Abstract
The limitations on the use of fluoride therapy in dental caries prevention has necessitated the development of newer preventive agents. This review focusses on the recent and significant studies on P11-4 peptide with an emphasis on different applications in dental hard tissue conditions. The self-assembling peptide P11-4 diffuses into the subsurface lesion assembles into aggregates throughout the lesion, supporting the nucleation of de novo hydroxyapatite nanocrystals, resulting in increased mineral density. P11-4 treated teeth shows more remarkable changes in the lesion area between the first and second weeks. The biomimetic remineralisation facilitated in conjunction with fluoride application is an effective and non-invasive treatment for early carious lesions. Despite, some studies have reported that the P11-4 group had the least amount of remineralised enamel microhardness and a significantly lower mean calcium/phosphate weight percentage ratio than the others. In addition, when compared to a low-viscosity resin, self-assembling peptides could neither inhibit nor mask the lesions significantly. Moreover, when it is combined with other agents, better results can be achieved, allowing more effective biomimetic remineralisation. Other applications discussed include treatment of dental erosion, tooth whitening and dentinal caries. However, the evidence on its true clinical potential in varied dental diseases still remains under-explored, which calls for future cohort studies on its in vivo efficacy.
1. Introduction
Tooth enamel is a complex structure comprising organic and inorganic components that produce the human body’s strongest mineralised structure. Hydroxyapatite crystals, which are calcium phosphate salts, make up the mineral composition of enamel [1], resulting in enamel prisms [2]. Dixit et al., in 2021 stated that dental caries results in focal disintegration of the mineralised tissues of teeth which is attributed to numerous cycles of demineralisation and remineralisation, with the intervening phases being either reversible or irreversible [3].
As early as 1982, Mizrahi stated that demineralisation is evident in early carious lesions known as white spot lesions (WSLs), and the presence of underlying porosities give them a milky appearance [4]. Demineralisation and remineralisation are natural processes that take place in the oral cavity. Boland, in 1999 found that during the caries process, there is increased removal of inorganic minerals from the periphery of the prisms due to its enhanced accessibility and solubility [5], which is followed by the dissolving of prism bodies [2]. Dental enamel is dissolved by the acids from meals, soft drinks, and bacteria found in plaque [6]. Madan et al. [7] in 2011 concluded that the saliva’s buffering effect aids in remineralisation by allowing calcium and phosphate ions to precipitate onto the tooth surface and produce new material. Another group of authors stated the most common early sign of dental caries progression is the appearance of WSLs or reversible regions of demineralisation [8].
Kidd, in 2004 stated that less invasive and more biological approaches such as remineralisation and regeneration of biological tissue have challenged the traditional treatment of cavitated lesions by drilling and filling. This merely involves replacing damaged tissue with foreign material, temporarily masking the process [9]. Hence, this raises the question of whether it is genuinely possible to reconstruct something which was made by nature and eventually destroyed during the caries process. Innes, as early as 2016, stated that there is emerging evidence and worldwide consensus in favour of utilising less invasive therapies that focus on lesion control rather than tissue removal [10]. Restorative treatment is limited to cavitated non-cleansable, carious lesions or restoring afflicted teeth function, form, and aesthetics [11].
When encountering demineralised WSLs, the primary goal must be to remineralise the lesions using a non-invasive method [12]. This necessitates the detection of these lesions in their early phases of development. Topical fluoride application has been popular for years to prevent enamel demineralisation and speed up the remineralisation process [13]. Gurunathan et al., in 2012 have reported that the utilisation of bioactive materials and calcium in the form of the Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) complex is one of the more recent innovations in the treatment of early carious lesions [14].
The self-assembling peptide was first discovered in 1989 due to curiosity-driven research similar to the chance discovery of X-ray and CRISPR for gene editing [15]. Since then, self-assembling peptides have been used in an array of applications ranging from surfactant materials to accelerated wound healing in regenerative medicine [15,16].
A self-assembling peptide molecule P11-4 has been studied extensively. It is a rationally designed synthetic peptide of 11 amino acids that undergoes hierarchical self-assembly into B-sheet tapes, ribbons, fibrils and fibres [17]. It exists as unimers of random coil conformations in water above pH 7.5 but at low pH adopts an antiparallel β-sheet conformation. It also self-assembles under physiological conditions in a concentration-dependent manner [18]. It has been demonstrated that incorporation of Glu- or Orn- into the primary structure could enable rapid and reversible self-assembly by simply changing the pH [17]. Li and colleagues studied the role of self-assembling peptides in enamel remineralisation based on a biomimetic approach [19], whose main goal is to replicate the natural process of enamel mineralisation. It was also reported in 2016 that non-collagenous proteins with a negative charge play a function in attracting positively charged calcium ions during the natural process of mineralisation [20] and that the negative charge acts as a nucleation site, and mineral crystal formation is accomplished through the growth and fusion of mineral nuclei. In addition to the use of P11-4 peptide on enamel, its applicability on dentin tissues has lately been investigated. De Sousa et al., in 2019 assessed P11-4’s interaction with organic dentin components, as well as its impact on proteolytic activity, mechanical aspects of the bonding interface, and nanoleakage evaluation in simulated caries-affected dentin. P11-4 interacts with collagen type I fibres, improving the integrity of the hybrid layer generated by artificial caries-affected dentin and enhancing collagen fibre resistance to proteolysis [21].
This review describes the technology of self-assembling peptides and the mechanism of 3D scaffold construction, emphasising the notion of de novo hydroxyapatite crystal formation in response to the administration of peptide P11-4, assisting in enamel/dentin remineralisation after the initiation of dental caries. In addition, its applicability in dentinal hypersensitivity and dental erosion is discussed. Bearing that in mind, the following review question was addressed ‘What are the different applications of P11-4 self-assembling peptide in dental hard tissue (Enamel/Dentin) conditions’?
For searching all relevant studies on the efficacy of P11-4 self-assembling peptide in various dental hard tissue conditions, PubMed, Scopus, Embase, Web of Science databases was searched as follows: (((“P11-4 peptide” [Supplementary Concept]) AND “Dental Enamel” [Mesh]) OR “Dentin Sensitivity” [Mesh]) OR “Tooth Erosion” [Mesh].
The literature search was carried out in the Journal of Dental Research, Journal of Endodontics, and Journal of Conservative Dentistry to ensure a complete screening process. Review articles were used as additional sources of references for further information. All included studies were checked for the availability of full text. The search was mainly focused on mapping existing literature. The search span had articles until January 2022 published in English. A total of 7845 full-text articles were found. Duplicate records were removed using Mendeley (Elsevier Inc., USA), and 868 free full-text articles were retrieved. Based on our inclusion criteria, 35 articles were included for review. The details of the included articles are presented in Table 1. Most of the articles focused on evaluating P11-4 peptides on remineralisation of enamel caries [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Only three articles were found to have studied its effects on dentin [21,50,51], while two others assessed remineralisation in dental erosion [52,53], and one assessed its efficacy in tooth whitening [54,55].

Table 1.
Functions of Self-assembling Peptide P11-4.
2. P11-4 Self-Assembling Peptide
Acar et al., in 2017 described the roles of proteins and peptides in the human body, which can fold into various shapes, making them valuable biomaterials. The building blocks of peptides are amino acids [56]. They also suggested that amino acid side chains with a terminal -COOH or -NH2 can be placed [16]. Aggeli et al., in 2003 stated that there is a controlled interaction between adjacent peptides [17], and this interaction can self-organise into different structures [56]. In 2017, Fan et al. referred to this as molecular self-assembly. In addition, they stated that specific peptides, such as the α-helical peptide, β-sheet peptide, amphiphilic peptide, cyclic peptide, and dipeptide, can self-assemble [57]. The lower occurrence of side effects and stable drug release are also advantages of peptide-based self-assembled structures [58,59]. Self-assembly of these structures can result in the formation of nanostructures, including nanofibers, nanotubes, and nanovesicles [60]. In 2007, Gelain et al. itemised the use of these self-assembled nanostructures as scaffolds in the field of regenerative medicine, 3-D tissue cell culture and drug delivery systems were reported [61]. The self-assembling peptide P11-4 is one of the promising biomimetic alternatives for enamel remineralisation. P11 peptide group performs one-dimensional, hierarchical self-assembly when the concentration of peptide monomer approaches the critical monomer concentration (C*). Within seconds, micrometre-long- β sheet nanotapes and ribbons are formed and then assembled to create fibrils and edge-to-edge fibres during the next 24 h [34,62].
The glutamine residues at the end of the chain increase hydrophobic interactions and hydrogen bonding. The presence of arginine in the chemical structure results in a positive net charge, allowing for antiparallel β-sheet formation. Since glutamate residues are negatively charged at higher pH in aqueous solution, electrostatic repulsion prevents efficient β -sheet production [18]. However, it was discovered that sodium ions could buffer the repulsive effects of negatively charged residues at physiological conditions (pH 7.4, NaCl 130 mM). P11-4 undergoes self-assembly as a result of the ionic interaction between the negatively charged glutamic acid and positively charged arginine [63].
P11-4 features four negatively charged Glu-residues that could act as Ca2+ binding sites when assembled into fibres. The distance between these sites is 9.4, which is close to the position of columnar Ca2+ ions in the HAP crystal lattice [24]. Furthermore, Firth et al. used energy-dispersive X-ray examination and revealed that the crystals’ Ca/P molar ratios are consistent with HAP-1.67 [64]. Therefore, this anionic peptide can be used as a low viscosity, injectable monomer solution that can infiltrate demineralised enamel and gel quickly at pH levels below 8.0 [64]. Furthermore, the freshly constructed three-dimensional scaffold has a strong chemical interaction with the tooth surface. Therefore, it could act as a template for HAP nucleation and deposition within the lesion, mimicking the role of enamel matrix proteins [65].
3. The Rationale behind the Use of P11-4
In the vast majority of instances, untreated or inadequately treated incipient caries results in the formation of a cavity in the tooth, which must be treated invasively. A filling might last anywhere from 10 to 15 years. Following that, a more extensive filling is routinely placed, which frequently results in tooth loss. It has been reported [35,66] that fluoride is the main reason for the reduction in caries because of its cariostatic potential. Despite its effectiveness in slowing the advancement of caries, it has several drawbacks. Fluoride does not entirely eliminate caries. Moreover, in-depth mineralisation does not occur [35]. This necessitates the use of therapy to regenerate a mineral deficiency in the tooth enamel due to a carious lesion [21,23,24,26,32,38,40,67,68] and could be termed as ‘Guided Enamel Remineralisation’ (GER). Dentin, too, has the potential to undergo such biomimetic remineralisation [21,50,51].
4. Method of Application of P11-4 Peptide
It is pertinent to remove the superficial pellicle using 2% sodium hypochlorite followed by the application of 35% phosphoric acid for 20 s. After cleaning and drying the teeth, the surface must be assessed for the presence of open pores. This is intended for allowing the material to penetrate the lesion and initiate the process. Fluorides and other remineralisation-promoting chemicals can also aid in this process, as observed in previous literature [25,46,69]. Fluoridation with products containing more than 5000 ppm, on the other hand, should not be carried out immediately after application. As the process of remineralisation is time-dependent, it is necessary to administer the peptide numerous times over the course of 3–6 months to achieve the beneficial effect. However, a study by Brunton found that a single application is associated with significant enamel regeneration, presumably by promoting mineral deposition within the subsurface tissue [34].
5. Mode of Action
Aggeli et al. described the mode of action of the P11-4 peptide. Its chemical structure (Ace-Gln-Gln-Arg-Phe-Glu-Trp- Glu-Phe-Glu-Gln-Gln-NH2) is made up of five amino acids: arginine, tryptophan, phenylalanine, glutamine, and glutamic acid [70]. It is also called oligopeptide 104. They also described that the 11 amino acid peptide P11-2 is modified to allow the self-assembly of peptides in response to pH changes. The glutamine (Gln) residues of peptide P11-2 are organised in a specific order. These residues have side chains that interact and cause β-sheet formations to form [71]. By replacing the glutamic acid residues at positions 5 and 7 with glutamine residues, peptide P11-4 was induced to remain monomeric at high pH and transform to a nematic gel at low pH [70].
As early as 2006, Davies et al. described the formation of peptide fibres from the β-sheet structure that self-assembles to generate nanotapes, the simplest kind of hierarchical structure. These tapes have a helical structure due to their twisting and bending. Nanoribbons are formed when nanotapes are intertwined. The nanoribbons do not have the same helical structure as nanotapes but instead have a saddle curvature. This occurs because the tapes’ bending, and twisting must be reduced to promote stacking [62]. Nanoribbon stacking results in the production of nanofibrils. The number of ribbons that can be stacked is determined by the balance between the untwisting of nanoribbons involved in stacking and the increase in the attraction energy of these ribbons [57]. A ribbon with a short twist angle will have a large pitch. If the magnitude of attraction energy is large enough, the ribbon will untwist completely, causing stacking and the production of a two-dimensional crystal [60,62]. A ribbon with significant twist angles and a smaller magnitude of attraction energy will not produce fibrils; instead, the equilibrium structure in the solution will be a ribbon. Low to moderate twisting angles and low to intermediate attraction energy are thus required to produce separate fibrils. Edge-to-edge entwining is possible with stable fibrils. Peptide fibres are thus formed [62], serving as a scaffold for the de novo creation of hydroxyapatite crystals [34].
The mechanism of action of the P11-4 peptide (Figure 1) shows the formation of a hierarchical self-assembly unit. Kirkham et al., in 2007 described its conversion to nanostructures, thereby constructing a scaffold under appropriate environmental circumstances [25]. The presence of cations and a low pH of 7.4 characterise a carious lesion. Peptide P11-4 self-assembles under these conditions [34]. By undergoing self-assembly in one dimension, it produces a β-sheet structure. Intermolecular hydrogen bonding and interactions between side chains are involved in this self-assembly process [25]. Fan et al., in 2017, described the β-sheet structure, which is amphiphilic because it has a peptide sequence with alternating hydrophobic and hydrophilic amino acids. The amphiphilic feature of the β-sheet structure drives its self-assembling property [57].
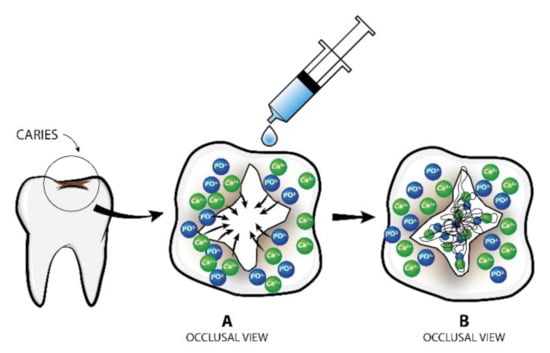
Figure 1.
Mode of action of P11-4 on dental caries. (A) Calcium hydroxyapatite crystals around the lesion are attracted inwards and interact with peptide molecules; (B) Formation of new hydroxyapatite crystals within the scaffold created.
It was in 2006 that the interaction between peptide aggregates leading to the formation of either flexible 3D structure or more rigid anisotropic gel was described [62]. As the peptide concentration grows, so does the number of aggregates generated and the average length of the peptides. At a given concentration (Cgel), the interaction between the aggregates can be seen. When the concentration of a solution exceeds that of Cgel, it is considered a semi-dilute state. The more flexible tapes and ribbons create a sponge-like 3D structure, which leads to gelation. On the other hand, the more rigid fibrils align into nematic domains and join to form an anisotropic gel structure. This method shifts the liquid from isotropic to the nematic condition [62].
Previous researchers have demonstrated that peptide P11-4 has a low viscosity and is an isotropic liquid at high pH [62]. It changes to a nematic gel state during the self-assembly process, occurring at a pH range of 6.8–7.2 [17]. They used rheological measures to investigate the transition of nematic to isotropic fluids. According to their findings, the self-assembling peptide P11-4 remains monomeric and shows Newtonian behaviour at higher pH values. It showed an intermittent behaviour between isotropic liquid and nematic gel in the pH range of 6.9 to 7.3, which is known as the biphasic region. The viscosity falls at pH 6.6, and it transforms into a nematic state with viscoelastic fluid features; at pH 2, it transforms into a nematic gel with low yield stress. Between pH 2.0 and pH 13.0, the self-assembling peptide exists in four distinct states [17]. They could also create an instantaneous changeover between the nematic gel phase and isotropic fluid phase by appropriately introducing an acid or a base. However, after four ‘pH jumps’, the gel flocculated due to increased ionic strength [48].
Wierichs et al., in 2017, discussed the disadvantages of the self-assembling peptide technique. They concluded that the nematic form of a self-assembling peptide undergoes flocculation in oral environmental settings, where the pH fluctuates due to alternate demineralising and remineralising cycles. This flocculated condition of the self-assembling peptide is relatively inert and may obstruct the remineralisation process [39]. Furthermore, they also stated that during the remineralisation process, incorporating these flocculates into the enamel impacts the diffusion of calcium, phosphate, and fluoride ions to the enamel surface. As a result, during later phases of demineralisation, the availability of fluoride ions is reduced [39].
6. Evaluation of Efficacy of P11-4 Peptide
In vivo clinical evaluation of successful treatment by P11-4 peptide is assessed by multiple methods. Welk et al. assessed using impedance measurement by CarieScan Pro and morphometric measurement (in mm2) of the lesion [38], tactile sensation using dental mirror and explorer. DIAGNOdent has also been used by some authors [27]. International Caries Detection and Assessment System (ICDAS) II was used to assess qualitative improvement in remineralisation [27].
In vitro studies on P11-4 peptide were assessed using Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM) with microindentation [72], DIAGNOdent, micro-CT [37,43,73], infrared (IR) spectroscopy, circular dichroism (CD) [74]. AFM based nanoindentation is a valuable tool to investigate the demineralisation and remineralisation of surface softened enamel with high accuracy [72]. Soares et al., 2017 described enamel remineralisation by P11-4 peptide using SEM as shown in Figure 2 [35].
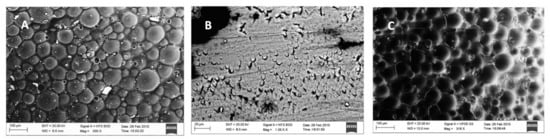
Figure 2.
Scanning electron microscopy images (A) Normal enamel surface; (B) Demineralised enamel; (C) P11-4 treated enamel surface after demineralisation [35] (Reproduced with permission).
Structural similarity between sound enamel and P11-4 treated enamel suggested biomimetic remineralisation by nucleating HA crystals. Soares et al. have also reported greater surface mean hardness values compared to Casein Phosphopeptide-Amorphous Calcium Phosphate Fluoride (CPP ACPF), Bioactive Glass (BAG) and fluoride enhanced hydroxyapatite (HA) gel [35]. Similar results were reported by Jablonski et al., in 2014 [44].
7. Uses of P11-4 Peptide in Dental Hard Tissue Conditions
7.1. Early Enamel Caries Remineralisation
Brunton et al., in 2013 [34] found porosities in a WSL of the enamel and reported that due to its low viscosity, monomeric peptide P11-4 penetrates these porosities when applied. The peptide self-assembles into a viscous fibrous scaffold under the influence of the circumstances seen in a carious environment. The anionic groups in peptide P11-4 attract calcium ions and can precipitate hydroxyapatite crystals from scratch. The nucleator pulls ions out of tissue fluids and organises them into a crystalline structure. The crystals will only grow once the crucial nuclei have been stabilised. The scaffold matrix is responsible for this stabilisation. This mechanism is similar to what happens naturally prior to tooth eruption when the enamel matrix proteins self-assemble to guide hydroxyapatite crystal precipitation [34].
Ex vivo research and clinical trials on class V early carious lesions have looked into the effect of self-assembling peptides. Brunton et al. also stated that early enamel smooth surface lesions have been shown to benefit from the use of self-assembling peptide P11-4 (Curodont®). The peptide’s main effects are seen within the first thirty days of treatment and are manifested as a reduction in the size of the lesion. However, the pitfall of this study is the absence of a control group [34]. After the peptide builds a new enamel matrix, calcium and phosphate ions from saliva are incorporated to remineralise the teeth [44]. This effect is maintained by using the remineralising paste regularly. Schlee et al., in 2018, studied the use of P11-4 peptide on proximal caries. They stated that once a proximal carious lesion reaches the dentin, the risk of cavitation increases dramatically. A restorative procedure for such a lesion would result in the tooth’s integrity being compromised. As a result, it’s critical for a clinician to try to prevent a proximal carious lesion from progressing into dentin. The use of self-assembling peptide P11-4 has resulted in the regression of initial proximal carious lesions, delaying or avoiding the necessity for restorative procedures [30].
A contrasting study by Golland et al., in 2017 on bovine teeth concluded that application of P11-4 peptide on demineralised bovine enamel did not lead to increased fluorescence measured using quantitative laser fluorescence, indicating either lack of remineralisation or irregular crystals [42]. Additionally, Memarpour et al., (2021) revealed P11-4 peptide treated primary teeth had the lowest percentage of surface enamel microhardness. In addition, the mean calcium/phosphate weight percentage ratio of P11-4 was significantly lower than the others (p < 0.001) [29]. Recently, a study by Wahba and colleagues reported on the inability of P11-4 peptide to remineralise caries in deciduous teeth [75]. However, some studies have concluded the beneficial effects of P11-4 peptide combined with fluoride varnish over the use of fluoride varnish alone [32,45]. Furthermore, another study reported that P11-4 peptide worked better when it was combined with either fluoride or CCP-ACPF than when used alone [46].
7.2. Orthodontic Treatment-Induced Caries
Demineralisation can occur near brackets during fixed orthodontic treatment. Early enamel alterations may progress, and WSLs may arise if appropriate preventative strategies are not used. According to a meta-analysis, 45.8% of patients developed new carious lesions while undergoing orthodontic treatment [76]. High treatment demand and the prevalence of biofilm-related problems, according to some experts [77], make orthodontic treatment a possible public health issue.
Although fluoride has been found to successfully prevent tooth cavities [78,79], when the caries lesion has developed to the clinically evident WSL, and the best probable outcome is an arrest of the lesion’s activity, there are restrictions to using fluoride [66]. In 2020, Jablonski et al. studied the use of the P11-4 peptide in patients with a high caries risk, such as those receiving orthodontic treatment. They came to the conclusion that the mineral gain effectiveness of fluoride varnish is superior to the one-time application of fluoride varnish. The benefit is that it improves enamel remineralisation, which is especially important in these patients because fluoride alone may not be enough [45]. Another study by Knaup et al., in 2021 concluded that the shear bond strength was not affected by using the caries-protecting P114 peptide before attaching brackets. As a result, preparation of the enamel surface with P114 peptide prior to bracket insertion is a viable option [26].
7.3. Dentinal Caries
A perusal of literature showed only a study by de Sousa et al. [21] that analysed the applicability of the self-assembling peptide P11-4 in the dentin tissue with organic components of the dentin matrix. The results demonstrated a new and promising ability of the self-assembling peptide P11-4 of binding type I collagen. Such characteristics increased the fibrils’ width and ultimately increased the immediate resistance of collagen type I fibres against collagenase activity. This could probably be due to P11-4 properties as a collagen binder, leading to the reinforcement of the bonding interface and the inhibition of collagen proteolysis at the hybrid layer. Atomic force microscopy of dentin samples showed dry P11-4 hydrogels with parallel nanofibrillar structures. The mean collagen type I fibre widths in the presence of P11-4 peptide increased from 30 to 330 nm [21].
7.4. Hypersensitivity of The Dentin
Two studies were found on hypersensitivity treatment using self-assembling peptides [50,51]. Dentine hypersensitivity (DH) is widely believed to occur as a result of fluid flow within exposed dentinal tubules in the tooth surface. Most treatments are designed to occlude these tubules. In order to determine the efficacy of self-assembling peptide P11-4 in treating dentinal hypersensitivity, many randomised clinical trials have been conducted. Due to hydroxyapatite binding sites, the self-assembling peptide P11-4 has a high affinity for the dentinal surface. As a result, electrostatic interactions bind the matrix to the tooth, due to which the dentinal tubules are occluded, and dentinal hypersensitivity is reduced due to these interactions [51]. Another in vitro study investigated the ability of a novel self-assembling peptide matrix gel with calcium phosphate in effectively occluding dentine tubules compared to selected desensitising toothpastes [50]. The ability of the desensitising gel and toothpastes to occlude the dentine tubules was assessed and compared before and after brushing using Scanning Electron Microscopy (SEM) on both etched and fractured dentine surfaces. The self-assembling peptide matrix gel demonstrated a more significant reduction in the number of open tubules compared to the other desensitising toothpastes. Reductions in the hydraulic conductance measurements were observed to be 55.1 (± 12.5%) [50].
7.5. Erosion of The Enamel
Early reference of causative factor for enamel erosion dates back to 1975 by Geddes, who suggested increased consumption of highly acidic soft drinks and fruit juices [80]. Under scanning electron microscopy, these erosions can be seen as surface roughness and abnormalities. The enamel is protected using the self-assembling peptide P11-4 before or after exposure to such acidic environments [52]. It slows the deterioration of enamel and helps with remineralisation [53]. However, Attin et al. reported that there was no anti-erosive effect and as well as no significant difference from the untreated control group [81].
8. Perspective and Conclusions
Recent examples of many successful clinical trials of self-assembling peptide P11-4 in initiating regenerative capacity for dental hard tissues have provided a glimpse of its widespread applications in future. As this peptide holds potential for a breakthrough in dentistry in guided enamel remineralisation, further research is to be directed towards the effects of self-assembling peptides on the dentinal structures of the tooth.
It is germane to remember that remineralisation in vitro can be substantially different from changes in the oral cavity in vivo. As a result, direct extrapolations to clinical settings must be carried out with caution. Hence, future in vivo cohort studies on treated surfaces may be undertaken to unfold the long-term effects of oral environment on the clinical longevity of treated tooth surfaces. It also underscores further exploration of P11-4 peptide in regenerative therapy of human periodontal tissue defects. The authors also suggest the use of this technology in enhancing the bond strength prior to orthodontic treatment.
The promising effect of P11-4 on early enamel caries by guided enamel remineralisation is evidenced from previous studies. Some reports have doubted its beneficial effects when used alone; however, it is also pertinent to note that some studies have reported better outcomes when combined with other agents than when used alone. This leads to the inference that the evidence to draw a concrete conclusion on its true clinical potential still remains under-explored.
Author Contributions
Writing—review&editing, A.A.D., R.A.T., Z.M., A.A. and K.T.P.; Writing—original draft, A.A.D., R.A.T., Z.M., A.A. and K.T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Universiti Sains Malaysia for facilitating the above review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mann, A.B.; Dickinson, M.E. Nanomechanics, Chemistry and Structure at the Enamel Surface. Monogr. Oral Sci. 2005, 19, 105–131. [Google Scholar] [CrossRef]
- Robinson, C.; Shore, R.; Brookes, S.; Strafford, S.; Wood, S.; Kirkham, J. The Chemistry of Enamel Caries. Crit. Rev. Oral Biol. Med. 2000, 11, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Gopakumar, R.; Mampilly, M.O.; Nallamothu, R.; Jayachandran, M.; Terence, N.M. Analysis of Remineralization Potential of Three Different Remineralizing Pastes on Demineralized Enamel: A Comparative Study. J. Contemp. Dent. Pract. 2021, 22, 939–942. [Google Scholar] [CrossRef]
- Mizrahi, E. Enamel demineralization following orthodontic treatment. Am. J. Orthod. 1982, 82, 62–67. [Google Scholar] [CrossRef]
- Boland, T.W. Dental erosion: More acid means fewer teeth. New South Wales Public Health Bull. 1999, 10, 35. [Google Scholar] [CrossRef]
- Cheng, Z.-J.; Wang, X.-M.; Cui, F.-Z.; Ge, J.; Yan, J.-X. The enamel softening and loss during early erosion studied by AFM, SEM and nanoindentation. Biomed. Mater. 2009, 4, 015020. [Google Scholar] [CrossRef]
- Madan, N.; Madan, N.; Sharma, V.; Pardal, D.; Madan, N. Tooth remineralization using bio-active glass—A novel approach. J. Adv. Oral Res. 2011, 2, 45–50. [Google Scholar] [CrossRef]
- Vashisht, R.; Indira, R.; Ramachandran, S.; Kumar, A.; Srinivasan, M.R. Role of casein phosphopeptide amorphous calcium phosphate in remineralization of white spot lesions and inhibition of Streptococcus mutans? J. Conserv. Dent. 2013, 16, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Kidd, E. How ‘Clean’ Must a Cavity Be before Restoration? Caries Res. 2004, 38, 305–313. [Google Scholar] [CrossRef]
- Innes, N.P.T.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Terminology. Adv. Dent. Res. 2016, 28, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Innes, N.; Schwendicke, F. Restorative Thresholds for Carious Lesions: Systematic Review and Meta-analysis. J. Dent. Res. 2017, 96, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwendicke, F.; Frencken, J.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Fontana, M. Enhancing Fluoride: Clinical Human Studies of Alternatives or Boosters for Caries Management. Caries Res. 2016, 50, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, D.; Somasundaram, S.; Kumar, S. Casein phosphopeptide-amorphous calcium phosphate: A remineralizing agent of enamel. Aust. Dent. J. 2012, 57, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Discovery and design of self-assembling peptides. Interface Focus 2017, 7, 20170028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-Assembling Peptides: From Design to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, A.; Bell, M.; Carrick, L.M.; Fishwick, C.W.G.; Harding, R.; Mawer, P.J.; Radford, S.E.; Strong, A.E.; Boden, N. pH as a Trigger of Peptide β-Sheet Self-Assembly and Reversible Switching between Nematic and Isotropic Phases. J. Am. Chem. Soc. 2003, 125, 9619–9628. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Aggeli, A.; Ingham, E.; McPherson, M.J. Recombinant self-assembling peptides as biomaterials for tissue engineering. Biomaterials 2010, 31, 9395–9405. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, J.; Joiner, A.; Chang, J. The remineralisation of enamel: A review of the literature. J. Dent. 2014, 42, S12–S20. [Google Scholar] [CrossRef]
- Caruso, S.; Bernardi, S.; Pasini, M.; Giuca, M.R.; Docimo, R.; Continenza, M.A.; Gatto, R. The process of mineralisation in the development of human tooth. Eur. J. Paediatr. Dent. 2016, 17, 322–326. [Google Scholar]
- de Sousa, J.; Carvalho, R.; Barbosa-Martins, L.F.; Torquato, R.; Mugnol, K.; Nascimento, F.; Tersariol, I.; Puppin-Rontani, R. The Self-Assembling Peptide P11-4 Prevents Collagen Proteolysis in Dentin. J. Dent. Res. 2019, 98, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Alkilzy, M.; Tarabaih, A.; Santamaria, R.; Splieth, C. Self-assembling Peptide P11-4 and Fluoride for Regenerating Enamel. J. Dent. Res. 2018, 97, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bröseler, F.; Tietmann, C.; Bommer, C.; Drechsel, T.; Heinzel-Gutenbrunner, M.; Jepsen, S. Randomised clinical trial investigating self-assembling peptide P11-4 in the treatment of early caries. Clin. Oral Investig. 2020, 24, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.; Brookes, S.; Aggeli, A. Self-assembling Peptide Scaffolds Promote Enamel Remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Knaup, T.; Korbmacher-Steiner, H.; Jablonski-Momeni, A. Effect of the caries-protective self-assembling peptide P11-4 on shear bond strength of metal brackets. J. Orofac. Orthop. 2021, 82, 329–336. [Google Scholar] [CrossRef]
- Kobeissi, R.; Badr, S.B.; Osman, E. Effectiveness of Self-assembling Peptide P11-4 Compared to Tricalcium Phosphate Fluoride Varnish in Remineralization of White Spot Lesions: A Clinical Randomized Trial. Int. J. Clin. Pediatr. Dent. 2020, 13, 451–456. [Google Scholar] [CrossRef]
- Sezici, Y.L.; Yetkiner, E.; Yetkiner, A.A.; Eden, E.; Attin, R. Comparative evaluation of fluoride varnishes, self-assembling peptide-based remineralization agent, and enamel matrix protein derivative on artificial enamel remineralization in vitro. Prog. Orthod. 2021, 22, 4. [Google Scholar] [CrossRef]
- Memarpour, M.; Razmjouei, F.; Rafiee, A.; Vossoughi, M. Remineralization effects of self-assembling peptide P11-4 associated with three materials on early enamel carious lesions: An in vitro study. Microsc. Res. Tech. 2021, 85, 630–640. [Google Scholar] [CrossRef]
- Schlee, M.; Schad, T.; Koch, J.H.; Cattin, P.C.; Rathe, F. Clinical performance of self-assembling peptide P 11 -4 in the treatment of initial proximal carious lesions: A practice-based case series. J. Investig. Clin. Dent. 2018, 9, e12286. [Google Scholar] [CrossRef]
- Schmidlin, P.; Zobrist, K.; Attin, T.; Wegehaupt, F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J. Appl. Oral Sci. 2016, 24, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedlakova Kondelova, P.; Mannaa, A.; Bommer, C.; Abdelaziz, M.; Daeniker, L.; Di Bella, E.; Krejci, I. Efficacy of P11-4 for the treatment of initial buccal caries: A randomized clinical trial. Sci. Rep. 2020, 10, 20211. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.D.; Wong, B.P.Y.; Sivagurunathan, K.S.; Abrams, S.H.; Kirkham, J.; Amaechi, B.T. Remineralization of natural early caries lesions in vitro by P11-4 monitored with photothermal radiometry and luminescence. J. Investig. Clin. Dent. 2017, 8, e12257. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.; Davies, R.P.W.; Burke, J.L.; Smith, A.G.; Aggeli, A.; Brookes, S.; Kirkham, J. Treatment of early caries lesions using biomimetic self-assembling peptides—A clinical safety trial. Br. Dent. J. 2013, 215, E6. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.; De Ataide, I.N.; Fernandes, M.; Lambor, R. Assessment of Enamel Remineralisation After Treatment with Four Different Remineralising Agents: A Scanning Electron Microscopy (SEM) Study. J. Clin. Diagn. Res. 2017, 11, ZC136–ZC141. [Google Scholar] [CrossRef]
- Tripathi, P.; Kochhar, A.S.; Mengi, R.; Gajare, S.M.; Nanda, S.S.; Wani, S.A. Evaluation of Remineralizing Capacity of P11-4, CPP-ACP, Silver Diamine Fluoride, and NovaMin: An In Vitro Study. J. Contemp. Dent. Pr. 2021, 22, 357–360. [Google Scholar] [CrossRef]
- Üstün, N.; Aktören, O. Analysis of efficacy of the self-assembling peptide-based remineralization agent on artificial enamel lesions. Microsc. Res. Tech. 2019, 82, 1065–1072. [Google Scholar] [CrossRef]
- Welk, A.; Ratzmann, A.; Reich, M.; Krey, K.F.; Schwahn, C. Effect of self-assembling peptide P11-4 on orthodontic treatment-induced carious lesions. Sci. Rep. 2020, 10, 6819. [Google Scholar] [CrossRef] [Green Version]
- Wierichs, R.J.; Kogel, J.; Lausch, J.; Esteves-Oliveira, M.; Meyer-Lueckel, H. Effects of Self-Assembling Peptide P11-4, Fluorides, and Caries Infiltration on Artificial Enamel Caries Lesions in vitro. Caries Res. 2017, 51, 451–459. [Google Scholar] [CrossRef]
- Deyhle, H.; Dziadowiec, I.; Kind, L.; Thalmann, P.; Schulz, G.E.; Müller, B. Mineralization of Early Stage Carious Lesions In Vitro—A Quantitative Approach. Dent. J. 2015, 3, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Doberdoli, D.; Bommer, C.; Begzati, A.; Haliti, F.; Heinzel-Gutenbrunner, M.; Juric, H. Randomized Clinical Trial investigating Self-Assembling Peptide P11-4 for Treatment of Early Occlusal Caries. Sci. Rep. 2020, 10, 4195. [Google Scholar] [CrossRef] [PubMed]
- Golland, L.; Schmidlin, P.R.; Schätzle, M. The Potential of Self-assembling Peptides for Enhancement of In Vitro Remineralisation of White Spot Lesions as Measured by Quantitative Laser Fluorescence. Oral Health Prev. Dent 2017, 2, 147–152. [Google Scholar]
- Jablonski-Momeni, A.; Korbmacher-Steiner, H.; Heinzel-Gutenbrunner, M.; Jablonski, B.; Jaquet, W.; Bottenberg, P. Randomised in situ clinical trial investigating self-assembling peptide matrix P11-4 in the prevention of artificial caries lesions. Sci. Rep. 2019, 9, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonski-Momeni, A.; Heinzel-Gutenbrunner, M. Efficacy of the self-assembling peptide P11-4 in constructing a remineralization scaffold on artificially-induced enamel lesions on smooth surfaces. J. Orofac. Orthop./Fortschr. Kieferorthopädie 2014, 75, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Jablonski-Momeni, A.; Nothelfer, R.; Morawietz, M.; Kiesow, A.; Korbmacher-Steiner, H. Impact of self-assembling peptides in remineralisation of artificial early enamel lesions adjacent to orthodontic brackets. Sci. Rep. 2020, 10, 1320. [Google Scholar] [CrossRef]
- Kamal, D.; Hassanein, H.; ElKassas, D.; Hamza, H. Complementary remineralizing effect of self-assembling peptide (P11-4) with CPP-ACPF or fluoride: An in vitro study. J. Clin. Exp. Dent. 2020, 12, e161–e168. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.A.; Marei, T.E.; Elmalt, M.A. Assessment of self-assembling peptide P 11-4 in the treatment of white spot lesions after orthodontic treatment. Egypt. Orthod. J. 2020, 50, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Gözetici, B.; Öztürk-Bozkurt, F.; Toz-Akalın, T. Comparative Evaluation of Resin Infiltration and Remineralisation of Noncavitated Smooth Surface Caries Lesions: 6-month Results. Oral Health Prev. Dent 2019, 2, 99–106. [Google Scholar]
- Stoleriu, S.; Iovan, G.; Pancu, G.; Nica, I.; Georgescu, A.; Tofan, N.; Andrian, S.; Buhatel, D. Study Regarding the Capacity of Self-assembling Peptides to Remineralize the Acute and Chronic Incipient Caries Lesions. Rev. Chim. 2019, 70, 3073–3076. [Google Scholar] [CrossRef]
- Hill, R.G.; Chen, H.; Lysek, D.A.; Gillam, D. An In Vitro Comparison of A Novel Self-Assembling Peptide Matrix Gel and Selected Desensitizing Toothpastes in Reducing Fluid Flow by Dentine Tubular Occlusion. J. Dent. Maxillofac. Res. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Schlee, M.; Rathe, F.; Bommer, C.; Broseler, F.; Kind, L. Self-assembling peptide matrix for treatment of dentin hypersensitivity: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ceci, M.; Mirando, M.; Beltrami, R.; Chiesa, M.; Colombo, M.; Poggio, C. Effect of self-assembling peptide P11-4 on enamel erosion: AFM and SEM studies. Scanning 2016, 38, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, S.; Takamizawa, T.; Takahashi, F.; Tsujimoto, A.; Akiba, S.; Nagura, Y.; Kurokawa, H.; Miyazaki, M. Application of the Self- Assembling Peptide P11-4 for Prevention of Acidic Erosion. Oper. Dent. 2018, 43, E166–E172. [Google Scholar] [CrossRef] [PubMed]
- Hojabri, N.; Kaisarly, D.; Kunzelmann, K.-H. Adhesion and whitening effects of P11-4 self-assembling peptide and HAP suspension on bovine enamel. Clin. Oral Investig. 2021, 25, 3237–3247. [Google Scholar] [CrossRef]
- Farhana, F.; Shetty, K.H.S. Evaluation of the Effect of Self Assembling Peptide-Curodont on Microhardness of Bleached Enamel Surface: An In Vitro Study. IOSR J. Dent. Med. Sci. 2020, 19, 51–54. [Google Scholar]
- Acar, H.; Srivastava, S.; Chung, E.J.; Schnorenberg, M.R.; Barrett, J.C.; LaBelle, J.L.; Tirrell, M. Self-assembling peptide-based building blocks in medical applications. Adv. Drug Deliv. Rev. 2017, 110–111, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Yu, X.; Shen, B.; Sun, L. Peptide Self-Assembled Nanostructures for Drug Delivery Applications. J. Nanomater. 2017, 2017, 4562474. [Google Scholar] [CrossRef] [Green Version]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Panda, J.J.; Chauhan, V.S. Short peptide based self-assembled nanostructures: Implications in drug delivery and tissue engineering. Polym. Chem. 2014, 5, 4418–4436. [Google Scholar] [CrossRef]
- Gelain, F.; Horii, A.; Zhang, S. Designer Self-Assembling Peptide Scaffolds for 3-D Tissue Cell Cultures and Regenerative Medicine. Macromol. Biosci. 2007, 7, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Aggeli, A.; Beevers, A.; Boden, N.; Carrick, L.; Fishwick, C.; McLeish, T.; Nyrkova, I.; Semenov, A. Self-assembling β-Sheet Tape Forming Peptides. Supramol. Chem. 2006, 18, 435–443. [Google Scholar] [CrossRef]
- Carrick, L.M.; Aggeli, A.; Boden, N.; Fisher, J.; Ingham, E.; Waigh, T.A. Effect of ionic strength on the self-assembly, morphology and gelation of pH responsive β-sheet tape-forming peptides. Tetrahedron 2007, 63, 7457–7467. [Google Scholar] [CrossRef]
- Firth, A.; Aggeli, A.; Burke, J.L.; Yang, X.; Kirkham, J. Biomimetic self-assembling peptides as injectable scaffolds for hard tissue engineering. Nanomedicine 2006, 1, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int. J. Clin. Pediatr. Dent. 2019, 12, 139–144. [Google Scholar] [CrossRef]
- Anderson, M.; Dahllöf, G.; Twetman, S.; Jansson, L.; Bergenlid, A.-C.; Grindefjord, M. Effectiveness of Early Preventive Intervention with Semiannual Fluoride Varnish Application in Toddlers Living in High-Risk Areas: A Stratified Cluster-Randomized Controlled Trial. Caries Res. 2016, 50, 17–23. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Pessan, J.P. New Preventive Approaches Part I: Functional Peptides and Other Therapies to Prevent Tooth Demineralization. Monogr. Oral Sci. 2017, 26, 88–96. [Google Scholar] [CrossRef]
- Mohamed, R.N.; Basha, S.; Al-Thomali, Y.; Alshamrani, A.S.; Alzahrani, F.S.; Enan, E.T. Self-assembling peptide P11-4 in remineralization of enamel caries—A systematic review of in-vitro studies. Acta Odontol. Scand. 2021, 79, 139–146. [Google Scholar] [CrossRef]
- Alkilzy, M.; Santamaria, R.; Schmoeckel, J.; Splieth, C. Treatment of Carious Lesions Using Self-Assembling Peptides. Adv. Dent. Res. 2018, 29, 42–47. [Google Scholar] [CrossRef]
- Aggeli, A.; Bell, M.; Boden, N.; Carrick, L.M.; Strong, A.E. Self-Assembling Peptide Polyelectrolyteβ-Sheet Complexes Form Nematic Hydrogels. Angew. Chem. 2003, 115, 5761–5764. [Google Scholar] [CrossRef]
- Bonchev, A.; Vasileva, R.; Dyulgerova, E.; Yantcheva, S. Self-assembling Peptide P11-4: A Biomimetic Agent for Enamel Remineralization. Int. J. Pept. Res. Ther. 2021, 27, 899–907. [Google Scholar] [CrossRef]
- Lippert, F.; Parker, D.M.; Jandt, K.D. In vitro demineralization/remineralization cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J. Colloid Interface Sci. 2004, 280, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ş.; Taran, P.K.; Mammadlı, N.; Altınova, İ.S.; Gazioğlu, I. Remineralization potential of P11-4 and fluoride on secondary carious primary enamel: A quantitative evaluation using microcomputed tomography. Microsc. Res. Tech. 2022, 85, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Qin, X.; Ren, Q.; Hu, D.; Tian, T.; He, T.; Li, W.; Zhang, L.L. Rational Design of β-sheet Peptides with Self-Assembly into Nanofibres on Remineralisation of Initial Caries Lesions. Chin. J. Dent. Res. 2020, 23, 131–141. [Google Scholar] [CrossRef]
- Wahba, N.; Schwendicke, F.; Mohamed, B.; Kamel, A.; Allam, G.; Paris, S.; Jost-Brinkmann, P.-G. Preventing and arresting primary tooth enamel lesions using self-assembling peptide P11-4 in vitro. J. Int. Soc. Prev. Community Dent. 2022, 12, 58. [Google Scholar]
- Sundararaj, D.; Venkatachalapathy, S.; Tandon, A.; Pereira, A. Critical evaluation of incidence and prevalence of white spot lesions during fixed orthodontic appliance treatment: A meta-analysis. J. Int. Soc. Prev. Community Dent. 2015, 5, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Jongsma, M.A.; Mei, L.; Van Der Mei, H.C.; Busscher, H.J. Orthodontic treatment with fixed appliances and biofilm formation—a potential public health threat? Clin. Oral Investig. 2014, 18, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.C.; Worthington, H.V.; Walsh, T.; Clarkson, J.E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013, 11, CD002279. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Logan, S.; Sheiham, A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002782. [Google Scholar] [CrossRef]
- Geddes, D.A. Acids Produced by Human Dental Plaque Metabolism in situ. Caries Res. 1975, 9, 98–109. [Google Scholar] [CrossRef]
- Attin, T.; Becker, K.; Wiedemeier, D.B.; Schmidlin, P.R.; Wegehaupt, F. Anti-erosive effect of a self-assembling peptide gel. Swiss Dent. J. 2017, 127, 857–864. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).