Effects of Non-Ionic Micelles on the Acid-Base Equilibria of a Weak Polyelectrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Materials
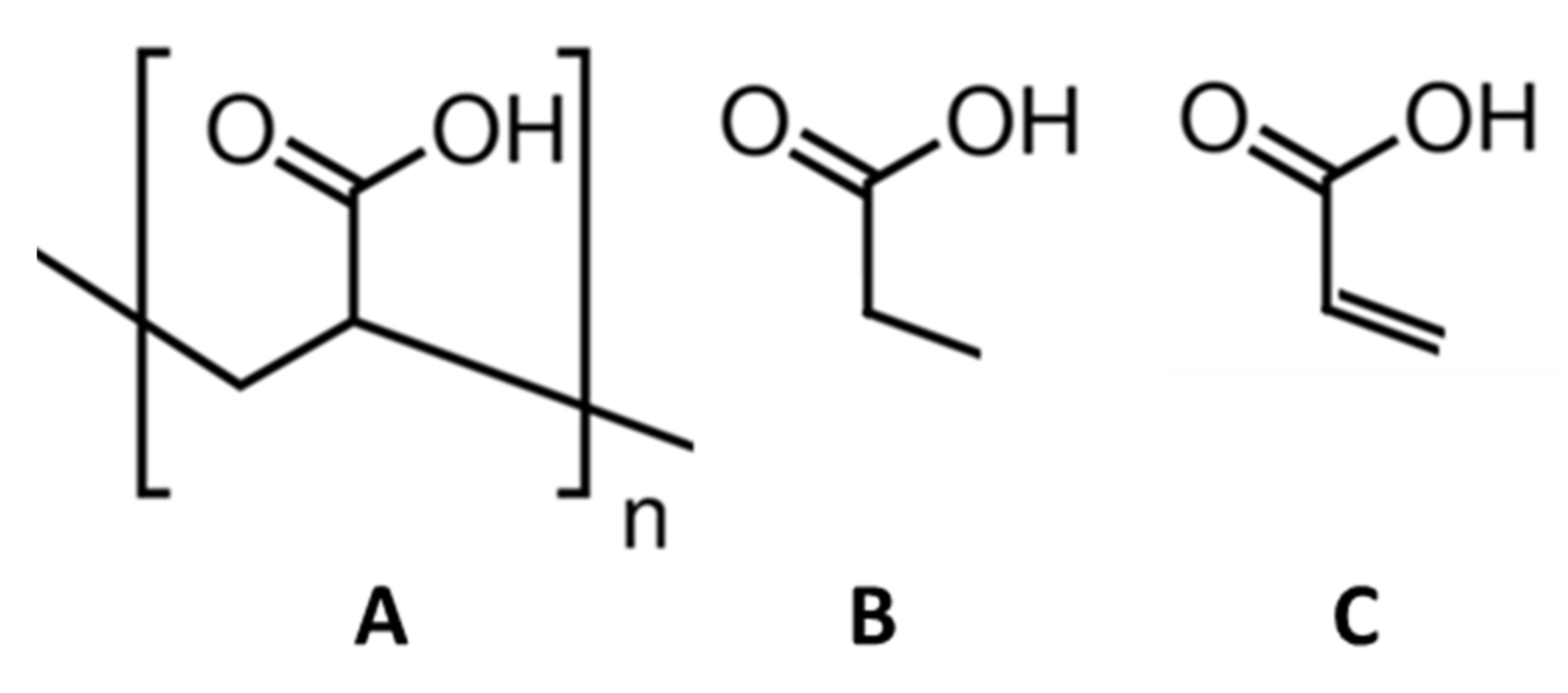
2.1.1. Polyelectrolytes
2.1.2. Acid Base
2.1.3. Non-Ionic Surfactant and Block-Copolymers
2.1.4. Solvents
2.2. Preparation of Solutions
2.3. Characterization
2.3.1. Automatic Potentiometric Titration
2.3.2. FTIR-ATR Measurement
2.3.3. Surface Tension
2.3.4. Small-Angle X-ray Scattering (SAXS)
2.3.5. Transmission Electron Microscopy (TEM)
2.3.6. Viscosity Measurements
3. Results
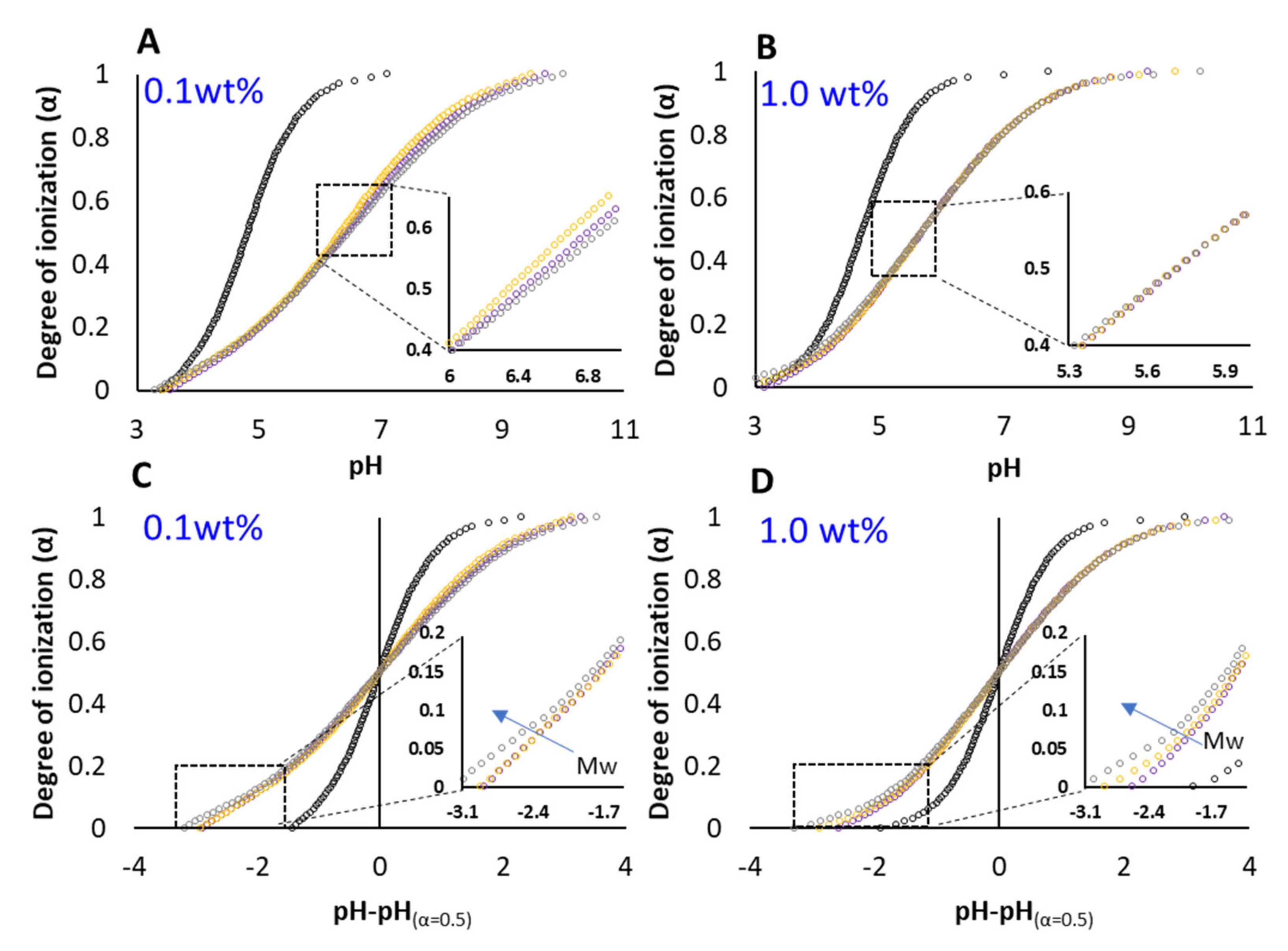
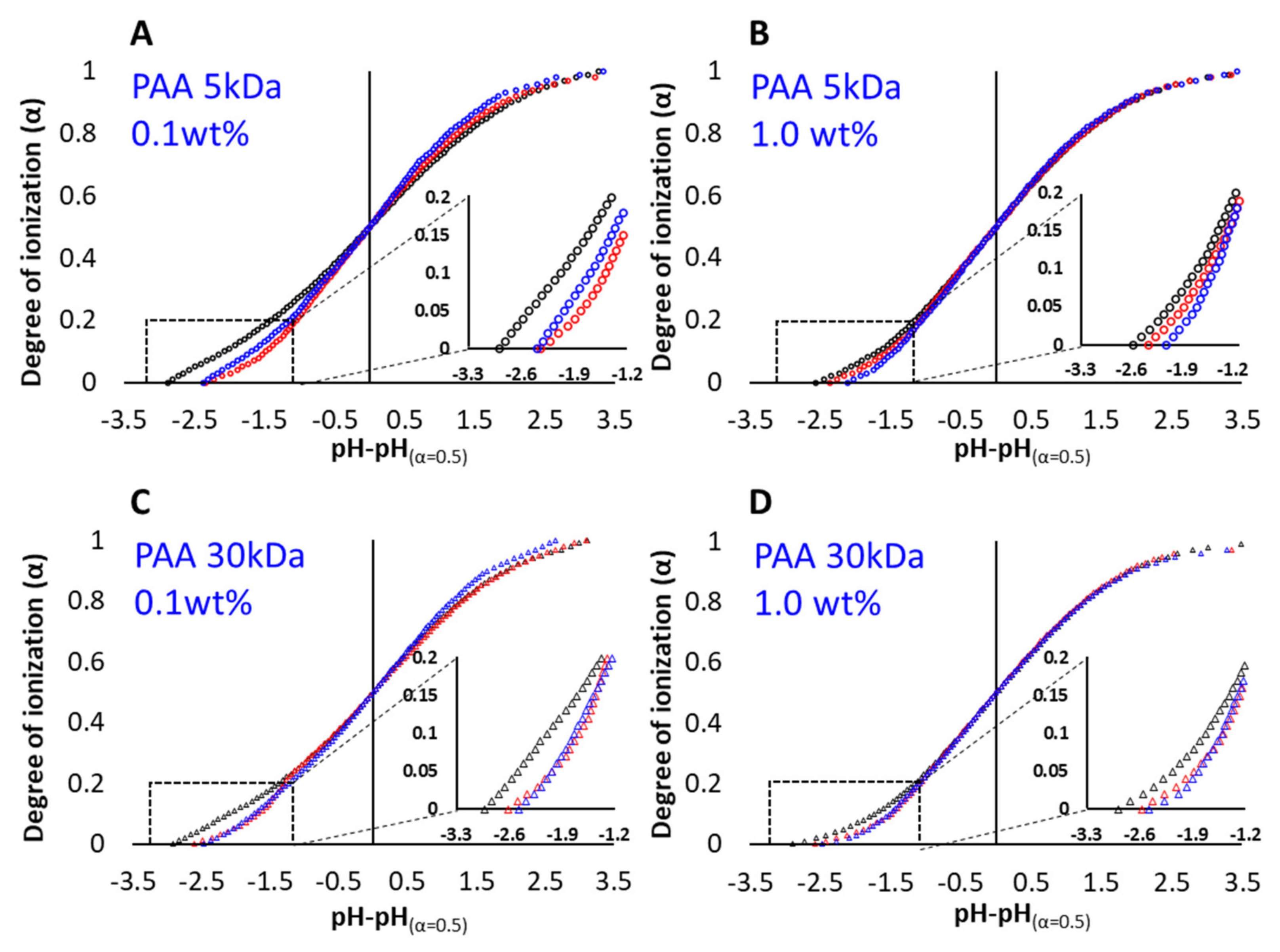
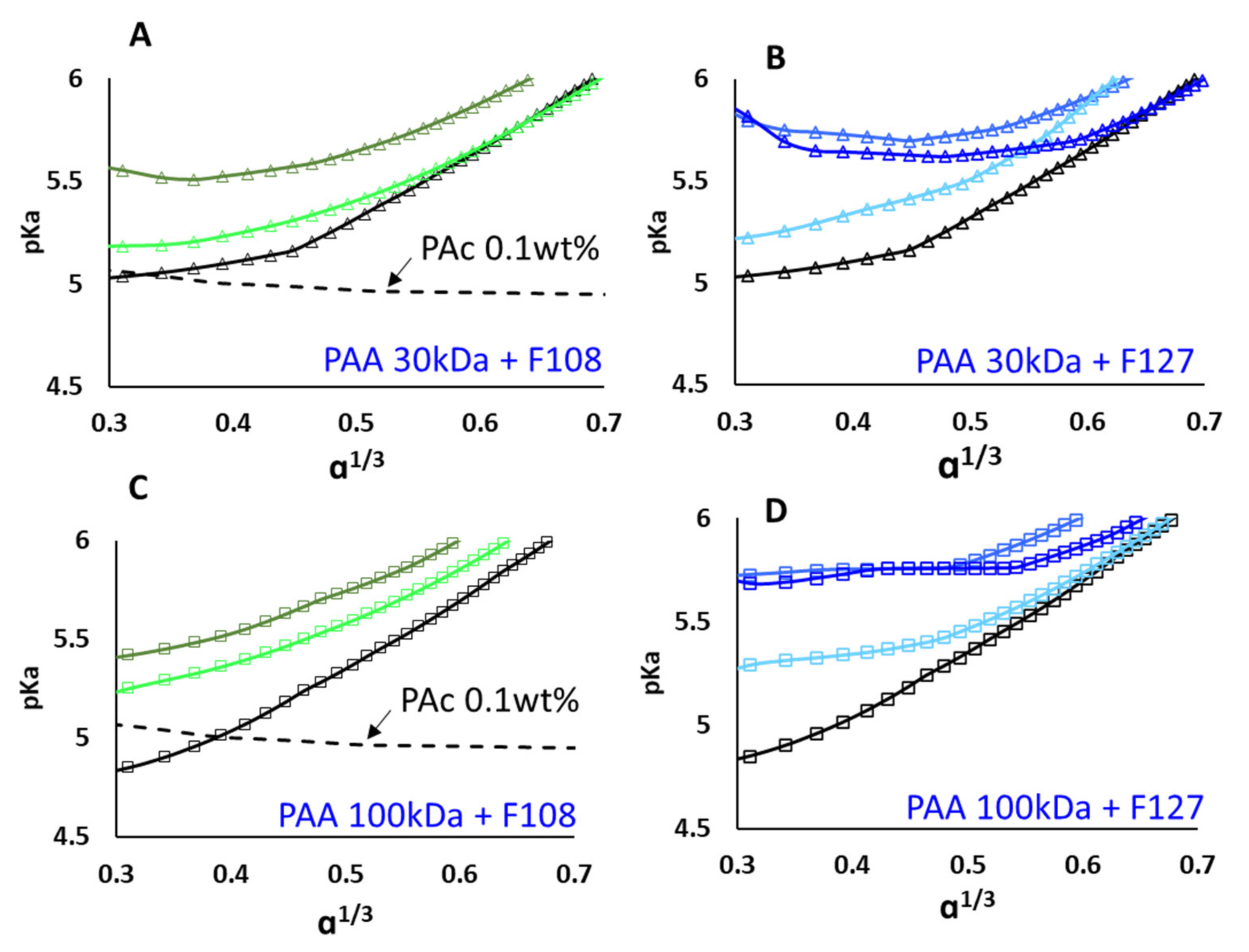
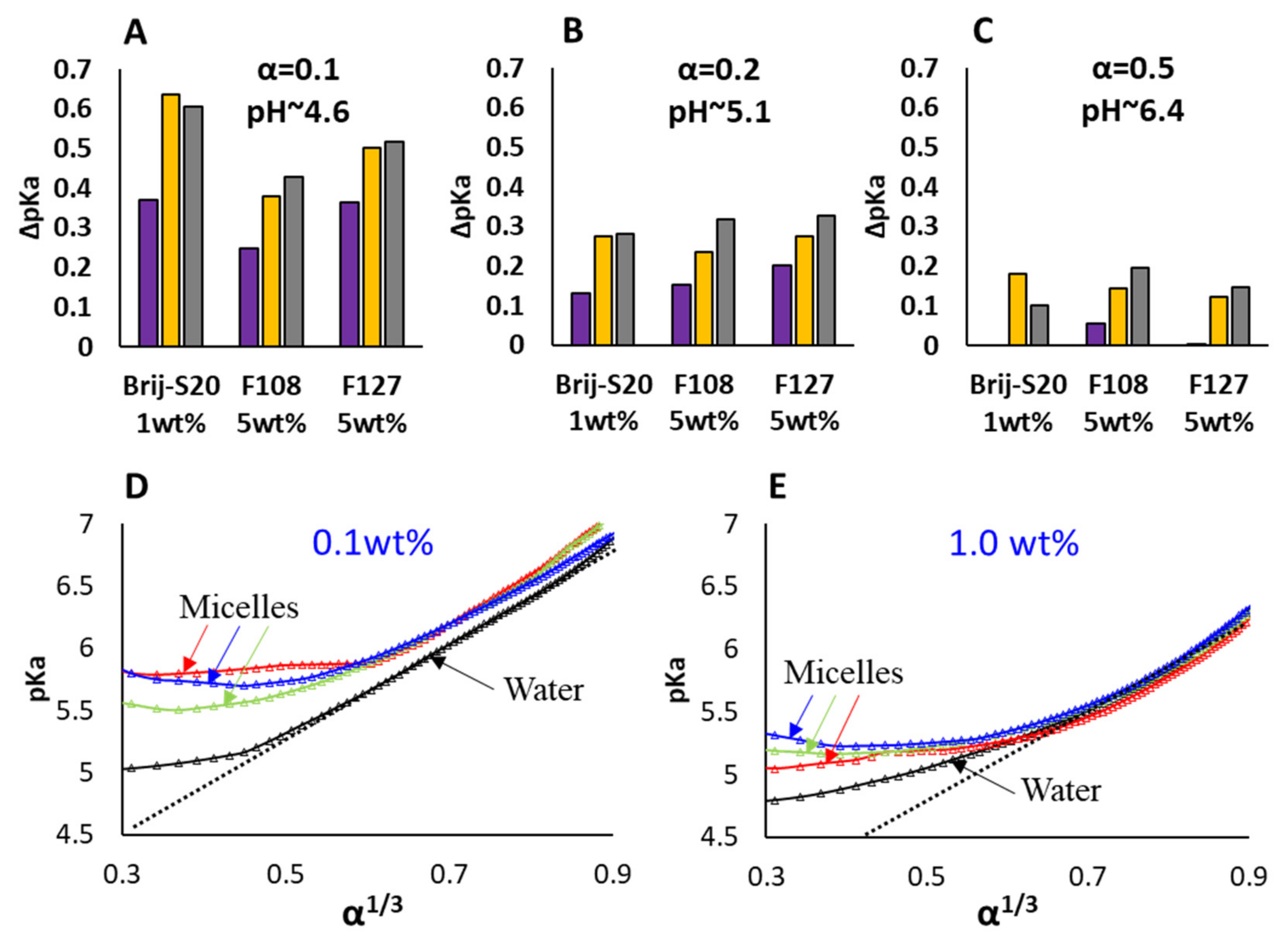
3.1. Titration Experiments
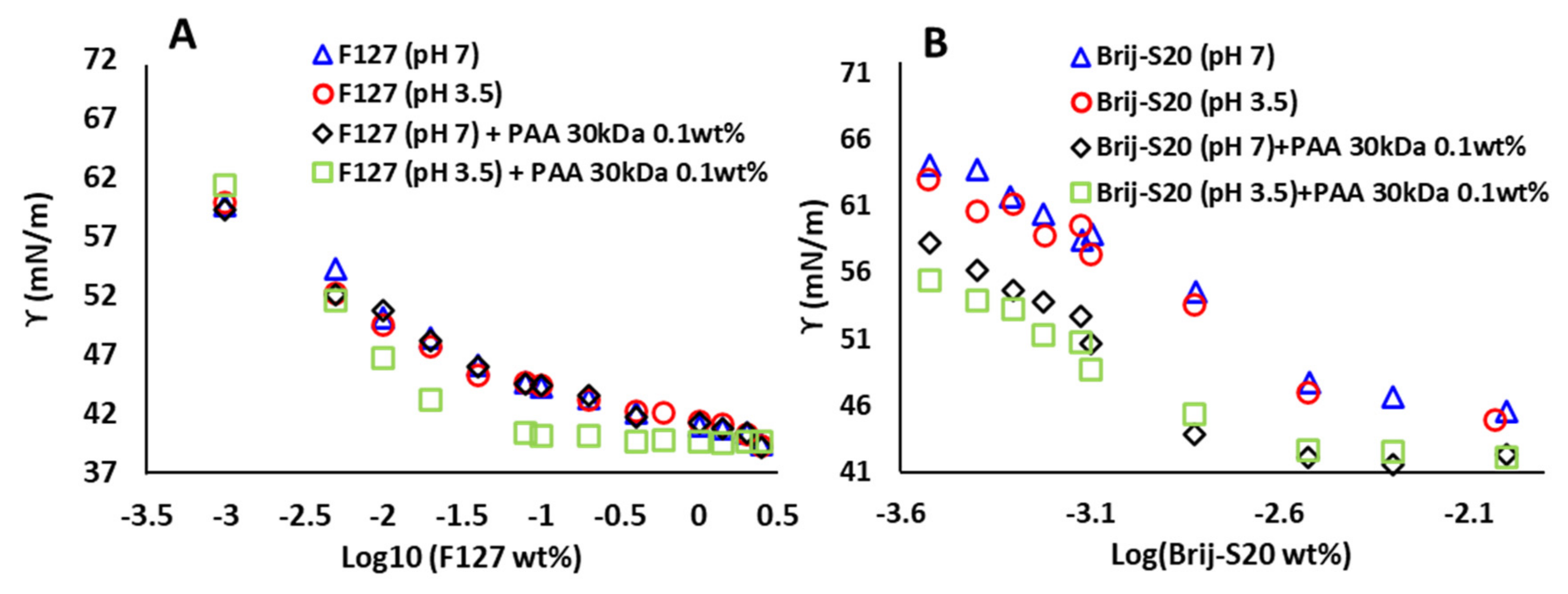
3.2. PAA–Micellar Interactions
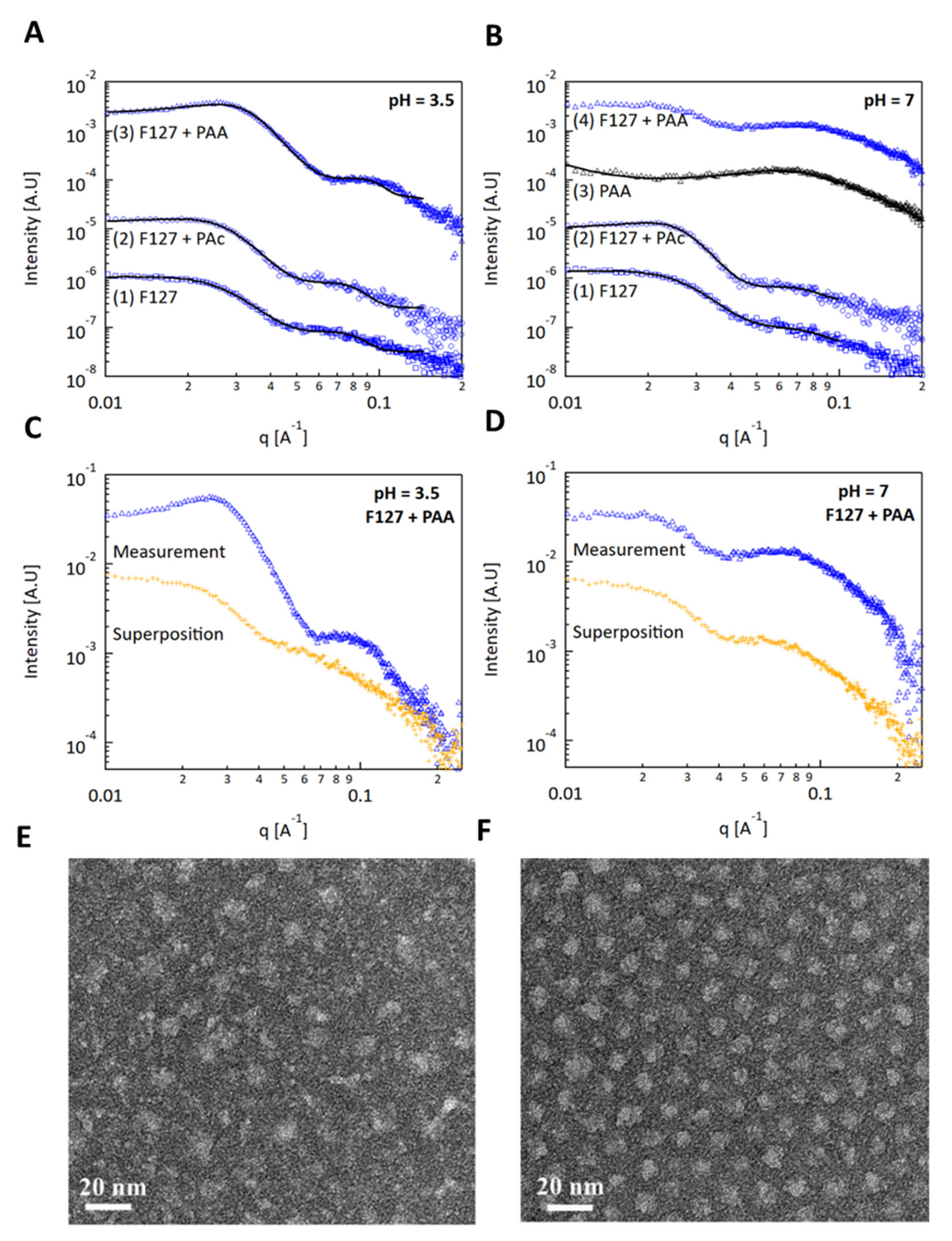
3.3. Characterization of PAA–Micelle Solutions Using SAXS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solveyra, E.G.; Nap, R.J.; Huang, K.; Szleifer, I. Theoretical Modeling of Chemical Equilibrium in Weak Polyelectrolyte Layers on Curved Nanosystems. Polymers 2020, 12, 2282. [Google Scholar] [CrossRef] [PubMed]
- Nap, R.; Gong, P.; Szleifer, I. Weak polyelectrolytes tethered to surfaces: Effect of geometry, acid-base equilibrium and electrical permittivity. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2638–2662. [Google Scholar] [CrossRef]
- Atkins, P.; De Paula, J. Physical Chemistry for the Life Sciences; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Muthukumar, M. 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katchalsky, A. Problems in the physical chemistry of polyelectrolytes. J. Polym. Sci. 1954, 12, 159–184. [Google Scholar] [CrossRef]
- Katchalsky, A.; Alexandrowicz, Z.; Kedem, O. Chemical Physics of Ionic Solutions; Wiley: New York, NY, USA, 1966; p. 295. [Google Scholar]
- Nightingale, E.R. Chemical Physics of Electrolyte Solutions; Conway, B.E., Barradas, R.G., Eds.; Wiley: New York, NY, USA, 1966. [Google Scholar]
- Ullner, M.; Jönsson, B.; Widmark, P. Conformational properties and apparent dissociation constants of titrating polyelectrolytes: Monte Carlo simulation and scaling arguments. J. Chem. Phys. 1994, 100, 3365–3366. [Google Scholar] [CrossRef]
- Katchalsky, A.; Gillis, J. Theory of the potentiometric titration of polymeric acids. Recl. Trav. Chim. Pays-Bas 1949, 68, 879–897. [Google Scholar] [CrossRef]
- Katchalsky, A.; Künzle, O.; Kuhn, W. Behavior of polyvalent polymeric ions in solution. J. Polym. Sci. 1950, 5, 283–300. [Google Scholar] [CrossRef]
- Nova, L.; Uhlik, F.; Kosovan, P. Local pH and effective pK(A) of weak polyelectrolytes—Insights from computer simulations. Phys. Chem. Chem. Phys. 2017, 19, 14376–14387. [Google Scholar] [CrossRef] [Green Version]
- Arnold, R. The titration of polymeric acids. J. Colloid Sci. 1957, 12, 549–556. [Google Scholar] [CrossRef]
- Gonzalez, A.E.; Muto, S. An approximate treatment of polymer gelation in a solvent. J. Chem. Phys. 1980, 73, 4668–4670. [Google Scholar] [CrossRef]
- Plamper, F.A.; Becker, H.; Lanzendorfer, M.; Patel, M.; Wittemann, A.; Ballauff, M.; Muller, A.H.E. Synthesis, characterization and behavior in aqueous solution of star-shaped poly(acrylic acid). Macromol. Chem. Phys. 2005, 206, 1813–1825. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Poisson-Boltzmann Theory of pH-Sensitive (Annealing) Polyelectrolyte Brush. Langmuir 2011, 27, 10615–10633. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Genzer, J.; Szleifer, I. Phase behavior and charge regulation of weak polyelectrolyte grafted layers. Phys. Rev. Lett. 2007, 98, 018302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, P.; Wu, T.; Genzer, J.; Szleifer, I. Behavior of surface-anchored poly(acrylic acid) brushes with grafting density gradients on solid substrates: 2. Theory. Macromolecules 2007, 40, 8765–8773. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Structure and interaction of weakly charged polyelectrolyte brushes: Self-consistent field theory. J. Chem. Phys. 1997, 107, 5952–5967. [Google Scholar] [CrossRef]
- Longo, G.S.; de la Cruz, M.O.; Szleifer, I. Molecular Theory of Weak Polyelectrolyte Gels: The Role of pH and Salt Concentration. Macromolecules 2011, 44, 147–158. [Google Scholar] [CrossRef]
- Tagliazucchi, M.; de la Cruz, M.O.; Szleifer, I. Self-organization of grafted polyelectrolyte layers via the coupling of chemical equilibrium and physical interactions. Proc. Natl. Acad. Sci. USA 2010, 107, 5300–5305. [Google Scholar] [CrossRef] [Green Version]
- de la Cruz, M.O.; Ermoshkin, A.V.; Carignano, M.A.; Szleifer, I. Analytical theory and Monte Carlo simulations of gel formation of charged chains. Soft Matter 2009, 5, 629–636. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.H.; Wei, Q.; Luo, M.; Huang, G.; Sumer, B.D.; Gao, J.M. Non-covalent interactions in controlling pH-responsive behaviors of self-assembled nanosystems. Polym. Chem. 2016, 7, 5949–5956. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, G.F.D.; Hailes, R.L.N.; Adali-Kaya, Z.; Robson, T.; Dahlin, A. Generic high-capacity protein capture and release by pH control. Chem. Commun. 2020, 56, 5889–5892. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Bravo, J.M.; Flores-Guillen, M.E.; Lugo-Medina, E.; Licea-Claverie, A. Drug release from complexes with a series of poly(carboxyalkyl methacrylates), a new class of weak polyelectrolytes. Int. J. Pharm. 2005, 305, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbari, E.; Nozari, S. Swelling behavior of acrylic acid hydrogels prepared by gamma-radiation crosslinking of polyacrylic acid in aqueous solution. Eur. Polym. J. 2000, 36, 2685–2692. [Google Scholar] [CrossRef]
- Schreyer, H.B.; Gebhart, N.; Kim, K.J.; Shahinpoor, M. Electrical activation of artificial muscles containing polyacrylonitrile gel fibers. Biomacromolecules 2000, 1, 642–647. [Google Scholar] [CrossRef]
- Li, T.H.; Robertson, M.L.; Conrad, J.C. Molecular weight and dispersity affect chain conformation and pH-response in weak polyelectrolyte brushes. Polym. Chem. 2021, 12, 6737–6744. [Google Scholar] [CrossRef]
- Pragatheeswaran, A.M.; Chen, S.B. The influence of poly(acrylic acid) on micellization and gelation characteristics of aqueous Pluronic F127 copolymer system. Colloid Polym. Sci. 2016, 294, 107–117. [Google Scholar] [CrossRef]
- Ikawa, T.; Abe, K.; Honda, K.; Tsuchida, E. Interpolymer Complex Between Poly(Ethylene Oxide) and Poly(Carboxylic Acid). J. Polym. Sci. Part A Polym. Chem. 1975, 13, 1505–1514. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Mun, G.A.; Nurkeeva, Z.S.; Dubolazov, A.V. pH and salt effects on interpolymer complexation via hydrogen bonding in aqueous solutions. Polym. Int. 2004, 53, 1382–1387. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Dubolazov, A.V.; Nurkeeva, Z.S.; Mun, G.A. pH effects in the complex formation and blending of poly(acrylic acid) with poly(ethylene oxide). Langmuir 2004, 20, 3785–3790. [Google Scholar] [CrossRef]
- Serjeant, E.P.; Dempsey, B. Ionisation Constants of Organic Acids in Aqueous Solution; Pergamon: Oxford, UK, 1979; Volume 23. [Google Scholar]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.P.; Begin, A. Inhibitory effect of organic acids upon meat spoilage bacteria. J. Food Prot. 1997, 60, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Gildberg, A.; Raa, J. Properties of a Propionic Acid-Formic Acid Preserved Silage of Cod Viscera. J. Sci. Food Agric. 1977, 28, 647–653. [Google Scholar] [CrossRef]
- Hackley, V.A. Colloidal processing of silicon nitride with poly(acrylic acid): I, Adsorption and electrostatic interactions. J. Am. Ceram. Soc. 1997, 80, 2315–2325. [Google Scholar] [CrossRef]
- Dolce, C.; Meriguet, G. Ionization of short weak polyelectrolytes: When size matters. Colloid Polym. Sci. 2017, 295, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Reith, D.; Muller, B.; Muller-Plathe, F.; Wiegand, S. How does the chain extension of poly (acrylic acid) scale in aqueous solution? A combined study with light scattering and computer simulation. J. Chem. Phys. 2002, 116, 9100–9106. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.; de Moura, C.L.; Vieira, M.G.S.; Gramosa, N.V.; Chaibundit, C.; de Mattos, M.C.; Attwood, D.; Yeates, S.G.; Nixon, S.K.; Ricardo, N. Solubilisation capacity of Brij surfactants. Int. J. Pharm. 2012, 436, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Nativ-Roth, E.; Shvartzman-Cohen, R.; Bounioux, C.; Florent, M.; Zhang, D.S.; Szleifer, I.; Yerushalmi-Rozen, R. Physical adsorption of block copolymers to SWNT and MWNT: A nonwrapping mechanism. Macromolecules 2007, 40, 3676–3685. [Google Scholar] [CrossRef]
- Bedrov, D.; Ayyagari, C.; Smith, G.D. Multiscale modeling of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer micelles in aqueous solution. J. Chem. Theory Comput. 2006, 2, 598–606. [Google Scholar] [CrossRef]
- Lee, J.; Martic, P.A.; Tan, J.S. Protein Adsorption on Pluronic Copolymer-Coated Polystyrene Particles. J. Colloid Interface Sci. 1989, 131, 252–266. [Google Scholar] [CrossRef]
- Zhao, M.M.; Eghtesadi, S.A.; Dawadi, M.B.; Wang, C.; Huang, S.Y.; Seymore, A.E.; Vogt, B.D.; Modarelli, D.A.; Liu, T.B.; Zacharia, N.S. Partitioning of Small Molecules in Hydrogen-Bonding Complex Coacervates of Poly(acrylic acid) and Poly(ethylene glycol) or Pluronic Block Copolymer. Macromolecules 2017, 50, 3819–3831. [Google Scholar] [CrossRef]
- Posa, M.; Pilipovic, A. Activity Coefficient of Triton X100 and Brij S20 in the Infinitely Diluted Micellar Pseudophase of the Binary Micelle Triton X100-Brij S20 in the Water Phase at the Temperature Interval T = (283.15–318.15) K. J. Chem. Eng. Data 2020, 65, 106–119. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Washington, C.; Davies, M.C.; Hadgraft, J. The Behavior of Pluronic-F127 in Aqueous-Solution Studied Using Fluorescent-Probes. Int. J. Pharm. 1987, 40, 93–99. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; van Bronswijk, W. In situ FTIR-ATR examination of poly(acrylic acid) adsorbed onto hematite at low pH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Tan, L.X.; Elkins, J.G.; Davison, B.H.; Kelley, E.G.; Nickels, J. Implementation of a self-consistent slab model of bilayer structure in the SasView suite. J. Appl. Crystallogr. 2021, 54, 363–370. [Google Scholar] [CrossRef]
- Sulatha, M.S.; Natarajan, U. Origin of the Difference in Structural Behavior of Poly(acrylic acid) and Poly(methacrylic acid) in Aqueous Solution Discerned by Explicit-Solvent Explicit-Ion MD Simulations. Ind. Eng. Chem. Res. 2011, 50, 11785–11796. [Google Scholar] [CrossRef]
- Thurn, T.; Couderc, S.; Sidhu, J.; Bloor, D.M.; Penfold, J.; Holzwarth, J.F.; Wyn-Jones, E. Study of mixed micelles and interaction parameters for ABA triblock copolymers of the type EOm-POn-EOm and ionic surfactants: Equilibrium and structure. Langmuir 2002, 18, 9267–9275. [Google Scholar] [CrossRef]
- Jadoon, Q.; Bibi, I.; Mehmood, K.; Sajjad, S.; Nawaz, M.; Ali, F.; Bibi, S.; Bano, S.; Usman, M. Interaction of surfactants with block-copolymer systems in the presence of Hofmeister anions. Mater. Res. Express 2017, 4, 035307. [Google Scholar] [CrossRef]
- Adamsom, A.W.; Gast, A.P. Physical Chemistry of Surfaces; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Schulz, G.V. Molecular weight determination in macromolecular compounds. VIII. Distribution function of multimolecular compounds and their determination by fractionation. Z. Phys. Chem. 1949, 47, 155–193. [Google Scholar]
- Valero, M.; Hu, W.J.; Houston, J.E.; Dreiss, C.A. Solubilisation of salicylate in F127 micelles: Effect of pH and temperature on morphology and interactions with cyclodextrin. J. Mol. Liq. 2021, 322, 114892. [Google Scholar] [CrossRef]
- Taylor, T.J.; Stivala, S.S. Small-angle X-ray scattering study of a weak polyelectrolyte in water. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1263–1272. [Google Scholar] [CrossRef]
- Doty, P.M. Classical theory of light scattering from solutions—A review. Polym. Bull. 1945, 1, 90. [Google Scholar]
- Borukhov, I.; Andelman, D.; Borrega, R.; Cloitre, M.; Leibler, L.; Orland, H. Polyelectrolyte titration: Theory and experiment. J. Phys. Chem. B 2000, 104, 11027–11034. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, W.; Künzle, O.; Katchalsky, A. Verhalten polyvalenter fadenmolekelionen in lösung. Helv. Chim. Acta 1948, 31, 1994–2037. [Google Scholar] [CrossRef] [PubMed]
- Bizley, S.C.; Williams, A.C.; Khutoryanskiy, V.V. Thermodynamic and kinetic properties of interpolymer complexes assessed by isothermal titration calorimetry and surface plasmon resonance. Soft Matter 2014, 10, 8254–8260. [Google Scholar] [CrossRef] [Green Version]
- Uhlik, F.; Kosovan, P.; Limpouchova, Z.; Prochazka, K.; Borisov, O.V.; Leermakers, F.A.M. Modeling of Ionization and Conformations of Starlike Weak Polyelectrolytes. Macromolecules 2014, 47, 4004–4016. [Google Scholar] [CrossRef]
- Mori, H.; Seng, D.C.; Lechner, H.; Zhang, M.F.; Muller, A.H.E. Synthesis and characterization of branched polyelectrolytes. 1. Preparation of hyperbranched poly(acrylic acid) via self-condensing atom transfer radical copolymerization. Macromolecules 2002, 35, 9270–9281. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Mortensen, K.; Talmon, Y. Cryo-TEM and SANS microstructural study of pluronic polymer solutions. Macromolecules 1995, 28, 8829–8834. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chattoraj, D. Adsorption and the Gibbs Surface Excess; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Debye, P. Molecular-weight determination by light scattering. J. Phys. Chem. 1947, 51, 18–32. [Google Scholar] [CrossRef]
- Horkay, F.; Hammouda, B. Small-angle neutron scattering from typical synthetic and biopolymer solutions. Colloid Polym. Sci. 2008, 286, 611–620. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Schurtenberger, P. Scattering functions of semiflexible polymers with and without excluded volume effects. Macromolecules 1996, 29, 7602–7612. [Google Scholar] [CrossRef]
- Taylor, T.J.; Stivala, S.S. Small-angle X-ray scattering of poly(acrylic acid) in solution: 1. Dioxane. Polymer 1996, 37, 715–719. [Google Scholar] [CrossRef]
- Percus, J.K.; Yevick, G.J. Analysis of classical statistical mechanics by means of collective coordinates. Physical Review 1958, 110, 1. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yekymov, E.; Attia, D.; Levi-Kalisman, Y.; Bitton, R.; Yerushalmi-Rozen, R. Effects of Non-Ionic Micelles on the Acid-Base Equilibria of a Weak Polyelectrolyte. Polymers 2022, 14, 1926. https://doi.org/10.3390/polym14091926
Yekymov E, Attia D, Levi-Kalisman Y, Bitton R, Yerushalmi-Rozen R. Effects of Non-Ionic Micelles on the Acid-Base Equilibria of a Weak Polyelectrolyte. Polymers. 2022; 14(9):1926. https://doi.org/10.3390/polym14091926
Chicago/Turabian StyleYekymov, Evgenee, David Attia, Yael Levi-Kalisman, Ronit Bitton, and Rachel Yerushalmi-Rozen. 2022. "Effects of Non-Ionic Micelles on the Acid-Base Equilibria of a Weak Polyelectrolyte" Polymers 14, no. 9: 1926. https://doi.org/10.3390/polym14091926
APA StyleYekymov, E., Attia, D., Levi-Kalisman, Y., Bitton, R., & Yerushalmi-Rozen, R. (2022). Effects of Non-Ionic Micelles on the Acid-Base Equilibria of a Weak Polyelectrolyte. Polymers, 14(9), 1926. https://doi.org/10.3390/polym14091926






