Construction of Fluorescent Conjugated Polytriazole Containing Double-Decker Silsesquioxane: Click Polymerization and Thermal Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
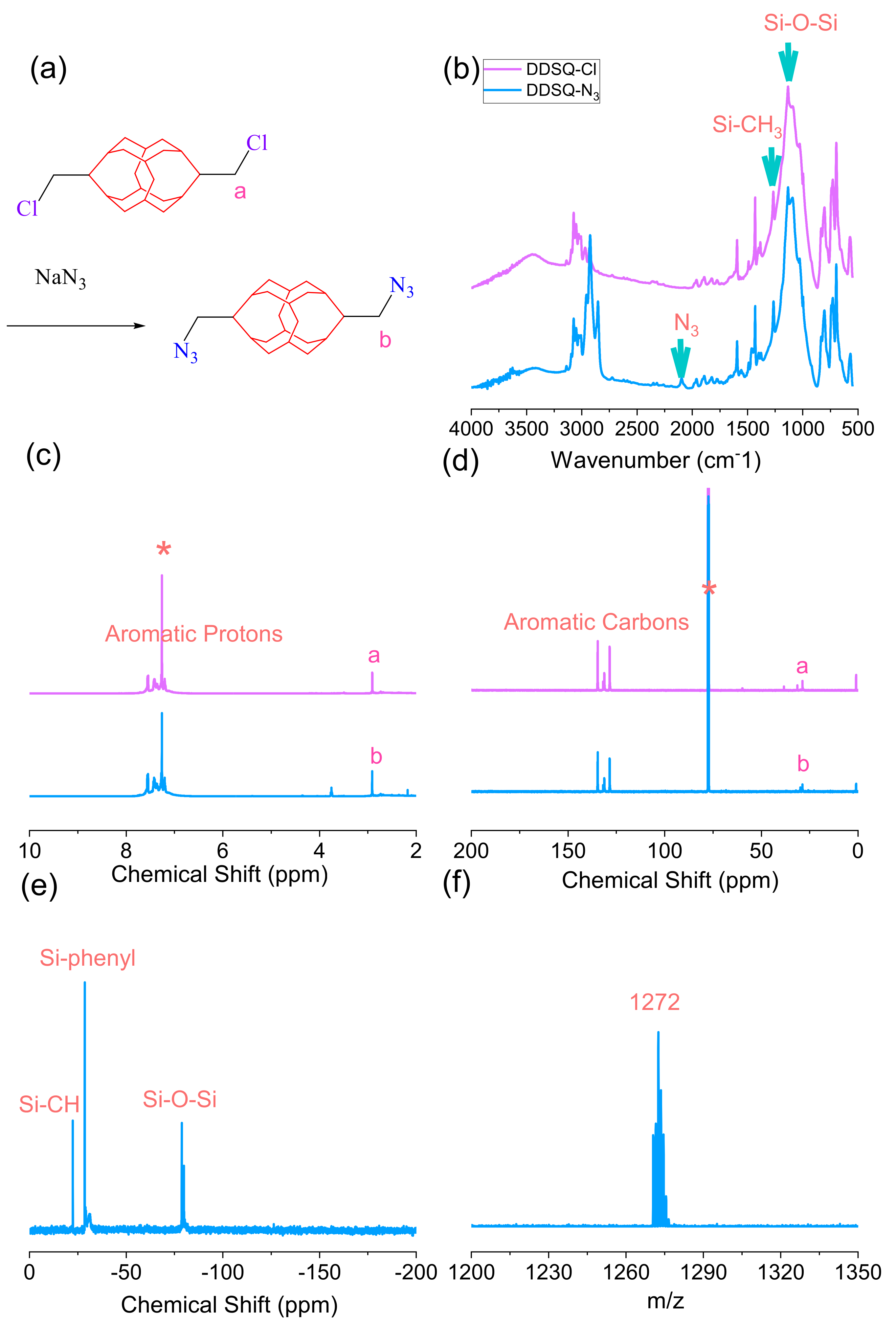
2.2. Synthesis of Double-Decker Silsesquioxane-Cl (DDSQ-Cl)
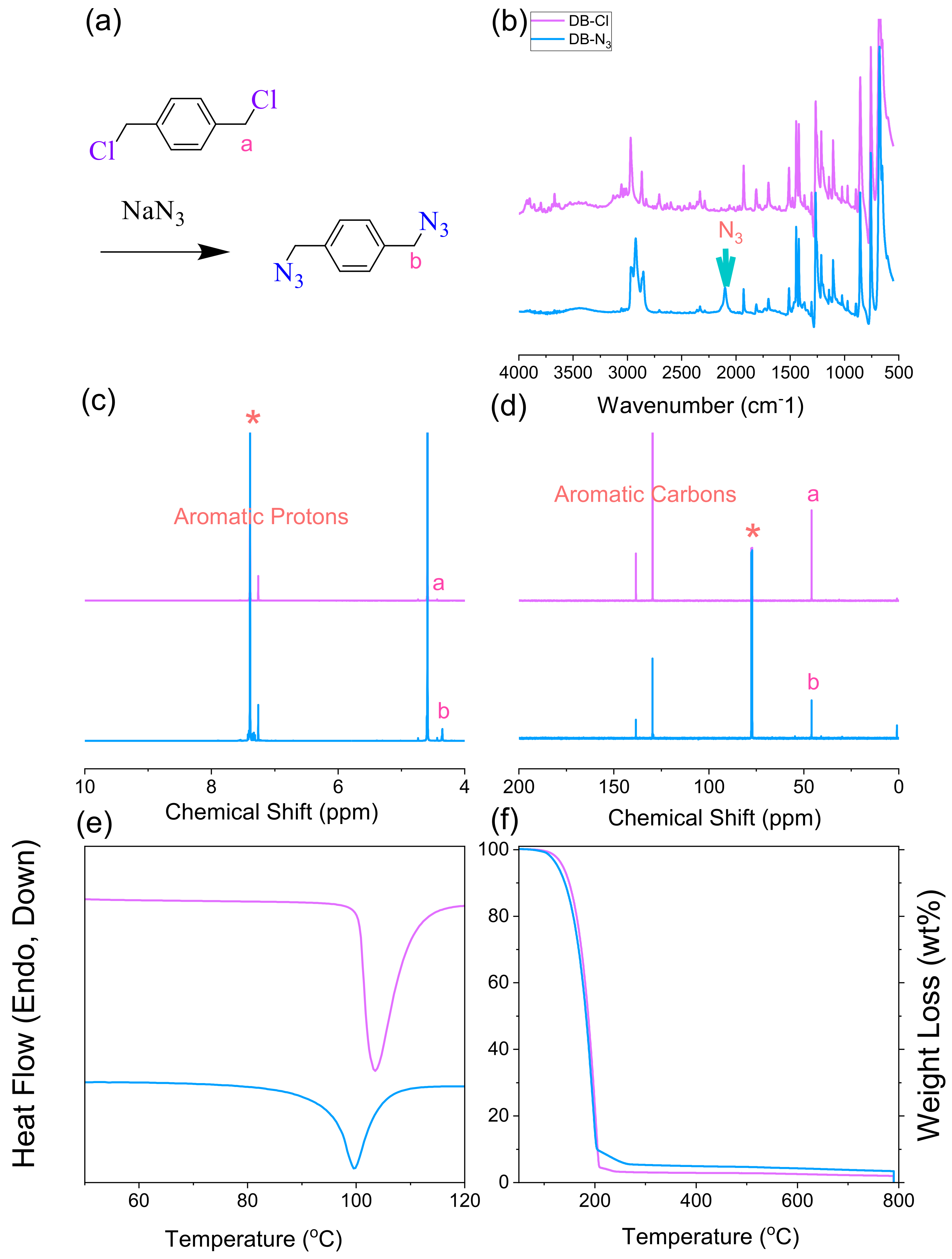
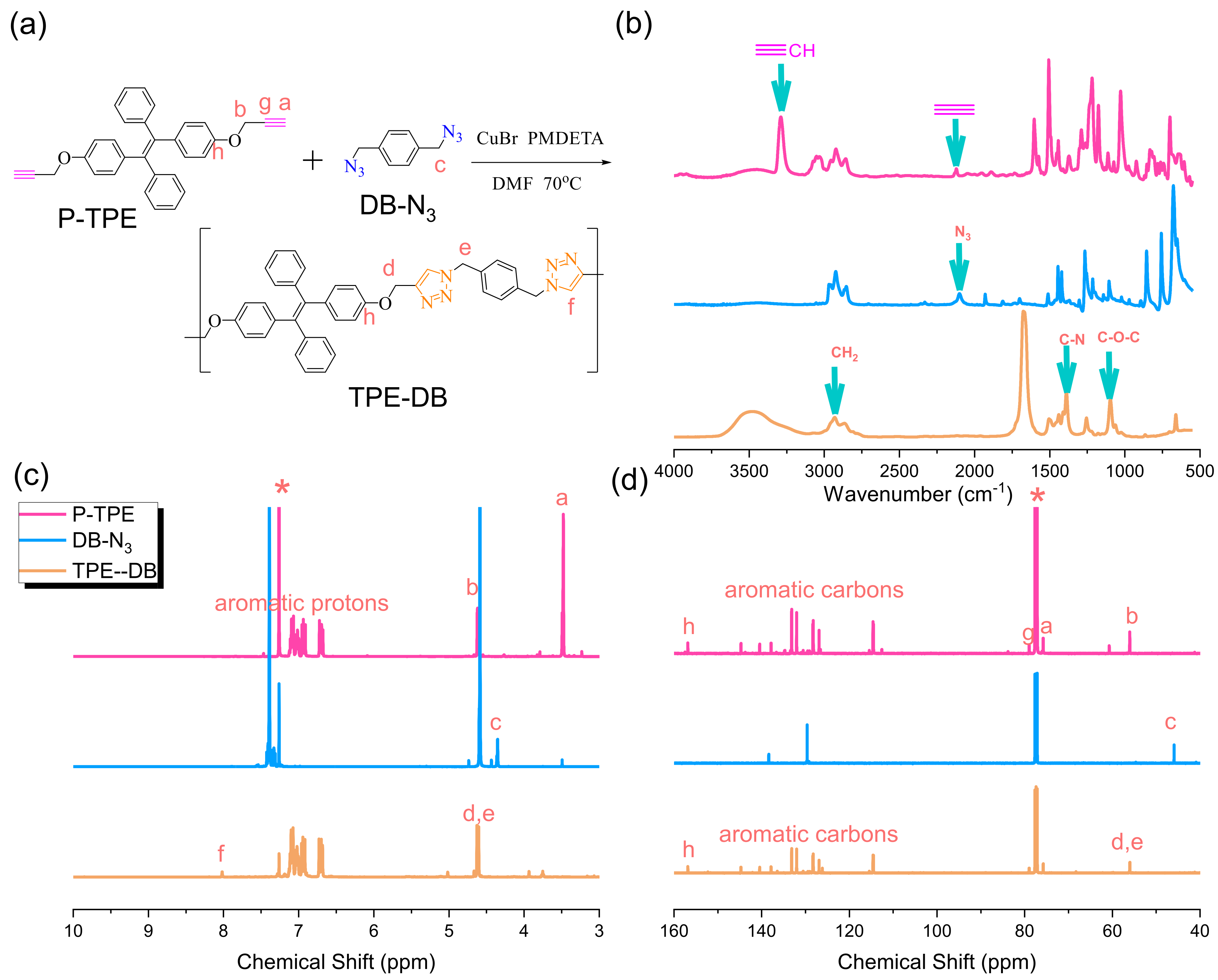
2.3. Synthesis of p-Xylylene Diazide (DB-N3) and Double-Decker Silsesquioxane-N3 (DDSQ-N3)
2.4. Synthesis of 1,4-bis(prop-2-yn-1-yloxy)benzene (P-B), 4,4′-(propane-2,2-diyl)bis((prop-2-yn-1-yloxy)benzene) (P-BPA), and bis(4-(prop-2-yn-1-yloxy)phenyl)methanone (P-CO)
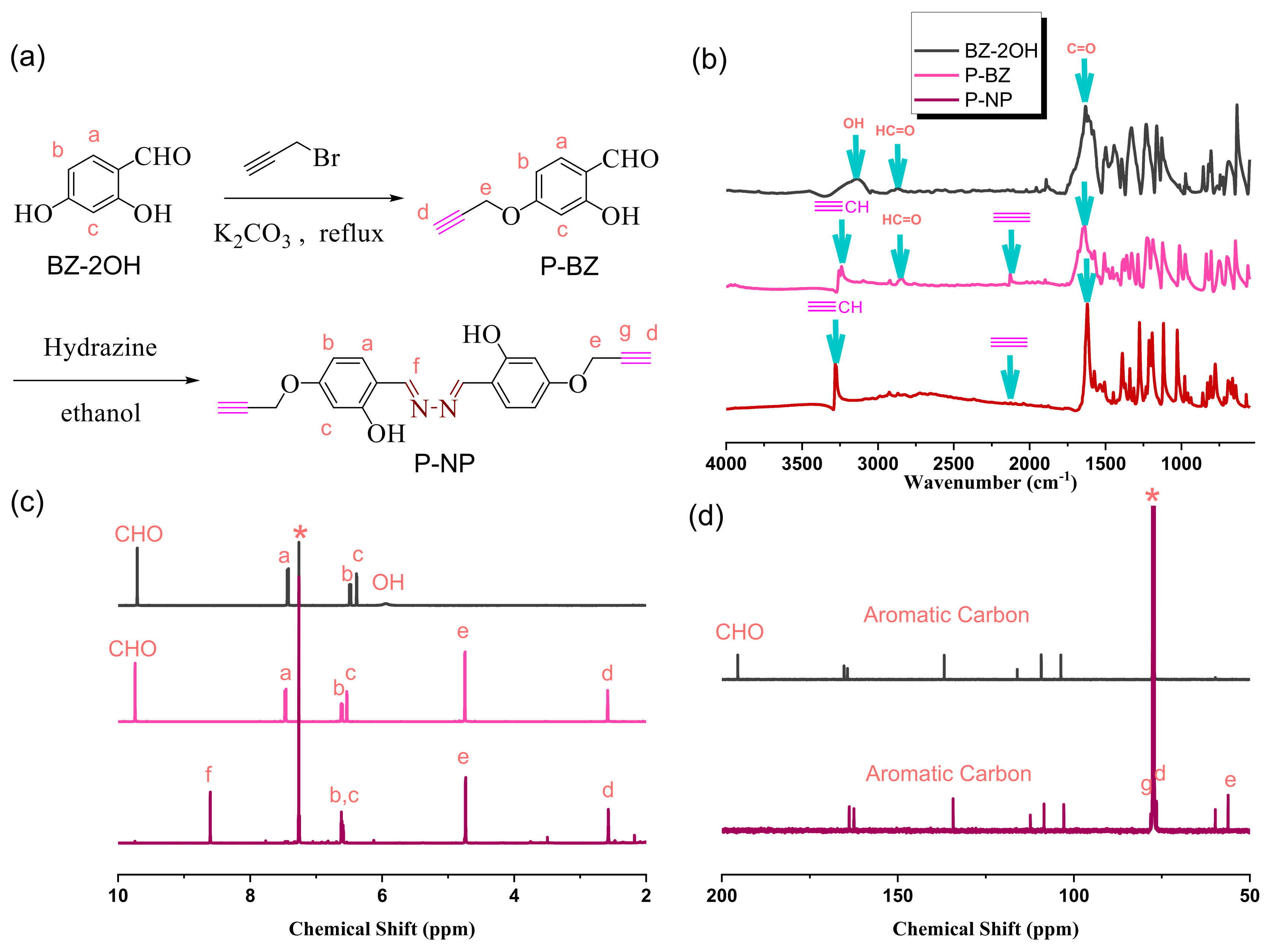
2.5. Synthesis of 1,2-Diylidenebis(methaneylelidene)bis(3-(prop-2-yn-1-yloxy)phenol) (P-NP)
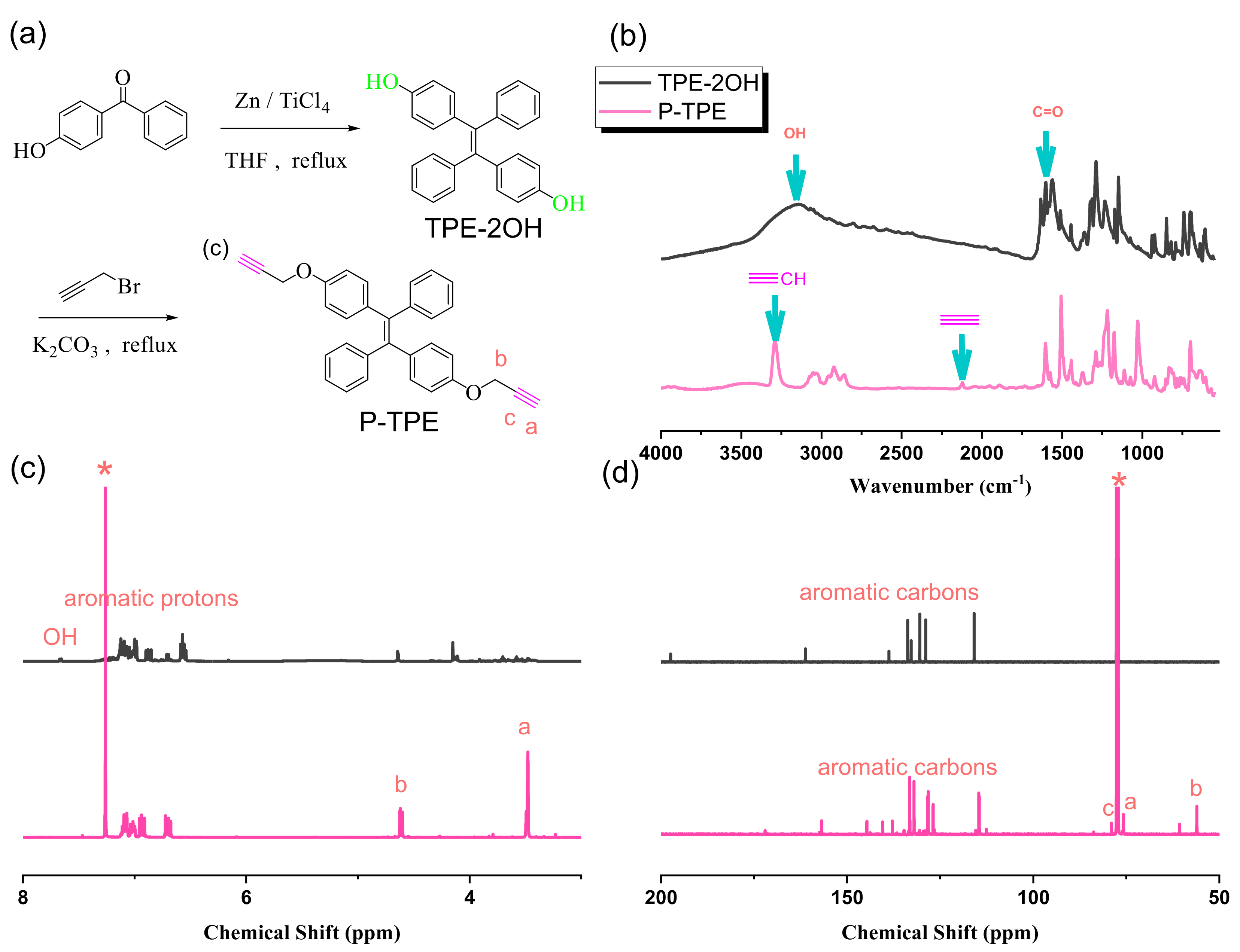
2.6. Synthesis of 1,2-bis(4-Hydroxyphenyl)-1,2-diphenylethene (TPE-2OH) and Propargylated-TPE (P-TPE)
2.7. Synthesis of DDSQ-Based Main Chain Type of Copolymer through Click Reaction
3. Results
3.1. Synthesis of DB-N3 and DDSQ-N3
3.2. Synthesis of P-B, P-BPA, and P-CO Monomers
3.3. Synthesis of P-NP Monomer
3.4. Synthesis of P-TPE Monomer
3.5. Synthesis of TPE-DB-Based Main Chain Type of Copolymer through Click Reaction
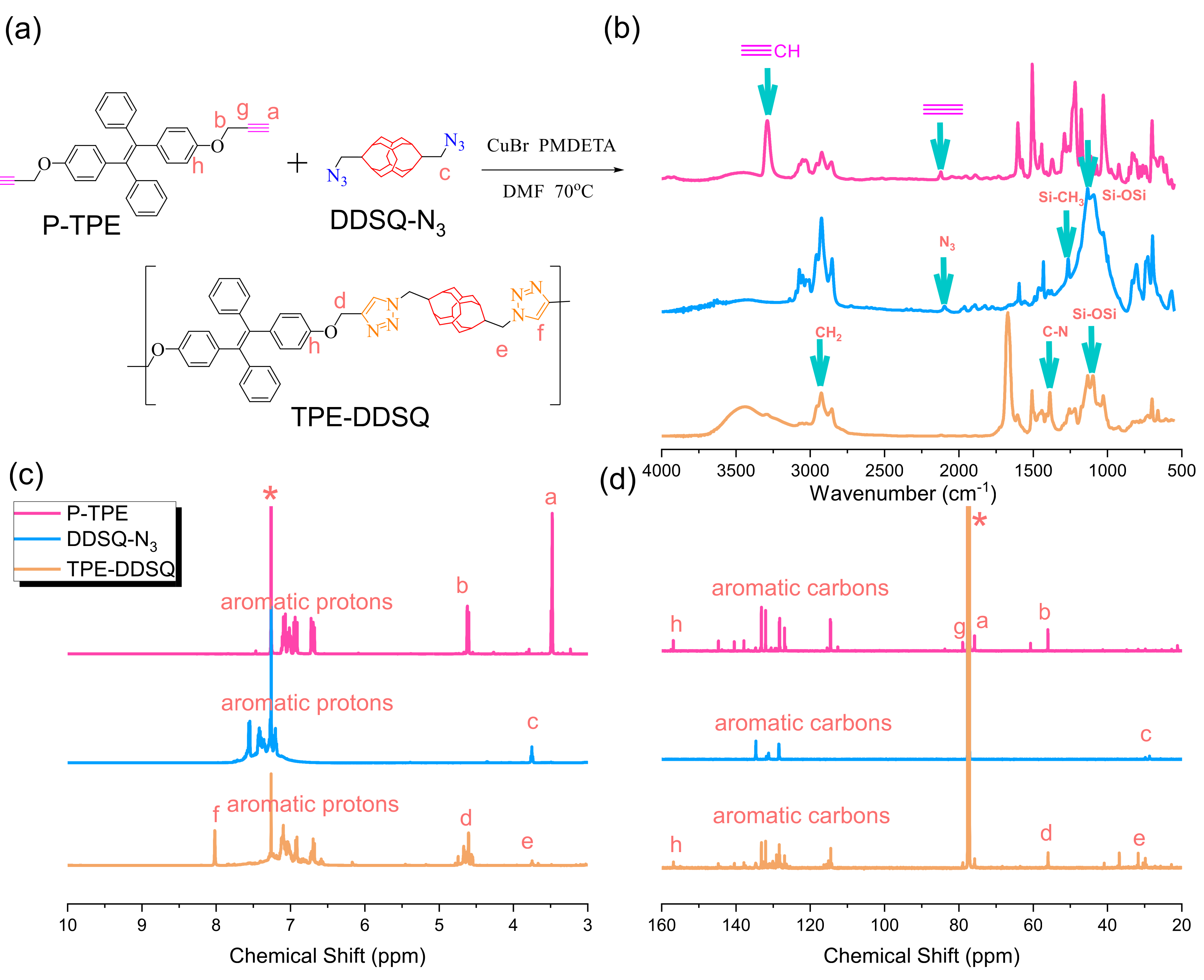
3.6. Synthesis of TPE-DDSQ-Based Main Chain Type of Copolymer through Click Reaction
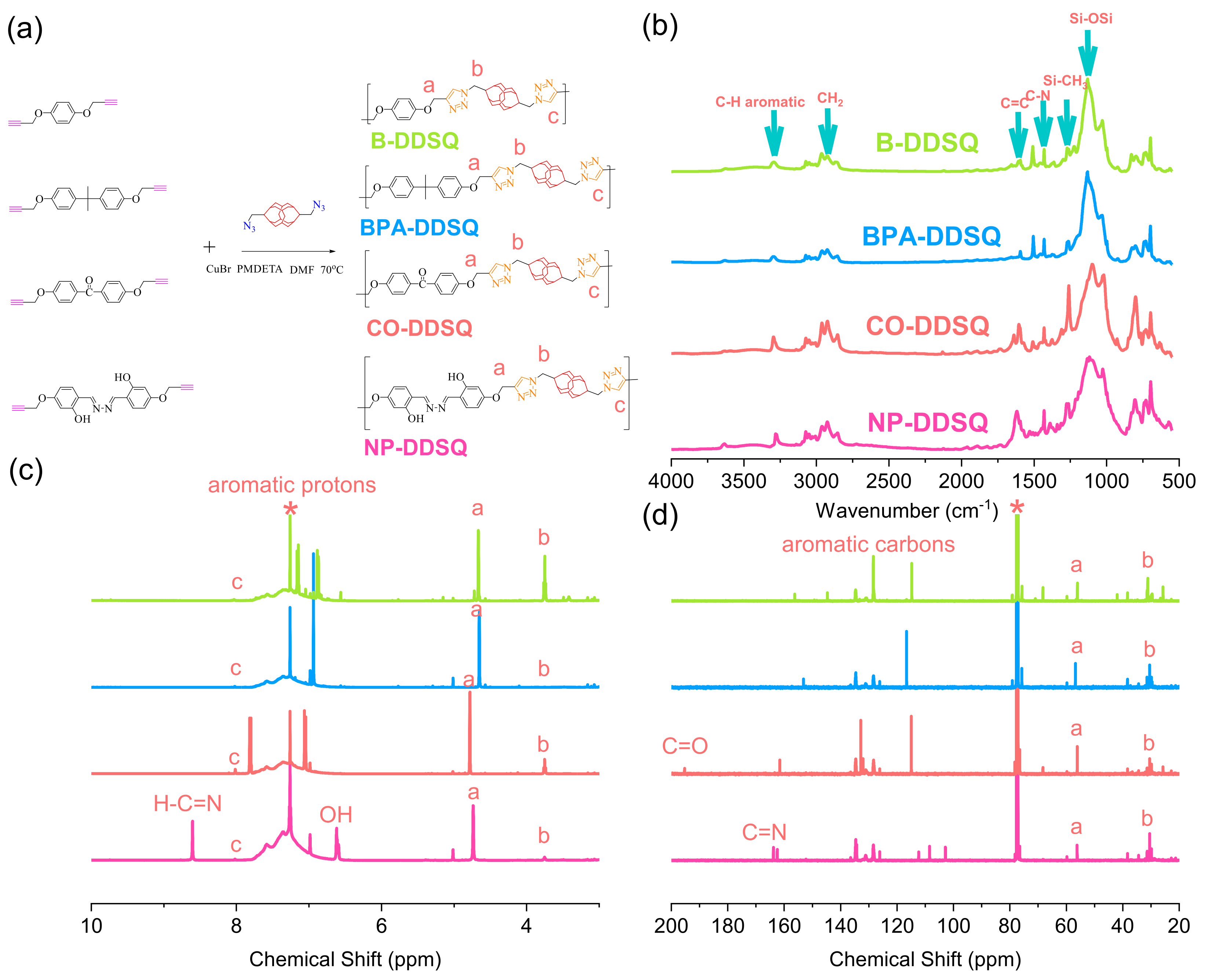
3.7. Synthesis of Other DDSQ-Based Main Chain Types of Copolymer through Click Reaction
3.8. Thermal Property and Morphology Analyses of DDSQ-Based Copolymers
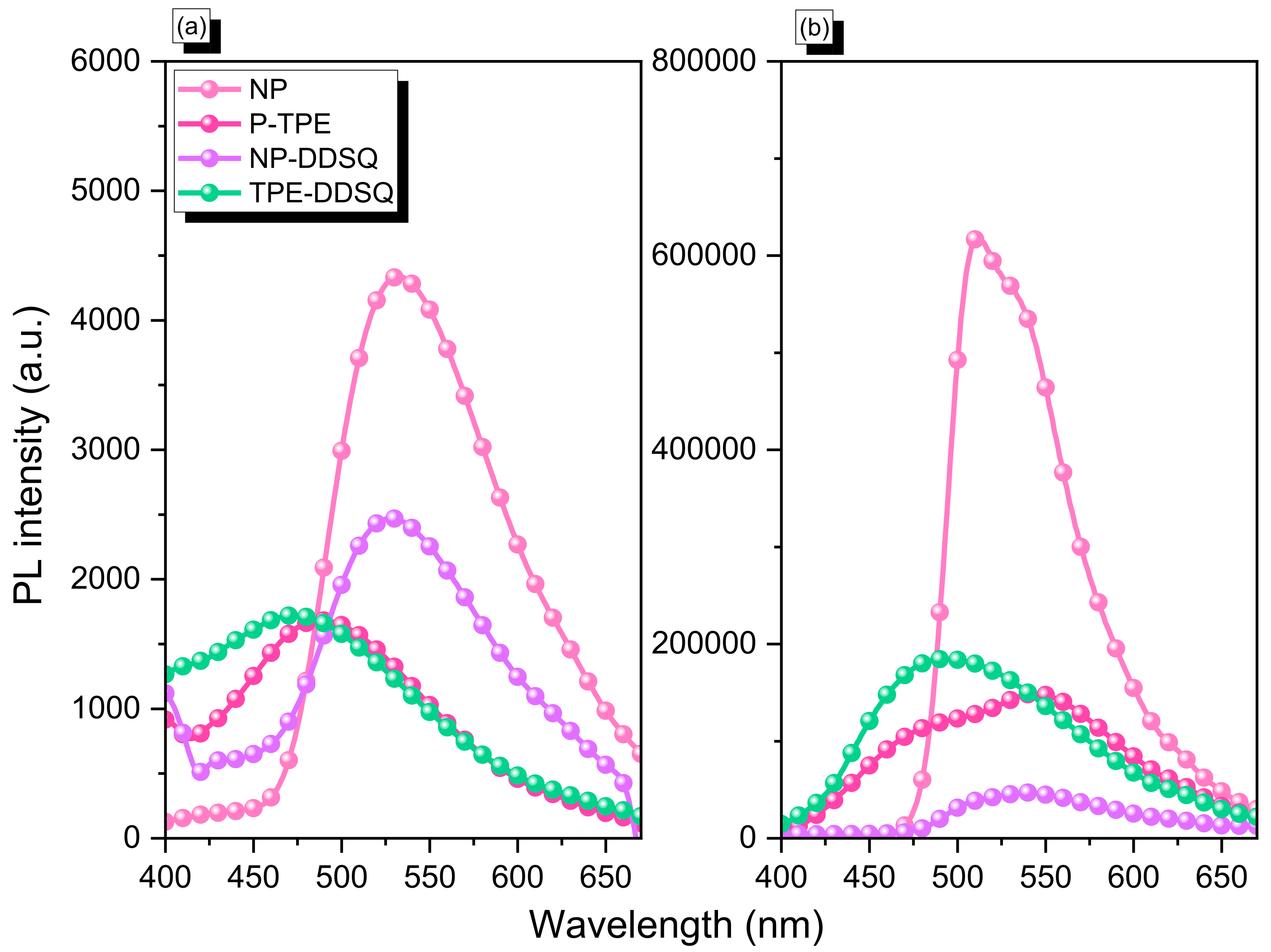
3.9. Photoluminescence Property of NP-DDSQ and TPE-DDSQ Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuo, S.-W. Hydrogen bonding interactions in polymer/polyhedral oligomeric silsesquioxane nanomaterials. J. Polym. Res. 2022, 29, 69. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, S.; Zhang, W. Macromolecular isomerism in giant molecules. Chem. A Eur. J. 2019, 26, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Kuo, S.-W. Progress in the self-assembly of organic/inorganic polyhedral oligomeric silsesquioxane (POSS) hybrids. Soft Matter 2022, 18, 5535–5561. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Mohamed, M.G.; Sharma, S.U.; Lee, J.T.; Huang, C.F.; Chen, T.; Kuo, S.W. An Ultrastable Porous Polyhedral Oligomeric Silsesquioxane/Tetraphenylthiophene Hybrid as a High-Performance Electrode for Supercapacitors. Molecules 2022, 27, 6238. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Elsayed, M.E.; Ye, Y.; Samy, M.M.; Hassan, A.E.; Mansoure, T.H.; Wen, Z.; Chou, H.H.; Chen, K.H.; Kuo, S.W. Construction of Porous Organic/Inorganic Hybrid Polymers Based on Polyhedral Oligomeric Silsesquioxane for Energy Storage and Hydrogen Production from Water. Polymers 2023, 15, 182. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional silica and carbon nanocomposites based on polybenzoxazines. Macromol. Chem. Phys. 2018, 220, 1800306. [Google Scholar] [CrossRef]
- Kowalewska, A. Self-Assembling Polyhedral Silsesquioxanes—Structure and properties. Curr. Org. Chem. 2017, 21, 1243–1264. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A. Self-healing silsesquioxane-based materials. Polymers 2022, 14, 1869. [Google Scholar] [CrossRef]
- Blanco, I. The rediscovery of POSS: A molecule rather than a filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Pramudya, I.; Rico, C.G.; Lee, C.; Chung, H. POSS-containing bioinspired adhesives with enhanced mechanical and optical properties for biomedical applications. Biomacromolecules 2016, 17, 3853–3861. [Google Scholar] [CrossRef]
- Hebda, E.; Bukowczan, A.; Michałowski, S.; Pielichowski, K. Flexible polyurethane foams reinforced by functionalized polyhedral oligomeric silsesquioxanes: Structural characteristics and evaluation of thermal/flammability properties. Polymers 2022, 14, 4743. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, R.; Yan, X.-Y.; Guo, Q.-Y.; Huang, J.; Shan, W.; Liu, Y.; Liu, T.; Huang, M.; Cheng, S.Z. The role of architectural engineering in macromolecular self-assemblies via non-covalent interactions: A molecular LEGO approach. Prog. Polym. Sci. 2020, 103, 101230. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Liu, W.; Pi, H.; Zeng, G. POSS hybrid robust biomass IPN hydrogels with temperature responsiveness. Polymers 2019, 11, 524. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional polyimide/polyhedral oligomeric silsesquioxane nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef]
- Żak, P.; Dudziec, B.; Dutkiewicz, M.; Ludwiczak, M.; Marciniec, B.; Nowicki, M. A new class of stereoregular vinylene-arylene copolymers with double-decker silsesquioxane in the main chain. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1044–1055. [Google Scholar] [CrossRef]
- Sodkhomkhum, R.; Ervithayasuporn, V. Synthesis of poly(siloxane/double-decker silsesquioxane) via dehydrocarbonative condensation reaction and its functionalization. Polymer 2016, 86, 113–119. [Google Scholar] [CrossRef]
- Liu, N.; Wei, K.; Wang, L.; Zheng, S. Organic-inorganic polyimides with double decker silsesquioxane in the main chains. Polym. Chem. 2016, 7, 1158–1167. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Liu, N.Y.; EL-Mahdy, A.F.M.; Kuo, S.W. Ultrastable luminescent hybrid microporous polymers based on polyhedral oligomeric silsesquioxane for CO2 uptake and metal ion sensing. Microporous Mesoporous Mater. 2021, 311, 110695. [Google Scholar] [CrossRef]
- Lin, I.-M.; Hsu, C.-C.; Yu, T.-C.; Kuo, S.-W.; Chuang, W.-T.; Chiang, Y.-W. Propagatable hierarchical architectures from dispersive fragments to periodic nanosheets within phase-separated nanostructures by controlling guest–Host interaction. Macromolecules 2022, 55, 9048–9056. [Google Scholar] [CrossRef]
- Chen, W.-C.; Kuo, S.-W. Ortho-imide and allyl groups effect on highly thermally stable polybenzoxazine/double-decker-shaped polyhedral silsesquioxane hybrids. Macromolecules 2018, 51, 9602–9612. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chen, W.-C.; Kuo, S.-W. Mesoporous phenolic/poss hybrids induced by microphase separation arising from competitive hydrogen bonding interactions. Macromolecules 2022, 55, 8918–8930. [Google Scholar] [CrossRef]
- Zhao, B.; Mei, H.; Wang, H.; Li, L.; Zheng, S. Organic-inorganic polyureas with POSS cages in the main chains via polycondensation of diamines with carbon dioxide. ACS Appl. Polym. Mater. 2021, 4, 509–520. [Google Scholar] [CrossRef]
- Chen, W.-C.; Chen, Z.-Y.; Ba, Y.; Wang, B.; Chen, G.; Fang, X.; Kuo, S.-W. Double-decker-shaped polyhedral silsesquioxanes reinforced epoxy/bismaleimide hybrids featuring high thermal stability. Polymers 2022, 14, 2380. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Mansoure, T.H.; Takashi, Y.; Samy, M.M.; Chen, T.; Kuo, S.-W. Ultrastable porous organic/inorganic polymers based on polyhedral oligomeric silsesquioxane (POSS) hybrids exhibiting high performance for thermal property and energy storage. Microporous Mesoporous Mater. 2021, 328, 111505. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Chen, W.-C.; El-Mahdy, A.F.M.; Hsu, M.-Y.; Lin, C.-H.; Kuo, S.-W. High thermal resistance of epoxy/cyanate ester hybrids incorporating an inorganic double-decker-shaped polyhedral silsesquioxane nanomaterial. Molecules 2022, 27, 5938. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Han, H.; Wang, C. Mechanical and thermal properties of epoxy-POSS reinforced-(biphenyl diol formaldehyde/epoxy hybrid resin) composites. Iran. Polym. J. 2014, 23, 609–617. [Google Scholar] [CrossRef]
- Pagacz, J.; Hebda, E.; Janowski, B.; Sternik, D.; Jancia, M.; Pielichowski, K. Thermal decomposition studies on polyurethane elastomers reinforced with polyhedral silsesquioxanes by evolved gas analysis. Polym. Degrad. Stab. 2018, 149, 129–142. [Google Scholar] [CrossRef]
- Montero, B.; Bellas, R.; Ramírez, C.; Rico, M.; Bouza, R. Flame retardancy and thermal stability of organic-inorganic hybrid resins based on polyhedral oligomeric silsesquioxanes and montmorillonite clay. Compos. Part B Eng. 2014, 63, 67–76. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Tsai, M.-Y.; Wang, C.-F.; Huang, C.-F.; Danko, M.; Dai, L.; Chen, T.; Kuo, S.-W. Multifunctional polyhedral oligomeric silsesquioxane (POSS) based hybrid porous materials for CO2 uptake and iodine adsoprtion. Polymers 2021, 13, 221. [Google Scholar] [CrossRef]
- Chi, H.; Wang, M.; Xiao, Y.; Wang, F.; Joshy, K.S. Self-assembly and applications of amphiphilic hybrid POSS copolymers. Molecules 2018, 23, 2481. [Google Scholar] [CrossRef]
- Hebda, E.; Bukowczan, A.; Michałowski, S.; Wroński, S.; Urbaniak, P.; Kaczmarek, M.; Hutnik, E.; Romaniuk-Drapała, A.; Wolun-Cholewa, M.; Pielichowski, K. Examining the influence of functionalized POSS on the structure and bioactivity of flexible polyurethane foams. Mater. Sci. Eng. C 2020, 108, 110370. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Mohamed, M.G.; Kuo, S.-W.; Xin, Z. Crystallization behaviors of poly(ethylene terephthalate) (PET) with monosilane isobutyl-polyhedral oligomeric silsesquioxanes (POSS). J. Mater. Sci. 2020, 55, 14642–14655. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, P.; Chen, T.; Jing, M. Study on the influence of nano-Ov POSS on the compatibility, molecular structure, and properties of SBS modified asphalt by molecular dynamics simulation. Polymers 2022, 14, 4121. [Google Scholar] [CrossRef]
- Strachota, B.; Matějka, L.; Hodan, J.; Kobera, L.; Mahun, A.; Dybal, J.; Šlouf, M. Polyhedral oligomeric silsesquioxane (POSS)-based epoxy nanocomposite involving a reversible diels—Alder-type network as a self-healing material. J. Adhes. Sci. Technol. 2021, 35, 2736–2757. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, L.; Du, Q.; Shan, T.; Zheng, K.; He, J.; He, H.; Chen, S.; Wang, X. Synthesis, properties and applications of well-designed hybrid polymers based on polyhedral oligomeric silsesquioxane. Polym. Int. 2022, 71, 379–392. [Google Scholar] [CrossRef]
- Ju, J.; Fejjari, K.; Cheng, Y.; Liu, M.; Li, Z.; Kang, W.; Liao, Y. Engineering hierarchically structured superhydrophobic PTFE/POSS nanofibrous membranes for membrane distillation. Desalination 2020, 486, 114481. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S. Polymeric nanocomposites—Polyhedral oligomeric silsesquioxanes (POSS) as hybrid nanofiller. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 389–410. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, B.; Adeel, M.; Zheng, S. Shape memory and self-healing properties of poly(acrylate amide) elastomers reinforced with polyhedral oligomeric silsesquioxanes. ACS Appl. Polym. Mater. 2019, 1, 359–368. [Google Scholar] [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral oligomeric silsesquioxane hybrid polymers: Well-defined architectural design and potential functional applications. Macromol. Rapid Commun. 2019, 40, e1900101. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Wang, F.; Nie, J.; Ma, C.; Gao, M. POSS hybrid hydrogels: A brief review of synthesis, properties and applications. Eur. Polym. J. 2020, 143, 110180. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, S.; Zheng, S. Synthesis, self-assembly and self-healing properties of organic-inorganic ABA triblock copolymers with poly(POSS acrylate) endblocks. Polym. Chem. 2019, 10, 2424–2435. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; You, M.; Li, Z.; He, C. Polyhedral oligomeric silsesquioxanes (POSS)-based hybrid soft gels: Molecular design, material advantages, and emerging applications. ACS Mater. Lett. 2020, 2, 296–316. [Google Scholar] [CrossRef]
- Duszczak, J.; Mituła, K.; Santiago-Portillo, A.; Soumoy, L.; Rzonsowska, M.; Januszewski, R.; Fusaro, L.; Aprile, C.; Dudziec, B. Double-decker silsesquioxanes self-assembled in one-dimensional coordination polymeric nanofibers with emission properties. ACS Appl. Mater. Interfaces 2021, 13, 22806–22818. [Google Scholar] [CrossRef]
- Tanaka, T.; Hasegawa, Y.; Kawamori, T.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of double-decker silsesquioxanes from substituted difluorosilane. Organometallics 2019, 38, 743–747. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Fu, Q.; Zhang, Q.; Xu, R. Synthesis of a novel bifunctional epoxy double-decker silsesquioxane: Improvement of the thermal stability and dielectric properties of polybenzoxazine. Polymers 2022, 14, 5154. [Google Scholar] [CrossRef]
- Sathiyaraj, M.; Pavithra, K.; Thiagarajan, V. Azine based Aiegens with multi-stimuli response towards picric acid. New J. Chem. 2020, 44, 8402–8411. [Google Scholar] [CrossRef]
- Gong, Q.; Li, Y.; Wang, H.; Wang, G.; Feng, Q.; Zhong, Y.; Liu, F. Unveiling the aggregation-induced emission (AIE) mechanism and the effect of substituents on luminescence properties for salicylaldehyde azine derivatives with intramolecular hydrogen bond. J. Phys. Chem. C 2022, 126, 18429–18438. [Google Scholar] [CrossRef]
- Wang, P.; Liang, B.; Xia, D. A Linear AIE supramolecular polymer based on a salicylaldehyde azine-containing pillararene and its reversible cross-linking by CuII and cyanide. Inorg. Chem. 2019, 58, 2252–2256. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Lin, R.-C.; Tu, J.-H.; Lu, F.-H.; Hong, J.-L.; Jeong, K.-U.; Wang, C.-F.; Kuo, S.-W. Thermal property of an aggregation-induced emission fluorophore that forms metal-ligand complexes with Zn(ClO4)2 of salicylaldehyde azine-functionalized polybenzoxazine. RSC Adv. 2015, 5, 65635–65645. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Yang, M.; Zhang, Y.; Li, H.; Zhang, X. A specific AIE and ESIPT fluorescent probe for peroxynitrite detection and imaging in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117230. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Kong, L.; Zhang, X.; Yang, J. Aggregation-induced emission-active tetraphenylethylene derivatives containing arylimidazole unit for reversible mechanofluorochromism and selective detection of picric acid. Dye. Pigment. 2020, 181, 108574. [Google Scholar] [CrossRef]
- Jia, J.; Wu, L. Stimuli-responsive fluorescence switching: Aggregation-induced emission (AIE), protonation effect and reversible mechanofluochromism of tetraphenylethene hydrazone-based dyes. Org. Electron. 2020, 76, 105466. [Google Scholar] [CrossRef]
- Ye, X.; Wang, H.; Yu, L.; Zhou, J. Aggregation-induced emission (AIE)-labeled cellulose nanocrystals for the detection of nitrophenolic explosives in aqueous solutions. Nanomaterials 2019, 9, 707. [Google Scholar] [CrossRef]
- Liu, F.; Ren, Y.; Lau, H.; Tang, B.Z.; Zhou, H. Aggregation induced emission (AIE) active cross-linked poly(N-isopropyl acrylamide-co-tetra(phenyl)ethene di-acrylates): Sensors for effective nitroaromatics detection in an aqueous environment. J. Mater. Chem. C 2022, 10, 5856–5863. [Google Scholar] [CrossRef]
- Weng, M.-T.; Elsyed, A.F.N.; Yang, P.-C.; Mohamed, M.G.; Kuo, S.-W.; Lin, K.-S. Fluorescent and thermoresponsive tetraphenylethene-based cross-linked poly(N-isopropylacrylamide)s: Synthesis, thermal/AIE properties, and cell viability. J. Taiwan Inst. Chem. Eng. 2022, 133, 104238. [Google Scholar] [CrossRef]
- Chou, L.-C.; Mohamed, M.G.; Kuo, S.-W.; Nakamura, Y.; Huang, C.-F. Synthesis of multifunctional poly(carbamoyl ester)s containing dual-cleavable linkages and an AIE luminogen via Passerini-type multicomponent polymerization. Chem. Commun. 2022, 58, 12317–12320. [Google Scholar] [CrossRef]
- Tong, J.; Zhang, K.; Wang, J.; Li, H.; Zhou, F.; Wang, Z.; Zhang, X.; Tang, B.Z. Keto-salicylaldehyde azine: Asymmetric substituent effect on their optical properties via electron-donating group insertion. J. Mater. Chem. C 2020, 8, 996–1001. [Google Scholar] [CrossRef]
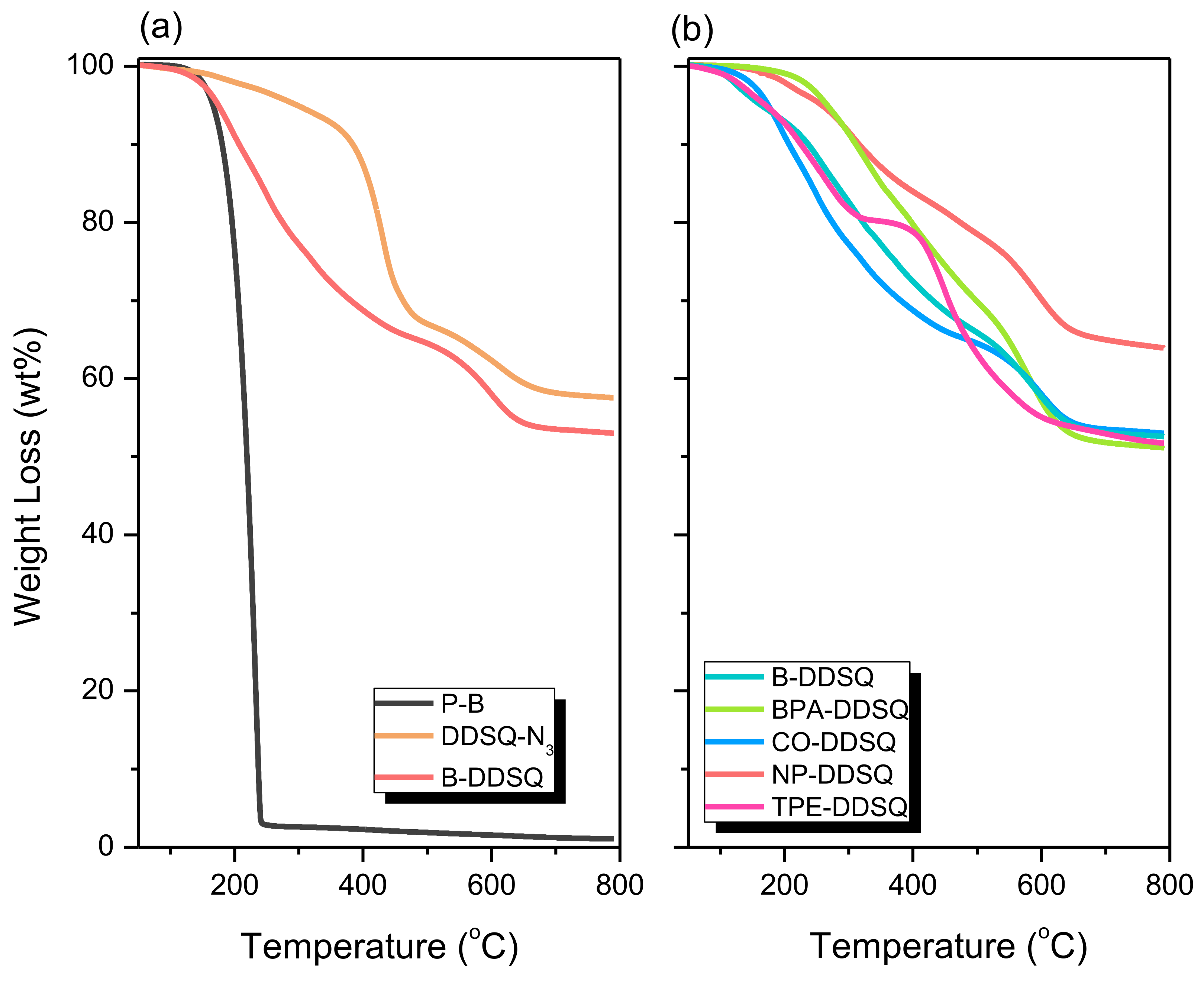
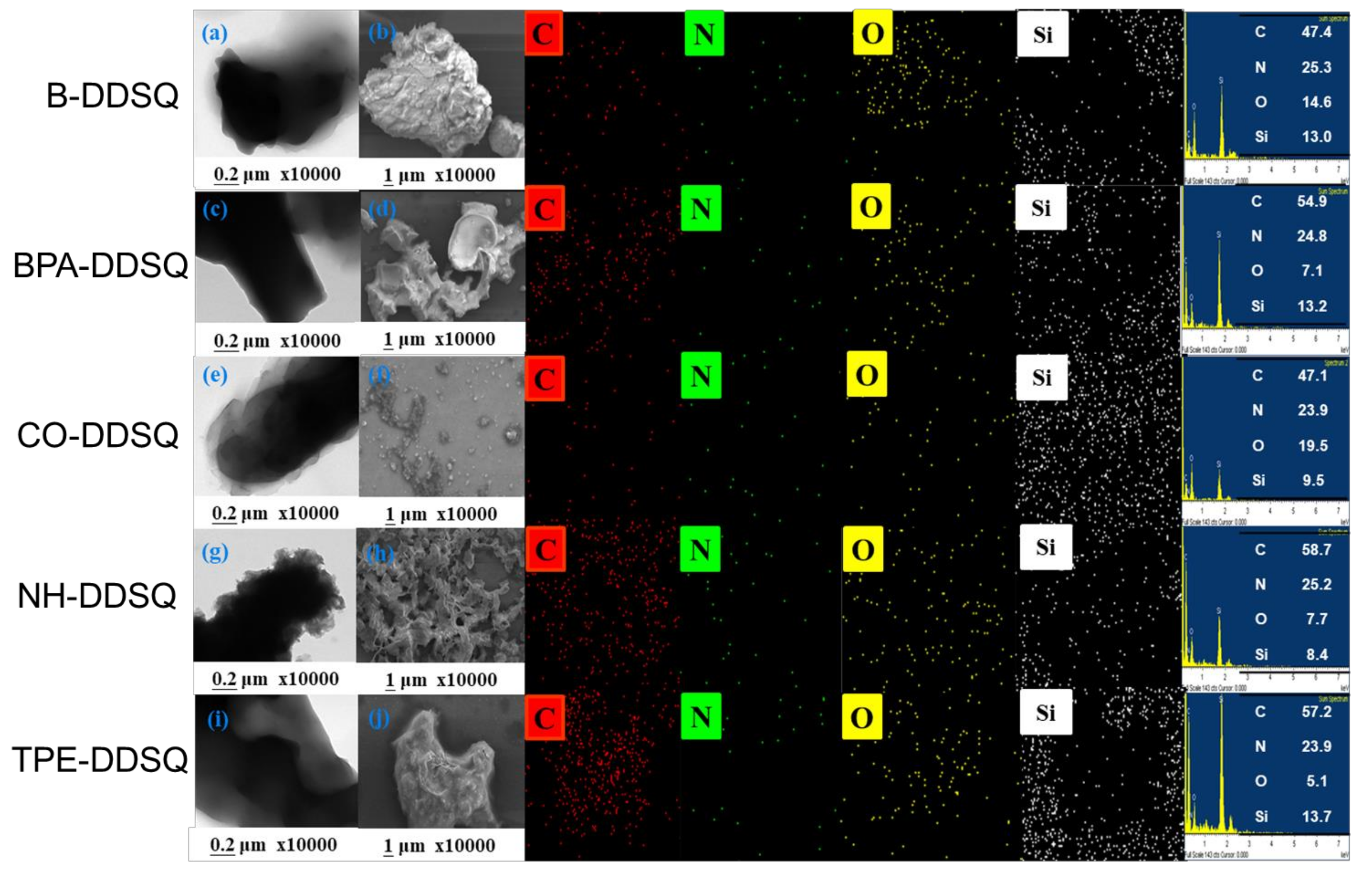
| Sample | Td10 (°C) | Char Yield (wt%) | Mn (g/mol) | PDI | Tg |
|---|---|---|---|---|---|
| B-DDSQ | 206 | 53.5 | 3270 | 1.30 | 94 |
| BPA-DDSQ | 235 | 53.0 | 2930 | 1.54 | 65 |
| CO-DDSQ | 309 | 51.6 | 3600 | 1.31 | 67 |
| NP-DDSQ | 315 | 65.1 | 4860 | 1.24 | 89 |
| TPE-DDSQ | 383 | 57.5 | 4720 | 1.10 | 168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-H.; Mohamed, M.G.; Chen, W.-C.; Madhu, M.; Tseng, W.-L.; Kuo, S.-W. Construction of Fluorescent Conjugated Polytriazole Containing Double-Decker Silsesquioxane: Click Polymerization and Thermal Stability. Polymers 2023, 15, 331. https://doi.org/10.3390/polym15020331
Chiang C-H, Mohamed MG, Chen W-C, Madhu M, Tseng W-L, Kuo S-W. Construction of Fluorescent Conjugated Polytriazole Containing Double-Decker Silsesquioxane: Click Polymerization and Thermal Stability. Polymers. 2023; 15(2):331. https://doi.org/10.3390/polym15020331
Chicago/Turabian StyleChiang, Chia-Husan, Mohamed Gamal Mohamed, Wei-Cheng Chen, Manivannan Madhu, Wei-Lung Tseng, and Shiao-Wei Kuo. 2023. "Construction of Fluorescent Conjugated Polytriazole Containing Double-Decker Silsesquioxane: Click Polymerization and Thermal Stability" Polymers 15, no. 2: 331. https://doi.org/10.3390/polym15020331
APA StyleChiang, C.-H., Mohamed, M. G., Chen, W.-C., Madhu, M., Tseng, W.-L., & Kuo, S.-W. (2023). Construction of Fluorescent Conjugated Polytriazole Containing Double-Decker Silsesquioxane: Click Polymerization and Thermal Stability. Polymers, 15(2), 331. https://doi.org/10.3390/polym15020331