Theoretical Insights into the Structures and Capacitive Performances of Confined Ionic Liquids
Abstract
1. Introduction
2. Models and Methods
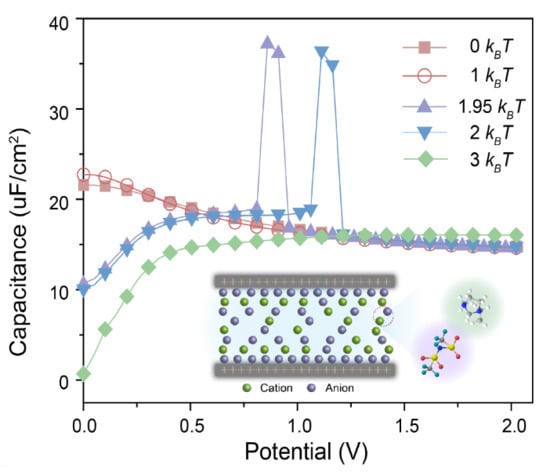
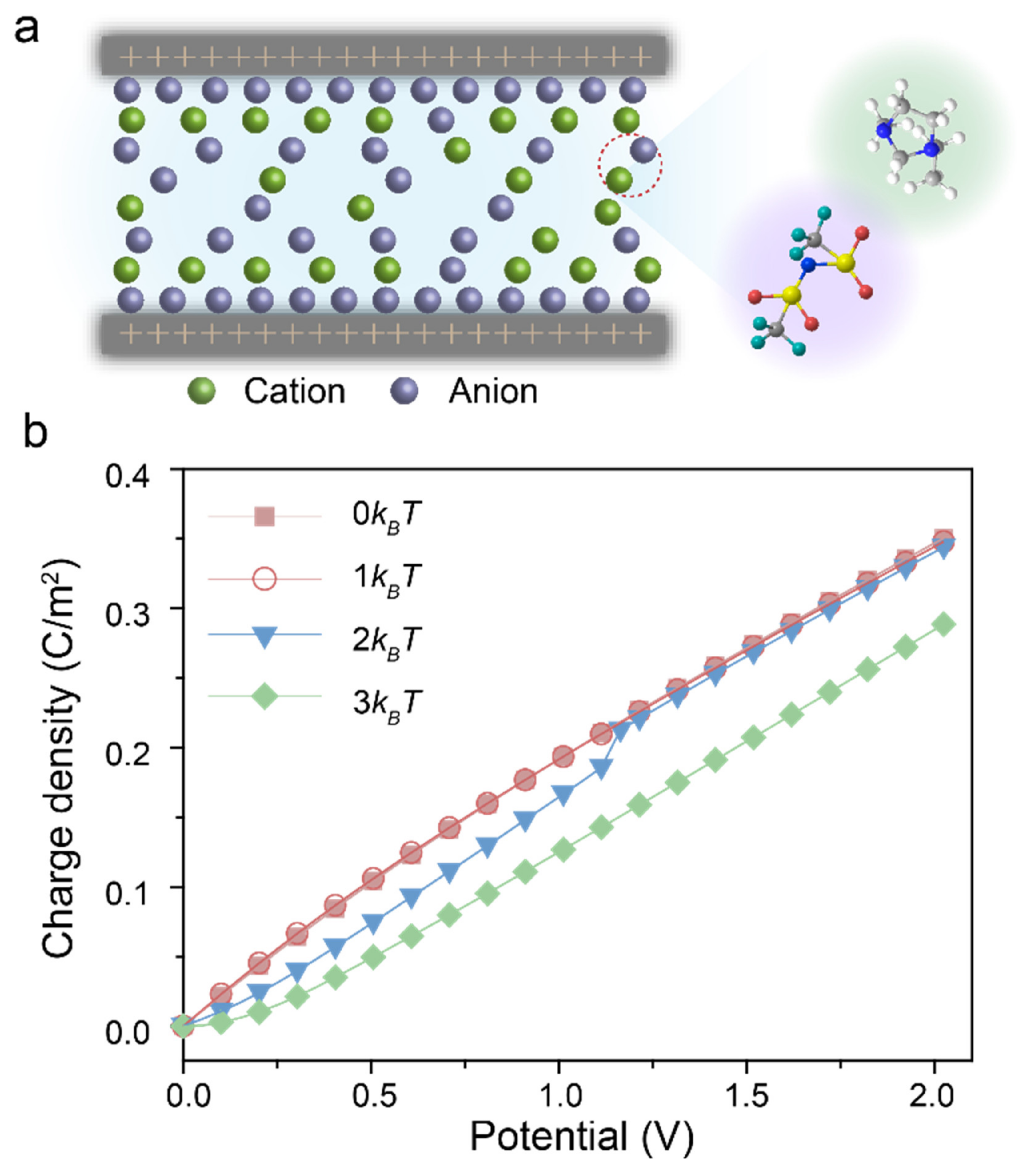
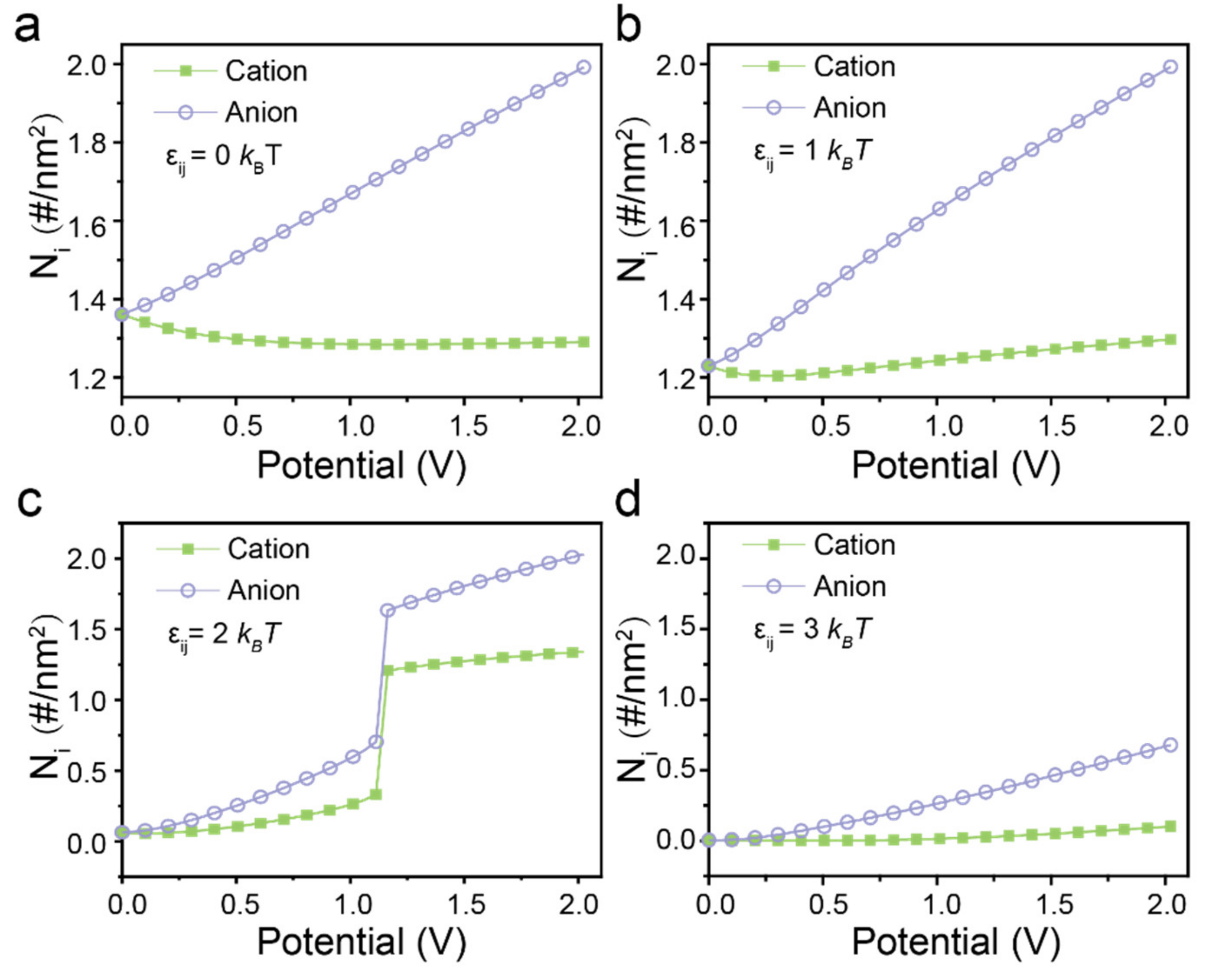
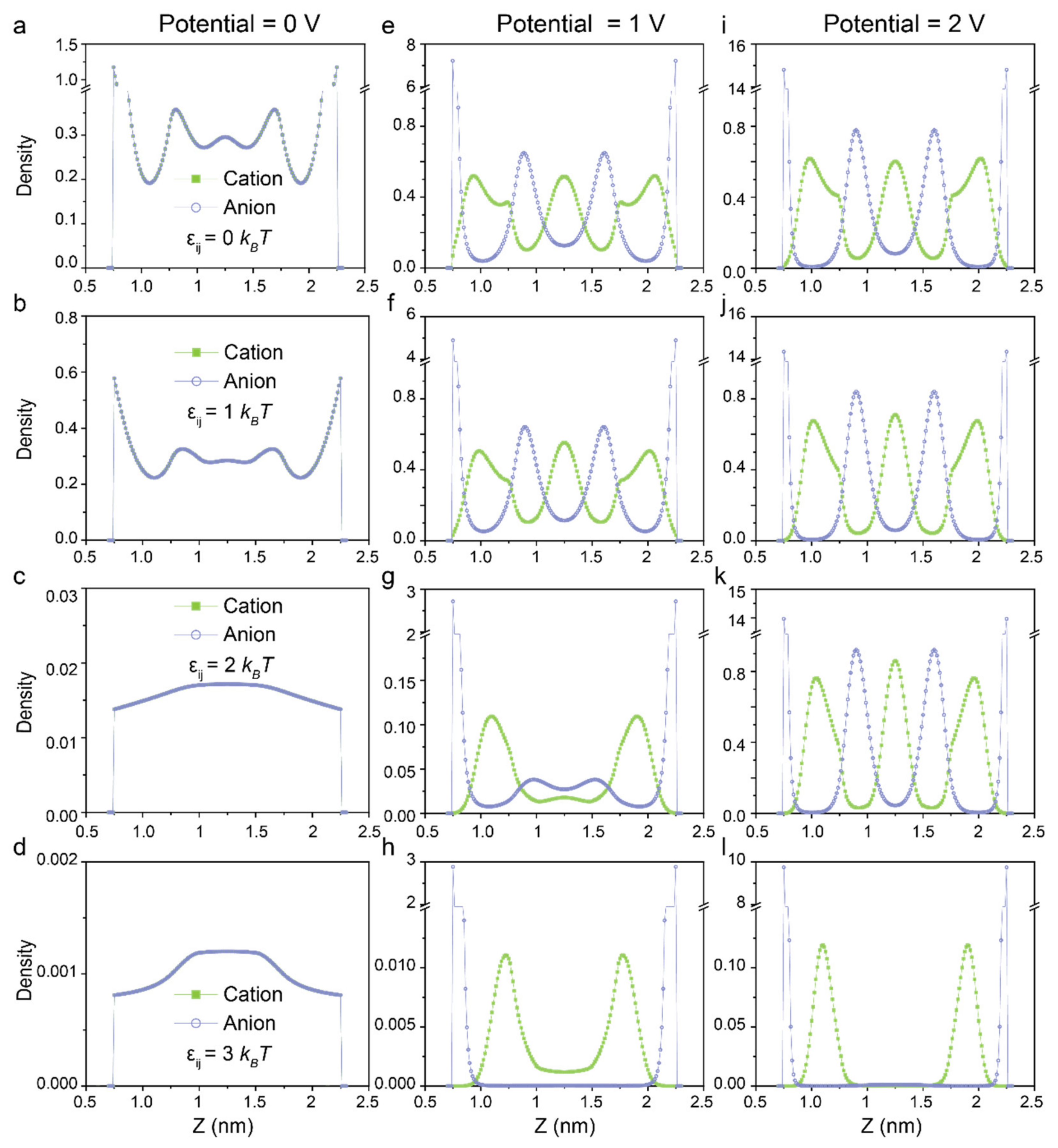
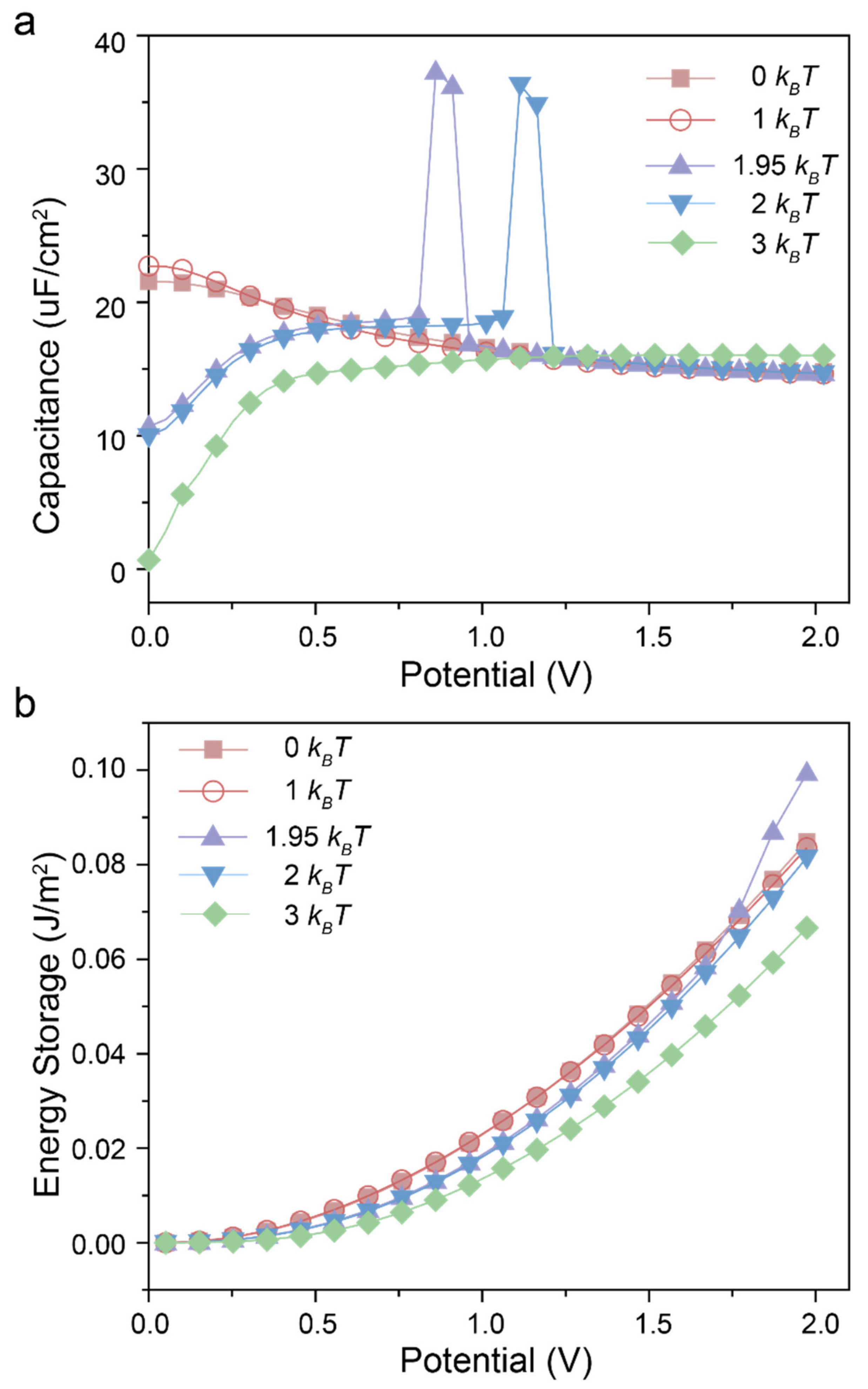
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.A.; Allam, N.K. First-principles roadmap and limits to design efficient supercapacitor electrode materials. Phys. Chem. Chem. Phys. 2019, 21, 17494–17511. [Google Scholar] [CrossRef] [PubMed]
- Burt, R.; Birkett, G.; Zhao, X.S. A review of molecular modelling of electric double layer capacitors. Phys. Chem. Chem. Phys. 2014, 16, 6519–6538. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.E.; Wu, J. Microscopic insights into the electrochemical behavior of nonaqueous electrolytes in electric double-layer capacitors. J. Phys. Chem. Lett. 2013, 4, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, M.V.; Kornyshev, A.A. Ionic liquids at electrified interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef]
- Forse, A.C.; Merlet, C.; Griffin, J.M.; Grey, C.P. New perspectives on the charging mechanisms of supercapacitors. J. Am. Chem. Soc. 2016, 138, 5731–5744. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Jiang, D.E.; Jin, Z.; Henderson, D.; Wu, J. Solvent effect on the pore-size dependence of an organic electrolyte supercapacito. J. Phys. Chem. Lett. 2012, 3, 1727–1731. [Google Scholar] [CrossRef]
- Kondrat, S.; Perez, C.R.; Presser, V.; Gogotsi, Y.; Kornyshev, A.A. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors. Energy Environ. Sci. 2012, 5, 6474–6479. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Capacitive energy storage in nanostructured carbon–electrolyte systems. Acc. Chem. Res. 2013, 46, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale studies on ionic liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef]
- Lian, C.; Liu, H.; Li, C.; Wu, J. Hunting ionic liquids with large electrochemical potential windows. AIChE J. 2019, 65, 804–810. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; King’ondu, C.K.; Holt, C.M.B.; Olsen, B.C.; et al. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Reddy, A.L.; Yu, J.; Vajtai, R.; et al. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of grapheme. Science 2011, 332, 1537. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, C.; Wang, Y.; Qiu, L.; Li, D. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 2013, 341, 534–537. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, J.S.; Qiao, R. Microstructure and capacitance of the electrical double layers at the interface of ionic liquids and planar electrodes. J. Phys. Chem. C 2009, 113, 4549–4559. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Meunier, V.; Sumpter, B.G.; Qiao, R. Complex capacitance scaling in ionic liquids-filled nanopores. ACS Nano 2011, 5, 9044–9051. [Google Scholar] [CrossRef] [PubMed]
- Kondrat, S.; Georgi, N.; Fedorov, M.V.; Kornyshev, A.A. A superionic state in nano-porous double-layer capacitors: Insights from Monte Carlo simulations. Phys. Chem. Chem. Phys. 2011, 13, 11359–11366. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Duay, J.; Lee, S.B. Heterogeneous nanostructured electrode materials for electrochemical energy storage. Chem. Commun. 2011, 47, 1384–1404. [Google Scholar] [CrossRef] [PubMed]
- Vatamanu, J.; Hu, Z.; Bedrov, D.; Perez, C.; Gogotsi, Y. Increasing energy storage in electrochemical capacitors with ionic liquid electrolytes and nanostructured carbon electrodes. J. Phys. Chem. Lett. 2013, 4, 2829–2837. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhang, Y.; Huo, F.; He, H.; Zhang, S. Molecular insights into the regulatable interfacial property and flow behavior of confined ionic liquids in graphene nanochannels. Small 2019, 15, e1804508. [Google Scholar] [CrossRef]
- Tong, J.; Xiao, X.; Liang, X.; von Solms, N.; Huo, F.; He, H.; Zhang, S. Insights into the solvation and dynamic behaviors of a lithium salt in organic- and ionic liquid-based electrolytes. Phys. Chem. Chem. Phys. 2019, 21, 19216–19225. [Google Scholar] [CrossRef]
- Trulsson, M.; Algotsson, J.; Forsman, J.; Woodward, C.E. Differential capacitance of room temperature Ionic liquids: The role of dispersion forces. J. Phys. Chem. Lett. 2010, 1, 1191–1195. [Google Scholar] [CrossRef]
- Forsman, J.; Woodward, C.E.; Trulsson, M. A classical density functional theory of ionic liquids. J. Phys. Chem. B 2011, 115, 4606–4612. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.T.; Islam, M.M.; Okajima, T.; Ohsaka, T. Measurements of differential capacitance at mercury/room-temperature ionic liquids interfaces. J. Phys. Chem. C 2007, 111, 18326–18333. [Google Scholar] [CrossRef]
- Alam, M.T.; Islam, M.M.; Okajima, T.; Ohsaka, T. Capacitance measurements in a series of room-temperature ionic liquids at glassy carbon and gold electrode interfaces. J. Phys. Chem. C 2008, 112, 16600–16608. [Google Scholar] [CrossRef]
- Lockett, V.; Sedev, R.; Ralston, J.; Horne, M.; Rodopoulos, T. Differential capacitance of the electrical Double layer in imidazolium-based ionic liquids: Influence of potential, cation size, and temperature. J. Phys. Chem. C 2008, 112, 7486–7495. [Google Scholar] [CrossRef]
- Shrivastav, G.; Remsing, R.C.; Kashyap, H.K. Capillary evaporation of the ionic liquid [EMIM][BF4] in nanoscale solvophobic confinement. J. Chem. Phys. 2018, 148, 193810. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, P.; Wu, J. Does capillary evaporation limit the accessibility of nonaqueous electrolytes to the ultrasmall pores of carbon electrodes? J. Chem. Phys. 2018, 149, 234708. [Google Scholar] [CrossRef]
- Szparaga, R.; Woodward, C.E.; Forsman, J. Theoretical prediction of the capacitance of ionic liquid films. J. Phys. Chem. C 2012, 116, 15946–15951. [Google Scholar] [CrossRef]
- Szparaga, R.; Woodward, C.E.; Forsman, J. Capillary condensation of ionic liquid solutions in porous electrodes. J. Phys. Chem. C 2013, 117, 1728–1734. [Google Scholar] [CrossRef][Green Version]
- Lian, C.; Liu, K.; Liu, H.; Wu, J. Impurity effects on charging mechanism and energy storage of nanoporous supercapacitors. J. Phys. Chem. C 2017, 121, 14066–14072. [Google Scholar] [CrossRef]
- Jiang, D.E.; Jin, Z.; Wu, J.Z. Oscillation of capacitance inside nanopores. Nano Lett. 2011, 11, 5373–5377. [Google Scholar] [CrossRef]
- Lian, C.; Jiang, D.E.; Liu, H.; Wu, J.A. Generic model for electric double layers in porous electrodes. J. Phys. Chem. C 2016, 120, 8704–8710. [Google Scholar] [CrossRef]
- Jiang, D.E.; Wu, J. Unusual effects of solvent polarity on capacitance for organic electrolytes in a nanoporous electrode. Nanoscale 2014, 6, 5545–5550. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Z. Density-functional theory for complex fluids. Annu. Rev. Phys. Chem. 2007, 58, 85–112. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, T.; Jiang, D.E.; Jin, Z.; Henderson, D. A classical density functional theory for interfacial layering of ionic liquids. Soft Matter 2011, 7, 11222–11231. [Google Scholar] [CrossRef]
- Lian, C.; Liu, K.; Van Aken, K.L.; Gogotsi, Y.; Wesolowski, D.J.; Liu, H.L.; Jiang, D.E.; Wu, J.Z. Enhancing the capacitive performance of electric double-layer capacitors with ionic liquid mixtures. ACS Energy Lett. 2016, 1, 21–26. [Google Scholar] [CrossRef]
- Boda, D.; Fawcett, W.R.; Henderson, D.; Sokołowski, S. Monte Carlo, density functional theory, and Poisson-Boltzmann theory study of the structure of an electrolyte near an electrode. J. Chem. Phys. 2002, 116, 7170–7176. [Google Scholar] [CrossRef]
- Gillespie, D.; Valiskó, M.; Boda, D. Density functional theory of the electrical double layer: The RFD functional. J. Phys. Condens. Matter 2005, 17, 6609–6626. [Google Scholar] [CrossRef]
- Yu, Y.X.; Wu, J.; Gao, G.H. Density-functional theory of spherical electric double layers and zeta potentials of colloidal particles in restricted-primitive-model electrolyte solutions. J. Chem. Phys. 2004, 120, 7223–7233. [Google Scholar] [CrossRef]
- Li, Z.; Wu, J. Density functional theory for planar electric double layers: Closing the gap between simple and polyelectrolytes. J. Phys. Chem. B 2006, 110, 7473–7484. [Google Scholar] [CrossRef]
- Lian, C.; Zhao, S.; Liu, H.; Wu, J. Time-dependent density functional theory for the charging kinetics of electric double layer containing room-temperature ionic liquids. J. Chem. Phys. 2016, 145, 204707. [Google Scholar] [CrossRef]
- Yu, Y.X.; Wu, J.Z. Structures of hard-sphere fluids from a modified fundamental-measure theory. J. Chem. Phys. 2002, 117, 10156–10164. [Google Scholar] [CrossRef]
- Fouad, W.A.; Haghmoradi, A.; Wang, L.; Bansal, A.; Hammadi, A.; Asthagiri, A.; Asthagiri, D.; Djamali, E.; Cox, K.R.; Chapman, W.G. Extensions of the SAFT model for complex association in the bulk and interface. Fluid Phase Equilib. 2016, 416, 62–71. [Google Scholar] [CrossRef]
- Lian, C.; Kong, X.; Liu, H.; Wu, J. On the hydrophilicity of electrodes for capacitive energy extraction. J. Phys. Condens. Matter 2016, 28, 464008. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ding, Y.; Lian, C.; Ying, S.; Liu, H. Theoretical Insights into the Structures and Capacitive Performances of Confined Ionic Liquids. Polymers 2020, 12, 722. https://doi.org/10.3390/polym12030722
Yang J, Ding Y, Lian C, Ying S, Liu H. Theoretical Insights into the Structures and Capacitive Performances of Confined Ionic Liquids. Polymers. 2020; 12(3):722. https://doi.org/10.3390/polym12030722
Chicago/Turabian StyleYang, Jie, Yajun Ding, Cheng Lian, Sanjiu Ying, and Honglai Liu. 2020. "Theoretical Insights into the Structures and Capacitive Performances of Confined Ionic Liquids" Polymers 12, no. 3: 722. https://doi.org/10.3390/polym12030722
APA StyleYang, J., Ding, Y., Lian, C., Ying, S., & Liu, H. (2020). Theoretical Insights into the Structures and Capacitive Performances of Confined Ionic Liquids. Polymers, 12(3), 722. https://doi.org/10.3390/polym12030722