Review of Soil Quality Improvement Using Biopolymers from Leather Waste
Abstract
:1. Introduction
- (a)
- untanned leather waste from the processing of raw and gray leather: wax (hypodermic layer) and gelatine skin (fringes and cuttings from the shaping of the leather contour), which represents the dermal layer without epidermis, hypodermis and hair;
- (b)
- tanned leather waste from the processing of tanned and finished hides, from the leather footwear and clothing industry (tanned leather and finished leather).
2. Comparison among Biopolymer-Based Fertilizers Obtained from Leather Waste and Other Types of Fertilizers (Chemical Fertilizer, Compost, etc.) Applied for Crop Growth
3. Nutrient Releasing Processes
4. Use of Biopolymers for Soil Remediation and Stabilization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Udayakumar, G.; Muthusamy, P.; Selvaganesh, S.B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, L.P. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Korhonen, J.; Snäkin, J.P. Quantifying the relationship of resilience and eco-efficiency in complex adaptive energy systems. Ecol. Econ. 2015, 120, 83–92. [Google Scholar] [CrossRef]
- Mattsson, B.; Cederberg, C.; Blix, I. Agricultural land use in life cycle assessment (LCA): Case studies of three vegetable oil crops. J. Clean. Prod. 2000, 8, 283–292. [Google Scholar] [CrossRef]
- Castro, C.; Logan, T.J. Liming effects on the stability and erodibility of some Brazilian oxisols. Am. J. Soil Sci. Soc. 1991, 55, 1407–1413. [Google Scholar] [CrossRef]
- Håkansson, I.; Medvedev, V.W. Protection of soils from mechanical overloading by establishing limits for stresses caused by heavy vehicles. Soil Tillage Res. 1995, 35, 85–97. [Google Scholar] [CrossRef]
- Awad, Y.M.; Lee, S.S.; Kim, K.-H.; Ok, Y.S.; Kuzyakov, Y. Carbon and nitrogen mineralization and enzyme activities in soil aggregate-size classes: Effects of biochar, oyster shells, and polymers. Chemosphere 2018, 198, 40–48. [Google Scholar] [CrossRef]
- Zainescu, G.; Voicu, P.; Constantinescu, R.; Barna, E. Biopolymers from protein wastes used in industry and agriculture. Rev. Ind. Text. 2011, 62, 34–37. [Google Scholar]
- Zainescu, G.A.; Stoian, C.; Constantinescu, R.R.; Voicu, P.; Arsene, M.; Mihalache, M. Innovative process for obtaining biopolymers from leather wastes for degraded soils remediation. In Proceedings of the 10th International Conference on Colloids and Surfaces Chemistry, Galati, Romania, 9–11 June 2011. [Google Scholar]
- Tal, A. Making Conventional Agriculture Environmentally Friendly: Moving beyond the Glorification of Organic Agriculture and the Demonization of Conventional Agriculture. Sustainability 2018, 10, 1078. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization. Available online: http://www.fao.org/faostat/en (accessed on 25 January 2022).
- Kılıc, O.; Boz, I.; Eryılmaz, G.A. Comparison of conventional and good agricultural practices farms: A socio-economic and technical perspective. J. Clean. Prod. 2020, 258, 120666. [Google Scholar] [CrossRef]
- Horue, M.; Berti, I.R.; Cacicedo, M.L.; Castro, G.R. Microbial production and recovery of hybrid biopolymers from wastes for industrial applications—A review. Bioresour. Technol. 2021, 340, 125671. [Google Scholar] [CrossRef]
- Rutz, A.L.; Shah, R.N. Protein Based Hydrogels in Polymeric Hydrogels as Smart Biomaterials, 1st ed.; Kalia, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 73–104. [Google Scholar]
- Ucar, B. Natural biomaterials in brain repair: A focus on collagen. Neurochem. Int. 2021, 146, 105033. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Osmalek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Gu, B.H.; Doner, H.E. The interaction of polysaccharides with silver hill illite. Clays Clay Miner. 1992, 40, 151–156. [Google Scholar] [CrossRef]
- Chang, I.; Cho, G.C. Strengthening of Korean residual soil with β-1,3/1,6-glucan biopolymer. Constr. Build. Mater. 2012, 30, 30–35. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.M.; Im, J.; Cho, G.C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, M.; Wang, Q.; Wang, Y.F.; Liu, J.; Cao, C.; Zheng, W.; Bao, Y.; Rocchi, I. Use of Sulfur-Free Lignin as a novel soil additive: A multi-scale experimental investigation. Eng. Geol. 2020, 269, 105551. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Cho, G.C. Introduction of microbial biopolymers in soil treatment for future environmentally-friendly and sustainable geotechnical engineering. Sustainability 2016, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Latifi, N.; Horpibulsuk, S.; Meehan, C.L.; Abd Majid, M.Z.; Md Tahir, M.; Tonnizam Mohamad, E. Improvement of Problematic Soils with Biopolymer—An Environmentally Friendly Soil Stabilizer. J. Mater. Civ. Eng. 2017, 29, 04016204. [Google Scholar] [CrossRef]
- Orts, W.J.; Roa-Espinosa, A.; Sojka, R.E.; Glenn, G.M.; Imam, S.H.; Erlacher, K.; Pedersen, J.S. Use of synthetic polymers and biopolymers for soil stabilization in agricultural, construction, and military applications. J. Mater. Civ. Eng. 2007, 19, 58–66. [Google Scholar] [CrossRef]
- Georgees, R.N.; Hassan, R.A.; Evans, R.P. A potential use of a hydrophilic polymeric material to enhance durability properties of pavement materials. Constr. Build. Mater. 2017, 148, 686–695. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, A.E.; Little, N.D. A state-of-the-art review of polymers used in soil stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durani, A.J.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Chen, G.; Yi, S.H.; Wang, Y.; Liu, Q.; Wang, R. Multifunctional porous hydrogel with nutrient controlled-release and excellent biodegradation. J. Environ. Chem. Eng. 2021, 9, 106146. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Wang, Y.; Hu, Z.; Wang, R. Characterization of slow-release collagen-g-poly(acrylic acid-co-2-acrylamido-2-methyl-1-propane sulfonic acid)–iron(III) superabsorbent polymer containing fertilizer. J. Appl. Polym. Sci. 2019, 136, 47178. [Google Scholar] [CrossRef]
- Ibrahim, S.; Nawwar, G.A.M.; Sultan, M. Development of bio-based polymeric hydrogel: Green, sustainable and low cost plant fertilizer packaging material. J. Environ. Chem. Eng. 2016, 4, 203–210. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; Abd El-Hai, F.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.Z.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–463. [Google Scholar] [CrossRef]
- Bremner, J.M. Problems in the use of urea as a nitrogen fertilizer. Soil Use Manag. 1990, 6, 70–71. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Benlloch, E.; Herrera, J. Effect of traditional and slow-release N fertilizers on growth of olive nursery plants and N losses by leaching. Sci. Hortic. 2004, 101, 39–49. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, Y.Z.; Du, C.W.; Zhou, J.M.; Wang, H.Y.; Chen, X.Y. Evaluation of waterborne coating for controlled-release fertilizer using wurster fluidized bed. Ind. Eng. Chem. Res. 2010, 49, 9644–9647. [Google Scholar]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef]
- Yang, L.; An, D.; Wang, T.J.; Kan, C.Y.; Jin, Y. Photodegradation of polymer materials used for film coatings of controlled-release fertilizers. Chem. Eng. Technol. 2017, 40, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Rajamani, S.; Chen, Z.G.; Zhang, S.H. Recent developments in cleaner production and environment protection in world leather sector. In Proceedings of the XXX Congress of the International Union of Leather Technologists and Chemists Societies, Beijing, China, 11–14 October 2009; pp. 69–73. [Google Scholar]
- Silva, J.D.C.; Leal, T.T.B.; Araújo, A.S.F.; Araujo, R.M.; Gomes, R.L.F.; Melo, W.J.; Singh, R.P. Effect of different tannery sludge compost amendment rates on growth, biomass accumulation and yield responses of Capsicum plants. Waste Manag. 2010, 30, 1976–1980. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Deng, W.; Wang, M.; Ma, S. Ecological utilization of leather tannery waste with circular economy model. J. Clean. Prod. 2011, 19, 221–228. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Ni, M.; Liu, S.; Lu, L.; Xie, Y. Environmentally friendly slow-release nitrogen fertilizer. J. Agric. Food Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef]
- Hassan, M.; Bai, J.; Dou, D.-Q. Biopolymers; definition, classification and applications. Egypt. J. Chem. 2019, 62, 1725–1737. [Google Scholar] [CrossRef]
- Sathish, M.; Madhan, B.; Raghava Rao, J. Leather solid waste: An eco-benign raw material for leather chemical preparation—A circular economy example. Waste Manag. 2019, 87, 357–367. [Google Scholar] [CrossRef]
- Majee, S.; Halder, G.; Mandal, T. Formulating nitrogen-phosphorous-potassium enriched organic manure from solid waste: A novel approach of waste valorization. Process Saf. Environ. Prot. 2019, 132, 160–168. [Google Scholar] [CrossRef]
- Al-Jabari, M.; Sawalha, H.; Pugazhendhi, A.; Rene, E.R. Cleaner production and resource recovery opportunities in leather tanneries: Technological applications and perspectives. Bioresour. Technol. Rep. 2021, 16, 100815. [Google Scholar] [CrossRef]
- Hansen, E.; Monteiro de Aquim, P.; Gutterres, M. Environmental assessment of water, chemicals and effluents in leather post-tanning process: A review. Environ. Impact Assess. Rev. 2021, 89, 106597. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrow, P.; Kułazynski, M. Progress in sustainable technologies of leather wastes valorization as solutions for the circular economy. J. Clean. Prod. 2021, 313, 127902. [Google Scholar] [CrossRef]
- Mutlu, M.M.; Crudu, M.; Maier, S.S.; Deselnicu, D.; Albu, L.; Gulumser, G.; Bitlisli, B.O.; Basaran, B.; Tosun, C.C.; Adiguzel Zengin, A.C. Eco-leather: Properties of chromium-free leathers produced with titanium tanning materials obtained from the wastes of metal industry. Ekoloji Int. J. Environ. 2014, 23, 83–90. [Google Scholar] [CrossRef]
- Dang, X.; Shan, Z.; Chen, H. The Preparation and Applications of One Biodegradable Liquid Film Mulching by Oxidized Corn Starch-Gelatin Composite. Appl. Biochem. Biotechnol. 2016, 180, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Enascuta, C.E.; Stanca, M.; Gaidau, C.C.; Alexe, C.; Gidea, M.; Becheritu, M. Complexes based on collagen and keratin for applications in agriculture. In Proceedings of the 8th International Conference on Advanced Materials and Systems ICAMS2020, Bucharest, Romania, 1–3 October 2020; pp. 219–224. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2017, 196, 28–38. [Google Scholar] [CrossRef]
- Stefan, D.S.; Bosomoiu, M.; Constantinescu, R.R.; Ignat, M. Composite Polymers from Leather Waste to Produce Smart Fertilizers. Polymers 2021, 13, 4351. [Google Scholar] [CrossRef]
- Adeoye, D.T.; Adeyi, O.A.; Kathir, I.B.; Ejila, A. De-chroming of Chrome Tanned Leather Solid Waste using Modified Alkaline Hydrolysis Process. Int. J. Eng. Res. Technol. 2014, 3, 620–623. [Google Scholar]
- Ferreira, M.J.; Almeida, M.F.; Pinho, S.C.; Gomes, J.R.; Rodrigues, J.L. Alkaline Hydrolysis of Chromium Tanned Leather Scrap Fibers and Anaerobic Biodegradation of the Products. Waste Biomass Valoriz. 2014, 5, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Tuomisto, H.L.; Teixeira De Mattos, M.J. Environmental impacts of cultured meat production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef]
- Nagaraju, R.K.; Khera, J.G.; Nielsen, P.H. The Combined Bioblasting Concept: Save Energy, Greenhouse Gases and Water. Int. Dye. Tech. Brief. 2013, 4, 36–38. [Google Scholar]
- Nielsen, P.H.; Oxenbøll, K.M.; Wenzel, H. Cradle-to-Gate Environmental Assessment of Enzyme Products Produced Industrially in Denmark by Novozymes A/S. Int. J. LCA 2007, 12, 432–438. [Google Scholar] [CrossRef]
- Rao, R.J.; Thanikaivelan, P.; Nair, B.U. An eco-friendly option for less-chrome and dye-free leather processing: In situ generation of natural colours in leathers tanned with Cr–Fe complex. Clean Technol. Environ. Policy 2002, 4, 115–121. [Google Scholar] [CrossRef]
- China, C.R.; Hilonga, A.; Nyandoro, S.S.; Schroepfer, M.; Kanth, S.V.; Meyer, M.; Njau, K.N. Suitability of selected vegetable tannins traditionally used in leather making in Tanzania. J. Clean. Prod. 2020, 251, 119687. [Google Scholar] [CrossRef]
- Carsote, C.; Sendrea, C.; Micu, M.-C.; Adams, A.; Badea, E. Micro-DSC, FTIR-ATR and NMR MOUSE study of the dose-dependent effects of gamma irradiation on vegetable-tanned leather: The influence of leather thermal stability. Radiat. Phys. Chem. 2021, 189, 109712. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, R.; Mi, Z.; Lyu, S.; Ma, J. Engineering a sustainable chrome-free leather processing based on novel lightfast wet-white tanning system towards eco-leather manufacture. J. Clean. Prod. 2021, 282, 124504. [Google Scholar] [CrossRef]
- Gao, D.; Cheng, Y.; Wang, P.; Li, F.; Wu, Y.; Lyu, B.; Ma, J.; Qin, J. An eco-friendly approach for leather manufacture based on P(POSS-MAA)-aluminum tanning agent combination tannage. J. Clean. Prod. 2020, 257, 120546. [Google Scholar] [CrossRef]
- Zuriaga-Agustí, E.; Galiana-Aleixandre, M.V.; Bes-Pia, A.; Mendoza-Roca, J.A.; Risueno-Puchades, V.; Segarra, V. Pollution reduction in an eco-friendly chrome-free tanning and evaluation of the biodegradation by composting of the tanned leather wastes. J. Clean. Prod. 2015, 87, 874–881. [Google Scholar] [CrossRef]
- Pradeep, S.; Sundaramoorthy, S.; Sathish, M.; Jayakumar, G.C.; Rathinam, A.; Madhan, B.; Saravanan, P.; Rao, J.R. Chromium-free and waterless vegetable-aluminium tanning system for sustainable leather manufacture. Chem. Eng. J. Adv. 2021, 7, 100108. [Google Scholar] [CrossRef]
- Beghetto, V.; Agostinis, L.; Gatto, V.; Samiolo, R.; Scrivanti, A. Sustainable use of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride as metal free tanning agent. J. Clean. Prod. 2019, 220, 864–872. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Sadulla, S.; Sehgal, P.K.; Mandal, A.B. Green chemistry approaches to leather tanning process for making chrome-free leather by unnatural amino acids. J. Hazard. Mater. 2012, 215–216, 173–182. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Sadulla, S.; Sehgal, P.K.; Mandal, A.B. Greener approach to leather tanning process: D-Lysine aldehyde as novel tanning agent for chrome-free tanning. J. Clean. Prod. 2013, 42, 277–286. [Google Scholar] [CrossRef]
- Wu, X.; Qiang, X.; Liu, D.; Yu, L.; Wang, X. An eco-friendly tanning process to wet-white leather based on amino acids. J. Clean. Prod. 2020, 270, 122399. [Google Scholar] [CrossRef]
- Stefan, D.S.; Zainescu, G.; Manea-Saghin, A.M.; Triantaphyllidou, I.E.; Tzoumani, I.; Tatoulis, T.I.; Syriopoulos, G.T.; Meghea, A. Collagen-Based Hydrogels Composites from Hide Waste to Produce Smart Fertilizers. Materials 2020, 13, 4396. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Zhang, S. A multifunctional eco-friendly fertilizer used keratin-based superabsorbent as coatings for slow-release urea and remediation of contaminated soil. Prog. Org. Coat. 2021, 154, 106158. [Google Scholar] [CrossRef]
- Dang, X.; Yang, M.; Zhang, B.; Chen, H.; Wang, Y. Recovery and utilization of collagen protein powder extracted from chromium leather scrap waste. Environ. Sci. Pollut. Res. 2019, 26, 7277–7283. [Google Scholar] [CrossRef]
- Cheruiyot, G.; Sirmah, P.; Ng’etich, W.; Mengich, E.; Mburu, F.; Kimaiyo, S.; Bett, E. Effects of Hydrogels on Soil Moisture and Growth of Cajanus cajan in Semi Arid Zone of Kongelai, West Pokot County. Open J. For. 2014, 4, 34–37. [Google Scholar]
- Majee, S.; Halder, G.; Mandal, D.D.; Tiwari, O.N.; Mandal, T. Transforming wet blue leather and potato peel into an eco-friendly bio-organic NPK fertilizer for intensifying crop productivity and retrieving value-added recyclable chromium salts. J. Hazard. Mater. 2021, 411, 125046. [Google Scholar] [CrossRef]
- Farneselli, M.; Tosti, G.; Onofri, A.; Benincasa, P.; Guiducci, M.; Pannacci, E.; Tei, F. Effects of N sources and management strategies on crop growth, yield and potential N leaching in processing tomato. Eur. J. Agron. 2018, 98, 46–54. [Google Scholar] [CrossRef]
- Constantinescu, R.R.; Zainescu, G.; Stefan, D.S.; Sirbu, C.; Voicu, P. Protein biofertilizer development and application on soybean cultivated degraded soil. Leather Footwear J. 2015, 15, 169–178. [Google Scholar] [CrossRef]
- Zainescu, G.; Deselnicu, D.C.; Ioannidis, I.; Crudu, M.; Voicu, P. New versatile conversion technology for wet white waste transformation to biofertiliser. In Proceedings of the 4th International Conference on Advanced Materials and Systems ICAMS2012, Bucharest, Romania, 27–29 September 2012; Volume 1, p. 8, ISSN 2068-0783. [Google Scholar]
- Lima, D.Q.; Oliveira, L.C.A.; Bastos, A.R.R.; Carvalho, G.S.; Marques, J.G.S.M.; Carvalho, J.G.; de Souza, G.A. Leather Industry Solid Waste as Nitrogen Source for Growth of Common Bean Plants. Appl. Environ. Soil Sci. 2010, 2010, 703842. [Google Scholar] [CrossRef]
- Simple fertilizers. Available online: https://www.azomures.com/en/fertilizers/ (accessed on 29 April 2022).
- Dang, X.; Shan, Z.; Chen, H. Biodegradable films based on gelatin extracted from chrome leather scrap. Int. J. Biol. Macromol. 2018, 107, 1023–1029. [Google Scholar] [CrossRef]
- Ravindran, B.; Lee, S.R.; Chang, S.W.; Nguyen, D.D.; Chung, W.J.; Balasubramanian, B.; Mupambwa, H.A.; Arasu, M.V.; Al-Dhabi, N.A.; Sekaran, G. Positive effects of compost and vermicompost produced from tannery waste-animal fleshing on the growth and yield of commercial crop-tomato (Lycopersicon esculentum L.) plant. J. Environ. Manag. 2019, 234, 154–158. [Google Scholar] [CrossRef]
- Vig, A.P.; Singh, J.; Wani, S.H.; Singh Dhaliwal, S. Vermicomposting of tannery sludge mixed with cattle dung into valuable manure using earthworm Eisenia fetida (Savigny). Bioresour. Technol. 2011, 102, 7941–7945. [Google Scholar] [CrossRef]
- Ravindran, B.; Dinesh, S.L.; Kennedy, L.J.; Sekaran, G. Vermicomposting of solid waste generated from leather industries using epigeic earthworm Eisenia foetida. Appl. Biochem. Biotechnol. 2008, 151, 480–488. [Google Scholar] [CrossRef]
- Singh, D.; Suthar, S. Vermicomposting of herbal pharmaceutical industry waste: Earthworm growth, plant-available nutrient and microbial quality of end materials. Bioresour. Technol. 2012, 112, 179–185. [Google Scholar] [CrossRef]
- Molina, M.J.; Soriano, M.D.; Ingelmo, F.; Llinares, J. Stabilisation of sewage sludge and vinasse bio-wastes by vermicomposting with rabbit manure using Eisenia fetida. Bioresour. Technol. 2013, 137, 88–97. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, A. Vermidegradation for faster remediation of chemical sludge and spent carbon generated. Soft Drink Ind. 2013, 1, 13–20. [Google Scholar]
- Singh, J.; Kaur, A.; Vig, A.P. Bioremediation of distillery sludge into soil-enriching material through vermicomposting with the help of Eisenia fetida. Appl. Biochem. Biotechnol. 2014, 174, 1403–1419. [Google Scholar] [CrossRef]
- Ravindran, B.; Contreras-Ramos, S.M.; Wong, J.W.C.; Selvam, A.; Sekaran, G. Nutrient and enzymatic changes of hydrolysed tannery solid waste treated with epigeic earthworm Eudrilus eugeniae and phytotoxicity assessment on selected commercial crops. Environ. Sci. Pollut. Res. Int. 2014, 21, 641–651. [Google Scholar] [CrossRef]
- Singh, S.; Bhat, S.A.; Singh, J.; Kaur, R.; Vig, A.P. Earthworms converting milk processing industry sludge into biomanure. Open Waste Manag. J. 2017, 10, 30–40. [Google Scholar] [CrossRef]
- Malińska, K.; Golańska, M.; Caceres, R.; Rorat, A.; Weisser, P.; Ślęzak, E. Biochar amendment for integrated composting and vermicomposting of sewage sludge—The effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour. Technol. 2017, 225, 206–214. [Google Scholar] [CrossRef]
- Nunes, R.R.; Pigatin, L.B.F.; Oliveira, T.S.; Bontempi, R.M.; Rezende, M.O.O. Vermicomposted tannery wastes in the organic cultivation of sweet pepper: Growth, nutritive value and production. Int. J. Recycl. Org. Waste Agric. 2018, 7, 313–324. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; de Melo, W.J.; Araujo, F.F.; Van den Brink, P.J. Long-term effect of composted tannery sludge on soil chemical and biological parameters. Environ. Sci. Pollut. Res. 2020, 27, 41885–41892. [Google Scholar] [CrossRef] [PubMed]
- Zainescu, G.; Barna, V.; Constantinescu, R.; Barna, E.; Voicu, P.; Sandru, L. High yield biopolymer systems obtained from leather wastes. Rev. Mater. Plast. 2011, 48, 295–298. [Google Scholar]
- Nogueira, F.G.E.; do Prado, N.T.; Oliveira, L.C.A.; Bastos, A.R.R.; Lopes, J.H.; de Carvalho, J.G. Incorporation of mineral phosphorus and potassium on leather waste (collagen): A new NcollagenPK-fertilizer with slow liberation. J. Hazard. Mater. 2010, 176, 374–380. [Google Scholar] [CrossRef]
- Nogueira, F.G.E.; Castro, I.A.; Bastos, A.R.R.; Souza, G.A.; de Carvalho, J.G.; Oliveira, L.C.A. Recycling of solid waste rich in organic nitrogen from leather industry: Mineral nutrition of rice plants. J. Hazard. Mater. 2011, 186, 1064–1069. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.C.A.; Dallago, R.M.; Nascimento Filho, I. Processo de Reciclagem dos Resíduos Sólidos de Curtumes por Extracão do Cromo e Recuperacão do Couro Descontaminado. BR PI 001538, 2004. [Google Scholar]
- Oliveira, D.Q.L.; Goncalves, M.; Oliveira, L.C.A.; Guilherme, L.R.G. Removal of As(V) and Cr(VI) from aqueous solutions using solid waste from leather industry. J. Hazard. Mater. 2008, 151, 280–284. [Google Scholar] [CrossRef]
- Dunne, K.S.; Holden, N.M.; Daly, K. A management framework for phosphorus use on agricultural soils using sorption criteria and soil test P. J. Environ. Manag. 2021, 299, 113665. [Google Scholar] [CrossRef]
- Bottinelli, N.; Maeght, J.L.; Pham, R.D.; Valentin, C.; Rumpel, C.; Pham, Q.V.; Nguyen, T.T.; Lam, D.H.; Nguyen, A.D.; Tran, T.M.; et al. Anecic earthworms generate more topsoil than they contribute to erosion—Evidence at catchment scale in northern Vietnam. Catena 2021, 201, 105186. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef]
- Treder, W.; Klamkowski, K.; Wójcik, K.; Tryngiel-Gać, A.; Sas-Paszt, L.; Mika, A.; Kowalczyk, W. Apple leaf macro- and micronutrient content as affected by soil treatments with fertilizers and microorganisms. Sci. Hortic. 2022, 297, 110975. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Chen, H.Y.H.; Ruan, H. Effects of elevated CO2 on the C:N stoichiometry of plants, soils, and microorganisms in terrestrial ecosystems. Catena 2021, 201, 105219. [Google Scholar] [CrossRef]
- Dong, L.; Changwen, D.; Fei, M.; Yazhen, S.; Ke, W.; Jianmin, Z. Interaction between polyacrylate coatings used in controlled-release fertilizers and soils in wheat-rice rotation fields. Agric. Ecosyst. Environ. 2019, 286, 106650. [Google Scholar]
- Xie, L.; Liu, M.; Ni, B.; Wang, Y. New Environment-Friendly Use of Wheat Straw in Slow-Release Fertilizer Formulations with the Function of Superabsorbent. Ind. Eng. Chem. Res. 2012, 51, 3855–3862. [Google Scholar] [CrossRef]
- Lubkowski, K.; Grzmil, B. Controlled release fertilizers. Pol. J. Chem. Technol. 2007, 9, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethyl cellulose and superabsorbent coated formulations. Chem. Eng. J. 2009, 155, 892–898. [Google Scholar] [CrossRef]
- Ge, J.J.; Wu, R.; Shi, X.H.; Yu, H.; Wang, M.; Li, W.J. Biodegradable polyurethane materials from bark and starch. II. Coating material for controlled-release fertilizer. J. Appl. Polym. Sci. 2002, 86, 2948–2952. [Google Scholar] [CrossRef]
- Tzoumani, I.; Lainioti, G.C.; Aletras, A.J.; Zainescu, G.; Stefan, S.; Meghea, A.; Kallitsis, J.K. Modification of Collagen Derivatives with Water-Soluble Polymers for the Development of Cross-Linked Hydrogels for Controlled Release. Materials 2019, 12, 4067. [Google Scholar] [CrossRef] [Green Version]
- Khatami, H.; O’Kelly, B. Improving mechanical properties of sand using biopolymers. J. Geotech. Geoenviron. Eng. 2012, 139, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Imeson, A. Food Stabilisers, Thickeners and Gelling Agents; John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- Chen, R.; Zhang, L.; Budhu, M. Biopolymer stabilization of mine tailings. J. Geotech. Geoenviron. Eng. 2013, 139, 1802–1807. [Google Scholar] [CrossRef]
- Acharya, R.; Pedarla, A.; Bheemasetti, T.V.; Puppala, A.J. Assessment of guar gum biopolymer treatment toward mitigation of desiccation cracking on slopes built with expansive soils. Transp. Res. Rec. 2017, 2657, 78–88. [Google Scholar] [CrossRef]
- Gupta, S.C.; Hooda, K.; Mathur, N.; Gupta, S. Tailoring of guar gum for desert sand stabilization. Indian J. Chem. Technol. 2009, 16, 507–512. [Google Scholar]
- Chang, I.; Prasidhi, A.K.; Im, J.; Cho, G.-C. Soil strengthening using thermo-gelation biopolymers. Constr. Build. Mater. 2015, 77, 430–438. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Lee, S.-W.; Cho, G.-C. Strength durability of gellan gum biopolymer treated Korean sand with cyclic wetting and drying. Constr. Build. Mater. 2017, 143, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- Pini, R.; Canarutto, S.; Guidi, G.V. Soil microaggregation as influenced by uncharged organic conditioners. Commun. Soil Sci. Plant Anal. 1994, 25, 2215–2229. [Google Scholar] [CrossRef]
- Emerson, W.W.; Greenland, D.J. Soil Aggregates—Formation and Stability. In Soil Colloids and Their Associations in Aggregates; De Boodt, M.F., Hayes, M.H.B., Herbillon, A., De Strooper, E.B.A., Tuck, J.J., Eds.; Springer: Boston, MA, USA, 1990; pp. 485–511. [Google Scholar]
- Bartoli, F.; Philippy, R.; Burtin, G. Aggregation in soils with small amounts of swelling clays. I. Aggregate stability. Eur. J. Soil Sci. 1988, 39, 593–616. [Google Scholar] [CrossRef]
- Im, J.; Tran, A.T.P.; Chang, I.; Cho, G.-C. Dynamic properties of gel-type biopolymer treated sands evaluated by Resonant Column (RC) tests. Geomech. Eng. 2017, 12, 815–830. [Google Scholar] [CrossRef]
- Lee, S.; Chang, I.; Chung, M.-K.; Kim, Y.; Kee, J. Geotechnical shear behavior of xanthan gum biopolymer treated sand from direct shear testing. Geomech. Eng. 2017, 12, 831–847. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Katzen, F.; Pühler, A.; Ielpi, L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Ayeldeen, M.K.; Negm, A.M.; El Sawwaf, M.A. Evaluating the physical characteristics of biopolymer/soil mixtures. Arab. J. Geosci. 2016, 9, 371. [Google Scholar] [CrossRef]
- Qureshi, M.U.; Chang, I.; Al-Sadarani, K. Strength and durability characteristics of biopolymer-treated desert sand. Geomech. Eng. 2017, 12, 785–801. [Google Scholar] [CrossRef]
- Lee, S.; Im, J.; Cho, G.-C.; Chang, I. Laboratory triaxial test behavior of xanthan gum biopolymer-treated sands. Geomech. Eng. 2019, 17, 445–452. [Google Scholar]
- Bouazza, A.; Gates, W.; Ranjith, P. Hydraulic conductivity of biopolymer-treated silty sand. Géotechnique 2009, 59, 71–72. [Google Scholar] [CrossRef]
- Cabalar, A.; Wiszniewski, M.; Skutnik, Z. Effects of Xanthan Gum Biopolymer on the Permeability, Odometer, Unconfined Compressive and Triaxial Shear Behavior of a Sand. Soil Mech. Found. Eng. 2017, 54, 356–361. [Google Scholar] [CrossRef]
- Chatterjee, T.; Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Coagulation of soil suspensions containing nonionic or anionic surfactants using chitosan, polyacrylamide, and polyaluminium chloride. Chemosphere 2009, 75, 1307–1314. [Google Scholar] [CrossRef]
- Reddy, I.; Seib, P.A. Paste properties of modified starches from partial waxy wheats. Cereal Chem. 1999, 76, 341–349. [Google Scholar] [CrossRef]
- Lentz, R.D.; Sojka, R.E.; Carter, D.L.; Shainberg, I. Preventing irrigation furrow erosion with small applications of polymers. Soil Sci. Soc. Am. J. 1992, 56, 1926–1932. [Google Scholar] [CrossRef] [Green Version]
- Fatehi, H.; Abtahi, S.M.; Hashemolhosseini, H.; Hejazi, S.M. A novel study on using protein based biopolymers in soil strengthening. Constr. Build. Mater. 2018, 167, 813–821. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Chung, M.-K.; Cho, G.-C. Bovine casein as a new soil strengthening binder from diary wastes. Constr. Build. Mater. 2018, 160, 1–9. [Google Scholar] [CrossRef]
- McAuliffe, K.W.; Scotter, D.R.; MacGregor, A.N.; Earl, K.D. Casein Whey Wastewater Effects on Soil Permeability. J. Environ. Qual. 1982, 11, 31–34. [Google Scholar] [CrossRef]
- Dang, X.; Yuan, H.; Shan, Z. An eco-friendly material based on graft copolymer of gelatin extracted from leather solid waste for potential application in chemical sand-fixation. J. Clean. Prod. 2018, 188, 416–424. [Google Scholar] [CrossRef]
- Chiroma, T.M.; Ebewele, R.O.; Hymore, F.K. Comparative Assessment of Heavy Metal Levels in Soil, Vegetables and Urban Grey Waste Water Used for Irrigation in Yola and Kano. Int. Ref. J. Eng. Sci. 2014, 3, 1–9. [Google Scholar]
- Soltani, A.; Deng, A.; Taheri, A.; O’Kelly, B.C. Engineering reactive clay systems by ground rubber replacement and polyacrylamide treatment. Polymers 2019, 11, 1675. [Google Scholar] [CrossRef] [Green Version]
- Georgees, R.N.; Hassan, R.A.; Evans, R.P.; Jegatheesan, P. An evaluation of performance-related properties for granular pavement materials using a polyacrylamide additive. Int. J. Pavement Eng. 2016, 19, 153–163. [Google Scholar] [CrossRef]
- Georgees, R.N.; Hassan, R.A.; Evans, R.P.; Jegatheesan, P. Performance Improvement of Granular Pavement Materials Using a Polyacrylamide-Based Additive. In Geo-China 2016: Advances in Pavement Engineering and Ground Improvement, Proceedings of the Fourth Geo-China International Conference, Shandong, China, 25–27 July 2016; Khabbaz, H., Hossain, Z., Nam, B.H., Chen, X., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2016; pp. 108–117. [Google Scholar]
- Lentz, R.D. Polyacrylamide and biopolymer effects on flocculation, aggregate stability, and water seepage in a silt loam. Geoderma 2015, 241–242, 289–294. [Google Scholar] [CrossRef]
- Tingle, J.S.; Newman, J.K.; Larson, S.L.; Weiss, C.A.; Rushing, J.F. Stabilization Mechanisms of Non-traditional Additives. J. Transp. Res. Board 2007, 1989, 59–67. [Google Scholar] [CrossRef]
- Tingle, J.S.; Santoni, R.L. Stabilization of clay soils with non-traditional additives. Transp. Res. Rec. 2003, 1819, 72–84. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Tabatabai, M.A. Inhibition of arylamidase activity in soils by toluene. Soil Biol. Biochem. 2002, 34, 229–237. [Google Scholar] [CrossRef]
- Nannipieri, P.; Kandeler, E.; Ruggiero, P. Enzyme activities and microbiological and biochemical processes in soil. In Enzymes in the Environment; Burns, R.G., Dick, R., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 1–33. [Google Scholar]
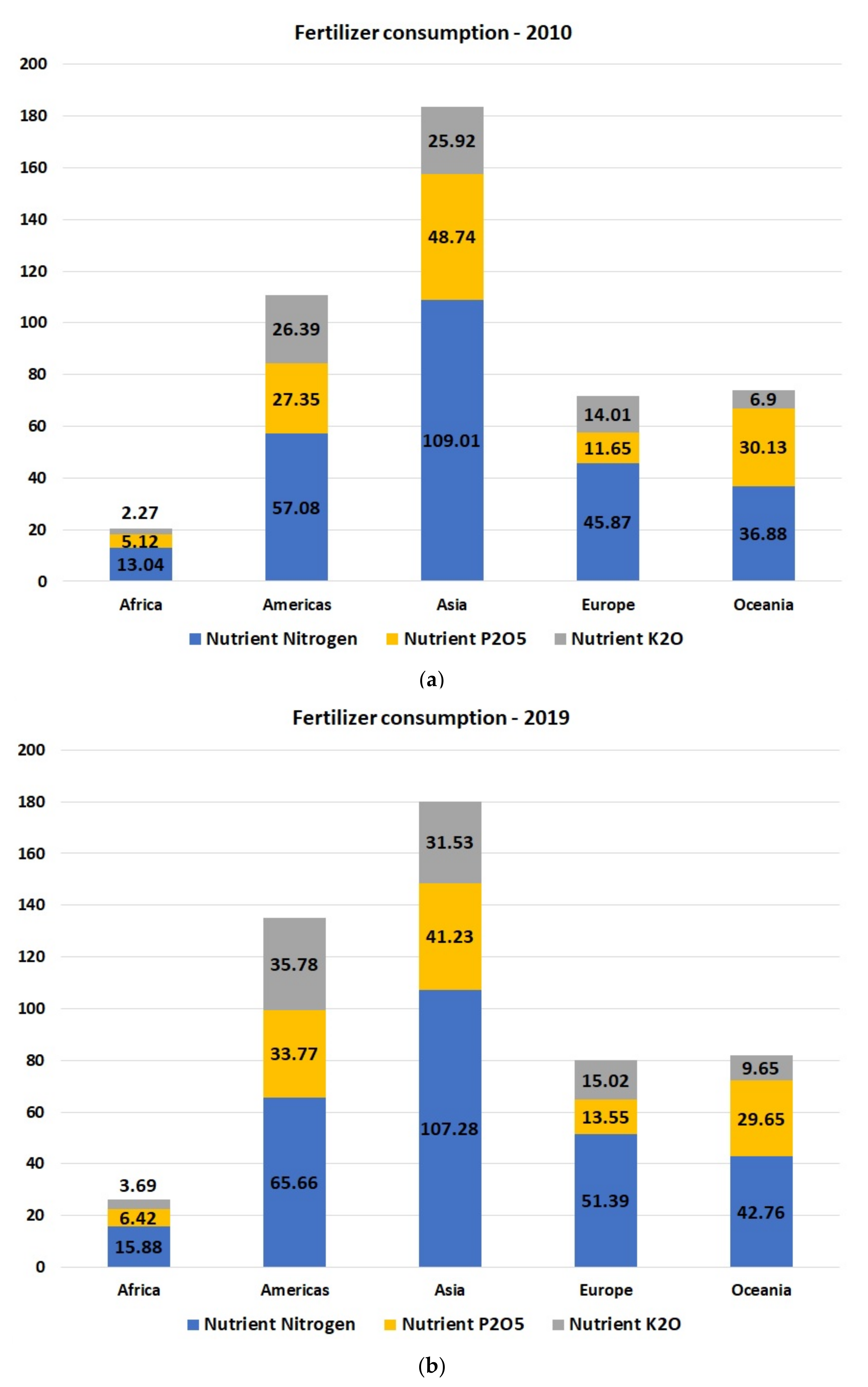
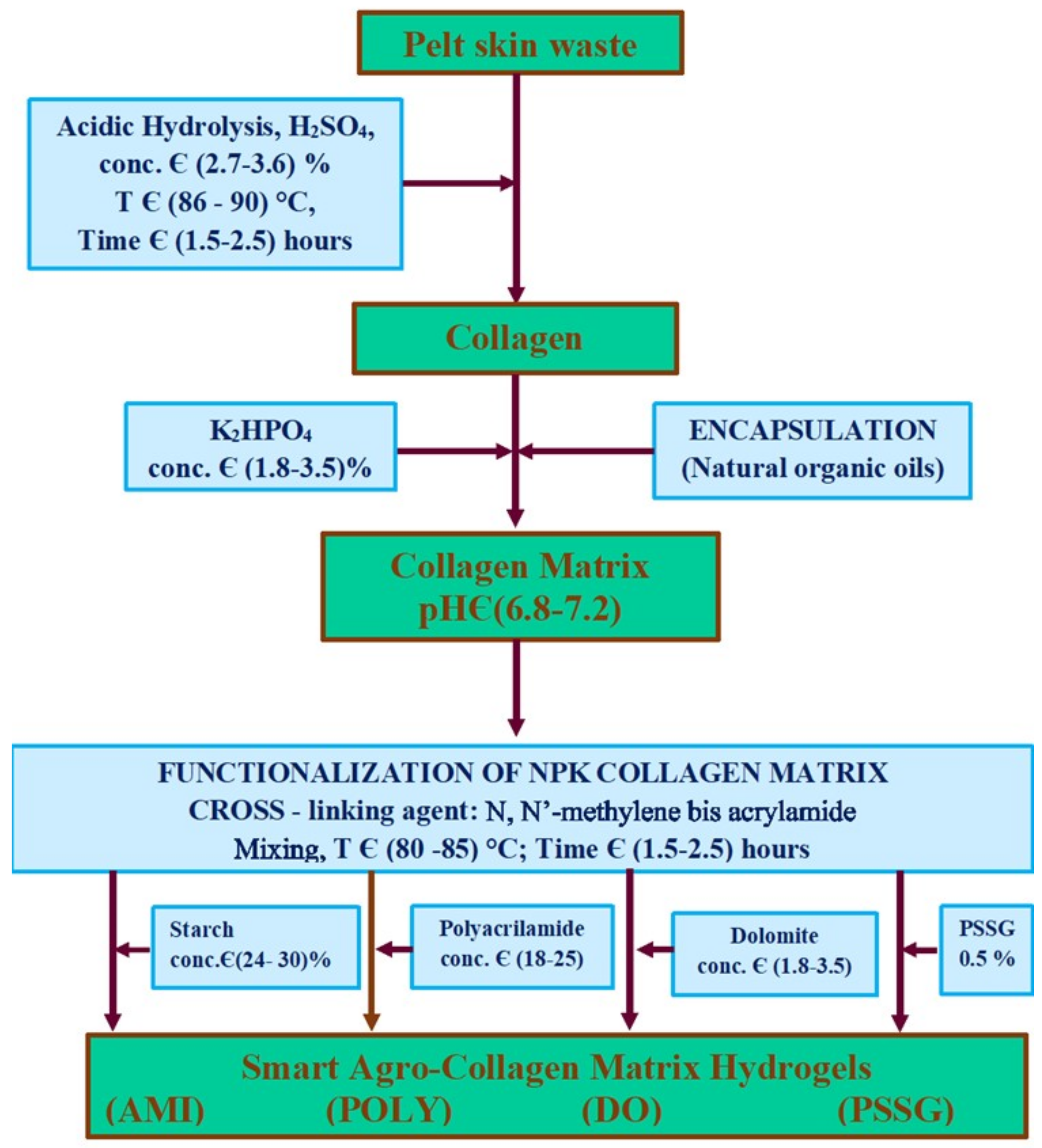



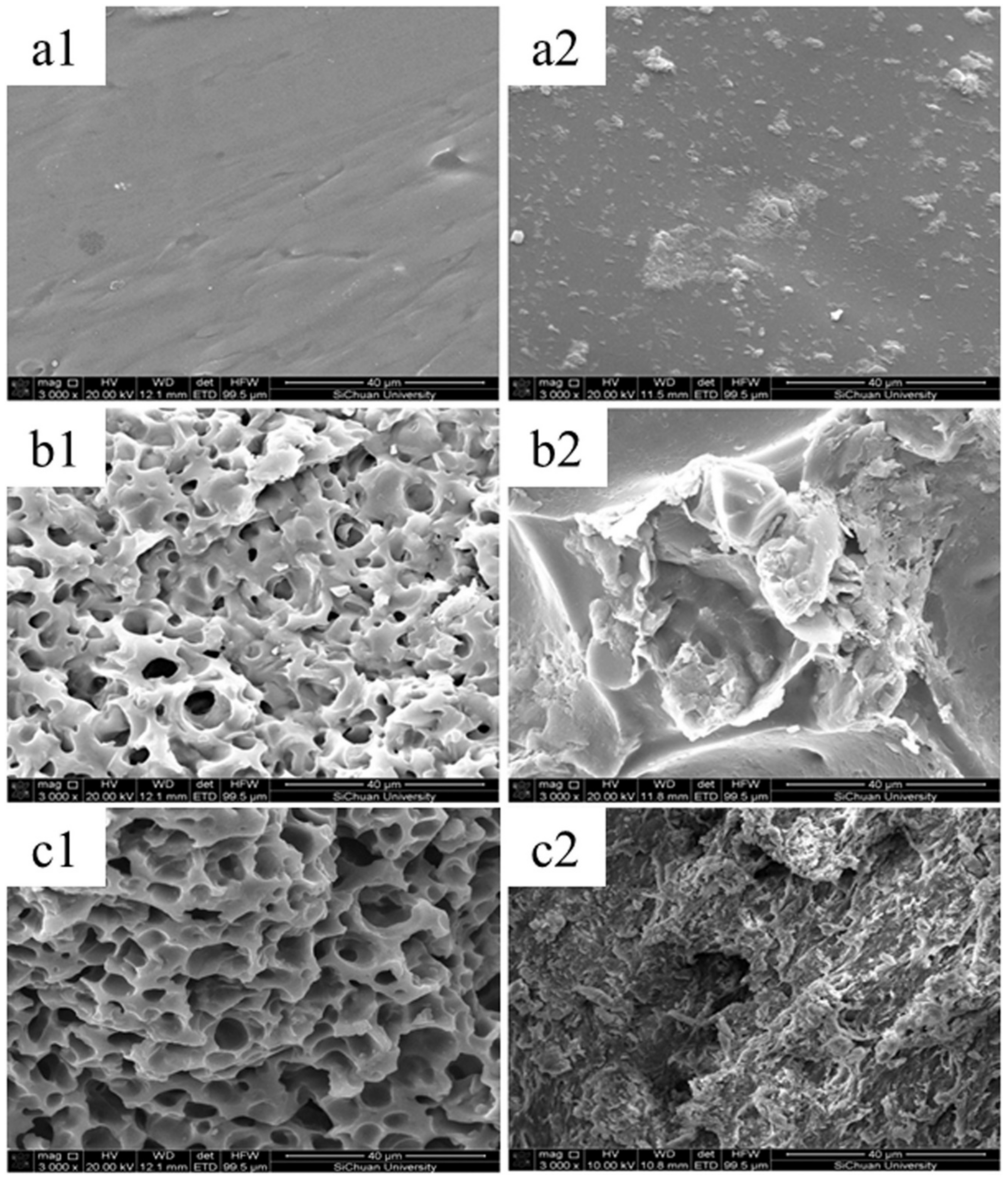
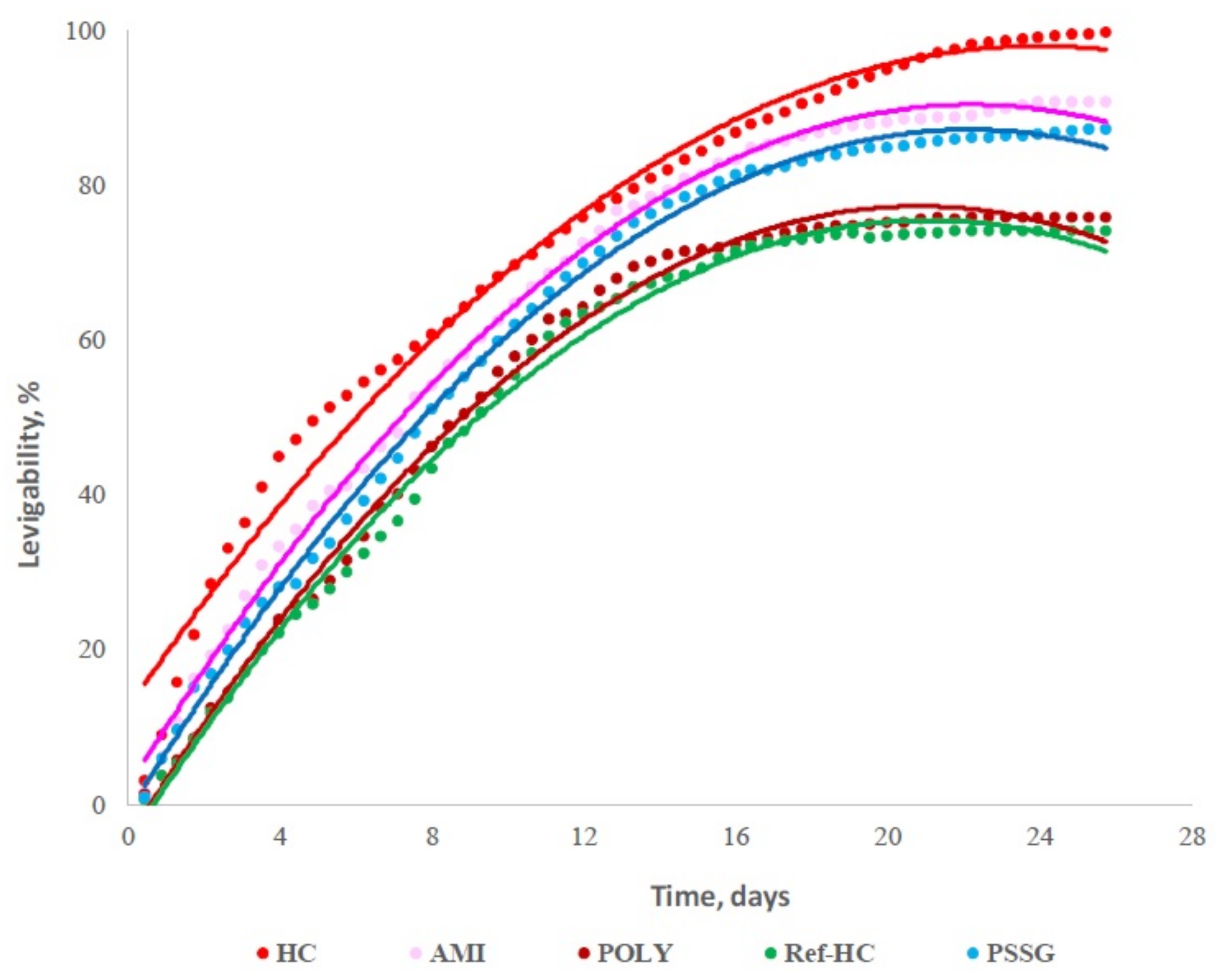
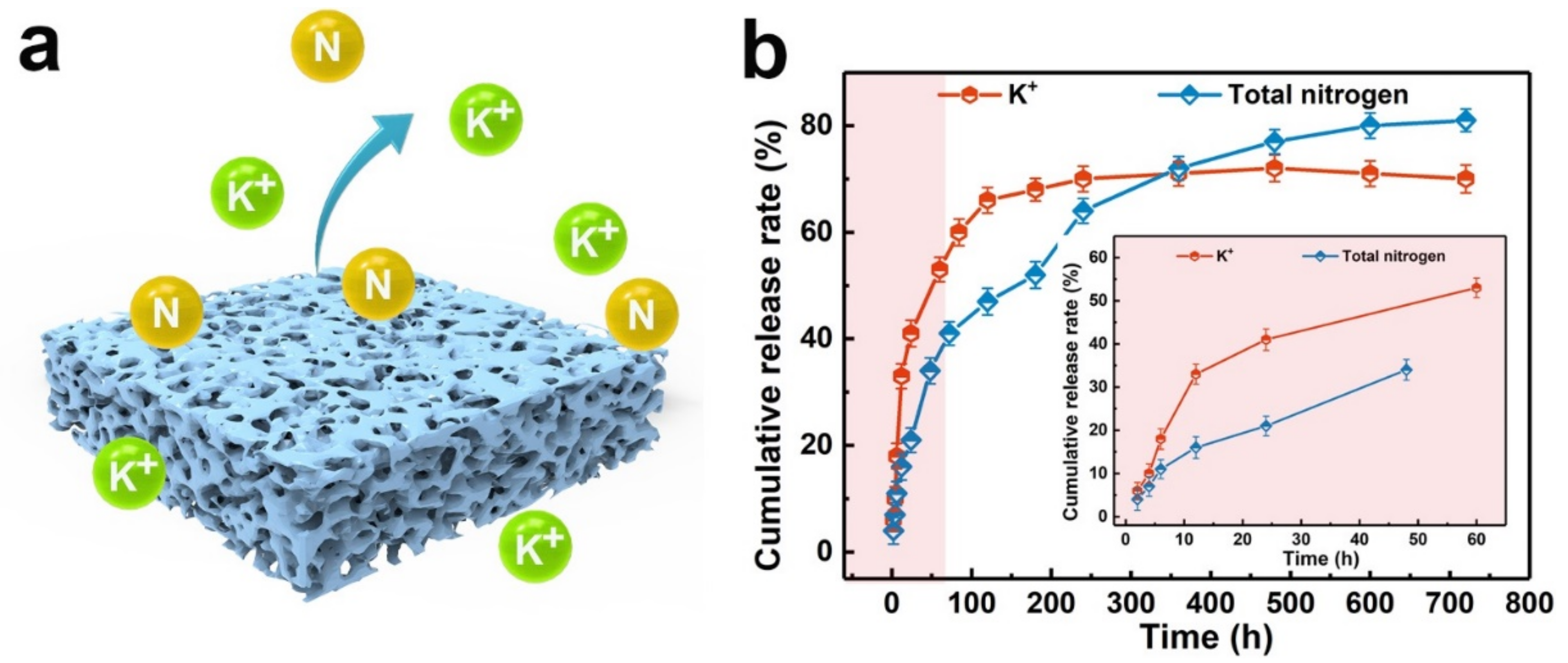
| Fertilizer Type | Soil Type | Crop | Rate | Comments |
|---|---|---|---|---|
| Poultry manure [82] | Central Italy—unspecified type | tomato | 100 kg N/ha | Does not fulfill the crop demand in nutrients |
| Poultry manure and by-product from leather factory [82] | Central Italy—unspecified type | tomato | 100 kg N/ha | The fertilizer gave the same efficacy as the mineral fertilizer. |
| Organic fertilizer by-product from leather factory [82] | Central Italy—unspecified type | tomato | 100 kg N/ha | The fertilizer gave the same efficacy as the mineral fertilizer. |
| Mineral fertilization [82] | Central Italy—unspecified type | tomato | 100 Kg N/ha and 200 Kg N/ha | The fertilizer gave the same efficacy as the fertilizers by-products from leather factory |
| Collagen-based biofertilizer [83] | stagnic albeluvisol, Romania; degraded soil classified as dusty clay soil | soybean | 10 kg fertilizer/m2 20 kg fertilizer/m2 | The second rate provided only a slightly higher production (about 0.2%), compared with the first-rate, and both gave about 20% more productivity, compared to unfertilized soil. |
| Collagen-based biofertilizer; collagen extracted from wet white leather waste [84] | Neutral or slightly alkaline soil | peas | 0.25–0.50 kg fertilizer/m2 | Good results on soil quality improvement and crop quantity. |
| Collagen extracted from wet blue leather [85] | Yellow-Red Latosol, clayey texture, Oxisol, pH = 5.9 | bean plants cultivated after the growth of elephant grass on the soil fertilized with collagen | 4, 8, 16, or 32 t collagen/ha | Results similar to mineral fertilization. |
| Fertilizer Type | % N | P, (Expressed as % P2O5) | K, (Expressed as % K2O) | Other Components | Comments | Reference |
|---|---|---|---|---|---|---|
| NPK, universal fertilizer | 26 | 13 | 6 | 0.004% Cu, 0.037% Fe, 0.03% Mn, 0.0015% Mo, 0.015% Zn | it is used for any type of culture | Produced by Azomures S.A. [86] |
| Radicon N30 | 30 | − | − | − | 7.5% NO3 − N + 7.5% NH4-N + 15% urea | [82] |
| Urea | 46 | − | − | − | − | [40] |
| Ammonium sulfate | 21 | − | − | − | − | [40] |
| Ammonium nitrate | 30.5 | − | − | − | − | [40] |
| Floranid | 32 | − | − | Low solubility material containing (3% urea-N; 29% IBDU—isobutilidenediurea -N) | [40] | |
| Fertilizer by-product from leather factory | 5 | − | − | C/N = 5.4 | The fertilizer with higher N content gave better results for tomato crop. | [82] |
| Organic fertilizer by-product from leather factory | 8 | − | C/N = 2.8 | [82] | ||
| Gelatine based fertilizer; gelatine extracted from leather waste | 43.84 (weight) | − | Not specified | 7.72% C; 40.26% O; 1.76% Na; 0.35% Al; 0.2% Si; 0.05% S; 5.28% Cl; 0.54% Ca | [87] | |
| Collagen-based biofertilizer; collagen extracted from leather waste | 11.14 | 2.43 | 3.77 | 0.127% Mg | pH of aqueous extract 7–7.5 | [83] |
| Collagen based fertilizer cross-linked with different polymers: (a) collagen hydrolysate with nutrients encapsulated as reference sample (b) collagen hydrolysate functionalized with P(SSNa-co-GMAx) copolymer (c) collagen hydrolysate functionalized with poly-acrylamide (d) collagen hydrolysate functionalized with functionalized with starch | 10.55 10.14 12.13 8.29 | 7.67 6.75 5.79 5.54 | 10.62 8.21 8.40 10.07 | (expressed as % TOC) 45.2 37.56 48.1 64.32 | pH = 7.2 pH = 6.87 pH = 6.76 pH = 6.20 | [77] |
| Collagen extracted from wet blue leather | 14.6 | 2.6 | 0.014 | Collagen was applied on a soil having the pH 5.9, and only minor changes in the soil pH were observed, in the range of 5.9–6.1 | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, D.S.; Bosomoiu, M.; Dancila, A.M.; Stefan, M. Review of Soil Quality Improvement Using Biopolymers from Leather Waste. Polymers 2022, 14, 1928. https://doi.org/10.3390/polym14091928
Stefan DS, Bosomoiu M, Dancila AM, Stefan M. Review of Soil Quality Improvement Using Biopolymers from Leather Waste. Polymers. 2022; 14(9):1928. https://doi.org/10.3390/polym14091928
Chicago/Turabian StyleStefan, Daniela Simina, Magdalena Bosomoiu, Annette Madelene Dancila, and Mircea Stefan. 2022. "Review of Soil Quality Improvement Using Biopolymers from Leather Waste" Polymers 14, no. 9: 1928. https://doi.org/10.3390/polym14091928
APA StyleStefan, D. S., Bosomoiu, M., Dancila, A. M., & Stefan, M. (2022). Review of Soil Quality Improvement Using Biopolymers from Leather Waste. Polymers, 14(9), 1928. https://doi.org/10.3390/polym14091928







