Oxygen-Containing Quaternary Phosphonium Salts (oxy-QPSs): Synthesis, Properties, and Cellulose Dissolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumental
2.2. Materials
2.3. General Procedure of the Synthesis of oxy-QPSs
2.4. Screening Dissolution of Microcrystalline Cellulose in oxy-QPSs
2.5. Antimicrobial and Antifungal Activity of oxy-QPSs
2.6. Toxicity of oxy-QPSs
2.6.1. Hemolytic Activity
2.6.2. Cell Toxicity Assay (MTT Assay)
2.6.3. Ecotoxicity to D. magna
3. Results and Discussion
3.1. Synthesis of oxy-QPSs
3.2. NMR Study
3.2.1. 31P NMR Spectral Data
3.2.2. 1H NMR Spectral Data
3.2.3. 13C NMR Spectral Data
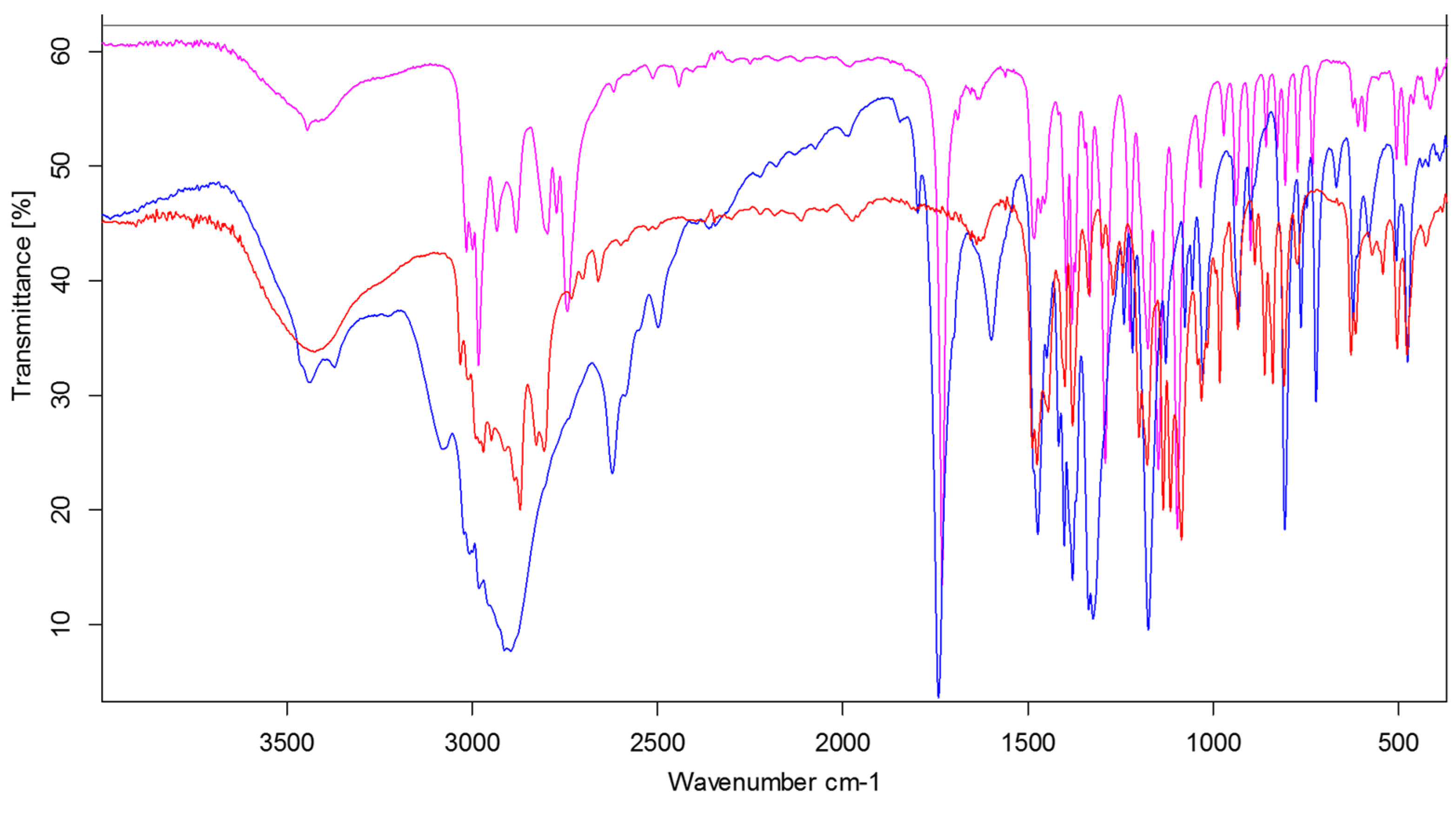
3.3. IR
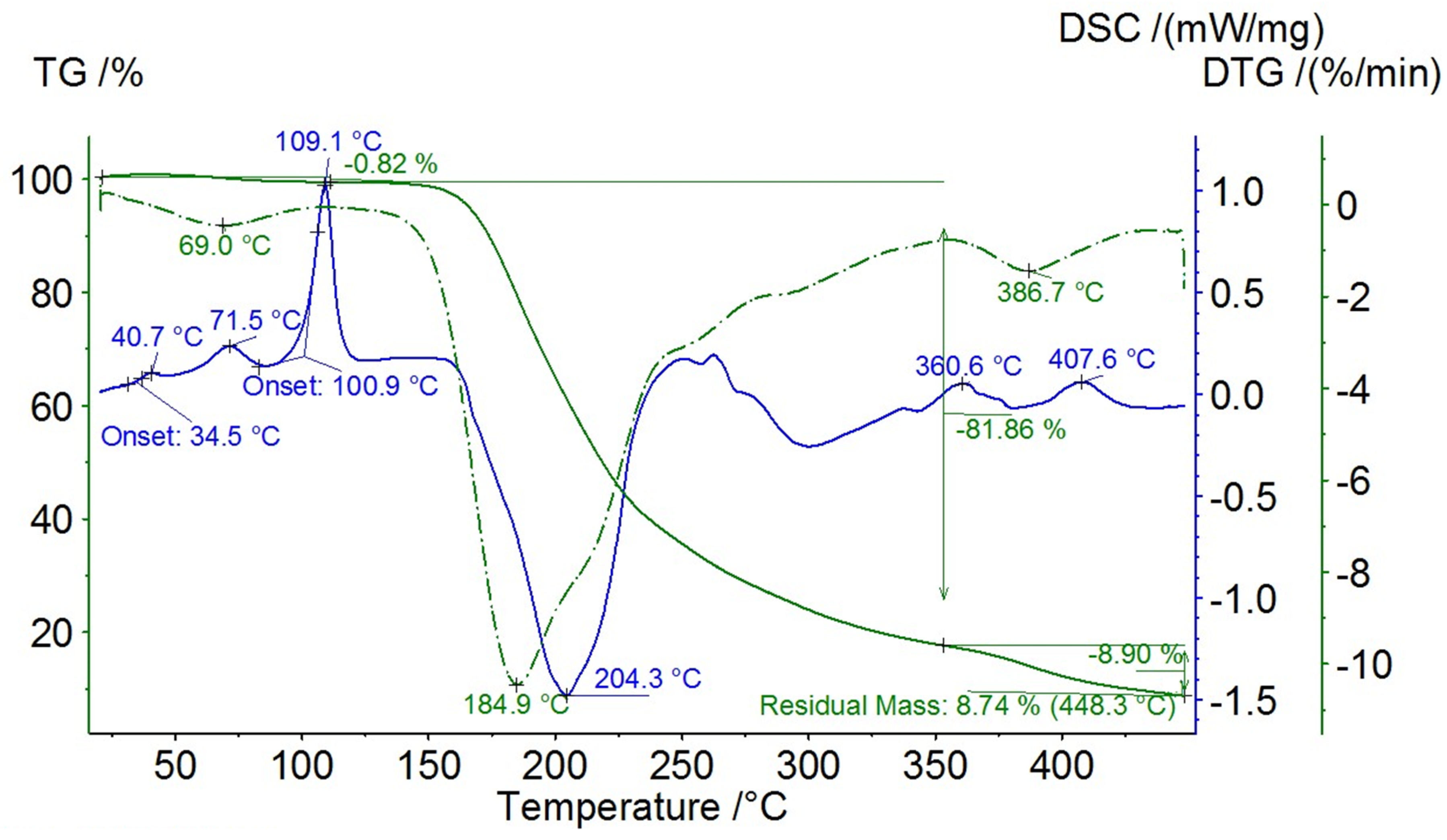
3.4. DSC Measurements and TG Analysis
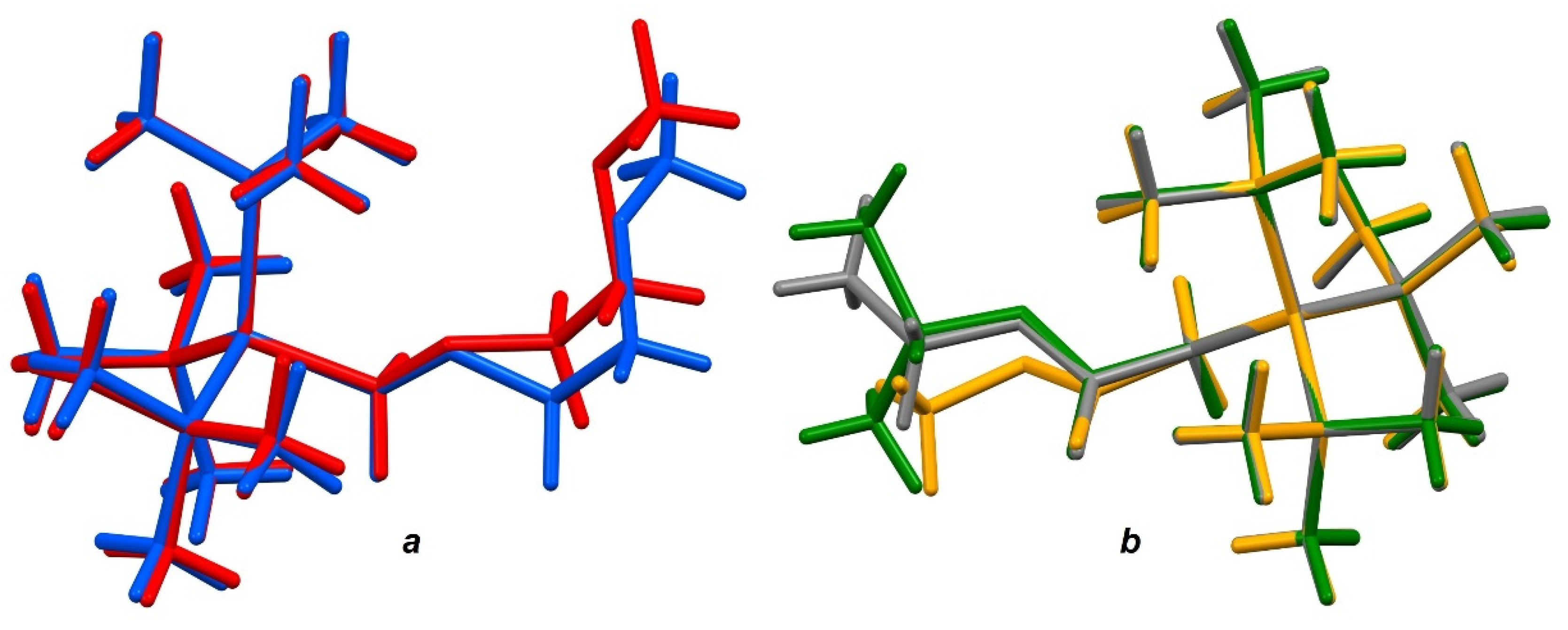
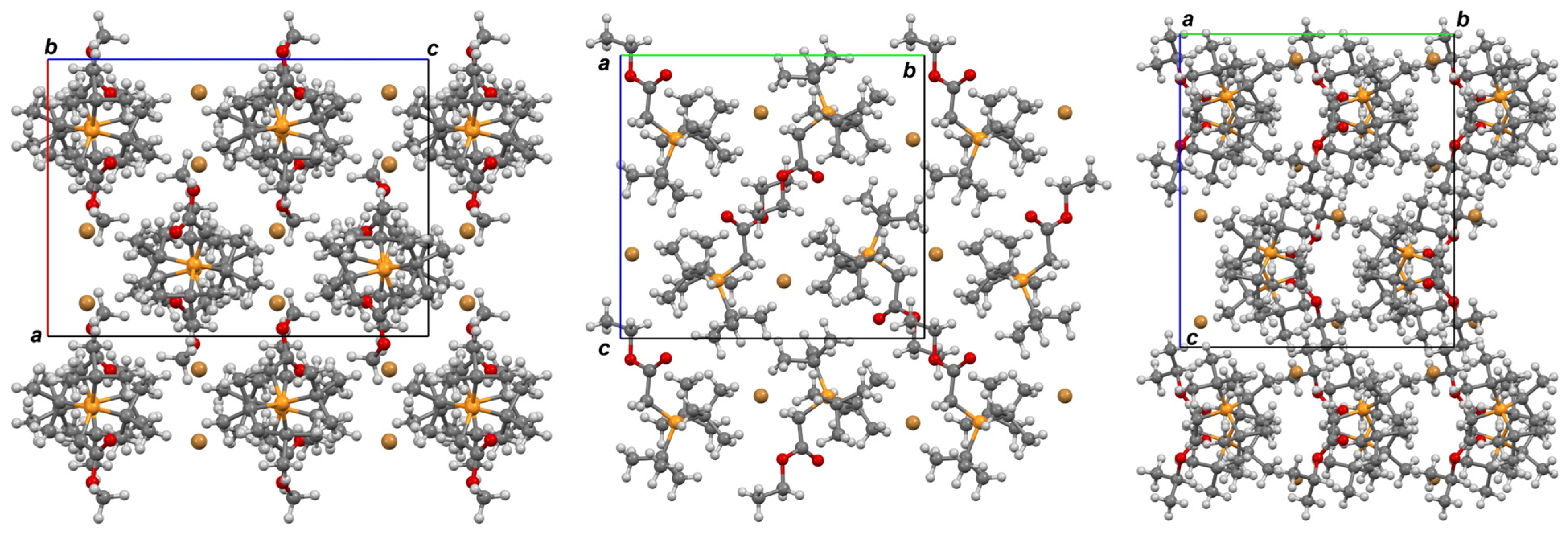
3.5. Single Crystal X-ray Analysis
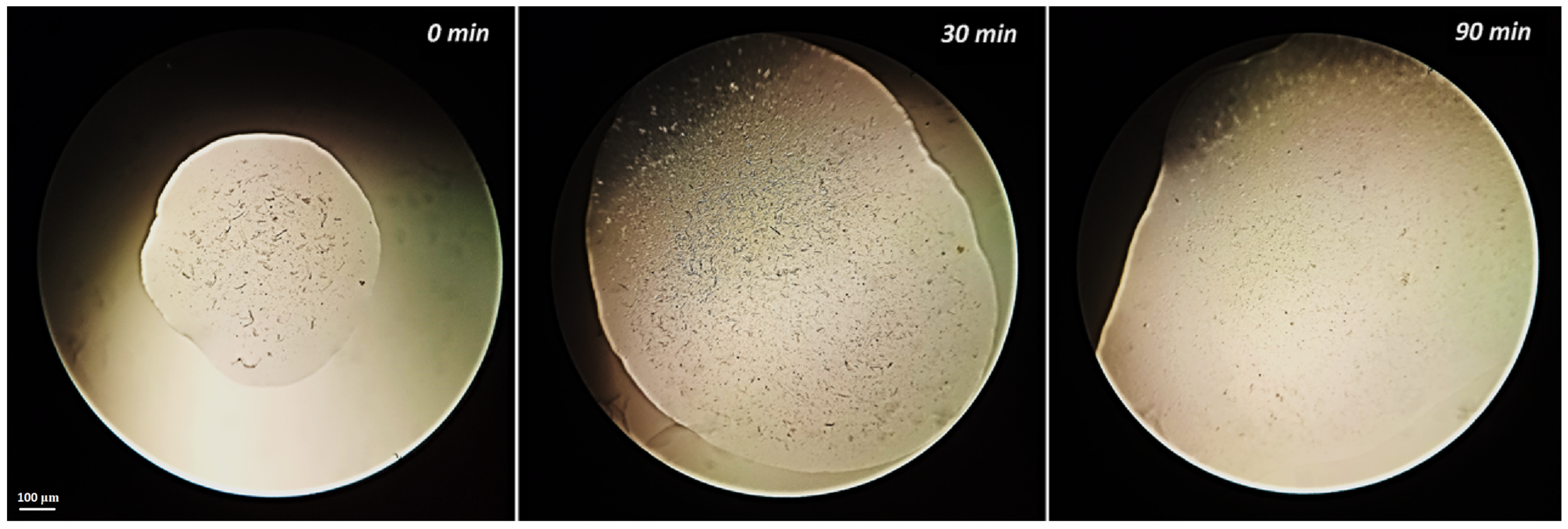
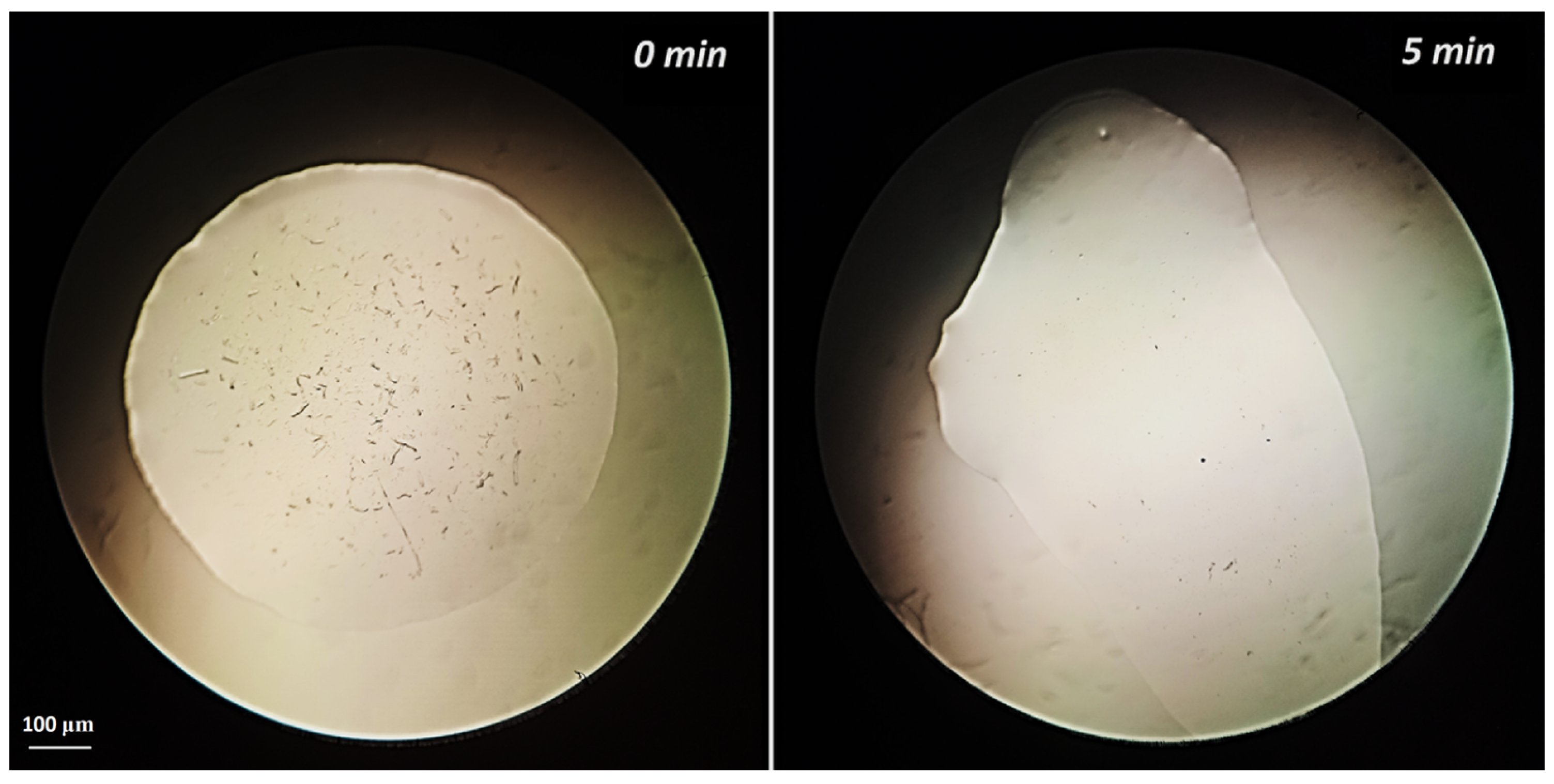
3.6. Microcrystalline Cellulose Dissolution
3.7. Antimicrobial Activity of oxy-QPSs
3.8. Cytotoxicity of oxy-QPSs
3.9. Ecotoxicity of oxy-QPSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Esperança, J.; Canongia Lopes, J.N.; Tariq, M.; Santos, L.M.; Magee, J.W.; Rebelo, L.P.N. Volatility of Aprotic Ionic Liquids A Review. J. Chem. Eng. Data 2010, 55, 3–12. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Samoylenko, D.E.; Lotsman, K.A.; Rodygin, K.S.; Ananikov, V.P. Metal nanoparticles in ionic liquids: Synthesis and catalytic applications. Coord. Chem. Rev. 2021, 445, 213982. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Saverina, E.A.; Frolov, N.A.; Kamanina, O.A.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. From Antibacterial to Antibiofilm Targeting: An Emerging Paradigm Shift in the Development of Quaternary Ammonium Compounds (QACs). ACS Infect. Dis. 2023, 9, 394–422. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Aremu, B.R.; Dehghandokht, S.; Salama, R.; Zhou, H.; Lackie, S.; Seifi, M.; Kennepohl, P.; Trant, J. Lethal weapon IL: A nano-copper/tetraalkylphosphonium ionic liquid composite material with potent antibacterial activity. RSC Sustain. 2023; accepted manuscript. [Google Scholar] [CrossRef]
- Francis, C.F.; Kyratzis, I.L.; Best, A.S. Lithium-ion battery separators for ionic-liquid electrolytes: A review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhang, Y.; Chen, Z.; Watanabe, M.; Deng, Y. Beyond solvents and electrolytes: Ionic liquids based advanced functional materials. Prog. Mater. Sci. 2016, 77, 80–124. [Google Scholar] [CrossRef]
- Giernoth, R. Task-specific ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef]
- Tang, S.; Baker, G.A.; Zhao, H. Ether-and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012, 41, 4030–4066. [Google Scholar] [CrossRef]
- Yang, J.; Chen, P.; Pan, Y.; Liang, Y. Fixation of Carbon Dioxide with Functionalized Ionic Liquids. Asian J. Org. Chem. 2022, 11, e202200527. [Google Scholar] [CrossRef]
- Wu, D.; Cai, P.; Zhao, X.; Kong, Y.; Pan, Y. Recent progress of task-specific ionic liquids in chiral resolution and extraction of biological samples and metal ions. J. Sep. Sci. 2018, 41, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Rennie, A.J.; Sanchez-Ramirez, N.D.; Torresi, R.M.; Hall, P.J. Ether-bond-containing ionic liquids as supercapacitor electrolytes. J. Phys. Chem. Lett. 2013, 4, 2970–2974. [Google Scholar] [CrossRef]
- Rauber, D.; Philippi, F.; Becker, J.; Zapp, J.; Morgenstern, B.; Kuttich, B.; Kraus, T.; Hempelmann, R.; Hunt, P.; Welton, T.; et al. Anion and ether group influence in protic guanidinium ionic liquids. Phys. Chem. Chem. Phys. 2023, 25, 6436–6453. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, D.V.; James, B.R. Tetrakis(hydroxymethyl)phosphonium salts: Their properties, hazards and toxicities. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 263–279. [Google Scholar] [CrossRef]
- Itoh, T.; Kude, K.; Hayase, S.; Kawatsura, M. Design of ionic liquids as a medium for the Grignard reaction. Tetrahedron Lett. 2007, 48, 7774–7777. [Google Scholar] [CrossRef]
- Philippi, F.; Rauber, D.; Zapp, J.; Präsang, C.; Scheschkewitz, D.; Hempelmann, R. Multiple Ether-Functionalized Phosphonium Ionic Liquids as Highly Fluid Electrolytes. ChemPhysChem 2019, 20, 443–455. [Google Scholar] [CrossRef]
- Abe, Y.; Yoshiyama, K.; Yagi, Y.; Hayase, S.; Kawatsura, M.; Itoh, T. A rational design of phosphonium salt type ionic liquids for ionic liquid coated-lipase catalyzed reaction. Green Chem. 2010, 12, 1976–1980. [Google Scholar] [CrossRef]
- Atefi, F.; Garcia, M.T.; Singer, R.D.; Scammells, P.J. Phosphonium ionic liquids: Design, synthesis and evaluation of biodegradability. Green Chem. 2009, 11, 1595–1604. [Google Scholar] [CrossRef]
- Cuthbert, T.J.; Guterman, R.; Ragogna, P.J.; Gillies, E.R. Contact active antibacterial phosphonium coatings cured with UV light. J. Mater. Chem. B 2015, 3, 1474–1478. [Google Scholar] [CrossRef]
- Cuthbert, T.J.; Hisey, B.; Harrison, T.D.; Trant, J.F.; Gillies, E.R.; Ragogna, P.J. Surprising antibacterial activity and selectivity of hydrophilic polyphosphoniums featuring sugar and hydroxy substituents. Angew. Chem. Int. Ed. 2018, 57, 12707–12710. [Google Scholar] [CrossRef]
- Glińska, K.; Gitalt, J.; Torrens, E.; Plechkova, N.; Bengoa, C. Extraction of cellulose from corn stover using designed ionic liquids with improved reusing capabilities. Process Saf. Environ. Prot. 2021, 147, 181–191. [Google Scholar] [CrossRef]
- Tao, J.; Kishimoto, T.; Hamada, M.; Nakajima, N. Novel cellulose pretreatment solvent: Phosphonium-based amino acid ionic liquid/cosolvent for enhanced enzymatic hydrolysis. Holzforschung 2016, 70, 911–917. [Google Scholar] [CrossRef]
- Mai, N.L.; Koo, Y.-M. Computer-Aided Design of Ionic Liquids for High Cellulose Dissolution. ACS Sustain. Chem. Eng. 2016, 4, 541–547. [Google Scholar] [CrossRef]
- Nguyen, H.V.T.; Faheem, A.B.; Kim, J.; Lee, J.; Jin, Q.; Koo, B.; Kim, W.; Lee, K.-K. Size Effect of Electrolyte Ions on the Electric Double-Layer Structure and Supercapacitive Behavior. ACS Appl. Energy Mater. 2023, 6, 3155–3166. [Google Scholar] [CrossRef]
- Phromphithak, S.; Onsree, T.; Tippayawong, N. Machine learning prediction of cellulose-rich materials from biomass pretreatment with ionic liquid solvents. Bioresour. Technol. 2021, 323, 124642. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, D.; Ermolaev, V.; Miluykov, V.; Nigmatullina, L.; Sinyashin, O. Functionalized Phosphonium Ionic Liquids: Synthesis and Application. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 899–901. [Google Scholar] [CrossRef]
- Arkhipova, D.M.; Ermolaev, V.V.; Miluykov, V.A.; Gubaidullin, A.T.; Islamov, D.R.; Kataeva, O.N.; Ananikov, V.P. Sterically Hindered Phosphonium Salts: Structure, Properties and Palladium Nanoparticle Stabilization. Nanomaterials 2020, 10, 2457. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, D.M.; Ermolaev, V.V.; Miluykov, V.A.; Valeeva, F.G.; Gaynanova, G.A.; Zakharova, L.Y.; Minyaev, M.E.; Ananikov, V.P. Tri-tert-butyl(n-alkyl)phosphonium Ionic Liquids: Structure, Properties and Application as Hybrid Catalyst Nanomaterials. Sustainability 2021, 13, 9862. [Google Scholar] [CrossRef]
- CrysAlisPro. Rigaku Oxford Diffraction, Version 1.171.41.106a; Rigaku: Woodlands, TX, USA, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility. Tests for Bacteria that Grow Aerobically, Approved Standard, M7-A5, 6th ed.; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium-Forming Filamentous Fungi: Proposed Standard, M38-P; NCCLS: Wayne, PA, USA, 1998. [Google Scholar]
- Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Belenok, M.G.; Strobykina, I.Y.; Lyubina, A.; Gumerova, S.K.; Kataev, V.E. Antimicrobia and cytotoxic effects of ammonium derivatives of diterpenoids steviol and isosteviol. Bioorg. Med. Chem. 2021, 32, 115974. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 15 February 2023).
- ISO 6341:2012; Water Quality. Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea). Acute Toxicity Test, IDT. International Organization for Standardization: Geneva, Switzerland, 2012.
- ABNT NBR 12713:2009; Aquatic Ecotoxicology—Acute Toxicity—Test with Daphnia spp. (Cladocera, Crustacea). ABNT: Sao Paulo, Brazil, 2009.
- OECD. Test No. 202: Daphnia sp. Acute Immobilization Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 15 February 2023).
- Jin, Y.; Fang, S.; Chai, M.; Yang, L.; Hirano, S.I. Ether-functionalized trialkylimidazolium ionic liquids: Synthesis, characterization, and properties. Ind. Eng. Chem. Res. 2012, 51, 11011–11020. [Google Scholar] [CrossRef]
- Morrissey, S.; Pegot, B.; Coleman, D.; Garcia, M.T.; Ferguson, D.; Quilty, B.; Gathergood, N. Biodegradable, non-bactericidal oxygen-functionalised imidazolium esters: A step towards ‘greener’ ionic liquids. Green Chem. 2009, 11, 475–483. [Google Scholar] [CrossRef]
- Mital, D.K.; Nancarrow, P.; Ibrahim, T.H.; Jabbar, N.A.; Khamis, M.I. Ionic Liquid Melting Points: Structure–Property Analysis and New Hybrid Group Contribution Model. Ind. Eng. Chem. Res. 2022, 61, 4683–4706. [Google Scholar] [CrossRef]
- Adamová, G.; Gardas, R.L.; Nieuwenhuyzen, M.; Puga, A.V.; Rebelo, L.P.N.; Robertson, A.J.; Seddon, K.R. Alkyltributylphosphonium chloride ionic liquids: Synthesis, physicochemical properties and crystal structure. Dalton Trans. 2012, 41, 8316–8332. [Google Scholar] [CrossRef]
- Fei, Z.; Ang, W.H.; Zhao, D.; Scopelliti, R.; Zvereva, E.E.; Katsyuba, S.A.; Dyson, P.J. Revisiting Ether-Derivatized Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2007, 111, 10095–10108. [Google Scholar] [CrossRef]
- Xuan, X.-P.; Chang, L.-L.; Zhang, H.; Wang, N.; Zhao, Y. Hydrogen bonds in the crystal structure of hydrophobic and hydrophilic COOH-functionalized imidazolium ionic liquids. CrystEngComm 2014, 16, 3040–3046. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 22, 962–979. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kucherov, F.A.; Gordeev, E.G.; Kashin, A.S.; Ananikov, V.P. Three-dimensional printing with biomass-derived PEF for carbon-neutral manufacturing. Angew. Chem. Int. Ed. Engl. 2017, 56, 15931–15935. [Google Scholar] [CrossRef] [PubMed]
- Seitkalieva, M.M.; Vavina, A.V.; Posvyatenko, A.V.; Egorova, K.S.; Kashin, A.S.; Gordeev, E.G.; Strukova, E.N.; Romashov, L.V.; Ananikov, V.P. Biomass-derived ionic liquids based on a 5-HMF platform chemical: Synthesis, characterization, biological activity, and tunable interactions at the molecular level. ACS Sustain. Chem. Eng. 2021, 9, 3552–3570. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Khoo, K.S.; Tan, X.; Ooi, C.W.; Chew, K.W.; Leong, W.H.; Chai, Y.H.; Ho, S.-H.; Show, P.L. How does ionic liquid play a role in sustainability of biomass processing? J. Clean. Prod. 2021, 284, 124772. [Google Scholar] [CrossRef]
- Tadesse, H.; Luque, R. Advances on biomass pretreatment using ionic liquids: An overview. Energy Environ. Sci. 2011, 4, 3913–3929. [Google Scholar] [CrossRef]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Li, Y.; Lu, Z.; Cao, S.; Fan, M.; Huang, L.; Chen, L. Facile Cellulose Dissolution and Characterization in the Newly Synthesized 1,3-Diallyl-2-ethylimidazolium Acetate Ionic Liquid. Polymers 2017, 9, 526. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Hu, Y.; Abidi, N. Cellulose Dissolution in Ionic Liquid under Mild Conditions: Effect of Hydrolysis and Temperature. Fibers 2021, 9, 5. [Google Scholar] [CrossRef]
- Burns, F.P.; Themens, P.A.; Ghandi, K. Assessment of phosphonium ionic liquid-dimethylformamide mixtures for dissolution of cellulose. Compos. Interfaces 2014, 21, 59–73. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Setiawan, A.; Atkin, R.; Parthasarthy, R.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Progress on the pre-treatment of lignocellulosic biomass employing ionic liquids. Renew. Sustain. Energy Rev. 2019, 105, 268–292. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. Cellulose in Ionic Liquids and Alkaline Solutions: Advances in the Mechanisms of Biopolymer Dissolution and Regeneration. Polymers 2019, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Andanson, J.-M.; Bordes, E.; Devémy, J.; Leroux, F.; Pádua, A.A.H.; Costa Gomes, M.F. Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 2528. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, W.; Lee, T.S.; Singer, S.W.; Simmons, B.A.; Singh, S.; Yuan, Q.; Cheng, G. Dimethyl sulfoxide assisted ionic liquid pretreatment of switchgrass for isoprenol production. ACS Sustain. Chem. Eng. 2018, 6, 4354–4361. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Padua, A.A.H.; Costa Gomes, M.F. Thermodynamics of cellulose dissolution in an imidazolium acetate ionic liquid. Chem. Commun. 2015, 51, 4485–4487. [Google Scholar] [CrossRef]
- Hunt, P.A.; Ashworth, C.R.; Matthews, R.P. Hydrogen bonding in ionic liquids. Chem. Soc. Rev. 2015, 44, 1257–1288. [Google Scholar] [CrossRef]
- Ammer, J.; Nolte, C.; Karaghiosoff, K.; Thallmair, S.; Mayer, P.; de Vivie-Riedle, R.; Mayr, H. Ion-Pairing of Phosphonium Salts in Solution: C-H⋅⋅⋅Halogen and C-H⋅⋅⋅π Hydrogen Bond. Chem. Eur. J. 2013, 19, 14612–14630. [Google Scholar] [CrossRef]
- Ermolaev, V.V.; Arkhipova, D.M.; Miluykov, V.A.; Lyubina, A.P.; Amerhanova, S.K.; Kulik, N.V.; Ananikov, V.P. Sterically hindered quaternary phosphonium salts (QPSs): Antimicrobial activity and hemolytic and cytotoxic properties. Int. J. Mol. Sci. 2021, 23, 86. [Google Scholar] [CrossRef] [PubMed]
- Bedard, J.; Caschera, A.; Foucher, D.A. Access to thermally robust and abrasion resistant antimicrobial plastics: Synthesis of UV-curable phosphonium small molecule coatings and extrudable additives. RSC Adv. 2021, 11, 5548–5555. [Google Scholar] [CrossRef]
- Cuthbert, T.J.; Harrison, T.D.; Ragogna, P.J.; Gillies, E.R. Synthesis, properties, and antibacterial activity of polyphosphonium semi-interpenetrating networks. J. Mater. Chem. B 2016, 4, 4872–4883. [Google Scholar] [CrossRef]
- Schifano, J.M.; Edifor, R.; Sharp, J.D.; Ouyang, M.; Konkimalla, A.; Husson, R.N.; Woychik, N.A. Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23S rRNA at the ribosomal A site. Proc. Natl. Acad. Sci. USA 2013, 110, 8501–8506. [Google Scholar] [CrossRef]
- Jardetzky, O. Studies on the mechanism of action of chloramphenicol. I. The conformation of chlioramphenicol in solution. J. Biol. Chem. 1963, 238, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.D.; Hahn, F.E. Mode of action of chloramphenicol IX. Effects of chloramphenicol upon a ribosomal amino acid polymerization system and its binding to bacterial ribosome. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1965, 95, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.E.; Wisseman, C.L.; Hopps, H.E. Mode of action of chloramphenicol. III. Action of chloramphenicol on bacterial energy metabolism. J. Bacteriol. 1955, 69, 215–223. [Google Scholar] [CrossRef]
- Yu, C.; Simmons, B.A.; Singer, S.W.; Thelen, M.P.; VanderGheynst, J.S. Ionic liquid-tolerant microorganisms and microbial communities for lignocellulose conversion to bioproducts. Appl. Microbiol. Biotechnol. 2016, 100, 10237–10249. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, D.; Singh, S.; Cheng, G. Recent developments in ionic liquid pretreatment of lignocellulosic biomass for enhanced bioconversion. Sustain. Energy Fuels 2021, 5, 1655–1667. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.V.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef]
- Tramer, F.; Da Ros, T.; Passamonti, S. Screening of Fullerene Toxicity by Hemolysis Assay. In Nanotoxicity. Methods in Molecular Biology; Reineke, J., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 926, pp. 203–217. [Google Scholar] [CrossRef]

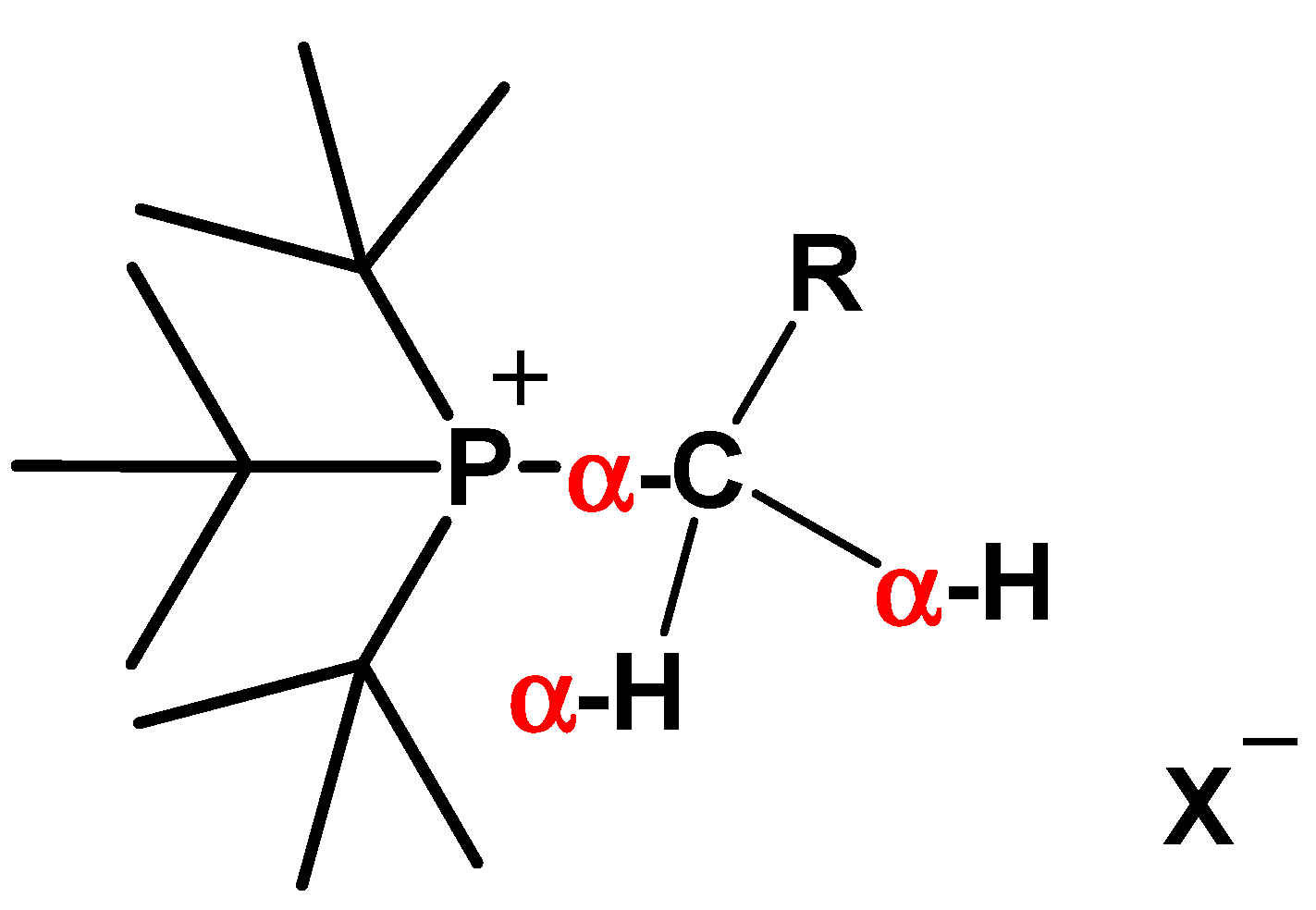

| oxy-QPSs | R | X |
|---|---|---|
| 1 | CH2-O-CH3 | Cl |
| 2 | CH2-O-CH3 | BF4 |
| 3 | CH2-CH2-O-CH2-CH3 | Br |
| 4 | CH2-O-CH2-CH2-O-CH3 | Cl |
| 5 | CH2-O-CH2-CH2-O-CH3 | BF4 |
| 6 | CH2-O-CH2-CH2-O-CH3 | PF6 |
| 7 | CH2-CH2-COOH | Br |
| 8 | CH2-CH2-CH2-CH2-CH2-COOH | Br |
| 9 | CH2-C(O)-O-CH3 | Br |
| 10 | CH2-C(O)-O-CH2-CH3 | Br |
| 11 | CH2-C(O)-O-CH(CH3)2 | Br |
| 12 | CH2-C(O)-O-CH2-CH2-CH2-CH3 | Br |
| oxy-QPSs | δ in 31P NMR Spectra (ppm) | δ of α-H in 1H NMR Spectra (ppm) | δ of α-C in 13C NMR Spectra (ppm) |
|---|---|---|---|
| 1 | 44.4 (45.8) * | 5.11 | 61.9 |
| 2 | 44.0 | 4.57 | 61.3 |
| 3 | 51.6 (52.4) | 2.95 | 20.2 |
| 4 | 44.7 (46.0) | 5.17 | 60.5 |
| 5 | 44.3 (45.5) | 4.62 | 59.8 |
| 6 | 44.2 | 4.55 | 59.8 |
| 7 | 50.0 (51.3) | 2.75 | 13.6 |
| 8 | 49.7 | 2.47 | 18.7 |
| 9 | 51.9 (53.4) | 4.27 | 25.5 |
| 10 | 51.9 (53.5) | 4.13 | 25.5 |
| 11 | 52.0 (53.6) | 4.10 | 25.8 |
| 12 | 52.0 (53.4) | 4.07 | 25.4 |
| oxy-QPSs | Tm, °C | Td, °C |
|---|---|---|
| 1 | 144 | 171 |
| 2 | 142 | 236 |
| 3 | 109 | 185 |
| 4 | 178 | 187 |
| 5 | 92 | 210 |
| 6 | 87 | 221 |
| 7 | 173 | 210 |
| 8 | 186 | 186 |
| 9 | 170 | 170 |
| 10 | 168 | 172 |
| 11 | 170 | 170 |
| 12 | 125 | 167 |
| The Mixture | Time of Cellulose Dissolution * | Note |
|---|---|---|
| 1 + DMSO | 5 min | - |
| 3 + DMSO | 90 min | oxy-QPS destruction ** |
| 4 + DMSO | 5 min | - |
| 5 + DMSO | >12 h | - |
| 7 + DMSO | >8 h | oxy-QPS crystallization |
| 8 + DMSO | >8 h | - |
| 9 + DMSO | >4 h | - |
| 10 + DMSO | >8 h | oxy-QPS destruction |
| 11 + DMSO | >4 h | - |
| 12 + DMSO | >8 h | oxy-QPS destruction |
| DMSO | >17 h | - |
| The Mixture (1:1) | The Temperature of the Dissolution Process, °C | ||
|---|---|---|---|
| 60 | 70 | 80 | |
| 1 + DMSO | >8 h | 5 min | 5 min |
| 3 + DMSO | >8 h | 5 h | 90 min |
| 4 + DMSO | oxy-QPS crystallization | 5 min | 5 min |
| oxy-QPSs | MIC—Minimal Inhibition Concentration, µg/mL | ||||
|---|---|---|---|---|---|
| Sa | Bc | Ef | Ec | Ca | |
| 1 | 250 ± 20 | >250 | >250 | >250 | >250 |
| 7 | 62.5 ± 5.3 | >250 | >250 | >250 | >250 |
| 9 | 250 ± 19 | >250 | >250 | >250 | >250 |
| 10 | 62.5 ± 5.2 | >250 | >250 | >250 | >250 |
| 11 | 250 ± 21 | >250 | >250 | >250 | >250 |
| 12 | 250 ± 18 | >250 | >250 | >250 | >250 |
| Chloramphenicol | 62.5 ± 5.5 | 62.5 ± 5.3 | 125 ± 11 | - | - |
| MBC and MFC—bactericide and fungicide activity, µg/mL | |||||
| 1 | 250 ± 21 | >250 | >250 | >250 | >250 |
| 7 | 250 ± 19 | >250 | >250 | >250 | >250 |
| 9 | 250 ± 19 | >250 | >250 | >250 | >250 |
| 10 | 62.5 ± 6.4 | >250 | >250 | >250 | >250 |
| 11 | 250 ± 22 | >250 | >250 | >250 | >250 |
| 12 | 250 ± 20 | >250 | >250 | >250 | >250 |
| oxy-QPSs | IC50, µg/mL | Hemolysis HC50, µg/mL | |
|---|---|---|---|
| Wi38 | Chang Liver | ||
| 1 | 206.1 | 132.7 | >500 |
| 4 | 238.4 | 113.4 | >500 |
| 5 | 230.9 | 208.9 | >500 |
| 7 | 276.8 | 260.8 | >500 |
| 8 | 295.9 | 237.8 | >500 |
| 9 | 144.3 | 407.8 | >500 |
| 10 | 470.8 | 351.9 | >500 |
| 11 | 208.4 | 223.8 | >500 |
| 12 | 207.5 | 203.1 | >500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipova, D.M.; Ermolaev, V.V.; Baembitova, G.R.; Samigullina, A.I.; Lyubina, A.P.; Voloshina, A.D. Oxygen-Containing Quaternary Phosphonium Salts (oxy-QPSs): Synthesis, Properties, and Cellulose Dissolution. Polymers 2023, 15, 4097. https://doi.org/10.3390/polym15204097
Arkhipova DM, Ermolaev VV, Baembitova GR, Samigullina AI, Lyubina AP, Voloshina AD. Oxygen-Containing Quaternary Phosphonium Salts (oxy-QPSs): Synthesis, Properties, and Cellulose Dissolution. Polymers. 2023; 15(20):4097. https://doi.org/10.3390/polym15204097
Chicago/Turabian StyleArkhipova, Daria M., Vadim V. Ermolaev, Gulnaz R. Baembitova, Aida I. Samigullina, Anna P. Lyubina, and Alexandra D. Voloshina. 2023. "Oxygen-Containing Quaternary Phosphonium Salts (oxy-QPSs): Synthesis, Properties, and Cellulose Dissolution" Polymers 15, no. 20: 4097. https://doi.org/10.3390/polym15204097
APA StyleArkhipova, D. M., Ermolaev, V. V., Baembitova, G. R., Samigullina, A. I., Lyubina, A. P., & Voloshina, A. D. (2023). Oxygen-Containing Quaternary Phosphonium Salts (oxy-QPSs): Synthesis, Properties, and Cellulose Dissolution. Polymers, 15(20), 4097. https://doi.org/10.3390/polym15204097






