Corrosion Inhibition Coating Based on the Self-Assembled Polydopamine Films and Its Anti-Corrosion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Coating Preparation and Characterization
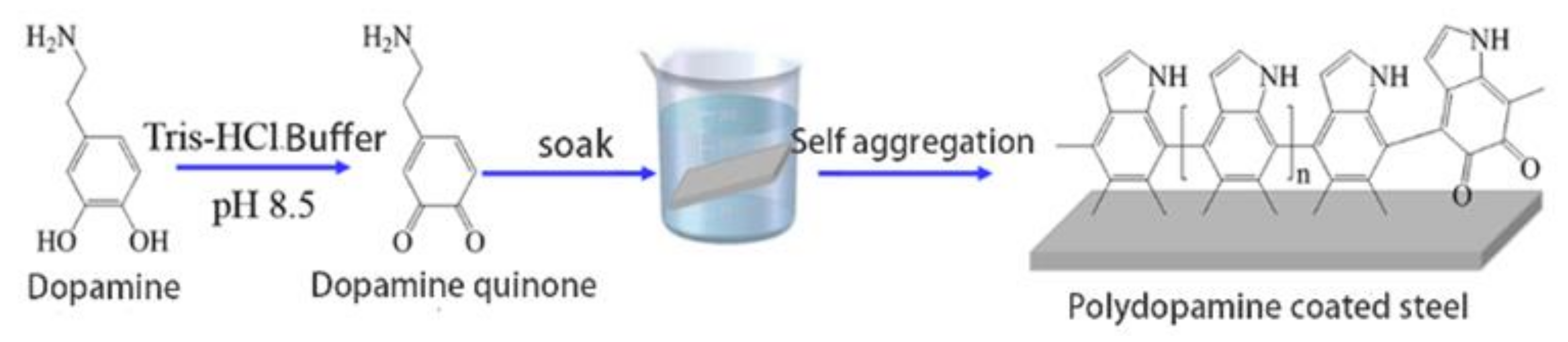
2.2.1. Coating Preparation
2.2.2. Evaluation of Coating Stability
- The corrosion testing
- Standard curve of dopamine absorbance
- 2.
- Test solution preparation and absorbance determination
- 3.
- Evaluation of coating performance
- 4.
- Electrochemical test
3. Results and Discussion
3.1. Optimization of Coating Preparation Parameters
3.2. Characterization of Coating Composition
3.3. Stability Test of Polydopamine Coating
3.4. Evaluation of Coating Performance
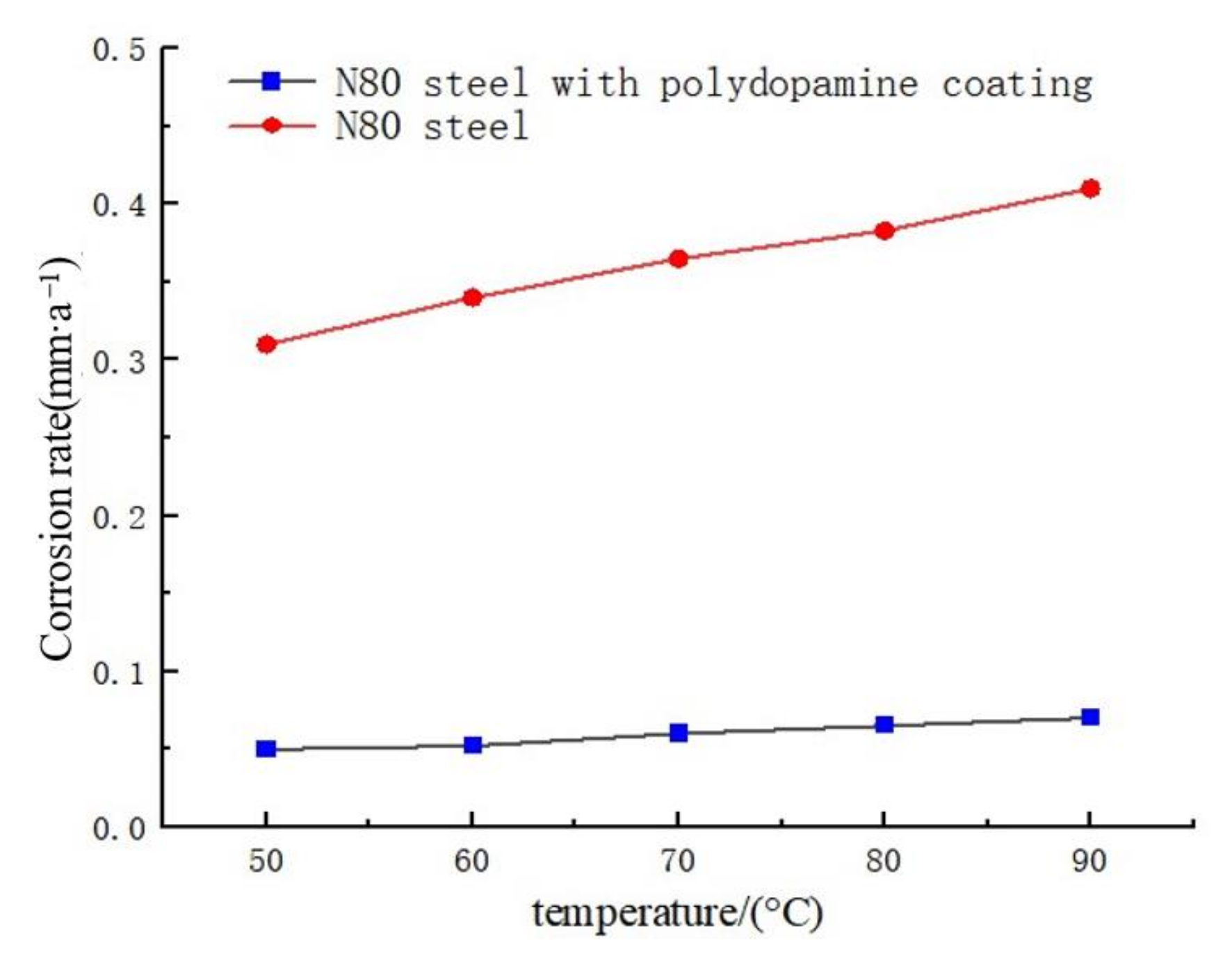
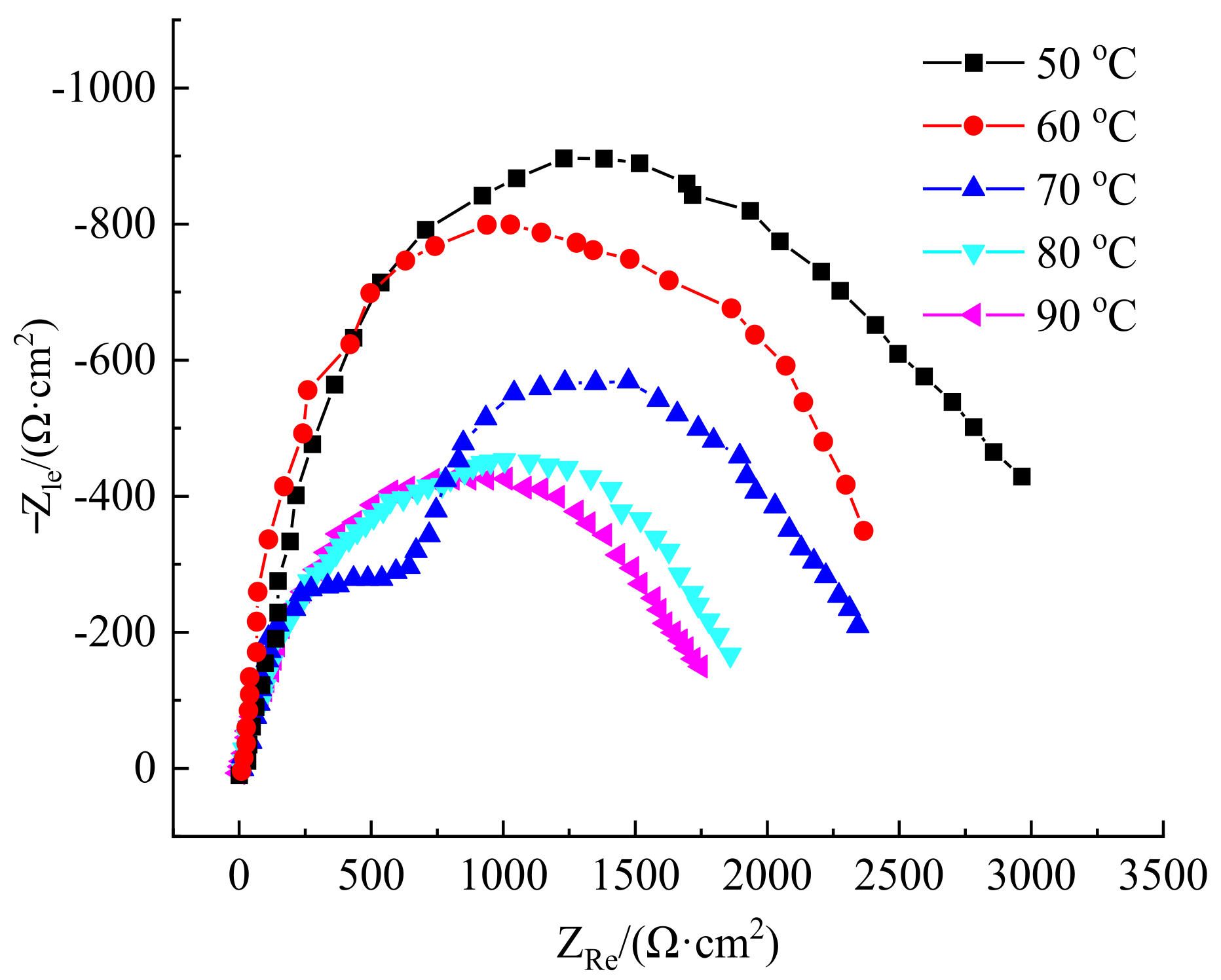
3.4.1. Impact of Corrosion Temperature
3.4.2. Impact of Corrosive Media
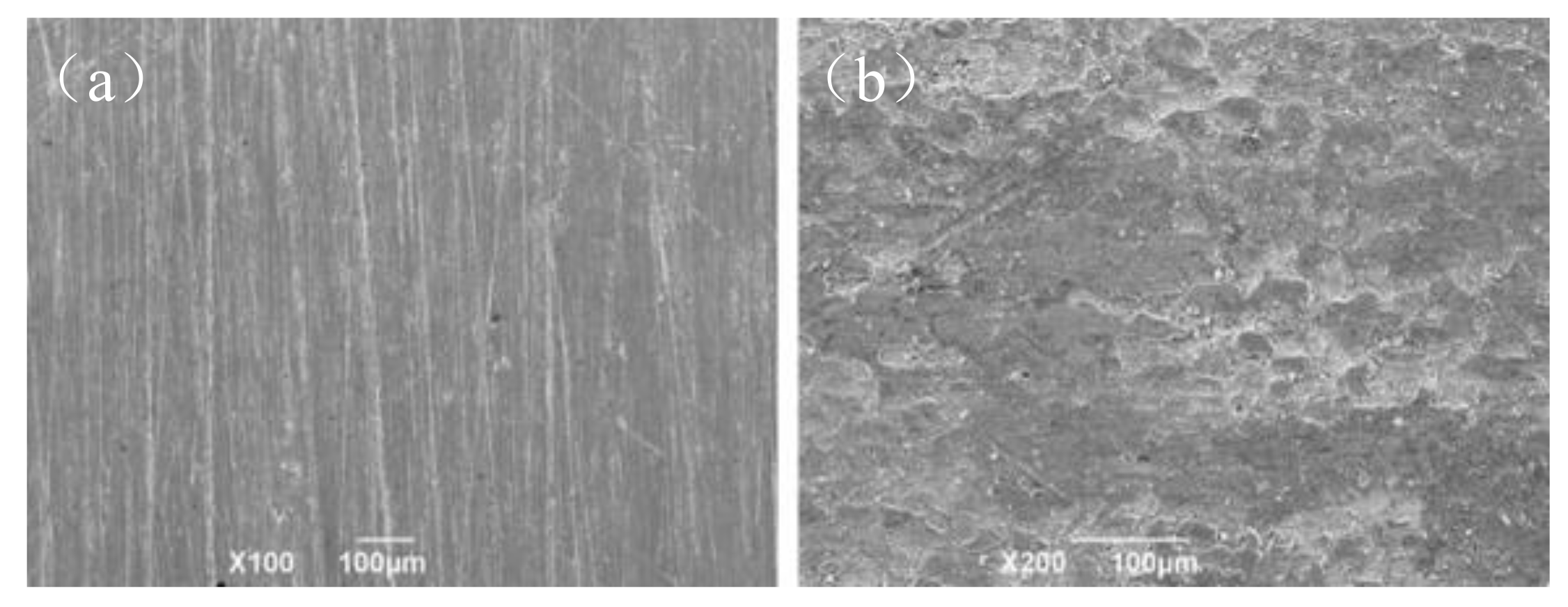
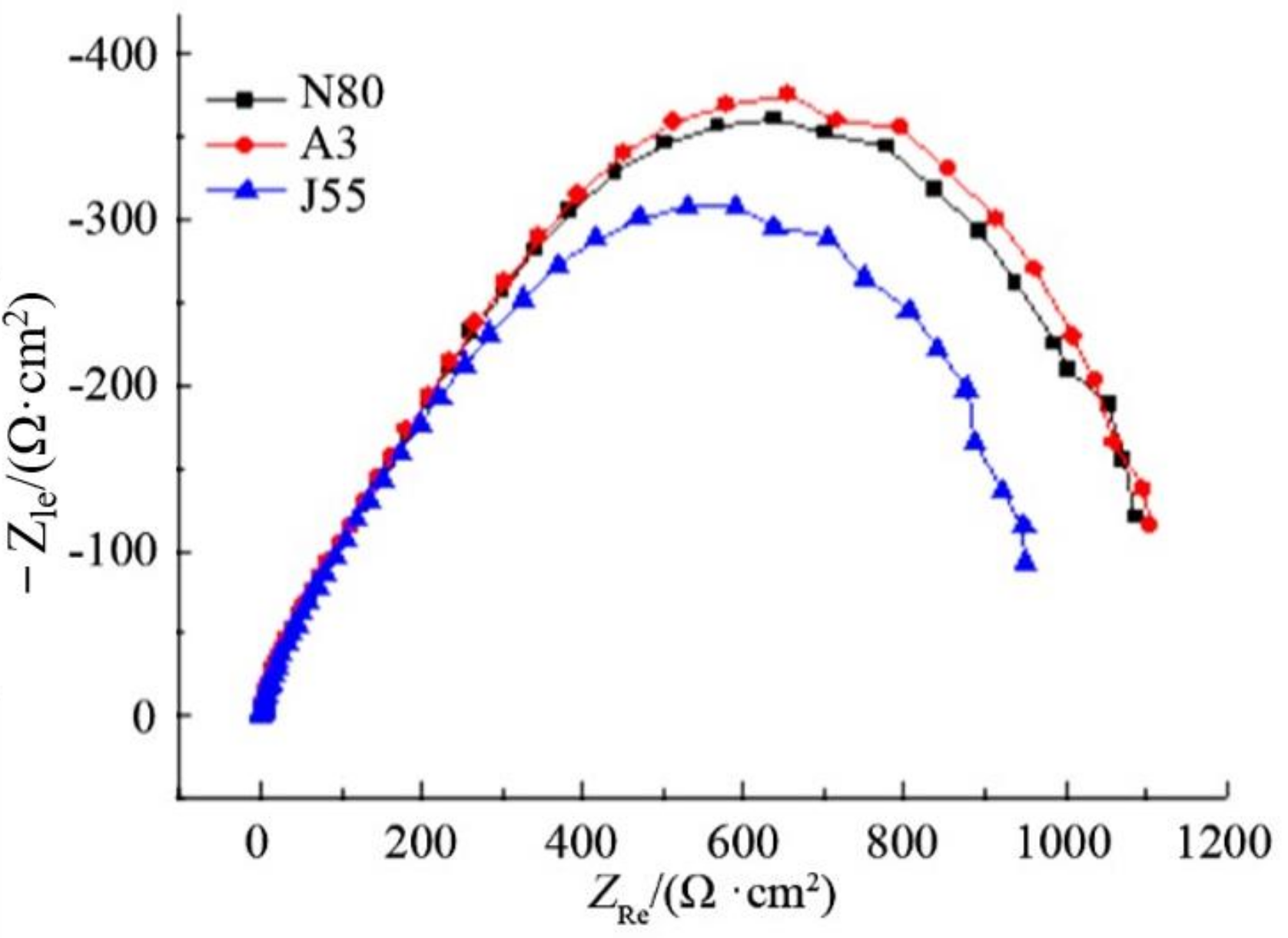
3.4.3. The influence of Test Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, C.Z.; Wan, M.W.; Li, K.K. Injection-production adjustment of typical wellgroup in companied fault-block reservoirs. Spec. Oil Gas Reserv. 2015, 22, 72–74. [Google Scholar]
- Yan, J.; Liu, Q.; Du, W.C.; Qu, C.T.; Song, Z.F.; Li, J.L.; Zhang, J.; Chen, G. Synthesis and properties of octadecyl trimethyl ammonium polyacrylic surfactants. Tenside Surfact. Det. 2020, 57, 122–128. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhang, Z.F.; Lin, J.; Qu, S.M.; Jia, X.G.; Zhu, S.D.; Chen, G. Investigation of aniline and N,N-dimethylaniline as acidic corrosion inhibitor: A structure-efficiency relationship study. J. Phys. Chem. B 2020, 14, 1007–1013. [Google Scholar]
- Chen, G.; Yan, J.; Bai, Y.; Liu, D.W.; Zhang, J.; Li, J.L.; Qu, C.T. Synthesis of two chiral surfactants by a simple method and the surface activity evaluation. J. Mol. Liq. 2020, 316, 113892. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, M.; Zhao, Y.; Li, J.; Qu, C.; Zhang, J.; Chen, G. Synthesis and interface activity of a new quaternary ammonium surfactant as an oil/gas field chemical. Tenside Surfact. Det. 2020, 57, 90–96. [Google Scholar] [CrossRef]
- Liu, Q.N.; Gao, M.L.; Qu, C.T.; Zhang, R.J.; Song, Z.F.; Li, J.L.; Chen, G. Synthesis and interface activity of cetyltrimethylammonium benzoate. Russ. J. Phys. Chem. B 2020, 14, 73–80. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.; Dong, S.B.; Li, J.L.; Zhang, R.J.; Pu, C.S.; Chen, G. Effect of anion on the corrosion inhibition of cationic surfactants and a mechanism study. Desalin. Water Treat. 2020, 188, 130–139. [Google Scholar] [CrossRef]
- Gao, J.F. Influence of the flow rate of natural gas on the performance of corrosion inhibitor injection for gathering and transporting pipelines. Oil-Gas Field Surf. Eng. 2018, 37, 82–86. [Google Scholar]
- Li, S.J.; Feng, L.J.; Dong, X.J. Synergistic effects of quinolone and thiourea in the produced water from saturated CO2-containing gas wells. Nat. Gas Ind. 2015, 35, 90–98. [Google Scholar]
- Li, Y.; Wang, Y.; Wang, Q.; Liu, Z.; Tang, L.; Liang, L.; Zhang, C.; Li, Q.; Xu, N.; Sun, J.; et al. Achieving the Super Gas-Wetting Alteration by Functionalized Nano-Silica for Improving Fluid Flowing Capacity in Gas Condensate Reservoirs. ACS Appl. Mater. Interfaces 2021, 13, 10996–11006. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q. Oxidant-induced plant phenol surface chemistry for multifunctional coatings: Mechanism and potential applications. J. Membr. Sci. 2019, 570, 176–183. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Elmi, F.; Gharakhani, A.; Ghasemi, S.; Alinezhad, H. Self-assemble l-glycine and l-cysteine/polydopamine nanohybrid films coated on 304 stainless steel for corrosion study in sterile seawater. Prog. Org. Coat. 2018, 119, 127–137. [Google Scholar] [CrossRef]
- Habibiyan, A.; Ramezanzadeh, B.; Mahdavian, M. Rational assembly of mussel-inspired polydopamine (PDA)-Zn (II) complex nanospheres on graphene oxide framework tailored for robust self-healing anti-corrosion coatings application. Chem. Eng. J. 2020, 391, 123630. [Google Scholar] [CrossRef]
- Bahremand, F.; Shahrabi, T.; Ramezanzadeh, B. Development of a nanostructured film based on samarium (III)/polydopamine on the steel surface with superior anti-corrosion and water-repellency properties. J. Colloid Interface Sci. 2021, 582, 342–352. [Google Scholar] [CrossRef]
- Joddar, B.; Albayrak, A.; Kang, J.; Nishihara, M.; Abe, H.; Ito, Y. Sustained delivery of siRNA from dopamine-coated stainless steel surface. Acta Biomater. 2013, 9, 6753–6761. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characterization of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Yao, Y.; Wang, Y.; Lai, N.; Wang, L. Preparation and characterization of polydopamine films on pure magnesium surface. J. South China Univ. Technol. (Nat. Sci. Ed.) 2018, 46, 16–23. [Google Scholar]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Miche, M.; Gracio, J.J.D.A.; Toniazzo, V.; Ruch, D. Dopamine-melanin film deposition depends on the used oxidant and buffer solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef]
- Shin, Y.M.; Jun, I.; Lim, Y.M.; Rhim, T.; Shin, H. Bio-inspired immobilization of cell-adhesive ligands on electrospun nanofibrous patches for cell delivery. Macromol. Mater. Eng. 2013, 298, 555–564. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Postma, A.; Yan, Y.; Wang, Y.J. Self-polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chem. Mater. 2009, 21, 3042–3044. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W.M. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Qu, J.F.; Wang, J.Q.; Liu, S. Microtribological and electrochemical corrosion behaviors of polydopamine coating on APTS-SAM modified Si substrate. Appl. Surf. Sci. 2009, 256, 894–899. [Google Scholar]
- Zhang, L.; Shi, J.; Jiang, Z.; Jiang, Y.; Meng, R.; Zhu, Y.; Liang, Y.; Zheng, Y. Facile preparation of robust microcapsules by manipulating metal-coordination interaction between biomineral layer and bioadhesive layer. ACS Appl. Mater. Interfaces 2011, 3, 597–605. [Google Scholar] [CrossRef]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Dai, Z.; Liu, T.; Zheng, W.; Zhao, H. Corrosion inhibition of polydopamine nanoparticles on mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2017, 12, 7469–7480. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [Green Version]





| Composition | Oil Well Produced Water (Changqing Oilfield) | Gas Well Produced Water (Yulin Gas Field) |
|---|---|---|
| Ca2+/(mg·L−1) | 1526.36 | 9028.02 |
| Na+/(mg·L−1) | 26,576.09 | 23,782.47 |
| Mg2+/(mg·L−1) | 383.91 | 302.32 |
| Cation content/(mg·L−1) | 28,486.36 | 33,150.51 |
| Cl-/(mg·L−1) | 42,154.48 | 52,959.11 |
| S2-/(mg·L−1) | 0.6 | 11.86 |
| SO42-/(mg·L−1) | 2228.79 | 338.13 |
| Material | C | Si | Mn | P | S | Cr | Mo | Ni |
|---|---|---|---|---|---|---|---|---|
| N80 | 0.240 | 0.220 | 1.190 | 0.013 | 0.003 | 0.036 | 0.021 | 0.028 |
| A3 | 0.140–0.220 | >0.300 | 0.300–0.650 | >0.045 | >0.005 | \ | \ | \ |
| J55 | 0.340 | 0.200 | 1.250 | 0.020 | 0.015 | 0.150 | \ | 0.200 |
| Soak Solution | Absorbance | Dopamine Concentration/wt% | Shedding Rate/% | Average Shedding Rate/% |
|---|---|---|---|---|
| Oil well produced water | 0.028 | 3.2184 | 1.95 | 1.88 |
| 0.025 | 2.8736 | 1.72 | ||
| 0.026 | 2.9885 | 1.88 | ||
| Gas well produced water | 0.029 | 3.3333 | 1.96 | 2.01 |
| 0.030 | 3.4483 | 2.08 | ||
| 0.028 | 3.2184 | 1.99 | ||
| Acid solution | 0.032 | 3.6782 | 2.15 | 2.20 |
| 0.032 | 3.6782 | 2.23 | ||
| 0.033 | 3.7931 | 2.22 |
| Soak Solution | Steel Plate | Corrosion Rate/mm·a−1 | Inhibition Rate/% |
|---|---|---|---|
| Oil well produced water | N80 | 0.3618 | 81.69 |
| Polydopamine-coated N80 | 0.0554 | ||
| Gas well produced water | N80 | 0.3694 | 86.14 |
| Polydopamine-coated N80 | 0.0512 | ||
| Acid solution | N80 | 0.3983 | 85.16 |
| Polydopamine-coated N80 | 0.0591 |
| Steel | Is It Coated with Polydopamine | Corrosion Rate/mm·a−1 | Inhibition Rate/% |
|---|---|---|---|
| N80 | No | 0.3894 | 86.11 |
| Yes | 0.0541 | ||
| A3 | No | 0.3626 | 86.27 |
| Yes | 0.0498 | ||
| J55 | No | 0.3362 | 86.47 |
| Yes | 0.0455 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Guo, C.; Wang, X.; Guan, C.; Chen, G. Corrosion Inhibition Coating Based on the Self-Assembled Polydopamine Films and Its Anti-Corrosion Properties. Polymers 2022, 14, 794. https://doi.org/10.3390/polym14040794
Li S, Guo C, Wang X, Guan C, Chen G. Corrosion Inhibition Coating Based on the Self-Assembled Polydopamine Films and Its Anti-Corrosion Properties. Polymers. 2022; 14(4):794. https://doi.org/10.3390/polym14040794
Chicago/Turabian StyleLi, Shanjian, Changwang Guo, Xin Wang, Chong Guan, and Gang Chen. 2022. "Corrosion Inhibition Coating Based on the Self-Assembled Polydopamine Films and Its Anti-Corrosion Properties" Polymers 14, no. 4: 794. https://doi.org/10.3390/polym14040794
APA StyleLi, S., Guo, C., Wang, X., Guan, C., & Chen, G. (2022). Corrosion Inhibition Coating Based on the Self-Assembled Polydopamine Films and Its Anti-Corrosion Properties. Polymers, 14(4), 794. https://doi.org/10.3390/polym14040794





