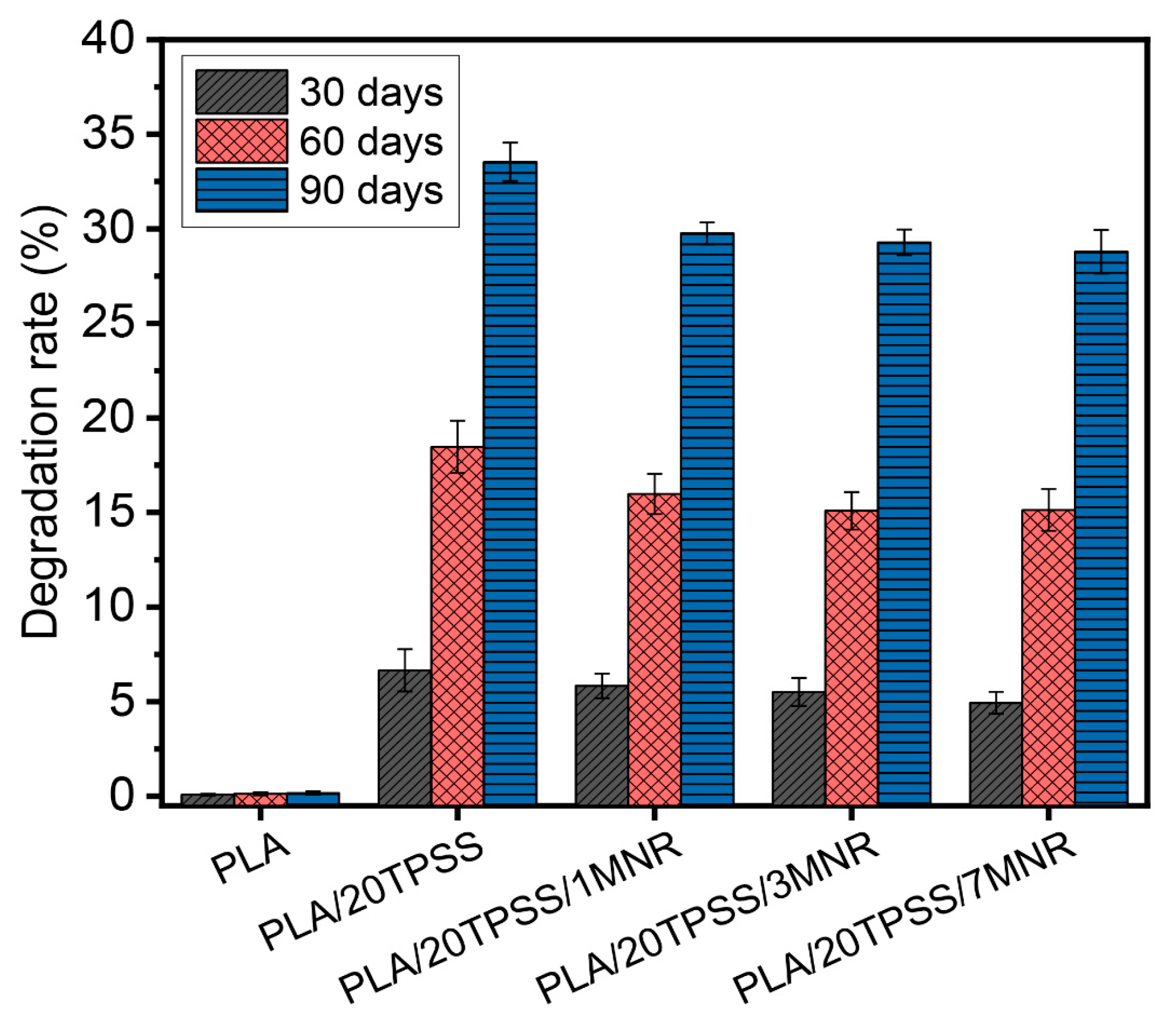
3.2.6. Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, S.; Samadhiya, A.; Kumar, A.; Majumdar, A.; Garza-Reyes, J.A.; Luthra, S. Achieving the sustainable development goals through net zero emissions: Innovation-driven strategies for transitioning from incremental to radical lean, green and digital technologies. Resour. Conserv. Recycl. 2023, 197, 107094. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.; Amaraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of starch/synthetic biodegradable polymer blends for packaging applications: A review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
- Kumar, R.; Sadeghi, K.; Jang, J.; Seo, J. Mechanical, chemical, and bio-recycling of biodegradable plastics: A review. Sci. Total Environ. 2023, 882, 163446. [Google Scholar] [CrossRef]
- Cakmak, O.K. Biodegradable polymers—A review on properties, processing, and degradation mechanism. Circ. Econ. Sust. 2023. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.W.; Huber, G.W. A review of biodegradable plastics: Chemistry, applications, properties, and future research needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical review on polylactic acid: Properties, structure, processing, biocomposites, and nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef] [PubMed]
- Tessanan, W.; Phinyocheep, P. Toughening modification of poly(lactic acid) using modified natural rubber. Iran. Polym. J. 2022, 31, 455–469. [Google Scholar] [CrossRef]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic starch (TPS)/polylactic acid (PLA) blending methodologies: A review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(lactic acid)-based blends: A comprehensive review. Appl. Sci. 2023, 13, 5148. [Google Scholar] [CrossRef]
- Kumar, V.; Dev, A.; Gupta, A.P. Studies of poly(lactic acid) based calcium carbonate nanocomposites. Compos. B Eng. 2014, 56, 184–188. [Google Scholar] [CrossRef]
- Infurna, G.; Botta, L.; Ingargiola, I.; Maniscalco, M.; Caputo, G.; Dintcheva, N.T. Biochar from digestate pyrolysis as a filler for biopolymer blends: Effect of blend composition. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Petinakis, E.; Liu, X.; Yu, L.; Way, C.; Sangwan, P.; Dean, K.; Bateman, S.; Edward, G. Biodegradation and thermal decomposition of poly(lactic acid)-based materials reinforced by hydrophilic fillers. Polym. Degrad. Stab. 2010, 95, 1704–1707. [Google Scholar] [CrossRef]
- Ruz-Cruz, M.A.; Herrera-Franco, P.J.; Flores-Johnson, E.A.; Moreno-Chulim, M.V.; Galera-Manzano, L.M.; Valadez-González, A. Thermal and mechanical properties of PLA-based multiscale cellulosic biocomposites. J. Mater. Res. Technol. 2022, 18, 485–495. [Google Scholar] [CrossRef]
- Banerjee, A.; Jha, K.; Petru, M.; Kumar, R.; Sharma, S.; Saini, M.S.; Mohammed, K.A.; Kumar, A.; Abbas, M.; Tag-Eldin, E.M. Fabrication and characterization of weld attributes in hot gas welding of alkali treated hybrid flax fiber and pine cone fibers reinforced poly-lactic acid (PLA) based biodegradable polymer composites: Studies on mechanical and morphological properties. J. Mater. Res. Technol. 2023, 27, 272–297. [Google Scholar] [CrossRef]
- Rahmadiawan, D.; Abral, H.; Yesa, W.H.; Handayani, D.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. White Ginger Nanocellulose as Effective Reinforcement and Antimicrobial Polyvinyl Alcohol/ZnO Hybrid Biocomposite Films Additive for Food Packaging Applications. J. Compos. Sci. 2022, 6, 316. [Google Scholar] [CrossRef]
- Rahmadiawan, D.; Abral, H.; Ilham, M.K.; Puspitasari, P.; Nabawi, R.A.; Shi, S.-C.; Sugiarti, E.; Muslimin, A.N.; Chandra, D.; Ilyas, R.A.; et al. Enhanced UV blocking, tensile and thermal properties of bendable TEMPO-oxidized bacterial cellulose powder-based films immersed in PVA/Uncaria gambir/ZnO solution. J. Mater. Res. Technol. 2023, 26, 5566–5575. [Google Scholar] [CrossRef]
- Nagy, B.; Török, F.; Tomasek, S.; Miskolczi, N. Vegetable oil based additives to enhance the properties of PLA/starch composites: The effect of reaction parameters. Ind. Crops. Prod. 2023, 191, 116025. [Google Scholar] [CrossRef]
- Tessanan, W.; Phinyocheep, P. Natural rubber-based mechanical modifiers for poly(lactic acid). Int. J. Sci. Innov. Technol. 2020, 3, 86–94. [Google Scholar]
- Fekete, I.; Ronkay, F.; Lendvai, L. Highly toughened blends of poly(lactic acid) (PLA) and natural rubber (NR) for FDM-based 3D printing applications: The effect of composition and infill pattern. Polym. Test. 2021, 99, 107205. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M. Mechanical, physical and thermal properties of sugar palm nanocellulose reinforced thermoplastic starch (TPS)/poly (lactic Acid) (PLA) blend bionanocomposites. Polymers 2020, 12, 2216. [Google Scholar] [CrossRef]
- Fonseca-García, A.; Osorio, B.H.; Aguirre-Loredo, R.Y.; Calambas, H.L.; Caicedo, C. Miscibility study of thermoplastic starch/polylactic acid blends: Thermal and superficial properties. Carbohydr. Polym. 2022, 293, 119744. [Google Scholar] [CrossRef] [PubMed]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Awale, R.J.; Ali, F.B.; Azmi, A.S.; Puad, N.I.M.; Anuar, H.; Hassan, A. Enhanced flexibility of biodegradable polylactic acid/starch blends using epoxidized palm oil as plasticizer. Polymers 2018, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Ghari, H.S.; Nazockdast, H. Morphology development and mechanical properties of PLA/differently plasticized starch (TPS) binary blends in comparison with PLA/dynamically crosslinked “TPS+EVA” ternary blends. Polymer 2022, 245, 124729. [Google Scholar] [CrossRef]
- Wu, C.S. Improving polylactide/starch biocomposites by grafting polylactide with acrylic acid—Characterization and biodegradability assessment. Macromol. Biosci. 2005, 5, 352–361. [Google Scholar] [CrossRef]
- Carmona, V.B.; Corrêa, A.C.; Marconcini, J.M.; Mattoso, L.H.C. Properties of a biodegradable ternary blend of thermoplastic starch (TPS), poly(ε-caprolactone) (PCL) and poly(lactic acid) (PLA). J. Polym. Environ. 2015, 23, 83–89. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, C.; Gao, L.; Jiao, L.; Xu, H.; Guo, W. Physical and degradation properties of binary or ternary blends composed of poly (lactic acid), thermoplastic starch and GMA grafted POE. Polym. Degrad. Stab. 2011, 96, 175–182. [Google Scholar] [CrossRef]
- Zhao, X.; Pelfrey, A.; Pellicciotti, A.; Koelling, K.; Vodovotz, Y. Synergistic effects of chain extenders and natural rubber on PLA thermal, rheological, mechanical and barrier properties. Polymer 2023, 269, 125712. [Google Scholar] [CrossRef]
- Phinyocheep, P. 3-Chemical modification of natural rubber (NR) for improved performance. In Chemistry, Manufacture and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2014; pp. 68–118. [Google Scholar]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Varyan, I.; Kolesnikova, N.; Xu, H.; Tyubaeva, P.; Popov, A. Biodegradability of Polyolefin-Based Compositions: Effect of Natural Rubber. Polymers 2022, 14, 530. [Google Scholar] [CrossRef]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crops. Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Tessanan, W.; Chanthateyanonth, R.; Yamaguchi, M.; Phinyocheep, P. Improvement of mechanical and impact performance of poly(lactic acid) by renewable modified natural rubber. J. Clean. Prod. 2020, 276, 123800. [Google Scholar] [CrossRef]
- ASTM D4274; Standard Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2021.
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2018.
- ASTM D256; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2023.
- ASTM D2240; Standard Test Method for Rubber Property—Durometer Hardness. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2021.
- Maroufkhani, M.; Katbab, A.; Liu, W.; Zhang, J. Polylactide (PLA) and acrylonitrile butadiene rubber (NBR) blends: The effect of ACN content on morphology, compatibility and mechanical properties. Polymer 2017, 115, 37–44. [Google Scholar] [CrossRef]
- Namphonsane, A.; Suwannachat, P.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M.; Amornsakchai, T. Toward a circular bioeconomy: Exploring pineapple stem starch film as a plastic substitute in single use applications. Membranes 2023, 13, 458. [Google Scholar] [CrossRef] [PubMed]
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Tessanan, W.; Daniel, P.; Phinyocheep, P. Mechanical properties’ strengthening of photosensitive 3D resin in lithography technology using acrylated natural rubber. Polymers 2023, 15, 4110. [Google Scholar] [CrossRef]
- Ibrahim, S.; Othman, N.; Baratha Nesan, K.V.; Mohd Rasdi, F.R. Photocatalytic degradation of epoxidized natural rubber latex using hydrogen peroxide and TiO2 nanocrystal. Iran. Polym. J. 2022, 31, 741–750. [Google Scholar] [CrossRef]
- Phetthong, C.; Nakaramontri, Y.; Marthosa, S.; Anancharoenwong, E. Influence of zirconium(IV) in polyurethane based on hydroxyl telechelic natural rubber for coating application. J. Coat. Technol. Res. 2021, 18, 1095–1107. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Müller, P.; Bere, J.; Fekete, E.; Móczó, J.; Nagy, B.; Kállay, M.; Gyarmati, B.; Pukánszky, B. Interactions, structure and properties in PLA/plasticized starch blends. Polymer 2016, 103, 9–18. [Google Scholar] [CrossRef]
- Zhang, C.; Man, C.; Pan, Y.; Wang, W.; Jiang, L.; Dan, Y. Toughening of polylactide with natural rubber grafted with poly(butyl acrylate). Polym. Int. 2011, 60, 1548–1555. [Google Scholar] [CrossRef]
- Collyer, A.A. Rubber Toughened Engineering Plastics; Springer: Dordrecht, The Netherlands, 1994; p. 366. [Google Scholar]
- Wang, Y.; Chen, K.; Xu, C.; Chen, Y. Supertoughened biobased poly(lactic acid)–epoxidized natural rubber thermoplastic vulcanizates: Fabrication, co-continuous phase structure, interfacial in situ compatibilization, and toughening mechanism. J. Phys. Chem. B 2015, 119, 12138–12146. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qiang, T. Fracture surface morphology and impact strength of cellulose/PLA composites. Materials 2017, 10, 624. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Huang, Y.; Pan, Y.; Jiang, L.; Dan, Y.; Luo, Y.; Peng, Z. Thermal, mechanical and rheological properties of polylactide toughened by expoxidized natural rubber. Mater. Des. 2013, 45, 198–205. [Google Scholar] [CrossRef]
- Kalogeras, I.M.; Brostow, W. Glass transition temperatures in binary polymer blends. J. Polym. Sci. B Polym. Phys. 2009, 47, 80–95. [Google Scholar] [CrossRef]
- Si, W.-J.; Yuan, W.-Q.; Li, Y.-D.; Chen, Y.-K.; Zeng, J.-B. Tailoring toughness of fully biobased poly(lactic acid)/natural rubber blends through dynamic vulcanization. Polym. Test. 2018, 65, 249–255. [Google Scholar] [CrossRef]
- Tábi, T.; Sajó, I.E.; Szabó, F.J.; Luyt, A.S.; Kovács, J.G. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Seib, P. Strengthening blends of poly(lactic acid) and starch with methylenediphenyl diisocyanate. J. Appl. Polym. Sci. 2001, 82, 1761–1767. [Google Scholar] [CrossRef]
- Yew, G.H.; Mohd Yusof, A.M.; Mohd Ishak, Z.A.; Ishiaku, U.S. Water absorption and enzymatic degradation of poly(lactic acid)/rice starch composites. Polym. Degrad. Stab. 2005, 90, 488–500. [Google Scholar] [CrossRef]
- Leu, Y.Y.; Chow, W.S. Kinetics of water absorption and thermal properties of poly(lactic acid)/organomontmorillonite/poly(ethylene glycol) nanocomposites. J. Vinyl Addit. Technol. 2011, 17, 40–47. [Google Scholar] [CrossRef]
- Mayekar, P.C.; Limsukon, W.; Bher, A.; Auras, R. Breaking it down: How thermoplastic starch enhances poly(lactic acid) biodegradation in compost─A comparative analysis of reactive blends. ACS Sustain. Chem. Eng. 2023, 11, 9729–9737. [Google Scholar] [CrossRef]
| Sample Code | PLA (wt.%) | TPSS (wt.%) | MNR (wt.%) |
|---|---|---|---|
| PLA | 100 | 0 | 0 |
| PLA/10TPSS | 100 | 10 | - |
| PLA/20TPSS | 100 | 20 | - |
| PLA/30TPSS | 100 | 30 | - |
| PLA/40TPSS | 100 | 40 | - |
| PLA/20TPSS/1MNR | 100 | 20 | 1 |
| PLA/20TPSS/3MNR | 100 | 20 | 3 |
| PLA/20TPSS/5MNR | 100 | 20 | 5 |
| PLA/20TPSS/7MNR | 100 | 20 | 7 |
| PLA/20TPSS/10MNR | 100 | 20 | 10 |
| Sample Code | Tensile Properties | Impact Strength (J/m) | Hardness (Shore D) | ||
|---|---|---|---|---|---|
| E * (GPa) | σ ** (MPa) | ε ***(%) | |||
| PLA | 2.1 ± 0.1 | 73.18 ± 4.37 | 4.84 ± 0.35 | 33.55 ± 1.80 | 80 ± 1 |
| PLA/10TPSS | 1.8 ± 0.2 | 52.82 ± 3.86 | 4.25 ± 0.44 | 29.49 ± 0.99 | 81 ± 1 |
| PLA/20TPSS | 1.7 ± 0.2 | 52.49 ± 3.66 | 4.12 ± 0.29 | 29.32 ± 0.46 | 82 ± 1 |
| PLA/30TPSS | 1.7 ± 0.1 | 40.63 ± 4.79 | 3.28 ± 0.32 | 26.67 ± 1.67 | 84 ± 2 |
| PLA/40TPSS | 1.4 ± 0.2 | 31.58 ± 3.14 | 2.87 ± 0.31 | 20.58 ± 1.26 | 84 ± 2 |
| PLA/20TPSS/1MNR | 1.7 ± 0.2 | 51.29 ± 3.66 | 4.34 ± 0.26 | 31.16 ± 1.15 | 78 ± 1 |
| PLA/20TPSS/3MNR | 1.7 ± 0.2 | 37.51 ± 2.37 | 6.44 ± 0.43 | 34.21 ± 1.25 | 75 ± 2 |
| PLA/20TPSS/5MNR | 1.6 ± 0.1 | 33.88 ± 1.29 | 8.01 ± 0.49 | 40.23 ± 1.18 | 74 ± 2 |
| PLA/20TPSS/7MNR | 1.6 ± 0.2 | 33.11 ± 1.07 | 10.27 ± 0.47 | 54.65 ± 1.53 | 72 ± 2 |
| PLA/20TPSS/10MNR | 1.3 ± 0.2 | 32.05 ± 1.43 | 8.82 ± 0.46 | 49.86 ± 1.97 | 69 ± 2 |
| Sample Code | Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| PLA | 62.7 | 126.4 | 11.5 | 153.8 | 17.76 | 7 |
| PLA/10TPSS | 61.1 | 108.8 | 19.5 | 148.1, 154.0 | 22.95 | 4 |
| PLA/20TPSS | 61.0 | 108.9 | 19.3 | 146.8, 153.1 | 22.47 | 4 |
| PLA/30TPSS | 61.0 | 108.2 | 19.2 | 146.4, 152.8 | 22.09 | 4 |
| PLA/40TPSS | 61.1 | 109.9 | 17.3 | 146.3, 152.0 | 19.15 | 3 |
| PLA/20TPSS/1MNR | 58.2 | 109.2 | 19.1 | 146.5, 152.6 | 22.83 | 5 |
| PLA/20TPSS/3MNR | 58.2 | 110.4 | 20.2 | 146.5, 151.9 | 23.54 | 4 |
| PLA/20TPSS/5MNR | 57.4 | 110.7 | 21.2 | 146.1, 152.1 | 23.04 | 4 |
| PLA/20TPSS/7MNR | 57.4 | 110.7 | 19.7 | 146.7, 151.7 | 22.59 | 4 |
| PLA/20TPSS/10MNR | 57.3 | 110.2 | 19.6 | 146.6, 152.1 | 22.17 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessanan, W.; Phinyocheep, P.; Amornsakchai, T. Sustainable Materials with Improved Biodegradability and Toughness from Blends of Poly(Lactic Acid), Pineapple Stem Starch and Modified Natural Rubber. Polymers 2024, 16, 232. https://doi.org/10.3390/polym16020232
Tessanan W, Phinyocheep P, Amornsakchai T. Sustainable Materials with Improved Biodegradability and Toughness from Blends of Poly(Lactic Acid), Pineapple Stem Starch and Modified Natural Rubber. Polymers. 2024; 16(2):232. https://doi.org/10.3390/polym16020232
Chicago/Turabian StyleTessanan, Wasan, Pranee Phinyocheep, and Taweechai Amornsakchai. 2024. "Sustainable Materials with Improved Biodegradability and Toughness from Blends of Poly(Lactic Acid), Pineapple Stem Starch and Modified Natural Rubber" Polymers 16, no. 2: 232. https://doi.org/10.3390/polym16020232
APA StyleTessanan, W., Phinyocheep, P., & Amornsakchai, T. (2024). Sustainable Materials with Improved Biodegradability and Toughness from Blends of Poly(Lactic Acid), Pineapple Stem Starch and Modified Natural Rubber. Polymers, 16(2), 232. https://doi.org/10.3390/polym16020232