Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps
Abstract
:1. Introduction
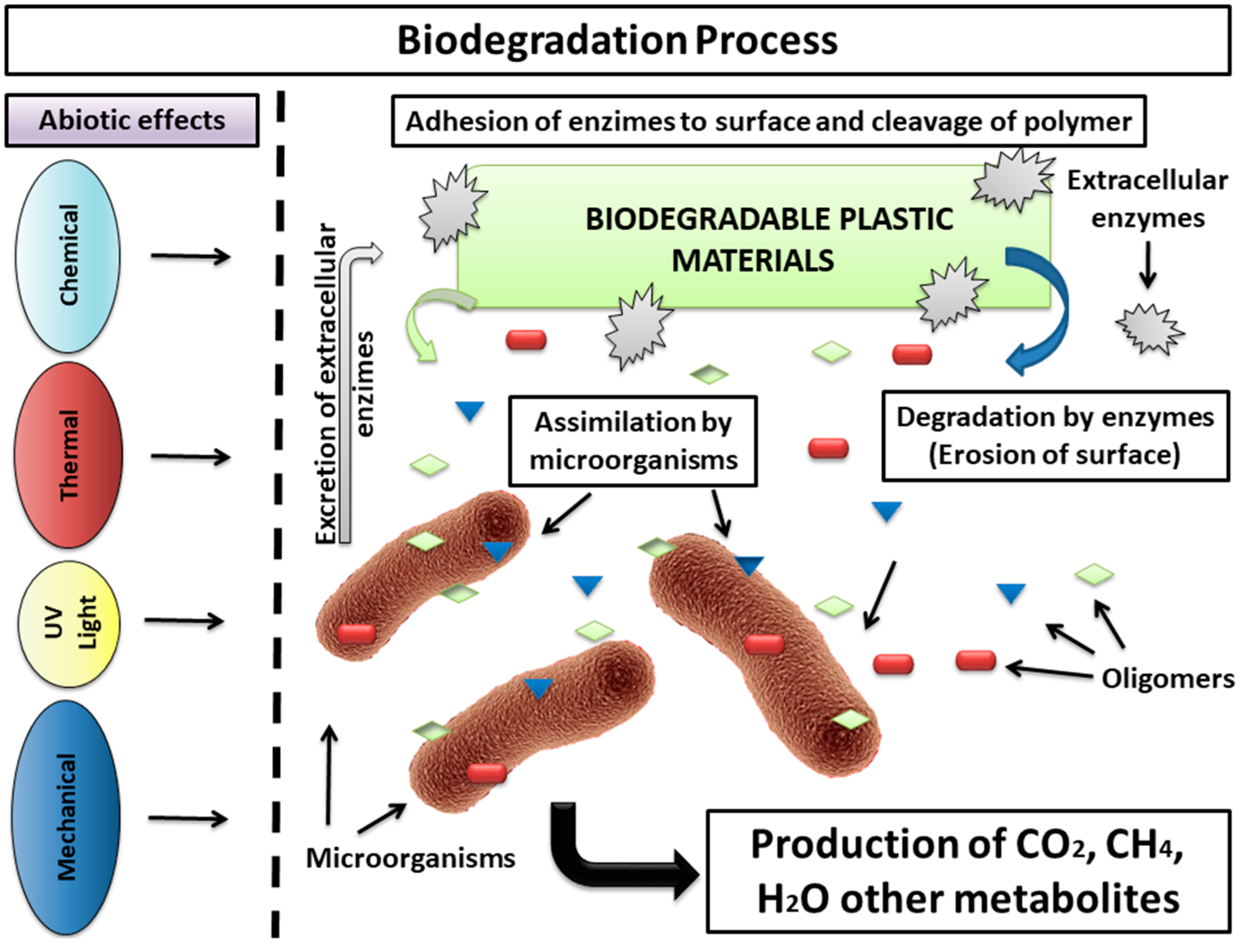
2. Current Biodegradation Assays Methodologies
2.1. Biodegradation Assays in Soil
| Specifications | ASTM D5988-18 | ISO 17556:2019 |
|---|---|---|
| Type | The soil should be natural and fertile, preferably “sandy loam” collected from fields and forests not exposed to pollutants. The soil used should be a mixture of natural and fertile soils collected from the surface layers of at least 3 diverse locations. | Natural soil from fields and/or forests may be used as an inoculum to simulate biodegradation in a specific natural environment. |
| Particle size | Less than 2 mm | Less than 5 mm |
| Room Temperature | 20 − 28 ± 2 °C | 20 − 28 ± 2 °C but in preference 25 °C |
| pH | 6–8 | 6–8 |
| Moisture holding Capacity (MHC) | 80–100% | 40–60% |
| Running Time | Should not exceed 6 months | Should not exceed 6 months but may be extended up to 24 months |
| Reference Material | Cellulose or starch | Microcrystalline cellulose powder, ashless cellulose filters or poly(3-hydroxybutyrate) |
| Validation Criteria | The test is considered valid if after 6 months more than 70% biodegradation is achieved for the reference material and if the amount of CO2 evolved from the control reactors is within 20% of the mean at the plateau phase or at the end of the test. | The test is considered valid if the degree of biodegradation of the reference material is more than 60% at the plateau phase or at the end of the test and if the BOD (biological oxygen demand) values of or the amount of CO2 evolved from the controls are within 20% of the mean at the plateau phase or at the end of the test. |
| Biopolymer * | Soil Conditions | Methodology | Ref. |
|---|---|---|---|
| Chitosan/Corn cob | Laboratory-controlled conditions | ASTM D5988 | [39] |
| PHBV/Olive Pomace | Laboratory-controlled conditions | ASTM D5988 | [40] |
| Cellulose based | Laboratory-controlled conditions | ASTM D5988 | [51] |
| PBAT/Nanocellulose | Laboratory-controlled conditions | ASTM D5988 | [52] |
| Polyurethane (PU)/Starch | Laboratory-controlled conditions | ASTM D5988, morphology and chemical characterization, visual analysis | [48] |
| Starch/Nanocellulose | Laboratory-controlled conditions | ISO 17556 | [53] |
| Poly(lactic acid) (PLA)/Glycerol | Laboratory-controlled conditions | ISO 17556 | [54] |
| Mater-Bi (Mixture of PBAT, starch, and additives) | Laboratory-controlled conditions | ISO 17556, ecotoxicological analysis | [55] |
| PHA, PBS, and PBAT/PLA | Laboratory-controlled conditions | ISO 17556, microrganismo characterization | [41] |
| PHB mixed with natural fillers | Laboratory-controlled conditions | Mass Loss | [47] |
| Starch/Nanocellulose | Outdoor soil conditions | Mass Loss | [42] |
| Poly(vinyl alcohol) (PVA)/Starch | Outdoor soil conditions | Mass Loss | [45] |
| PVA/Starch | Laboratory-controlled conditions | Mass loss, biofilm area, soil characterization, and visual analysis | [37] |
| PCL, PHB, PLA, and PBS | Laboratory-controlled conditions and outdoor soil conditions | Mass loss, mechanical properties, and microorganism characterization | [49] |
| Starch based | Laboratory-controlled conditions | Mass loss, morphology analysis, and mechanical properties | [56] |
| PBS/Sugarcane Fiber | Laboratory-controlled conditions | Mass loss, morphology analysis, and thermal characterization | [57] |
| PHA | Outdoor soil conditions | Mass loss and morphology and chemical analysis | [50] |
| PLA and PLA/Starch | Outdoor soil conditions | Mass loss, morphology and chemical analysis, and thermal characterization | [18] |
| PVA/Starch and PVA/Starch mixed with different natural fillers | Laboratory-controlled conditions | Mass loss, morphology and chemical analysis, and soil characterization | [58] |
2.2. Biodegradation Assays in Compost
| Biopolymer * | Compost Origin | Methodologies to Monitor Biodegradation | Ref. |
|---|---|---|---|
| PLA, PLA/Chitosan, PLA/Nanocellulose, and PLA/Gum | Food waste | ASTM D5338, mass loss, morphology and chemical analysis, thermal characterization, contact angle, and microorganism characterization | [63] |
| PVA PVA/Nanocellulose | MSW | ASTM D5338, visual analysis, mass loss, morphology and chemical analysis, thermal characterization, and compost quality (including physico-chemical parameters and ecotoxicological analysis) | [17] |
| PHA-based | MSW | ASTM D5338 | [70] |
| Starch/Carboxymethyl Chitosan | Comercial | ASTM D5338 | [71] |
| Chitosan | Not Specified | ASTM D-5338 and ISO 14855-1, visual analysis, morphology and chemical analysis, thermal characterization, and microorganism characterization | [61] |
| PVA/Starch | MSW | ISO 14855-1, ISO 20200, visual analysis, morphology analysis, thermal characterization | [72] |
| Nano-reinforced PLA | MSW | ISO 14855-1, ISO 20200 with adaptations of ISO 16929, visual analysis, and compost quality (including physico-chemical parameters and ecotoxicological analysis) | [73] |
| Gelatin, Chitosan, and/or Sodium Caseinate based | Mature compost (10%) mixed with synthetic biowaste (contained sawdust (40%), rabbit food (30%), starch (10%), sugar (5%), oil (4%), and urea (1%)) | ISO 20200, visual analysis, mass loss, and chemical analysis | [62] |
| PHA based | Mature compost (10%) mixed with synthetic biowaste (contained sawdust (40%), rabbit food (30%), starch (10%), sugar (5%), oil (4%), and urea (1%)) | ISO 20200 | [70] |
| PLA and PLA/Silica | Not speficied compost fermented with biomass (2:1) | Modified ISO 17556 | [74] |
| PLA, PLA/TAC, and PLA/PHB/TAC | MSW | CO2 concentration, mass loss, chemical analysis, and thermal characterization | [75] |
| PCL, PHB, PLA, and PBS | Comercial | Mass loss, mechanical properties, and microorganism characterization | [49] |
| Starch/Montmorillonite | Not Specified | Mass loss and ecotoxicological analysis | [76] |
| Nano-reinforced PLA | MSW | Mass loss and microorganism characterization | [77] |
| Specifications | ASTM D5338:15 | ISO 14855-1:2012 |
|---|---|---|
| Inoculum | Industrial compost soil that has a maturity level of 2–4 months | Stabilized, mature compost derived (four-month old), if possible, from composting the organic fraction of solid municipal waste |
| Particle size | - | 0.5 cm to 1 cm |
| Room temperature | Incubated in the dark at 58 °C ± 2 °C | Incubated in the dark at 58 °C ± 2 °C |
| pH | 7.0–8.2 | 7.0–9.0 |
| Total dry solids content | between 50% and 55% of the wet solids | between 50% and 55% of the wet solids |
| Volatile solids content | To produce more than 50 mg but less than 150 mg of CO2 per gram of volatile solids after 10 days of incubation | No more than about 15% of the wet or 30% of the dry solids |
| Moisture holding capacity (MHC) | 50% | 50–60% |
| Running time | Minimum of 90 days and should not exceed 6 months | Minimum of 90 days and should not exceed 6 months |
| Reference material | Celullose | TLC (thin-layer chromatography)-grade cellulose with a particle size of less than 20 μm |
| Validation criteria | If: (a) more than 2 g of volatile fatty acids per kilogram of dry matter in the composting vessel is formed, the test must be regarded as invalid; (b) a minimum of 70% for cellulose within 45 days is not observed with the positive reference, the test must be regarded as invalid and should be repeated, using new inoculum; (c) the deviation of the percentage of biodegradation of the positive reference is greater than or equal to 20% at the end of the test, then the test shall be regarded as invalid. | The test is considered as valid if: (a) the degree of biodegradation of the reference material is more than 70% after 45 days; (b) the difference between the percentage biodegradation of the reference material in the different vessels is less than 20% at the end of the test; (c) the inoculum in the blank has produced more than 50 mg but less than 150 mg of carbon dioxide per gram of volatile solids after 10 days of incubation. |
2.3. Biodegradation Assays in Aquatic Systems
3. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Production of Nanocellulose from Lignocellulosic Biomass Wastes: Prospects and Limitations. In Innovation, Engineering and Entrepreneurship; Machado, J., Soares, F., Veiga, G., Eds.; Lecture Notes in Electrical Engineering; Springer International Publishing: Cham, Switzerland, 2019; Volume 505, pp. 719–725. ISBN 9783319913339. [Google Scholar]
- Kawashima, N.; Yagi, T.; Kojima, K. How do bioplastics and fossil-based plastics play in a circular economy? Macromol. Mater. Eng. 2019, 304, 1900383. [Google Scholar] [CrossRef]
- Kubowicz, S.; Booth, A.M. Biodegradability of plastics: Challenges and misconceptions. Environ. Sci. Technol. 2017, 51, 12058–12060. [Google Scholar] [CrossRef] [PubMed]
- D570-98(2018); Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2018.
- Mangaraj, S.; Yadav, A.; Dash, S.K.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2018, 3, 77–96. [Google Scholar] [CrossRef]
- Rosenboom, J.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An overview of plastic waste generation and management in food packaging industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Yang, W.; Kenny, J.M.; Torre, L.; Puglia, D. Bio-based nanocomposites in food packaging. In Nanomaterials for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2018; pp. 71–110. ISBN 9780323512718. [Google Scholar]
- Pires, J.; De Paula, C.D.; Gomes, V.; Souza, L.; Fernando, A.L. Understanding the barrier and mechanical behavior of different nanofillers in chitosan films for food packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental behaviors of microplastics in aquatic systems: A systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef]
- Wieland, S.; Balmes, A.; Bender, J.; Kitzinger, J.; Meyer, F.; Frm, A.; Roeder, F.; Tengelmann, C.; Wimmer, B.H.; Laforsch, C.; et al. From properties to toxicity: Comparing microplastics to other airborne microparticles. J. Hazard. Mater. 2022, 428, 128151. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J. Bionanocomposite films for food packaging applications. Ref. Modul. Food Sci. 2018, 1–10. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Sundqvist-Andberg, H.; Åkerman, M. Sustainability governance and contested plastic food packaging—An integrative review. J. Clean. Prod. 2021, 306, 127111. [Google Scholar] [CrossRef]
- Use of Recycled Plastics in Food Packaging (Chemistry Considerations): Guidance for Industry; U.S. Food & Drug Administration: Rockville, MD, USA, 2021.
- Salehpour, S.; Jonoobi, M.; Ahmadzadeh, M.; Siracusa, V.; Rafieian, F.; Oksman, K. Biodegradation and ecotoxicological impact of cellulose nanocomposites in municipal solid waste composting. Int. J. Biol. Macromol. 2018, 111, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, Y.; Gu, J.; Tan, H. Soil burial-induced chemical and thermal changes in starch/poly (lactic acid) composites. Int. J. Biol. Macromol. 2018, 113, 338–344. [Google Scholar] [CrossRef]
- Qin, Z.-H.; Mou, J.; Chao, C.Y.H.; Chopra, S.S.; Daoud, W.; Leu, S.; Ning, Z.; Tso, C.Y.; Chan, C.K.; Tang, S.; et al. Biotechnology of plastic waste degradation, recycling, and valorization: Current advances and future perspectives. ChemSusChem 2021, 14, 4103–4114. [Google Scholar] [CrossRef]
- Fernando, A.L.; Duarte, M.P.; Vatsanidou, A.; Alexopoulou, E. Environmental aspects of fiber crops cultivation and use. Ind. Crop. Prod. 2015, 68, 105–115. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Brigham, C. Biopolymers: Biodegradable alternatives to traditional plastics. In Green Chemistry: An Inclusive Approach; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 753–770. ISBN 9780128095492. [Google Scholar]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- D6866-21; Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis. ASTM International: West Conshohocken, PA, USA, 2021.
- D883-20b; Standard Terminology Relating to Plastics. ASTM International: West Conshohocken, PA, USA, 2020.
- European Bioplastics. What are Bioplastics? Available online: https://www.european-bioplastics.org/bioplastics/ (accessed on 17 February 2020).
- Andrzejewska, A.; Wirwicki, M.; Andryszczyk, M. Procedure for determining aqueous medium absorption in biopolymers. AIP Conf. Proc. 2017, 1902, 020060. [Google Scholar] [CrossRef]
- Souza, V.G.L. Thesis Plan Proposal: Development of a Noval Bionanocomposite Based on Chitosan/MMT with Antioxidant Activity for Food Appliance. Ph.D. Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2015. [Google Scholar]
- Briassoulis, D.; Innocenti, F.D. Standards for soil biodegradable plastics. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Malinconico, M., Ed.; Springer: Cham, Switzerland, 2017; pp. 139–168. ISBN 9783662541302. [Google Scholar]
- Rudnik, E. Biodegradation of compostable polymers in various environments. In Compostable Polymer Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 255–287. [Google Scholar]
- Agarwal, S. Biodegradable polymers: Present opportunities and challenges in providing a microplastic-free environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef] [Green Version]
- Pattanasuttichonlakul, W.; Sombatsompop, N.; Prapagdee, B. Accelerating biodegradation of PLA using microbial consortium from dairy wastewater sludge combined with PLA-degrading bacterium. Int. Biodeterior. Biodegrad. 2018, 132, 74–83. [Google Scholar] [CrossRef]
- Rudnik, E.; Briassoulis, D. Degradation behaviour of poly (lactic acid) films and fibres in soil under Mediterranean field conditions and laboratory simulations testing. Ind. Crop. Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- Sen, C.; Das, M. Biodegradability of starch based self-supporting antimicrobial film and its effect on soil quality. J. Polym. Environ. 2018, 26, 4331–4337. [Google Scholar] [CrossRef]
- Pischedda, A.; Tosin, M.; Degli-Innocenti, F. Biodegradation of plastics in soil: The effect of temperature. Polym. Degrad. Stab. 2019, 170, 109017. [Google Scholar] [CrossRef]
- Chan, M.Y.; Koay, S.C. Biodegradation and thermal properties of crosslinked chitosan/corn cob biocomposite films by electron beam irradiation. Polym. Eng. Sci. 2019, 59, E59–E68. [Google Scholar] [CrossRef]
- Lammi, S.; Gastaldi, E.; Gaubiac, F. How olive pomace can be valorized as fillers to tune the biodegradation of PHBV based composites. Polym. Degrad. Stab. 2019, 166, 325–333. [Google Scholar] [CrossRef]
- Šerá, J.; Serbruyns, L.; De Wilde, B.; Koutný, M. Accelerated biodegradation testing of slowly degradable polyesters in soil. Polym. Degrad. Stab. 2020, 171, 109031. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Geethamma, V.G.; Gopi, S.; George, M.; Huski, M.; Kalarikkal, N.; Volova, T.; Rouxel, D.; Thomas, S. Thermal, biodegradation and theoretical perspectives on nanoscale confinement in starch/cellulose nanocomposite modified via green crosslinker. Int. J. Biol. Macromol. 2019, 134, 781–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D5988-18; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil. ASTM International: West Conshohocken, PA, USA, 2018.
- 17556:19; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. ISO: Geneva, Switzerland, 2019.
- Kaur, K.; Jindal, R.; Maiti, M.; Mahajan, S. Studies on the properties and biodegradability of PVA/Trapanatans starch(N-st) composite films and PVA/N-st-g-poly(EMA) composite films. Int. J. Biol. Macromol. 2018, 123, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, F.; Gori, R.; Lubello, C. Methodologies to assess biodegradation of bioplastics during aerobic composting and anaerobic digestion: A review. Waste Manag. Res. 2019, 37, 959–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.; Shumilova, A.A.; Kiselev, E.G.; Baranovsky, S.V.; Vasiliev, A.D.; Nemtsev, I.V.; Kuzmin, A.P.; Sukovatyi, A.G.; Pai, R.; Avinash; et al. Thermal, mechanical and biodegradation studies of biofiller based poly-3-hydroxybutyrate biocomposites. Int. J. Biol. Macromol. 2019; in press. [Google Scholar] [CrossRef]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Aerobic biodegradation of starch—Polyurethane flexible films under soil burial condition: Changes in physical structure and chemical composition. Int. Biodeterior. Biodegrad. 2019, 145, 104793. [Google Scholar] [CrossRef]
- Al Hosni, A.S.; Pittman, J.K.; Robson, G.D. Microbial degradation of four biodegradable polymers in soil and compost demonstrating polycaprolactone as an ideal compostable plastic. Waste Manag. 2019, 97, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, P.C.; Devaraj, S.; Devaraj, S.; Kathirvel, P. Polyhydroxyalkanoate production from statistically optimized media using rice mill effluent as sustainable substrate with an analysis on the biopolymer´s degradation potential. Int. J. Biol. Macromol. 2019, 126, 977–986. [Google Scholar] [CrossRef]
- Otoni, C.G.; Lodi, B.D.; Lorevice, M.V.; Leitão, R.C.; Ferreira, M.D.; De Moura, M.R.; Mattoso, L.H.C. Optimized and scaled-up production of cellulose-reinforced biodegradable composite films made up of carrot processing waste. Ind. Crop. Prod. 2018, 121, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, I.F.; Ferreira, F.V.; Souza, D.H.S.; Gouveia, R.F.; Lona, L.M.F.; Morales, A.R.; Mei, L.H.I. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur. Polym. J. 2017, 97, 356–365. [Google Scholar] [CrossRef]
- Bagde, P.; Nadanathangam, V. Mechanical, antibacterial and biodegradable properties of starch film containing bacteriocin immobilized crystalline nanocellulose. Carbohydr. Polym. 2019, 222, 115021. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-sadowska, J.; Isbrandt, M.; Grzybowski, Ł. Glycerolysis of poly (lactic acid) as a way to extend the “life cycle” of this material. Polymers 2019, 11, 1963. [Google Scholar] [CrossRef] [Green Version]
- Sforzini, S.; Oliveri, L.; Chinaglia, S.; Viarengo, A. Application of biotests for the determination of soil ecotoxicity after exposure to biodegradable plastics. Front. Environ. Sci. 2016, 4, 68. [Google Scholar] [CrossRef]
- Ibrahim, H.; Mehanny, S.; Darwish, L.; Farag, M. A comparative study on the mechanical and biodegradation characteristics of starch-based composites reinforced with different lignocellulosic fibers. J. Polym. Environ. 2018, 26, 2434–2447. [Google Scholar] [CrossRef]
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability studies of poly (butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Aleixo Moreira, A.; Mali, S.; Yamashita, F.; Bilck, A.P.; de Paula, M.T.; Merci, A.; Oliveira, A.L.M. de Biodegradable plastic designed to improve the soil quality and microbiological activity. Polym. Degrad. Stab. 2018, 158, 52–63. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Popa, E.E.; Popescu, P.A.; Rapa, M.; Popa, M.E. Soil ecotoxicity assessment after biodegradation of some polymeric materials. Agron. Ser. Sci. Res. Stiint. Ser. Agron. 2019, 61, 538–543. [Google Scholar]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind. Crop. Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Altun, E.; Çelik, E.; Ersan, H.Y. Tailoring the microbial community for improving the biodegradation of chitosan films in composting environment. J. Polym. Environ. 2020, 28, 1548–1559. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Disintegrability under composting conditions of films based on gelatin, chitosan and/or sodium caseinate containing boldo-of-Chile leafs extract. Int. J. Biol. Macromol. 2020, 151, 178–185. [Google Scholar] [CrossRef]
- Kalita, N.K.; Kumar, M.; Mudenur, C.; Kalamdhad, A. Biodegradation of modified poly (lactic acid) based biocomposite films under thermophilic composting conditions. Polym. Test. 2019, 76, 522–536. [Google Scholar] [CrossRef]
- 14855-1:2012; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method. ISO: Geneva, Switzerland, 2012.
- D5338-15; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions, Incorporating Thermophilic Temperatures. ASTM International: West Conshohocken, PA, USA, 2015.
- 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory. ISO: Geneva, Switzerland, 2018.
- DIN EN 13432; Requirements for Packaging Recoverable Through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. Australian Standards: Sydney, NSW, Australia, 2000.
- AS 5810-2010; Biodegradable Plastics—Biodegradable Plastics Suitable for Home Composting. Australian Standards: Sydney, NSW, Australia, 2010.
- 16929:2021; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Defined Composting Conditions in a Pilot-Scale Test. ISO: Geneva, Switzerland, 2021.
- Cinelli, P.; Seggiani, M.; Mallegni, N.; Gigante, V.; Lazzeri, A. Processability and degradability of PHA-based composites in terrestrial environments. Int. J. Mol. Sci. 2019, 20, 284. [Google Scholar] [CrossRef] [Green Version]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of mechanical properties and thermal stability of biodegradable rice starch—Based films blended with carboxymethyl chitosan. Ind. Crop. Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Biodegradation behavior of starch-PVA films as affected by the incorporation of different antimicrobials. Polym. Degrad. Stab. 2016, 132, 11–20. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly (lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Prapruddivongs, C.; Apichartsitporn, M.; Wongpreedee, T. Effect of silica resources on the biodegradation behavior of poly (lactic acid) and chemical crosslinked poly (lactic acid) composites. Polym. Test. 2018, 71, 87–94. [Google Scholar] [CrossRef]
- Sedničková, M.; Pekařová, S.; Kucharczyk, P.; Janigová, I.; Kleinová, A.; Jochec, D.; Omaníková, L.; Perďochová, D.; Sedlařík, V.; Alexy, P.; et al. Changes of physical properties of PLA-based blends during early stage of biodegradation in compost. Int. J. Biol. Macromol. 2018, 113, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Toro-Márquez, L.A.; Merino, D.; Mendieta, J.R. Hydrogen-bonding interactions and compostability of bionanocomposite films prepared from corn starch and nano-fillers with and without added Jamaica flower extract. Food Hydrocoll. 2019, 89, 283–293. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Enhancing the biodegradation rate of poly(lactic acid) films and PLA bionanocomposites in simulated composting through bioaugmentation. Polym. Degrad. Stab. 2018, 154, 46–54. [Google Scholar] [CrossRef]
- D6400-21; Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities. ASTM International: West Conshohocken, PA, USA, 2021.
- 17088:2021; Plastics–Organic Recycling–Specifications for Compostable Plastics. ISO: Geneva, Switzerland, 2021.
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Goller, C.C.; Venditti, R.A. Aerobic biodegradation in freshwater and marine environments of textile micro fibers generated in clothes laundering: Effects of cellulose and polyester-based microfibers on the microbiome. Mar. Pollut. Bull. 2020, 151, 110826. [Google Scholar] [CrossRef]
- Harrison, J.P.; Boardman, C.; Callaghan, O.; Delort, A.; Song, J.; Harrison, J.P. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 171792. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of so-called biodegradable polymers in seawater and freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Unger, B.; Mortier, N. Assessing marine biodegradability of plastic—Towards an environmentally relevant international standard test scheme. Proc. Int. Conf. Microplastic Pollut. Mediterr. Sea 2018, 189–193. [Google Scholar] [CrossRef]
- Kjeldsen, A.; Price, M.; Lilley, C.; Guzniczak, E.; Archer, I. A Review of Standards for Biodegradable Plastics. 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/817684/review-standards-for-biodegradable-plastics-IBioIC.pdf (accessed on 12 February 2022).
- Briassoulis, D.; Pikasi, A.; Papardaki, N.G.; Mistriotis, A. Aerobic biodegradation of bio-based plastics in the seawater/sediment interface (sublittoral) marine environment of the coastal zone—Test method under controlled laboratory conditions. Sci. Total Environ. 2020, 722, 137748. [Google Scholar] [CrossRef] [PubMed]
- Lott, C.; Eich, A.; Unger, B.; Makarow, D.; Battagliarin, G.; Schlegel, K.; Lasut, M.T.; Weber, M. Field and mesocosm test methods to assess the performance of biodegradable plastic under marine conditions. bioRxiv 2020. [Google Scholar] [CrossRef]
- 18830:2016; Plastics—Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sandy Sediment Interface—Method by Measuring the Oxygen Demand in Closed Respirometer. ISO: Geneva, Switzerland, 2016.
- 19679:2016; Plastics—Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sediment Interface—Method by Analysis of Evolved Carbon Dioxide. ISO: Geneva, Switzerland, 2016.
- 14853:2016; Plastics—Determination of the Ultimate Anaerobic Biodegradation of Plastic Materials in an Aqueous System—Method by Measurement of Biogas Production. ISO: Geneva, Switzerland, 2016.
- 23977-1:2020; Plastics—Determination of the Aerobic Biodegradation of Plastic Materials Exposed to Seawater—Part 1: Method by Analysis of Evolved Carbon Dioxide. ISO: Geneva, Switzerland, 2020.
- 23977-2:2020; Plastics—Determination of the Aerobic Biodegradation of Plastic Materials Exposed to Seawater—Part 2: Method by Measuring the Oxygen Demand in Closed Respirometer. ISO: Geneva, Switzerland, 2020.
- 15314:2018; Plastics—Methods for Marine Exposure. ISO: Geneva, Switzerland, 2018.
- 22766:2020; Plastics—Determination of the Degree of Disintegration of Plastic Materials in Marine Habitats under Real Field Conditions. ISO: Geneva, Switzerland, 2020.
- D6691-17; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum. ASTM International: West Conshohocken, PA, USA, 2017.
- D7473/D7473M-21; Standard Test Method for Weight Attrition of Non-Floating Plastic Materials by Open System Aquarium Incubations. ASTM International: West Conshohocken, PA, USA, 2021.
- D7991-15; Standard Test Method for Determining Aerobic Biodegradation of Plastics Buried in Sandy Marine Sediment under Controlled Laboratory Conditions. ASTM International: West Conshohocken, PA, USA, 2015.
- Dilkes-Hoffman, L.S.; Lane, J.L.; Grant, T.; Pratt, S.; Lant, P.A.; Laycock, B. Environmental impact of biodegradable food packaging when considering food waste. J. Clean. Prod. 2018, 180, 325–334. [Google Scholar] [CrossRef]
- D5229/D5229M-20; Standard Test Method for Moisture Absorption Properties and Equilibrium Conditioning of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2020.
- 62:2008; Plastics—Determination of Water Absorption. ISO: Geneva, Switzerland, 2008.
- Hassan, M.M.; Le Guen, M.J.; Tucker, N.; Parker, K. Thermo-mechanical, morphological and water absorption properties of thermoplastic starch/cellulose composite foams reinforced with PLA. Cellulose 2019, 26, 4463–4478. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239, 117994. [Google Scholar] [CrossRef]
- Kasmuri, N.; Safwan, M.; Zait, A. Enhancement of bio-plastic using eggshells and chitosan on potato starch based. Int. J. Eng. Technol. 2018, 7, 110–115. [Google Scholar] [CrossRef]
- Kumar Thiagamani, S.M.; Krishnasamy, S.; Muthukumar, C.; Tengsuthiwat, J.; Nagarajan, R.; Siengchin, S.; Ismail, S.O. Investigation into mechanical, absorption and swelling behaviour of hemp/sisal fibre reinforced bioepoxy hybrid composites: Effects of stacking sequences. Int. J. Biol. Macromol. 2019, 140, 637–646. [Google Scholar] [CrossRef]
- Morohoshi, T.; Oi, T.; Aiso, H.; Suzuki, T.; Okura, T.; Sato, S. Biofilm formation and degradation of commercially available biodegradable plastic films by bacterial consortiums in freshwater environments. Microbes Environ. 2018. [Google Scholar] [CrossRef] [Green Version]
- Rameshkumar, S.; Shaiju, P.; Connor, K.E.O. Bio-based and biodegradable polymers—State-of-the- art, challenges and emerging trends. Curr. Opin. Green Sustain. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Luyta, A.S.; Malik, S.S. Can biodegradable plastics solve plastic solid waste accumulation. In Plastics to Energy Fuel, Chemicals, and Sustainability Implications; Al-Salem, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–423. [Google Scholar]
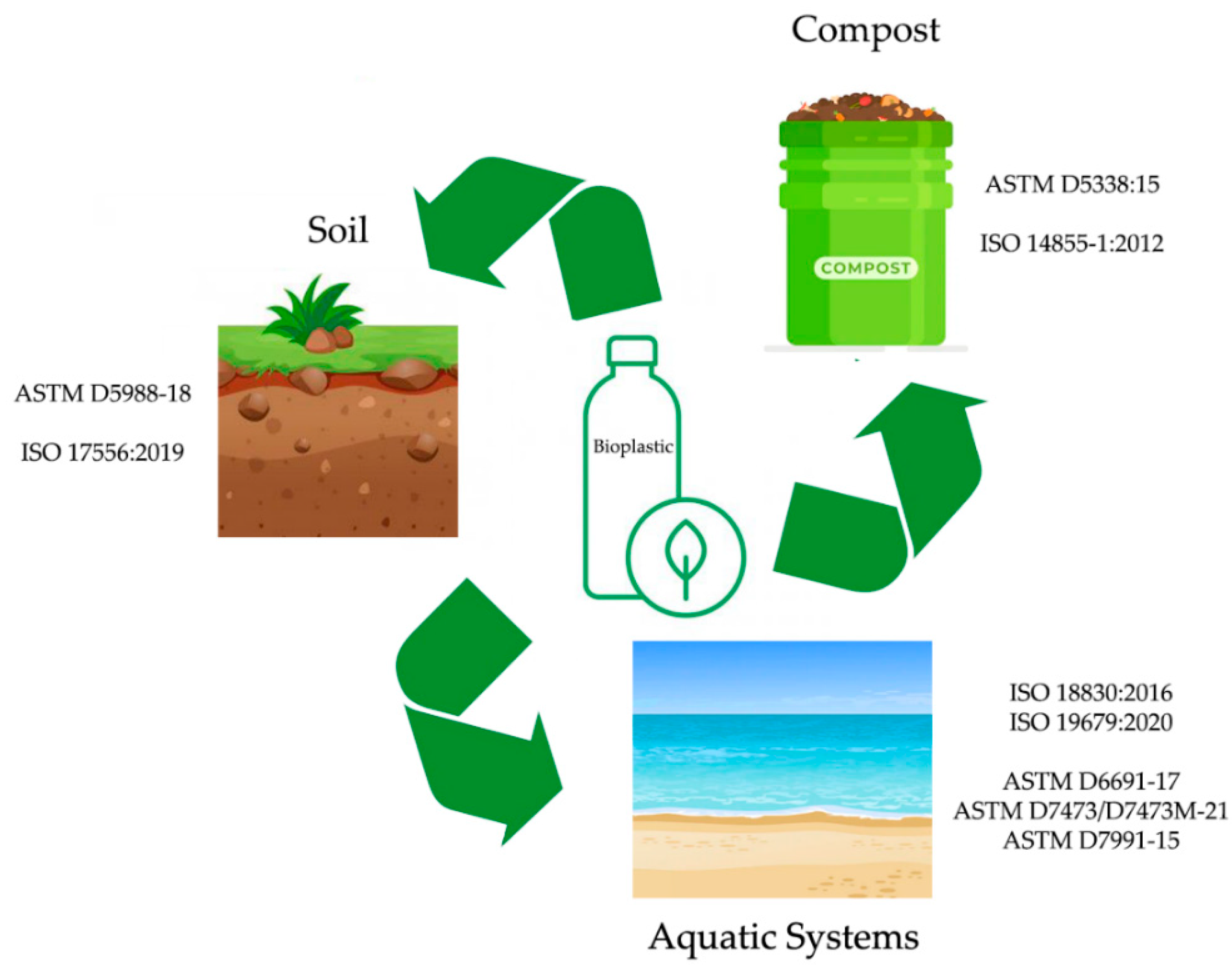
| Standard | Name | Scope |
|---|---|---|
| ASTM D5338:15 | Standard test method for determining aerobic biodegradation of plastic materials under controlled composting conditions, incorporating thermophilic temperatures | Determination of the ultimate aerobic biodegradability |
| ISO 14855-1:2012 | Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions—method by analysis of evolved carbon dioxide—Part 1: general method | |
| ISO 14855-2:2018 | determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions—method by analysis of evolved carbon dioxide—Part 2: gravimetric measurement of carbon dioxide evolved in a laboratory-scale test | |
| ISO 16929:2021 | Determination of the degree of disintegration of plastic materials under defined composting conditions in a pilot-scale test | Determination of the disintegration degree of plastic materials in composting |
| ISO 20200:2015 | Plastics—determination of the degree of disintegration of plastic materials under simulated composting conditions in a laboratory-scale test | |
| ASTM D6400-21 | Standard specification for labeling of plastics designed to be aerobically composted in municipal or industrial facilities | Establishes the requirements for identifying plastics and products made from plastics that compost satisfactorily in industrial and municipal aerobic composting facilities. Includes biodegradation, disintegration, and environmental safety testing requirements and criteria |
| ISO 17088:2021 | Plastics—organic recycling—specifications for compostable plastics |
| Standard | Name | Scope |
|---|---|---|
| ISO 18830:2016 | Plastics—determination of aerobic biodegradation of non-floating plastic materials in a seawater/sandy sediment interface—method by measuring the oxygen demand in closed respirometer | Determination of the degree and rate of aerobic biodegradation of plastic materials when settled on marine sandy sediment at the interface between seawater and the seafloor by measuring the oxygen demand in a closed respirometer. |
| ISO 19679:2016 | Plastics—determination of aerobic biodegradation of non-floating plastic materials in a seawater/sediment interface—method by analysis of evolved carbon dioxide | Determination of the degree and rate of aerobic biodegradation of plastic materials when settled on marine sandy sediment at the interface between seawater and the seafloor by measuring the evolved carbon dioxide. |
| ISO 14853:2016 | Plastics—determination of the ultimate anaerobic biodegradation of plastic materials in an aqueous system—method by measurement of biogas production | Determination of the ultimate anaerobic biodegradability of plastics by anaerobic microorganisms by exposing the test material to sludge for a period of up to 90 days, which is longer than the normal sludge retention time (25 to 30 days) in anaerobic digesters. |
| ISO 23977-1:2020 | Plastics—determination of the aerobic biodegradation of plastic materials exposed to seawater—Part 1: method by analysis of evolved carbon dioxide | Determination of the degree and rate of the aerobic biodegradation level of plastic materials. Biodegradation is determined by measuring the CO2 evolved from plastic materials when exposed to seawater sampled from coastal areas under laboratory conditions. |
| ISO 23977-2:2020 | Plastics—determination of the aerobic biodegradation of plastic materials exposed to seawater—Part 2: method by measuring the oxygen demand in closed respirometer | Determination of the degree and rate of the aerobic biodegradation level of plastic materials. Biodegradation of plastic materials is determined by measuring the oxygen demand in a closed respirometer when exposed to seawater sampled from coastal areas under laboratory conditions. |
| ISO 15314:2018 | Plastics—methods for marine exposure | Description of three methods for the exposure of plastics in a marine environment. Method A covers exposures where specimens float on the surface, method B covers exposures where specimens are partially immersed method C covers exposures where specimens are completely immersed. |
| ISO 22766:2020 | Plastics—determination of the degree of disintegration of plastic materials in marine habitats under real field conditions | Determination of the degree of disintegration of plastic materials exposed to marine habitats under real field conditions. The marine areas under investigation are the sandy sublittoral and the sandy eulittoral zone where plastic materials can either be placed intentionally. |
| ISO 62:2008 | Plastics—determination of water absorption | Determination of the moisture absorption properties in the “through-the-thickness” direction of flat or curved-form solid plastics. Determination of the amount of water absorbed by plastic specimens of defined dimensions when immersed in water or when subjected to humid air under controlled conditions. |
| ASTM D6691-17 | Standard test method for determining aerobic biodegradation of plastic materials in the marine environment by a defined microbial consortium or natural sea water inoculum | Determination of the degree and rate of aerobic biodegradation of plastic materials (including formulation additives) exposed to pre-grown population of at least ten aerobic marine microorganisms of known genera or the indigenous population existing in natural seawater. |
| ASTM D7473/D7473M-21 | Standard test method for weight attrition of non-floating plastic materials by open system aquarium incubations | Determination of the weight loss as a function of time of non-floating plastic materials (including formulation additives) when incubated under changing, open marine aquarium conditions, which is representative of aquatic environments near the coasts and near the bottom of a body of water in the absence of sunlight, particularly UV and visible portions of the spectrum. |
| ASTM D7991-15 | Standard test method for determining aerobic biodegradation of plastics buried in sandy marine sediment under controlled laboratory conditions | Determination of the biodegradation level of plastic materials exposed to laboratory conditions that simulate the environment found in the sandy tidal zone. The tidal zone, that is, the part of the coast affected by the tides and movement of the waves, is the borderline between sea and land, frequently a sandy area that is kept constantly damp by the lapping of the waves. |
| ASTM D570-98(2018) | Standard test method for water absorption of plastics | Determination of the relative rate of absorption of water by plastics when immersed. This test method is intended to apply to the testing of all types of plastics, including cast, hot-molded, and cold-molded resinous products, and both homogeneous and laminated plastics in rod and tube form and in sheets 0.13 mm (0.005 in.) or greater in thickness. |
| ASTM D5229/D5229M-20 | Standard test method for moisture absorption properties and equilibrium conditioning of polymer matrix composite materials | Determination of moisture absorption or desorption properties in the “through-the-thickness” direction for single-phase Fickian solid materials in flat or curved panel form. Procedures for conditioning test coupons prior to use in other test methods are also covered, either to an essentially moisture-free state to equilibrium in a standard laboratory atmosphere environment, or to equilibrium in a non-laboratory environment. Procedures for determining the moisture loss during elevated temperature testing are also included, as well as moisture loss resulting from thermal exposure after removal from the conditioning environment, such as during strain gauge bonding. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, J.R.A.; Souza, V.G.L.; Fuciños, P.; Pastrana, L.; Fernando, A.L. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers 2022, 14, 1359. https://doi.org/10.3390/polym14071359
Pires JRA, Souza VGL, Fuciños P, Pastrana L, Fernando AL. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers. 2022; 14(7):1359. https://doi.org/10.3390/polym14071359
Chicago/Turabian StylePires, João Ricardo Afonso, Victor Gomes Lauriano Souza, Pablo Fuciños, Lorenzo Pastrana, and Ana Luísa Fernando. 2022. "Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps" Polymers 14, no. 7: 1359. https://doi.org/10.3390/polym14071359
APA StylePires, J. R. A., Souza, V. G. L., Fuciños, P., Pastrana, L., & Fernando, A. L. (2022). Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers, 14(7), 1359. https://doi.org/10.3390/polym14071359











