Abstract
A mixed aqueous solution of hydrophilic poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and poly(acrylic acid) (PAAc) becomes cloudy under acidic conditions at room temperature. The pendant carboxylic acid groups in PAAc form hydrogen bonds with the ester and phosphate groups in PMPC. While the polymers aggregate under acidic conditions, neither one associate under basic conditions because of the deprotonation of the pendant carboxy groups in PAAc. We observed that the interpolymer complex formed from PMPC, and PAAc was dissociated in aqueous solutions with increasing temperature, which is an upper critical solution temperature behavior. With increasing temperature, the molecular motion increased to dissociate the interpolymer complex. The phase transition temperature increased with increasing polymer and salt concentrations, and with decreasing pH.
1. Introduction
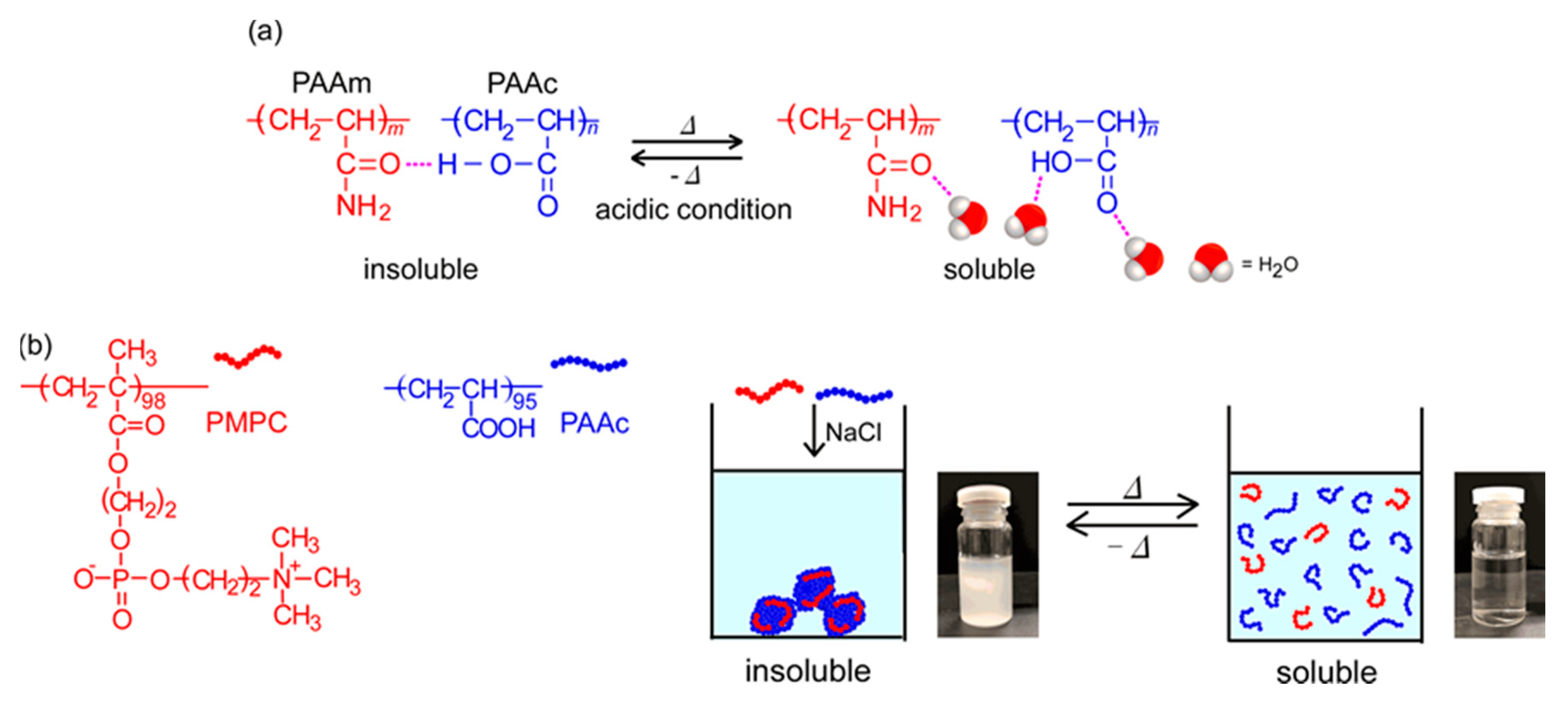
Stimuli-responsive polymers can change their physical and/or chemical properties on exposure to external conditions, such as temperature, pH, salt concentration, light, and magnetic fields [1,2,3,4]. A thermo-responsive polymer, poly(N-isopropylacrylamide) (PNIPAM), has been found to become insoluble in water owing to dehydration above the lower critical solution temperature (LCST) [5]. PNIPAM is used widely in the biomedical and bioengineering fields because the phase transition temperature (Tp; ~32 °C) is close to the human body temperature at physiological concentrations [6,7,8]. Generally, the Tp of PNIPAM increases and decreases to copolymerize hydrophilic and hydrophobic monomers, respectively [9]. It is well-known that another class of thermo-responsive polymers with the upper critical solution temperature (UCST) shows phase separation below the UCST. Above the UCST, the polymer is soluble in the solvent. However, thus far, only a few UCST water-soluble polymers have been reported, compared to LCST polymers [10]. UCST-type phase separation behavior in aqueous media can be observed by electrostatic [11] and/or hydrogen bonding interactions [12]. Zwitterionic sulfobetaine polymers are insoluble in water below the UCST because of the formation of aggregates by electrostatic attractive interactions. Conversely, above the UCST, the sulfobetaine polymers are soluble because the molecular motion of the polymer chains overcomes the charge interactions. Random copolymers composed of acrylamide and acrylonitrile become insoluble in water at low temperatures because of the hydrogen bonding interactions between the polymer chains, and they become soluble with increasing temperature because of the breakdown of hydrogen bonds [13,14]. The above examples of UCST polymers are single-component homopolymers or random copolymers in aqueous solutions. It is known that a mixture of two water-soluble polymers shows the UCST [15]. When poly(acrylamide) (PAAm) and poly(acrylic acid) (PAAc) homopolymers are mixed in water under acidic conditions at room temperature, the pendant carbonyl group in PAAm forms a hydrogen bond with the pendant carboxylic acid in PAAc and precipitates (Figure 1a). The molecular motion increases, and the polymer aggregates are dissociated with increasing temperature because the hydrogen bonds between PAAm and PAAc break [16,17,18]. The proton in the carboxylic acid in PAAc acts as a hydrogen donor. Therefore, the UCST behavior of PAAm/PAAc can be observed only in acidic conditions. PAAc shows pH-responsive behavior owing to hydration and dehydration of the pendant carboxy groups [19,20,21]. Poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) is a biomimetic material possessing a pendant hydrophilic phosphorylcholine group, which has the same structure as the surface of cell membranes. PMPC can be applied to biological and medical technology in the field of nanomaterials. PMPC is applied in medical fields for artificial joints, catheters, contact lenses, etc., [22,23,24].
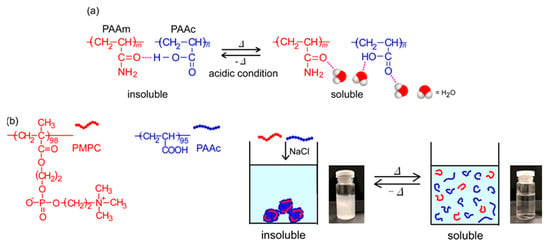
Figure 1.
(a) Complex formation of poly(acrylamide) (PAAm) and poly(acrylic acid) (PAAc) owing to hydrogen bonding interactions, and the dissociation of aggregates upon heating; and (b) conceptual illustration of the UCST behavior of the mixed aqueous solutions of poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and PAAc.
In this study, by reversible addition-fragmentation chain transfer (RAFT) radical polymerization, we prepared PMPC and PAAc with degrees of polymerization (DP) of 98 and 95, respectively. At pH 3, the pendant carboxy groups were protonated. To monitor the interactions between PMPC and PAAc, the values of percent transmittance (%T) were measured for the mixed aqueous solutions of PMPC and PAAc with varying molar ratios (fAAc = [AAc]/([MPC] + [AAc])), where [AAc] and [MPC] are the molar concentrations of the acrylic acid (AAc) and 2-methacryloyloxyethyl phosphorylcholine (MPC) units in the aqueous solution, respectively. The PMPC/PAAc complex was formed at pH 3 because the pendant carbonyl and phosphate groups in PMPC formed hydrogen bonds with the pendant carboxylic acid in PAAc. At fAAc = 0.85, PMPC and PAAc formed a complex and precipitated. We studied the UCST behavior of PMPC/PAAc complex aqueous solutions (Figure 1b). The Tp of the PMPC/PAAc complex aqueous solutions increased with increasing NaCl concentration ([NaCl]) and polymer concentration (Cp), and Tp decreased with increasing pH.
2. Materials and Methods
MPC (NOF Co., Tokyo, Japan) was recrystallized from acetonitrile. AAc (98%) from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan) was dried with a 4A molecular sieve (Kanto Chemical Co. Tokyo, Japan) and distilled under reduced pressure. 4,4′-Azobis-(4-cyanovaleric acid) (V-501; 98%) and 2,2′-azobisisobutyronitrile (AIBN; Wako, 98%) from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan) were recrystallized from methanol. 4-Cyanopentanoic acid dithiobenzoate (CPD) was synthesized according to a procedure reported previously [25]. Methanol (99.9%) was dried with a 4A molecular sieve (Kanto Chemical Co. Tokyo, Japan) and distilled before use. Water with an ion-exchange column was used throughout the study.
2.1. Preparation of PMPC
MPC (5.08 g, 17.2 mmol), CPD (47.5 mg, 0.170 mmol), and V-501 (19.1 mg, 0.0681 mmol) (mole ratio 100:1:0.4) were dissolved in a water/methanol solvent (17.0 mL, 9/1, v/v). The solution was purged with argon gas, stirring for 30 min to remove the oxygen. After that, the solution was heated at 70 °C for 4 h. The monomer conversion was 98.5%, as estimated from proton nuclear magnetic resonance (1H NMR, Bruker, Billerica, MA, USA) before purification (Figure S1). The reaction mixture was dialyzed against pure water for one day. The polymer (PMPC) was recovered by a freeze-drying technique (4.34 g, 85.4%). The values of the number-average molecular weight (Mn(GPC)) and the molecular weight distribution (Mw/Mn), as estimated from gel-permeation chromatography (GPC, Tosoh Co. Tokyo, Japan) measurements were 27.9 kDa and 1.37, respectively. The degree of polymerization calculated from 1H NMR (DP(NMR)) was 98.
2.2. Preparation of PAAc
AAc (5.86 g, 81.4 mmol), CPD (87.5 mg, 0.343 mmol), and AIBN (22.7 mg, 0.138 mmol) (mole ratio 237:1:0.4) were dissolved in methanol (80.0 mL). The solution was purged with argon gas, stirring for 30 min to remove the oxygen. After that, the solution was heated at 60 °C for 44 h. The monomer conversion was 39.8%, estimated from 1H NMR before purification (Figure S2). After polymerization, the solution was dialyzed against pure water for one week. The polymer (PAAc) was recovered by a freeze-drying technique (0.936 g, 16.2%). The Mn(GPC) and Mw/Mn were 9.10 kDa and 1.42, respectively. The theoretical degree of polymerization (DP(theo)) calculated from the conversion was 95.
2.3. Measurements
1H NMR spectra were obtained using a Bruker (Billerica, MA, USA) DRX-500 spectrometer. GPC measurements were performed using a Tosoh (Tokyo, Japan) RI-8020 reflective index detector, Tosoh DP-8020 pump, and Shodex (Tokyo, Japan) GF-7M column. A phosphate buffer (50 mM) at pH 9 and acetonitrile mixed solvent (9/1, v/v) was used as the eluent at 40 °C. The Mn(GPC) and Mw/Mn values for the polymers were calibrated using standard sodium poly(styrene sulfonate) samples. pH titration was performed using a Hiranuma Sangyo (Osaka, Japan) COM-1600 auto-titrator equipped with a glass electrode in 4.0 M KCl. PAAc was dissolved in 0.1 M NaOH at Cp = 5.0 g/L, which was titrated using 0.1 M HClaq. Ultraviolet–visible spectra were obtained using a Jasco (Tokyo, Japan) V-730 UV–vis spectrophotometer at 700 nm. To determine the UCST, the temperature was controlled using a Jasco ETC-717 temperature controller at a cooling rate of 1.0 °C/min. The Tp was defined as the temperature at which the %T at 700 nm starts to decrease.
3. Results and Discussion
3.1. Preparation and Characterization of the Polymers
To prepare PMPC and PAAc with similar DP values, we used a RAFT technique using a dithiobenzoate chain transfer agent (CTA) for controlled radical polymerization. The conversions (p) of PMPC and PAAc were 98.5% and 39.8%, respectively, estimated from 1H NMR after polymerization. The DP(theo) and theoretical Mn (Mn(theo)) were calculated using the following formulas:
where [M]0 and [CTA]0 are the initial monomer and CTA concentrations, and Mm and MCTA are the molecular weights of the monomer and CTA, respectively. The DP(theo) values for PMPC and PAAc were 99 and 95, respectively. 1H NMR spectra for PMPC and PAAc were measured in D2O at 20 °C. (Figure S3). The DP(NMR) for PMPC was 98, which was calculated from the integral intensity ratio of the pendant methylene protons at 3.7 ppm and the terminal phenyl protons attributed to CTA at 7.4–8.2 ppm. The DP(NMR) = 98 was close to the theoretical value, DP(theo) = 99. The DP(NMR) of PAAc was 104, as estimated from the integral intensity ratio of the main chain protons at 1.4–2.6 ppm and the terminal phenyl protons at 7.4–8.2 ppm, which was close to the DP(theo) = 95. GPC for PMPC and PAAc were measured using phosphate buffer as the eluent (Figure S4). The GPC elution curves for PMPC and PAAc were unimodal, and the Mw/Mn values were 1.37 and 1.42, respectively, which indicated that the polymers had well-controlled structures. The DP, Mn, and Mw/Mn for PMPC and PAAc are summarized in Table 1.

Table 1.
Degree of polymerization (DP), number-average molecular weight (Mn), and molecular weight distribution (Mw/Mn).
PAAc was dissolved in 0.1 M NaOH (Cp = 5.0 g/L) and titrated against 0.1 M HCl to obtain the titration curve (Figure S5). The x- and y-axes show the volume of HCl added (VolHCl) and the pH value of the solution, respectively. The end point (EP) indicates the neutralization point. The acid dissociation constant (pKa) value of PAAc was determined from the following equation [26]:
where VolEP is the volume difference of the HCl added at the two-step inflection point, [COOH] is the pendant carboxylic acid concentration in PAAc, [HCl] is the concentration of HCl, the titrant used, and Volsol is the initial volume of the polymer solution being titrated. In this measurement, the titration curve with two inflection points was obtained. As HCl was titrated, the first inflection point was due to the neutralization of NaOH, and the second was due to the protonation of PAAc. The HCl volumes used for the titration at the two inflection points were 1.2 and 3.7 mL, respectively, indicating that VolEP was 2.5 mL. The value of VolEP1/2 was 3.3 mL, estimated from Equation (3). As the pH at VolEP1/2 was pHEP1/2, this value corresponded to pKa. Therefore, from the titration curve, pHEP1/2 was 4.46 when VolEP1/2 was 3.3 mL. The pKa value of PAAc was determined to be 4.46. The estimated pKa value of PAAc was close to the literature value (pKa = 4.5) [27,28]. Hereafter, all experiments were performed at pH 3, where PAAc was protonated, unless otherwise noted. The protonation degree (δ) of PAAc in the aqueous solution was calculated based on the following equations [29,30]:
According to the equation, 96.6% of the pendant carboxy groups in PAAc were protonated.
3.2. Mole Ratio Dependence on Solubility after Mixing Polymers
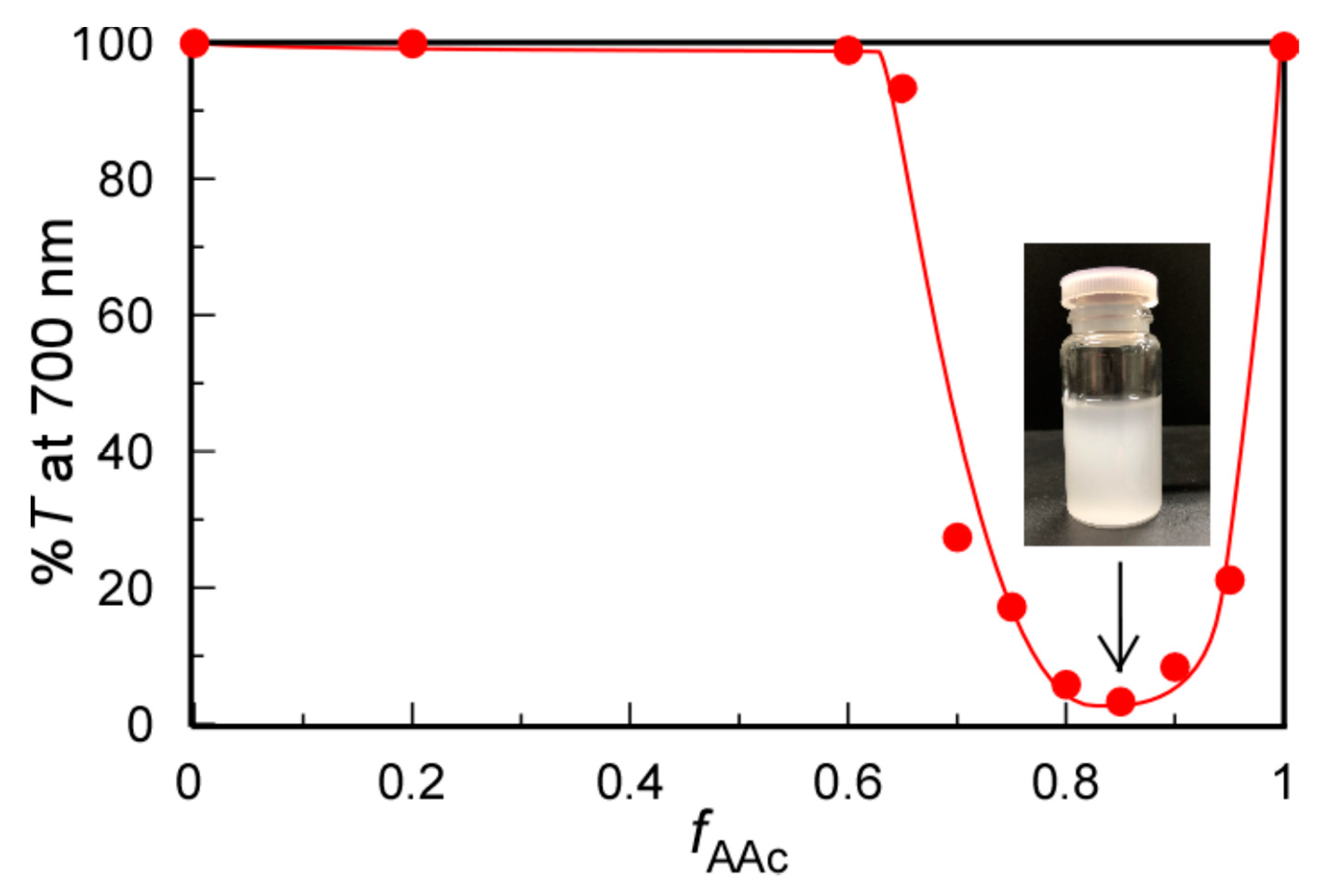
At pH 3, the pendant carboxy groups in PAAc were protonated in 0.1 M NaCl aqueous solutions at 20 °C. We studied the solubility changes of the mixed aqueous solutions of PMPC and PAAc with varying fAAc values using %T (Figure 2). At fAAc ≤ 0.6, %T was constant at 100%. At fAAc > 0.6, %T started to decrease, suggesting the formation of an insoluble PMPC/PAAc complex because the pendant carbonyl acceptor in the PMPC formed hydrogen bonds with the pendant carboxylic acid donor in PAAc. At fAAc = 0.85, %T decreased to the minimum value of 4.1%, suggesting the formation of the largest PMPC/PAAc complex with the strongest interpolymer interactions. Furthermore, 5.7 pendant carboxylic acids in PAAc interacted with one pendant group in PMPC at fAAc = 0.85, that is, [MPC]/[AAc] = 1/1.8. Surprisingly, interactions between PMPC and PAAc were not observed from %T at fAAc = 0.5, that is, [MPC]/[AAc] = 1/1. This is because the pendant oxygen atoms in the ester and oxygen atoms in the phosphorylcholine group in PMPC can act as proton acceptors. Tupikina et al. reported that one P=O acceptor in phosphoric acid binds two hydrogen donors [31]. Hereafter, all experiments were performed at fAAc = 0.85, unless otherwise noted.
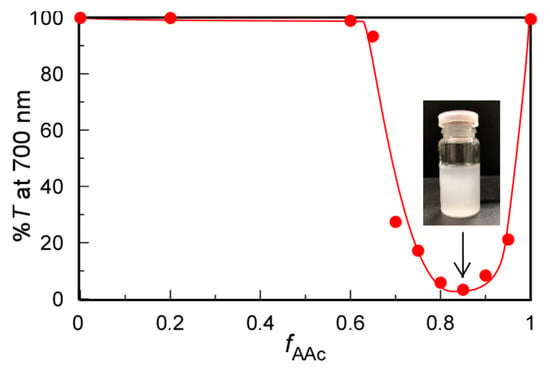
Figure 2.
Percent transmittance (%T) at 700 nm for PMPC/PAAc mixed aqueous solutions as a function of fAAc (= [AAc]/([MPC]+[AAc])) at Cp = 0.5 g/L, [NaCl] = 0.1 M, pH = 3, and 20 °C. The insert is a picture of the solution at fAAc = 0.85.
3.3. pH Dependence on Solubility
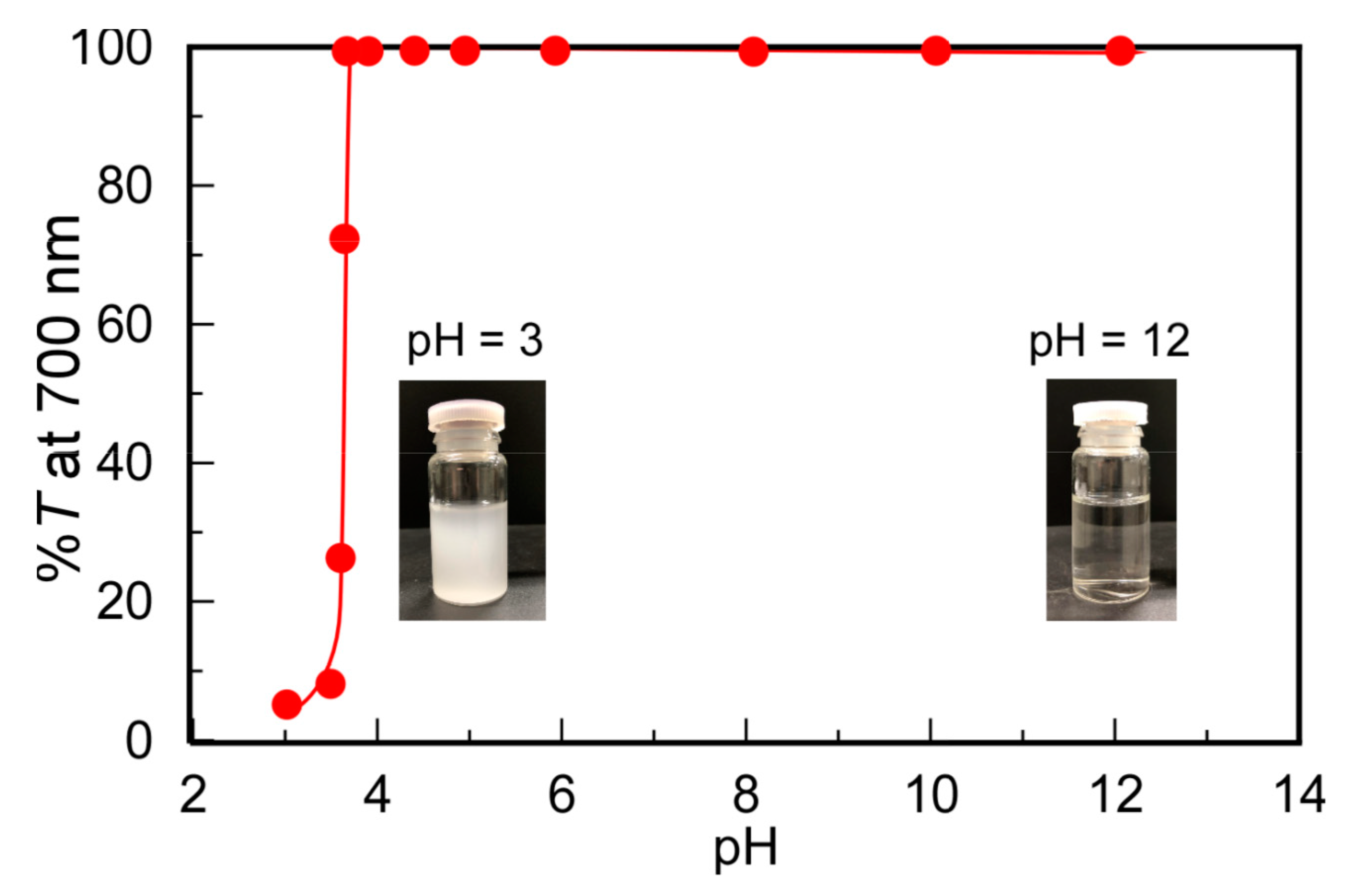
The changes in solubility depending on pH were investigated for PMPC/PAAc with fAAc = 0.85 (Figure 3). At pH 3, %T decreased to 5.2%, suggesting that the protonated pendant carboxylic acids in PAAc formed hydrogen bonds with the pendant ester and phosphorylcholine groups in PMPC to form insoluble PMPC/PAAc complexes. As the pH value increased from 3, the hydrogen bonding interactions of PMPC with PAAc were weakened because of the deprotonation of the pendant carboxylic acid groups in PAAc. Above pH 4, %T became 100%. The %T values of each aqueous solution of PMPC and PAAc were 100% from pH 3 to 12. These observations indicate that the complex formation of PMPC and PAAc originated from the hydrogen bonding interactions between the pendant groups in PMPC and PAAc below pH 4.
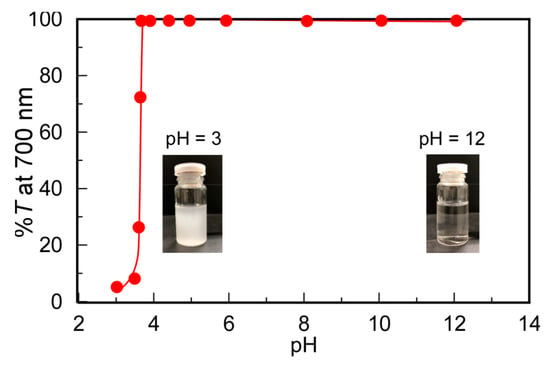
Figure 3.
Percent transmittance (%T) at 700 nm for PMPC/PAAc mixed aqueous solutions with fAAc = 0.85 as a function of pH at Cp = 0.5 g/L, [NaCl] = 0.1 M, and 20 °C. Inserts are pictures of the PMPC/PAAc aqueous solutions at pH 3 and 12.
3.4. pH, Cp, and [NaCl] Dependence on UCST
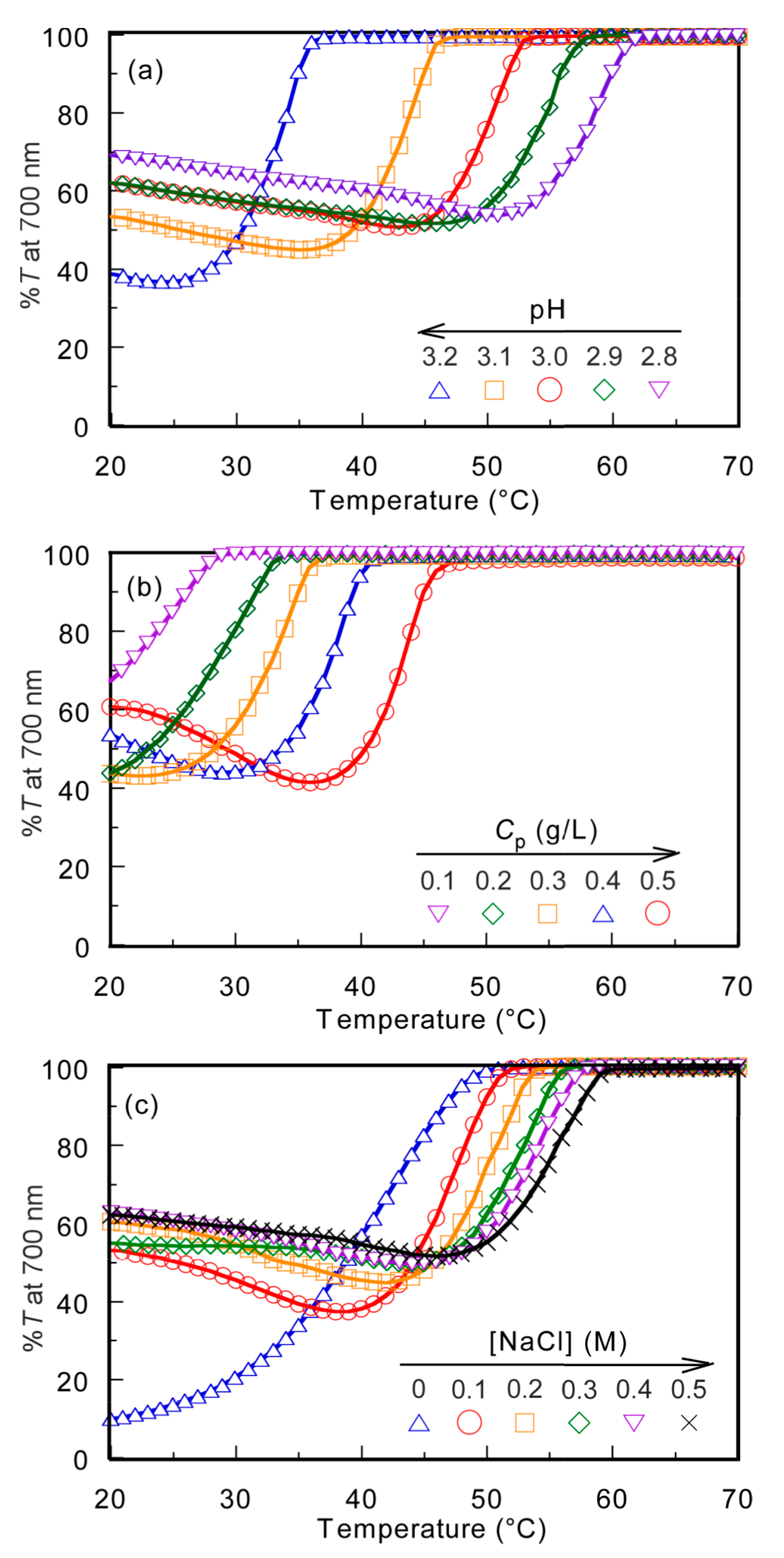
The PMPC/PAAc aqueous solution with fAAc = 0.85 showed the UCST behavior at pH 3.0 and Cp = 0.5 g/L. The %T of the PMPC/PAAc aqueous solution with fAAc = 0.85 was 100% at [NaCl] = 0.1 M, which started to decrease with the cooling process. Large hysteresis was observed for the plots of %T vs temperature with the heating and cooling processes (Figure S6). However, the plot of %T vs temperature with the cooling process always overlapped without hysteresis. Therefore, in this study, we focused on the cooling process of the UCST. The temperature at which %T decreased from 100% was defined as Tp. We studied the effect of pH, Cp, and [NaCl] on Tp. The PMPC/PAAc aqueous solution with fAAc = 0.85 at pH 3, Cp = 0.5 g/L, and [NaCl] = 0.1 M was used as the standard condition. Tp was determined from the change in %T as a function of temperature during the cooling process when one condition was changed and the other two were fixed (Figure 4).
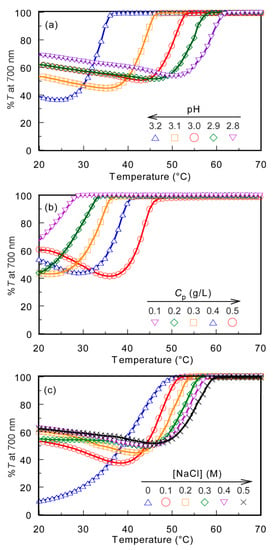
Figure 4.
Percent transmittance (%T) at 700 nm for PMPC/PAAc mixed aqueous solutions with fAAc = 0.85 as a function of temperature at pH 3, Cp = 0.5 g/L, and [NaCl] = 0.1 M, which is a standard condition: (a) pH, (b) Cp, and (c) [NaCl] dependence on the phase transition behavior.
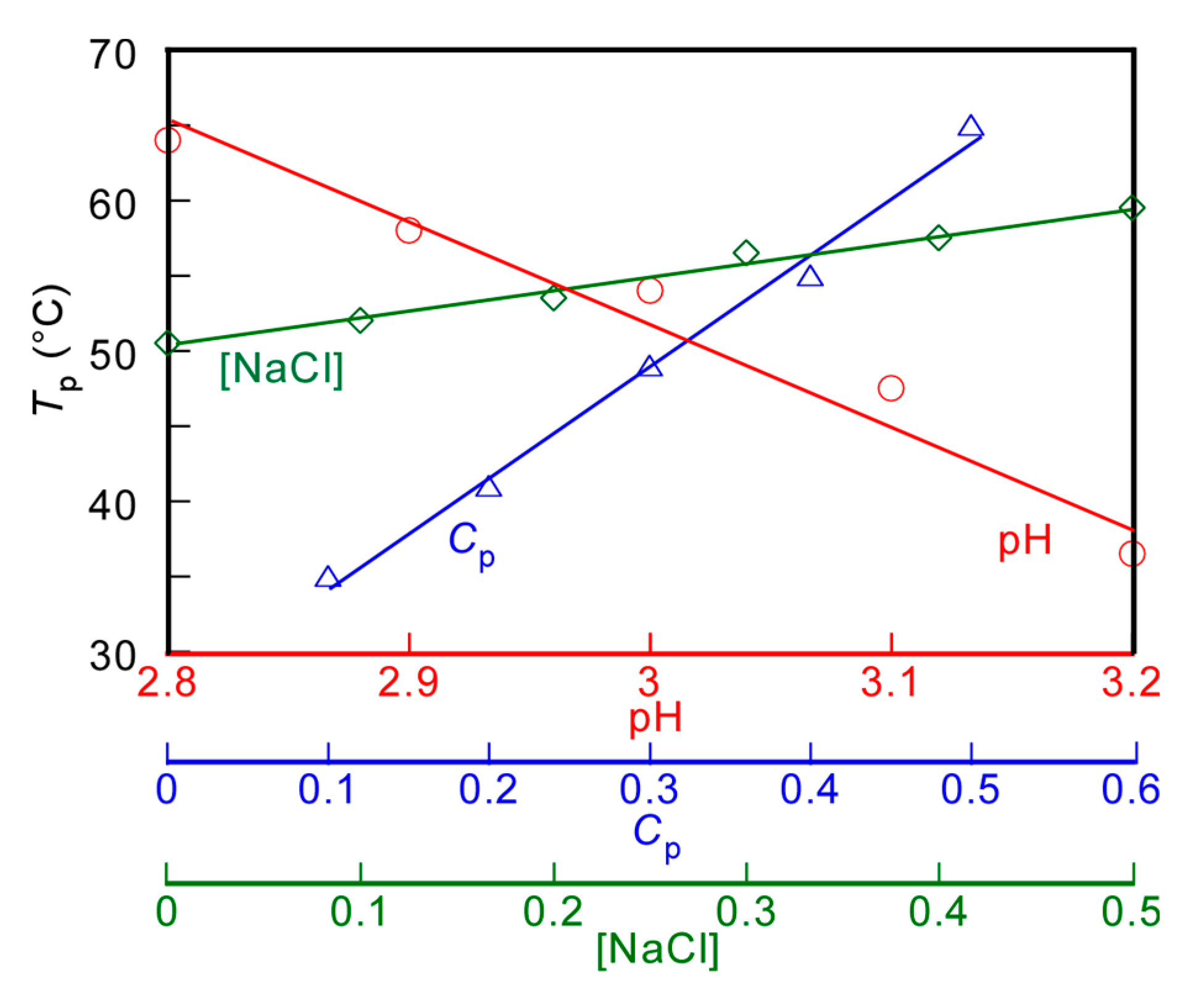
The Tp values of the PMPC/PAAc aqueous solution with fAAc = 0.85 at Cp = 0.5 g/L and [NaCl] = 0.1 M were measured at varying pH values (Figure 4a). Tp became relatively low with increasing pH. With increasing pH, the deprotonation of PAAc hindered the formation of hydrogen bonds with PMPC, and low energy was enough to break the interpolymer interactions, leading to a decrease in Tp. The Tp values were measured at varying Cp values (Figure 4b). The Tp value became relatively high with increasing Cp. At high Cp, there were numerous polymer chains, which resulted in a high probability of the formation of the PMPC/PAAc complex owing to hydrogen bonding interactions between polymer chains. Therefore, with increasing Cp, more energy was required to break the hydrogen bonding interactions, which resulted in a significant increase in the Tp value. The trend where Tp increases with increasing Cp was observed for other UCST polymers, such as random copolymers of acrylamide and styrene in water [32]. The Tp values were measured in the range of 0–0.5 M [NaCl] (Figure 4c). The Tp value became relatively high with increasing [NaCl]. At low [NaCl], each polymer chain was hydrated with water molecules, increasing the polymer solubility in the aqueous solution. Conversely, at high [NaCl], dehydration of the polymer chains occurred; this was because NaCl took water molecules from the polymer chains. The dehydrated polymer chains easily formed relatively strong hydrogen bonds. With increasing [NaCl] content, more energy was required to break the hydrogen bonds. This resulted in a significant increase in the Tp value with increasing [NaCl]. The relationships of Tp with pH, Cp, and [NaCl] are summarized in Figure 5.
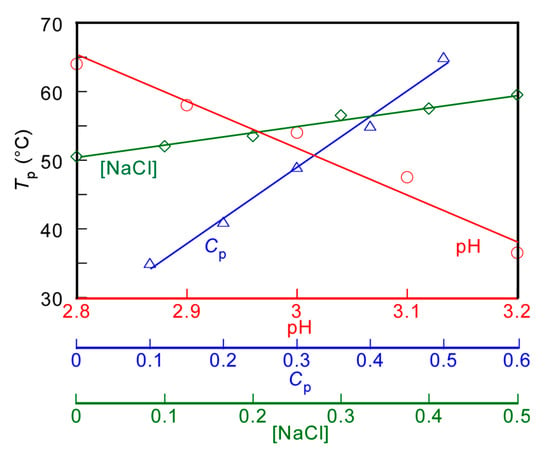
Figure 5.
pH (○), polymer concentration (Cp, △), and salt concentration ([NaCl], ◇) dependence on the phase transition temperature (Tp) for aqueous PMPC/PAAc solutions with fAAc = 0.85.
3.5. Effect of Urea on the UCST
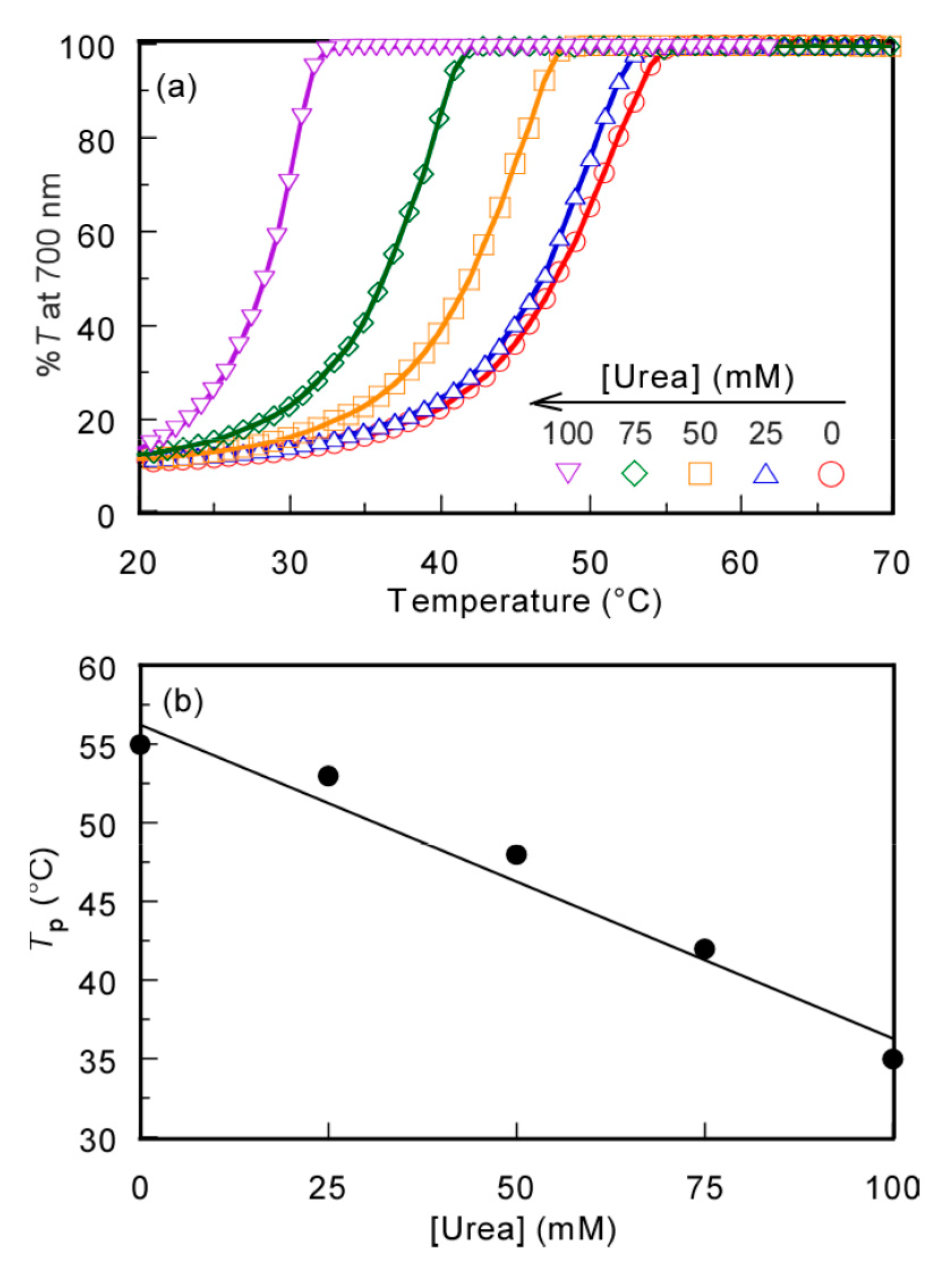
To confirm that the mechanism of the UCST behavior was due to the hydrogen bonding interactions, the effect of the hydrogen bond inhibitor, urea, on Tp was studied. The %T of the PMPC/PAAc aqueous solution with fAAc = 0.85 at Cp = 0.5 g/L, [NaCl] = 0.1 M, and pH 3.0 was measured at varying urea concentrations ([Urea]) (Figure 6). The Tp values became relatively low with increasing [Urea]. The formation of hydrogen bonds between polymer chains was restricted by urea, and the polymer chains were hydrated to increase their solubility in aqueous solution [33]. This finding indicates that the hydrogen bonding interaction is the main driving force of the UCST behavior of the PMPC/PAAc complex in aqueous solutions.
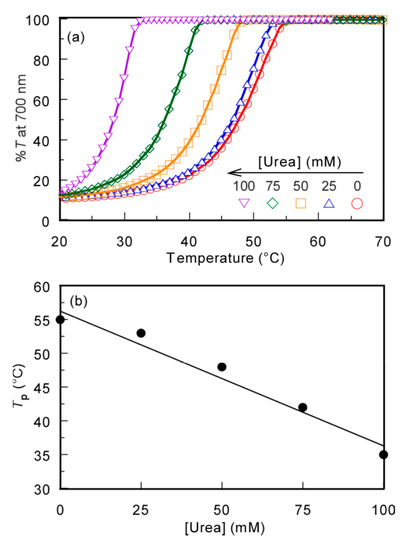
Figure 6.
(a) Percent transmittance (%T) at 700 nm for the PMPC/PAAc mixed aqueous solution with fAAc = 0.85 at Cp = 0.5 g/L, pH 3, and [NaCl] = 0.1 M as a function of temperature in the presence of urea; and (b) Tp as a function of urea concentration ([Urea]).
4. Conclusions
PMPC and PAAc were prepared by RAFT radical polymerization. PMPC and PAAc formed complexes in aqueous solutions at pH 3 because of the hydrogen bonding interactions between the pendant ester and phosphorylcholine groups in PMPC and the pendant carboxylic acid group in PAAc. The strongest interactions between PMPC and PAAc can be observed at fAAc = 0.85. The Tp value of the aqueous PMPC/PAAc solution increased with increasing Cp and [NaCl] and with decreasing pH. When urea, which is a hydrogen bond inhibitor, was added to the PMPC/PAAc aqueous solution, the Tp became relatively low. The main factor determining the behavior of the UCST is hydrogen bonding interactions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4360/13/1/148/s1: Figure S1. 1H NMR spectra for MPC (a) before and (b) after polymerization in a water/methanol mixed solvent (17.0 mL, 9/1, v/v). The solution was added D2O to lock the NMR equipment. The NMR measurements were performed at room temperature; Figure S2. 1H NMR spectra for acrylic acid (a) before and (b) after polymerization in methanol. The solution was added D2O to lock the NMR equipment. The NMR measurements were performed at room temperature; Figure S3. 1H NMR spectra for (a) PMPC and (b) PAAc in D2O at 20 °C. Assignments are indicated for the resonance peaks; Figure S4. GPC elution curves for PMPC (―) and PAAc (---) using a mixed solvent of 50 mM phosphate buffer at pH 9 and acetonitrile (9/1, v/v) as an eluent at 40 °C; Figure S5. EP and EP1/2 positions on the PAAc titration curve at Cp = 5.0 g/L titrated against HCl in 0.1 M aqueous solution at 25 °C: Firstly, PAAc was dissolved in 0.1 M NaOH at Cp = 5.0 g/L; Figure S6. Percent transmittance (%T) at 700 nm for PMPC/PAAc with fAA = 0.85 mixed aqueous solutions at pH 3, Cp = 0.5 g/L, and [NaCl] = 0.1 M as a function of temperature with the 2nd (circle) and 3rd (triangle) heating (red) and cooling processes (blue).
Author Contributions
Conceptualization, K.I. and S.-I.Y.; Data curation, H.F. and S.-I.Y.; Funding acquisition, S.-I.Y.; Investigation, H.F. and S.-I.Y.; Methodology, H.F. and S.-I.Y.; Project administration, S.-I.Y.; Writing—original draft, H.F. and S.-I.Y.; Writing—review & editing, K.I. and S.-I.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by a Grant-in-Aid for Scientific Research (17H03071) from the Japan Society for the Promotion of Science (JSPS), JSPS Bilateral Joint Research Projects (JPJSBP120203509), and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices (20204034).”
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Green, M.D.; Chen, K.; Daengngam, C.; Kotsuchibashi, Y. Stimuli-responsive polymers: Design, synthesis, characterization, and applications. Int. J. Polym. Sci. 2016, 2016, 6480259. [Google Scholar] [CrossRef]
- Khimani, M.; Patel, H.; Patel, V.; Parekh, P.; Vekariya, R.L. Self-assembly of stimuli-responsive block copolymers in aqueous solutions: An overview. Polym. Bull. 2020, 77, 5783–5810. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Allan, S.H.; Patrick, S.S.; Volga, B.; Guohua, C.; Jingping, C.; Chuck, C.; Atsutosh, C.; Zhongli, D.; Liangchang, D.; Robin, F.; et al. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586. [Google Scholar]
- Eve, R.G.; Jean-Christophe, L. In situ-forming hydrogels―Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar]
- Dirk, S. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar]
- Priest, J.H.; Murray, S.L.; Nelson, R.J.; Hoffman, A.S. Lower critical solution temperatures of aqueous copolymers of N-isopropylacrylamide and other N-substituted acrylamides. ACS Symp. Ser. 1987, 18, 255–264. [Google Scholar]
- Hanneke, M.L.L.; Friso, S.W.; Anja, B.; Lies, B.; Filip, E.D.P.; Ulrich, S.S.; Richard, H. Linear poly(ethylene imine)s by acidic hydrolysis of poly(2-oxazoline)s: Kinetic screening, thermal properties, and temperature-induced solubility transitions. Macromolecules 2010, 43, 927–933. [Google Scholar]
- Doncom, K.E.B.; Willcock, H.; O’reilly, R.K. The direct synthesis of sulfobetaine-containing amphiphilic block copolymers and their self-assembly behavior. Eur. Polym. J. 2017, 87, 497–507. [Google Scholar] [CrossRef]
- Fujihara, A.; Shimada, N.; Maruyama, A.; Ishihara, K.; Nakai, K.; Yusa, S. Preparation of upper critical solution temperature (UCST) responsive diblock copolymers bearing pendant ureido groups and their micelle formation behavior in water. Soft Matter 2015, 11, 5204–5213. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Contreras, B.A.; Schmalz, H.; Agarwal, S. pH dependent thermoresponsive behavior of acrylamide-acrylonitrile UCST-type copolymers in aqueous media. Polym. Chem. 2016, 7, 1979–1986. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. First example of a universal and cost-effective approach: Polymers with tunable upper critical solution temperature in water and electrolyte solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. UCST behavior of polyampholytes based on stoichiometric RAFT copolymerization of cationic and anionic monomers. Chem. Commun. 2015, 51, 70–73. [Google Scholar] [CrossRef]
- Abe, K.; Koide, M.; Tsuchida, E. Selective complexation of macromolecules. Macromolecules 1977, 10, 1259–1264. [Google Scholar] [CrossRef]
- Eustace, D.J.; Siano, D.B.; Drake, E.N. Polymer compatibility and interpolymer association in the poly(acrylic acid)-polyacrylamide-water ternary system. J. Appl. Polym. Sci. 1988, 35, 707–716. [Google Scholar] [CrossRef]
- Echeverria, C.; Lopez, D.; Mijangos, C. UCST responsive microgels of poly(acrylamide-acrylic acid) copolymers: Structure and viscoelastic properties. Macromolecules 2009, 42, 9118–9123. [Google Scholar] [CrossRef]
- Li, F.; Xing, Q.; Han, Y.; Li, Y.; Wang, W.; Perera, T.S.H.; Dai, H. Ultrasonically assisted preparation of poly(acrylic acid)/calcium phosphate hybrid nanogels as pH-responsive drug carriers. Mater. Sci. Eng. 2017, 80, 688–697. [Google Scholar] [CrossRef]
- Becerra-Bracamontes, F.; Sanchez-Diaz, J.C.; Gonzalez-Alvarez, A.; Ortega-Gudino, P.; Michel-Valdivia, E.; Martinez-Ruvalcaba, A. Design of a drug delivery system based on poly(acrylamide-co-acrylic acid)/chitosan nanostructured hydrogels. J. Appl. Polym. Sci. 2007, 106, 3939–3944. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs. Sci. Technol. Adv. Mater. 2000, 1, 131–138. [Google Scholar] [CrossRef]
- Ishihara, K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed. Mater. Res. Part A 2019, 107A, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Takatori, Y.; Ishihara, K.; Konno, T.; Takigawa, Y.; Matsushita, T.; Chung, U.I.; Nakamura, K.; Kawaguchi, H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004, 3, 829–836. [Google Scholar] [CrossRef]
- Yusa, S.; Yokoyama, Y.; Morishima, Y. Synthesis of oppositely charged block copolymers of poly(ethylene glycol) via reversible addition−fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. Macromolecules 2009, 42, 376–383. [Google Scholar] [CrossRef]
- Abel, B.A.; Sims, M.B.; McCormick, C.L. Tunable pH- and CO2-responsive sulfonamide-containing polymers by RAFT polymerization. Macromolecules 2015, 48, 5487–5495. [Google Scholar] [CrossRef]
- Geismann, C.; Tomicki, F.; Ulbricht, M. Block copolymer photo-grafted poly(ethylene terephthalate) capillary pore membranes distinctly switchable by two different stimuli. Sep. Sci. Technol. 2009, 44, 3312–3329. [Google Scholar] [CrossRef]
- Paek, K.; Yang, H.; Lee, J.; Park, J.; Kim, B.J. Efficient colorimetric pH sensor based on responsive polymer-quantum dot integrated graphene oxide. ACS Nano 2014, 8, 2848–2856. [Google Scholar] [CrossRef]
- Yan, B.; Han, D.; Boissiere, O.; Ayotte, P.; Zhao, Y. Manipulation of block copolymer vesicles using CO2: Dissociation or “breathing”. Soft Matter 2013, 9, 2011–2016. [Google Scholar] [CrossRef]
- Yin, H.; Feng, Y.; Liu, H.; Mu, M.; Fei, C. Insights into the relationship between CO2 switchability and basicity: Examples of melamine and its derivatives. Langmuir 2014, 30, 9911–9919. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Bodensteiner, M.; Tolstoy, P.M.; Denisov, G.S.; Shenderovich, I.G. P=O moiety as an ambidextrous hydrogen bond acceptor. J. Phys. Chem. C 2018, 122, 1711–1720. [Google Scholar] [CrossRef]
- Pineda-Contreras, B.A.; Liu, F.; Agarwal, S. Importance of compositional homogeneity of macromolecular chains for UCST-type transitions in water: Controlled versus conventional radical polymerization. J. Polym. Sci. A Polym. Chem. 2014, 52, 1878–1884. [Google Scholar] [CrossRef]
- Sagle, L.B.; Zhang, Y.; Litosh, V.A.; Chen, X.; Cho, Y.; Cremer, P.S. Investigating the hydrogen-bonding model of urea denaturation. J. Am. Chem. Soc. 2009, 131, 9304–9310. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).