3.1. Analysis of Copolymer Composition
Samples analyzed for copolymer composition were synthesized in the PLP setup at 50 °C, with a few samples produced at 80 °C also characterized. In accord with previous studies [
16,
21], copolymer composition was independent of temperature in this range. Experiments were conducted for bulk HEMA/BMA, as well as for comonomer mixtues diluted with DMF, xylenes, and BUOH to 10 vol%, 20 vol%, and 50 vol% monomer content.
Each data set is fit by the well-known Mayo–Lewis terminal model [
22], which relates copolymer molar composition (F
1) to the corresponding comonomer molar composition (f
1) in terms of the reactivity ratios (r
1 and r
2) that describe the relative monomer reactivity in the system.
with
and k
p,ij the propagation coefficient for the addition of monomer j to radical i.
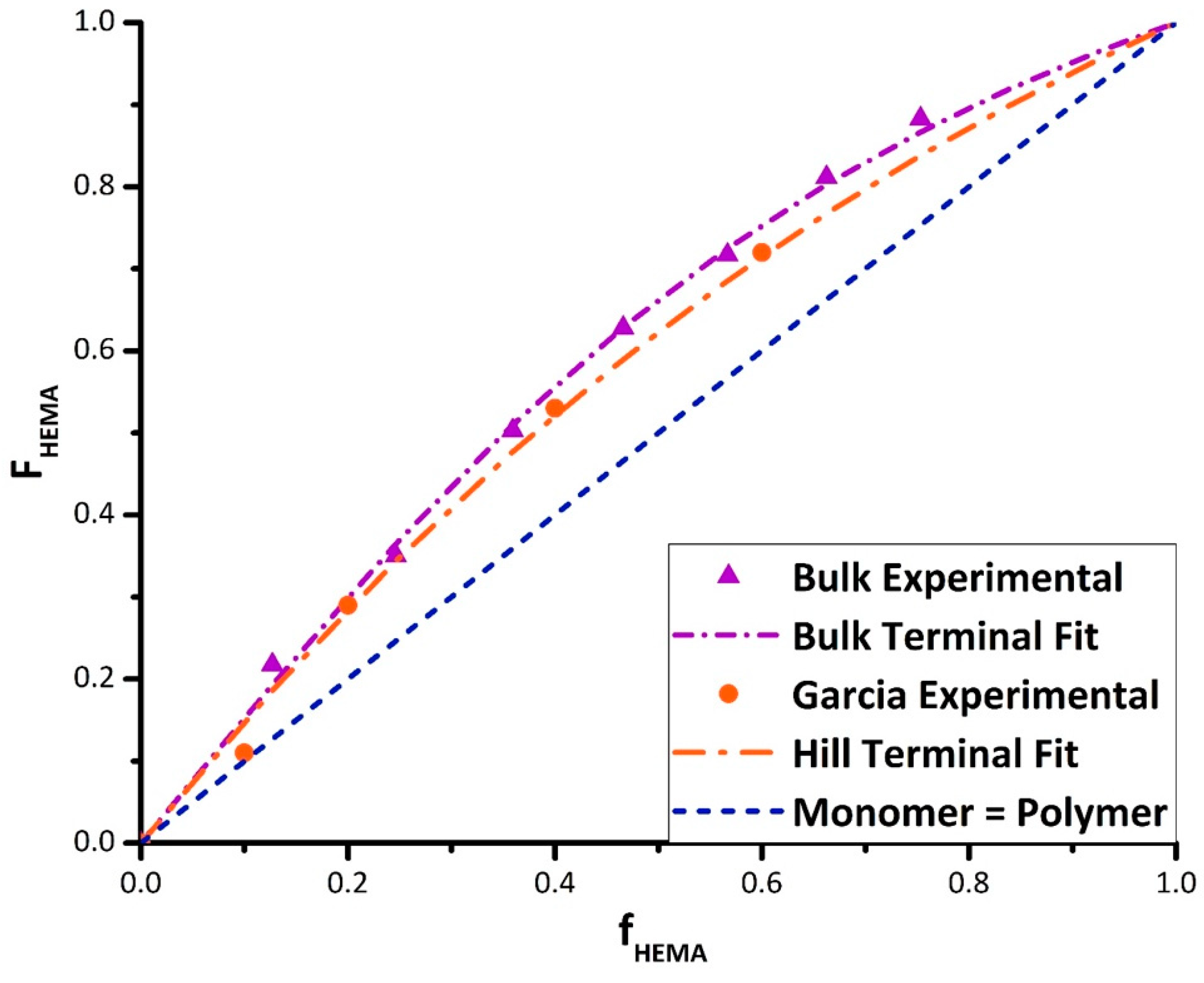
A set of experiments were run in bulk to establish a baseline relationship between comonomer and copolymer composition in the absence of solvent. The reactivity ratios were calculated using non-linear parameter estimation in Origin Lab software and confirmed with parameter estimation routines in MATLAB. The current data are in reasonable agreement with results reported by Fernández-García et al. [
23] and Hill et al. [
24] for bulk BMA/HEMA, as demonstrated in
Figure 2. The best fit reactivity ratios (with 95% confidence intervals) from the data obtained in this study are r
HEMA = 2.27 ± 0.18 and r
BMA = 0.66 ± 0.06, whereas the values from Hill et al., based on fewer data points and reported without confidence intervals, are r
HEMA = 1.73 and r
BMA = 0.65. The fit of the combined dataset yields estimates of r
HEMA = 2.25 ± 0.20 and r
BMA = 0.72 ± 0.07, within confidence limits of the values estimated using the new data alone.
In line with previous studies, the polar HEMA has higher incorporation into the copolymer than the non-polar BMA. While this enhanced reactivity is explained by H-bonding between HEMA molecules, it is somewhat surprising, as discussed by Rooney and Hutchinson [
18], that HEMA does not also enhance BMA reactivity to the same extent through H-bonding with the BMA carbonyl group; previous work has shown that BUOH as a solvent enhances BMA reactivity both during homopolymerization [
12] and when copolymerized with styrene [
15]. The result suggests that the intermolecular bonding between HEMA units is stronger than that between HEMA and BMA.
Recognizing the influence of H-bonding on HEMA relative reactivity, previous studies have studied the possibility of modifying this effect by introducing solvents that introduce competitive H-bonding or disrupt H-bonding in the system. This work goes further, varying not only solvent type but also monomer concentration (solvent fraction) to systematically explore the extent of H-bond influences on reactivity. The solvents used in this work—
n-butanol (BUOH), dimethylformamide (DMF), and xylene—were chosen based on previous research that highlighted their impact on related systems [
15,
16,
17].
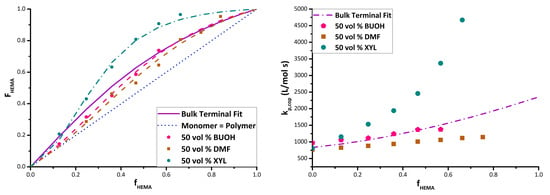
Using the bulk copolymer composition results as a reference, experiments with BMA/HEMA in the three solvents were carried out at comonomer/solvent volume ratios of 50/50, 20/80, and 10/90. Although in some cases it was not possible to cover the complete composition range because of heterogeneity (i.e., polymer precipitation) in the system, the data were used to estimate reactivity ratios for all conditions.
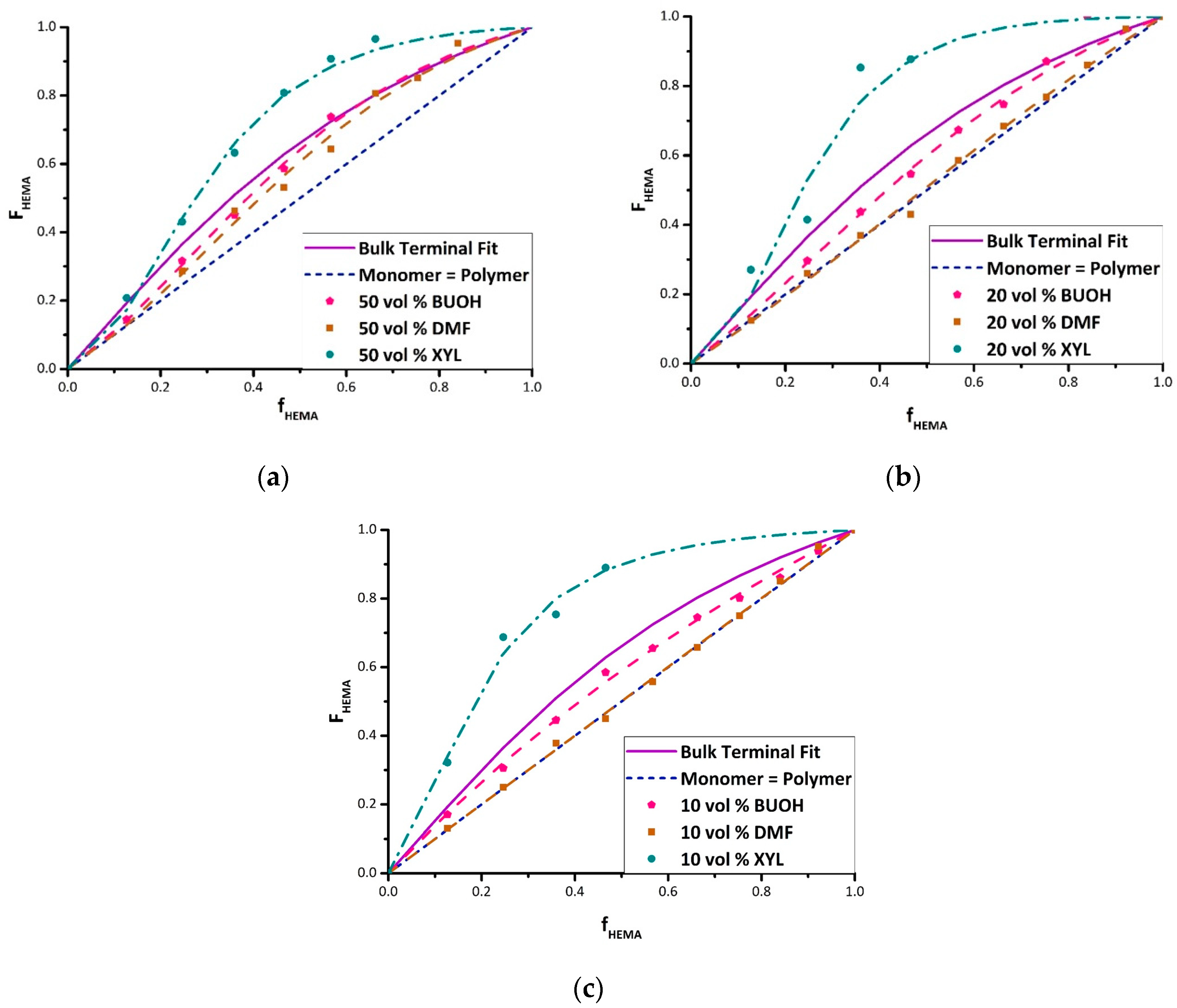
Figure 3 summarizes the complete set of experimental results in the form of Mayo–Lewis plots, with data tabulated as
Supporting Information (Tables S1 and S2). The figure also presents the best-fit representation of the data according to the terminal model, with the corresponding estimates of reactivity ratios summarized in
Table 2. The values were determined with some high HEMA data points excluded during the fit because of concerns regarding NMR integrations and the precipitation of copolymer from the solvent/monomer mixture [
25].
The data shows the expected dampening effect of polar solvents BUOH and DMF on HEMA incorporation [
15,
19], with the extent of the variation from the bulk system dependent on the solvent level. In addition, a boosting effect of xylene on HEMA reactivity is observed. For example, with 20 vol% monomer in solution (
Figure 3b) and f
HEMA = 0.47, the HEMA fraction incorporated into the copolymer increases from its bulk value of 0.63 to 0.88 in xylene, but decreases to 0.55 in BUOH and 0.43 in DMF. Previous studies [
15,
16,
17] have attributed the decrease in HEMA incorporation (relative to bulk) to the role of the solvent in enhancing (BUOH) or disrupting (DMF) H-bonding, although a clear interpretation of xylene on relative reactivity has not been established. Further discussion of the data, organized by solvent, follows.
As studied by Beuermann [
12,
13], the OH group in BUOH undergoes hydrogen bonding with the BMA carbonyl group, decreasing the electron density of the double bond, thereby increasing its reactivity towards radical attack, as demonstrated by the increase in its homopropagation rate coefficient. The role of BUOH in the presence of HEMA is less clear, as both species can participate in H-bonding. At 50 vol% solvent, the addition of BUOH enhances the reactivity of BMA to a greater extent than HEMA does, as the fraction of HEMA incorporated into the copolymer decreases towards the diagonal compared with in bulk for f
HEMA < 0.5; at higher HEMA fractions, however, the copolymer composition is the same as measured for the bulk system. Increasing the BUOH fraction to 80 vol% and 90 vol% lowers the HEMA incorporation over the complete composition range, such that the best-fit curves lie entirely below that of the bulk case. The decrease in HEMA incorporation is largely captured by a decrease in the HEMA reactivity ratio (see
Table 2), although HEMA monomer addition to a HEMA radical is still favored over BMA monomer addition even at a high BUOH fraction. Though the solvent contribution towards H-bonding boosts the reactivity of BMA, it is not selective and also increases the probability of increased H-bonding for HEMA, allowing the preferential incorporation of HEMA to persist.
Adding DMF as solvent also decreases the HEMA incorporation into the copolymer relative to bulk. DMF has a greater influence than BUOH, however, such that the reactivity of BMA becomes equal to that of HEMA (i.e., copolymer composition along the diagonal in
Figure 3) for 20 vol% and 10 vol% monomer in solution; the corresponding reactivity ratio estimates decrease to unity within experimental error (
Table 2). This result can be attributed to the aprotic nature of DMF that serves to disrupt the H-bonding between HEMA molecules rather than increasing BMA reactivity [
15,
19]. DMF levels the playing field for the two monomers, completely eliminating the higher relative reactivity for HEMA seen in bulk copolymerization with BMA.
As a non-polar solvent, xylene might be expected to be inert and hence yield the same copolymer composition as measured in bulk. However, there is a substantial increase in the relative reactivity of HEMA in xylene compared with the bulk case seen at all solvent levels. Schier and Hutchinson [
16] and Ito et al. [
26] also observed that HEMA (or HEA) has an abnormally high relative reactivity when copolymerized in aromatic compounds such as toluene and xylene that is not seen in other non-polar solvents. The reason for this behavior is not evident. Aromatic compounds are capable of forming weak hydrogen bonds owing to their π electrons, with substitution by a methyl group increasing the donor ability of the ring [
27]. It has been hypothesized that this reduces the electron density of the HEMA double bond and further enhances its reactivity, as suggested in a recent computational study [
28]. However, it is unlikely that this effect is stronger than that introduced by intermolecular H-bonding between HEMA units.
Others [
23,
29] propose that solvent effects in HEMA copolymerization result from a bootstrap effect, where the preferential solvation of HEMA next to the growing chain causes a higher localized concentration. This microphase separation isolates pockets of monomer, which leads to preferential HEMA incorporation without any change in the actual reactivity ratios. However, a careful study by Ito et al. [
26] argues that the magnitude of the change is too large to be explained by the bootstrap effect alone. Their investigation of HEMA copolymerized with dodecyl methacrylate (DMA) reports very similar results to what is found in the current study for BMA/HEMA; with all experiments conducted at a total monomer concentration of 0.5 mol/L, the reactivity of HEMA is abnormally high in benzene (r
HEMA = 11, r
DMA = 0.7), HEMA is more reactive than DMA in
tert-BUOH (r
HEMA = 1.6, r
DMA = 0.5), and copolymerization in DMF leads to equal incorporation of the two monomers (r
HEMA ≅ r
DMA ≅ 1.0). These reported reactivity ratio values for HEMA/DMA are in excellent agreement with the
Table 2 best-fit values for HEMA/BMA with 10 vol% and 20 vol% monomer in solution. The study by Ito et al. [
26] determined that the more than ten-fold increase in r
HEMA greatly surpassed the increased mean degree of HEMA aggregation in benzene, which was measured to be a factor of two based on cryoscopic measurements. Thus, it was concluded that the bootstrap effect alone cannot explain the magnitude of the increased HEMA incorporation. Rooney and Hutchinson [
18] propose that the increased reactivity of HEMA results not from monomer aggregation, but rather from HEMA hydrogen bonding between monomer and a HEMA unit located on the growing chain close to the radical end in the non-polar solvent, increasing the incorporation rate of the HEMA into the copolymer relative to the bulk system.
While the origin of the enhanced HEMA incorporation in xylene remains a matter of debate, the series of plots in
Figure 3 demonstrate that the magnitude of the increased reactivity is accentuated as the solvent fraction is increased from 50 vol% to 90 vol%. However, the change in reactivity is not necessarily proportional to solvent level in general; in DMF and BUOH, a decreased HEMA incorporation is seen as the solvent level is increased from 50% to 80%, but little further change is observed for 90% solvent.
3.2. Analysis of Composition-Averaged Propagation Rate Coefficient
Copolymer composition provides a means to examine the effect of solvent on relative reactivity. Further insights can be gained by also considering k
p,cop values, which measure the solvent influence on absolute (rather than relative) monomer addition rates. Using the IUPAC recommended PLP-SEC method, k
p,cop data were collected at two temperatures (50 and 80 °C) and two repetition rates per condition.
Figure 4 illustrates the typical PLP structures achieved. With several maxima observed on the corresponding first derivative plots, the first inflection point is taken as the position of MW
1 used to calculate k
p,cop according to Equation (2). To check the validity of the data, the second inflection point is also analyzed to verify that MW
2 is at about twice the value of the first. The complete set of data are tabulated as
Supporting Information (Tables S3–S6).
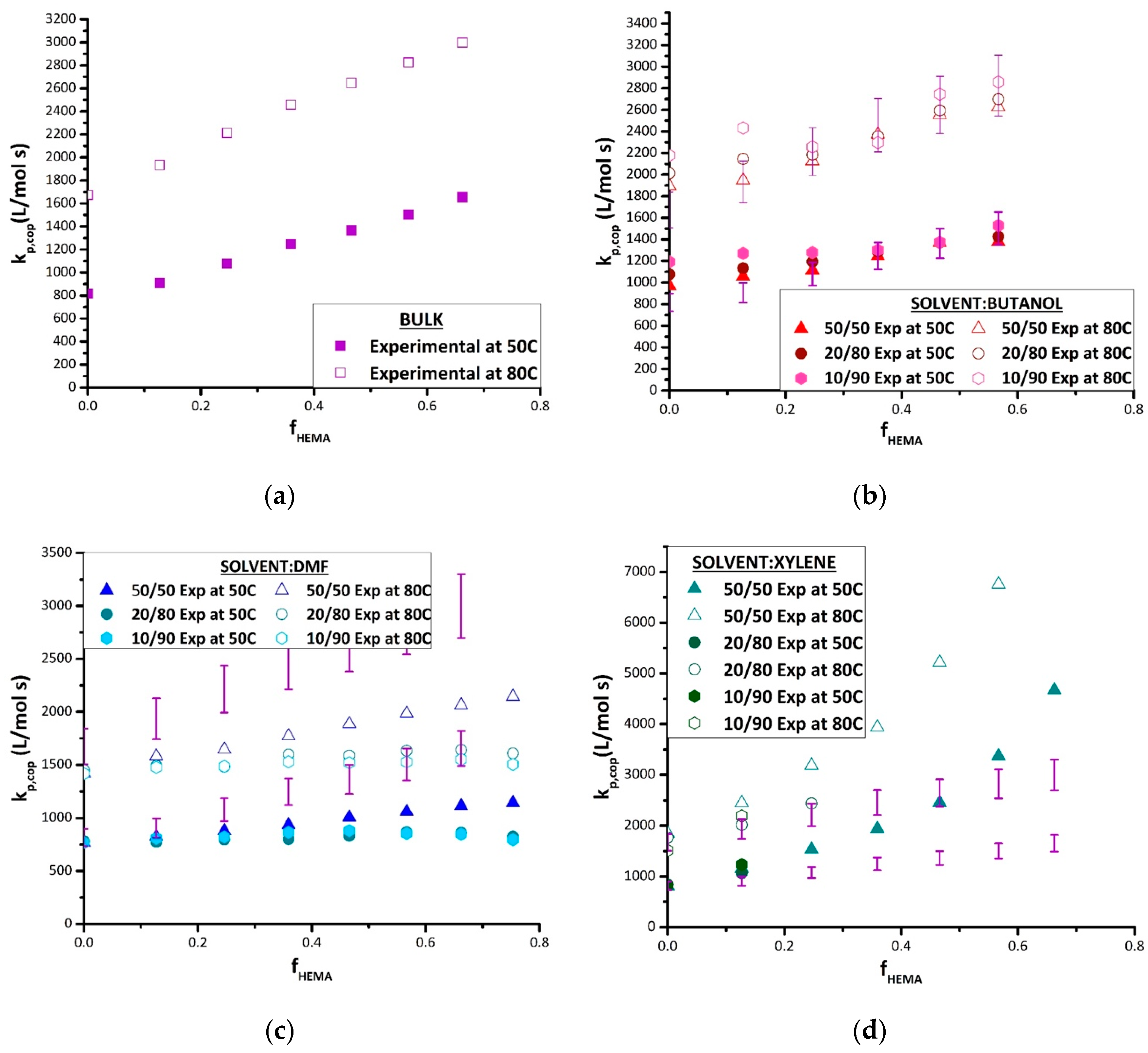
The complete set of experimental data is plotted in
Figure 5, comparing k
p,cop results found in each solvent at both 50 and 80 °C against values obtained for the bulk system, to examine for differences that may be attributed to solvent effects. The values displayed are averaged from the two repetition results per temperature. As previously mentioned, data are not available for HEMA homopolymerization or HEMA-rich copolymers, as the samples were not soluble in THF for SEC analysis. In bulk (
Figure 5a), the values of k
p,cop increase in a close-to-linear fashion from the BMA homopolymerization value as HEMA is added, as also evidenced by the shift of the distributions (and corresponding inflection points) to higher MWs seen in
Figure 4. The same general trend is seen in the various solutions, with the exception of DMF. The plots for the solution data include the bulk values with ±10% error bars, the generally accepted uncertainty in PLP-determined k
p values [
4,
5,
6,
7,
8,
9]; deviations outside this range can be attributed to solvent effects rather than experimental error. As H bonds are weaker at higher temperatures, the difference (if any) between the bulk and solvent values may be decreased at 80 °C compared with at 50 °C [
12,
13].
In BUOH, k
p,cop of the BMA/HEMA system is boosted compared with bulk at lower (
fHEMA < 0.25) HEMA content (
Figure 5b). This increase in k
p,cop is more pronounced as solvent concentration increases (monomer content lowered from 50 vol% to 10 vol%). In agreement with previously published data [
12,
15], the increase is dependent on the ratio of BUOH to BMA in the system, and is attributed to an increase in BMA reactivity due to the interactions of its carbonyl group with BUOH. This increased reactivity persists when small amounts of HEMA are added to the system, but disappears completely when HEMA fraction in the monomer mixture is raised to 0.3 (at 50 °C) or 0.2 (at 80 °C). There is no significant difference in k
p,cop values between bulk and BUOH solution for higher HEMA contents, although there is some indication that the values in BUOH solution are decreasing to lower than bulk for the higher HEMA fractions of 0.5 and 0.6. These results suggest that BUOH promotes the reactivity of BMA, but may reduce the reactivity of HEMA through its competitive H-bonding, a result also consistent with the reduced HEMA incorporation seen in the copolymer composition data (
Figure 3).
As also seen in the copolymer composition results, the relative reactivity of HEMA is greatly reduced in BMA/HEMA copolymerization in DMF relative to the bulk system (
Figure 5c). Although k
p,cop still increases with increasing HEMA level in 50 vol% DMF, the enhanced reactivity of HEMA completely disappears once DMF becomes the predominant species; for 20 vol% and 10 vol% monomer mixtures in solvent, the k
p,cop values are within 10% of the BMA experimental homopropagation value at both 50 and 80 °C. While this disruption of HEMA reactivity has been previously observed in copolymerization with ST [
15,
30], the complete flattening of the curve in this methacrylate/methacrylate study relative to the bulk system is particularly illuminating, illustrating both the strong influence of HEMA H-bonding on its reactivity in the absence of solvent and its equal reactivity to a “normal” methacrylate such as BMA when that H-bonding is disrupted.
Though an increase in k
p,cop values in xylene relative to bulk can be clearly seen (
Figure 5d), poor copolymer solubility in the mixtures restricted the experimental range that could be studied at higher HEMA levels. Higher xylene levels (80 vol% and 90 vol%) resulted in turbidity and eventual separation during the low conversion PLP experiment, a result perhaps also exacerbated by the increased incorporation rates of HEMA into the copolymer (see
Figure 3). Even at 50 vol% solvent, the reaction cell became cloudy at increased HEMA levels. A second issue limiting the xylene data set was that, even for concentrations for which the solution remained homogeneous, the resultant polymer (containing a high HEMA fraction) was insoluble in THF and thus could not be analyzed by SEC. Previous studies have also found a significant increase in the reactivity of hydroxyfunctional monomers HPMA (hydroxypropyl methacrylate) [
13] and HEA [
17] when polymerized in xylenes. While all studies relate the behavior to the hydroxyl functional group, the literature has been unable to reach a consensus on the cause of the increased reactivity. It has been hypothesized that a donor–acceptor complex between the growing polymer radical and the aromatic solvent affects reactivity [
28,
31], and that the non-polar solvent may lead to partial solubility that results in localization of the monomer species and preferential solvation [
23,
26] or to enhanced H-bonding involving HEMA units in the polymer chain [
18]. As discussed for the copolymer composition results, perhaps all of these factors contribute to the behavior observed.
3.3. Fitting of Propagation Data
Propagation kinetics in a two monomer system can be represented by the terminal model (Equation (5)) or by the implicit unit model (IPUE) (Equation (6)). While the terminal model assumes that the reactivity of a growing chain is dependent only on the last monomer unit added, the IPUE model assumes that the identity of the unit preceding the radical influences its reactivity (k
p,cop) but not its selectivity (i.e., copolymer composition) [
32]. Thus, the need to use the IPUE to represent k
p,cop does not discredit the terminal model representation of composition, and the monomer reactivity ratios estimated from the terminal-model fit of composition data are still employed in the IPUE treatment of k
p,cop.
with
The new parameters in the IPUE model, radical reactivity ratios s
1 and s
2, capture the influence of the penultimate unit on the reactivity of the radical taking part in the homopropagation of the monomer [
32].
For the analysis of k
p,cop, it is good practice to start by comparing terminal model predictions to the experimental data. While shown not to be valid for many mixed monomer systems (e.g., styrene/methacrylate, styrene/acrylate, acrylate/methacrylate) [
14,
16,
32,
33,
34,
35], the terminal model has provided a reasonable estimate of k
p,cop data in the few methacrylate/methacrylate studies that could be found in the literature [
32]. However, in order to compare predictions to the data, homopolymerization k
p values for each monomer in each solvent are required. This task is not straightforward because, as seen by the data of
Figure 5, the homopolymerization endpoints are not only dependent on the monomer/solvent pairing, but also vary with the solvent fraction in the system. Though k
p values are experimentally determined for BMA directly, the insolubility of poly(HEMA) in THF made it impossible to measure the corresponding k
p,HEMA values. Thus, non-linear parameter estimation was used to estimate the necessary homopolymerization values, assuming that the terminal model (Equation (5)) provides a valid description of the k
p,cop datasets and using the reactivity ratios fit from the composition data summarized in
Table 2.
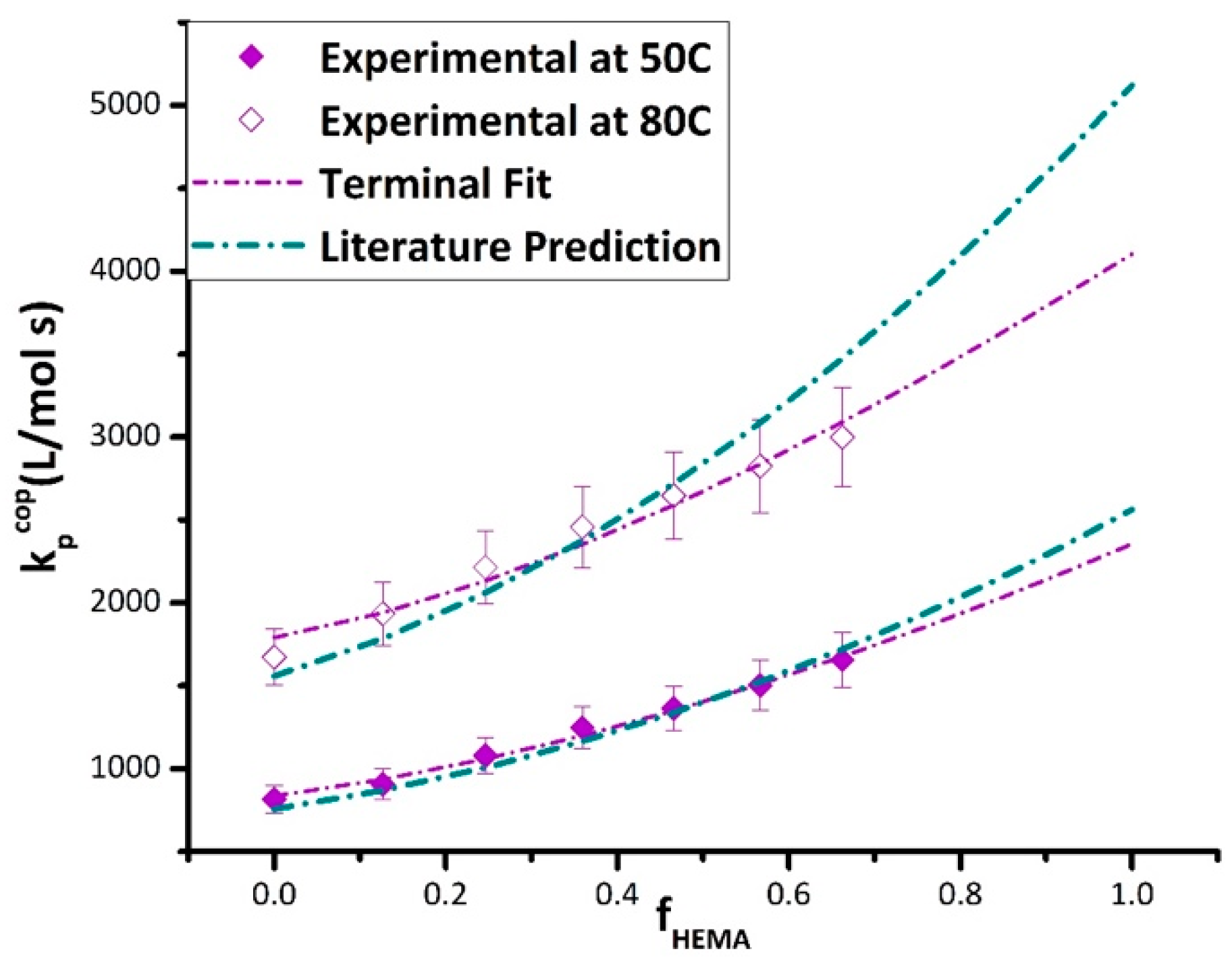
The fitting methodology was first tested using the bulk k
p,cop experimental data plotted in
Figure 6, using the bulk reactivity ratios reported in
Table 2. The resulting estimates of the BMA (k
p,BMA) and HEMA (k
p,HEMA) endpoint values are compared to literature and experimental (available for BMA only) values in
Table 3. The agreement with literature values for k
p,BMA at both temperatures and with k
p,HEMA at 50 °C is within the 10% uncertainty generally associated with the method. However, the discrepancy in the k
p,HEMA value estimated at 80 °C with literature [
10] is closer to 20%. The corresponding predictions of the terminal model are compared to the experimental bulk k
p,cop in
Figure 6 over the complete composition range. While the curves generated using the literature k
p values go through most of the data points, at 80 °C, the deviation between predicted and experimental becomes greater than the error bars at a higher HEMA content (f
HEMA > 0.6). This deviation might be attributed to uncertainty in the HEMA homopropagation k
p value, available from a single literature study [
10], but could also indicate that the terminal model does not provide a good representation of k
p,cop at the higher temperature level. Experimental data measured at a high HEMA content are required to resolve this issue.
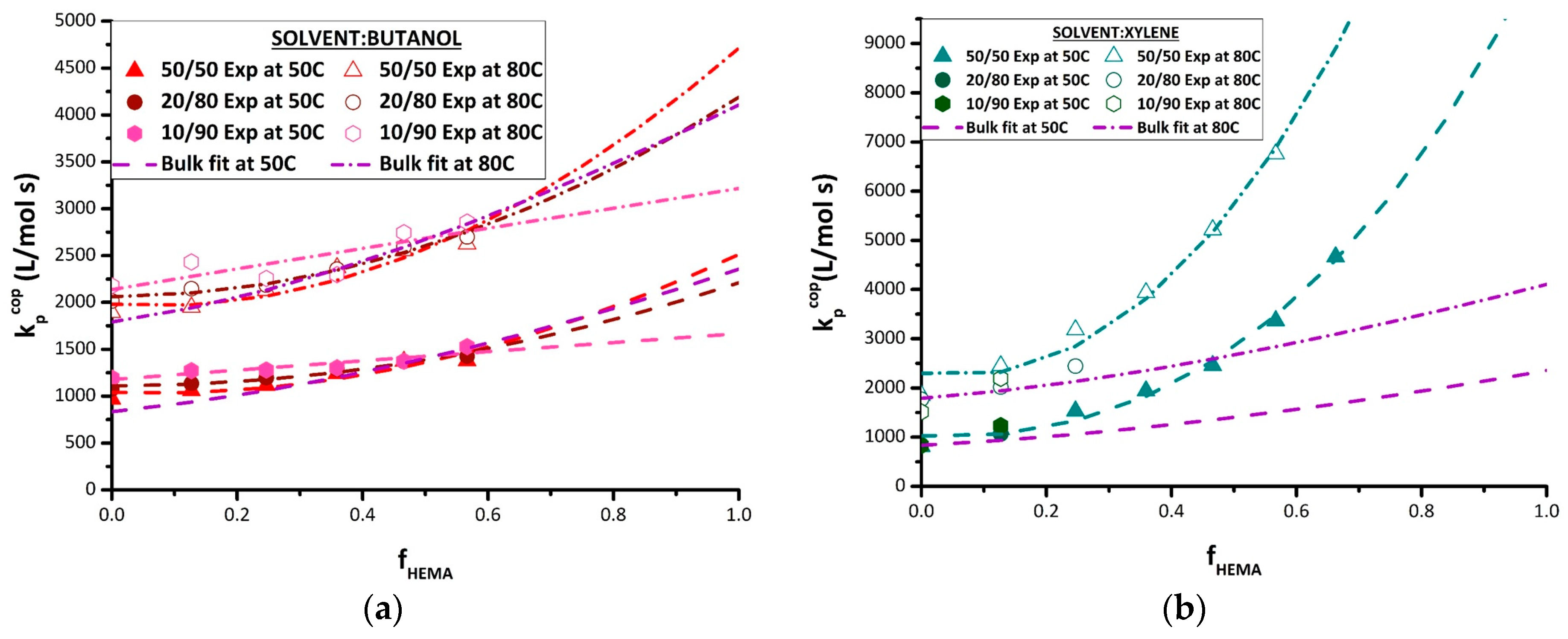
The strategy developed to fit the terminal model to estimate homopolymer k
p values from experimental k
p,cop data was then applied to the various sets of solution k
p,cop data measured in this study. The best-fit k
p,BMA and k
p,HEMA values for BUOH and xylene solvents are summarized in
Table 4, with the corresponding terminal model curves compared to experimental data in
Figure 7. As there were insufficient data for the experiments conducted with 10 vol% and 20 vol% monomer in xylene, the fit was conducted using only the 50 vol% xylene data. While the curve generated by the terminal model fit provides a good representation of the k
p,cop data measured in xylene (
Figure 7b), it should be noted the best-fit k
p,BMA endpoint is higher than the experimental values measured at 80 °C; other studies have also shown that xylene does not influence the value of k
p,BMA [
15,
16]. What is most remarkable is that the value of k
p,HEMA required to represent the k
p,cop dataset in xylene is greater than the bulk value by a factor of 4–5. While this value is estimated assuming the terminal model, the actual experimental k
p,cop values measured at f
HEMA = 0.6 already exceed the literature values for bulk HEMA. Although the reason for this behavior is not clear, it cannot be explained by the bootstrap model; the required monomer concentration around the radical would exceed that in a bulk monomer system, a physically impossible situation.
The k
p,cop data obtained in BUOH are also well-represented by the terminal model (
Figure 7a). Consistent with previous investigations [
12,
13,
15,
16] and also matching the experimental data obtained in this study, the estimate of k
p,BMA is affected by H-bonding of the monomer with BUOH, with the best-fit value (
Table 4) systematically increasing as the monomer fraction is decreased from 50 vol% to 10 vol%. At the other end of the k
p,cop curve, the opposite trend is required to fit the data to the terminal model; that is, k
p,HEMA is predicted to decrease from the bulk value with increasing BUOH fraction. It is also interesting to note that while higher BUOH content slightly increases the values of k
p,cop for BMA-rich mixtures (f
HEMA < 0.5), the three curves converge at an f
HEMA value of ~0.6, with the increased BUOH content then predicted to decrease the averaged propagation rate coefficient in the system as f
HEMA increases further. The ability to analyze PLP-generated HEMA-rich polymer samples is necessary to verify these predictions.
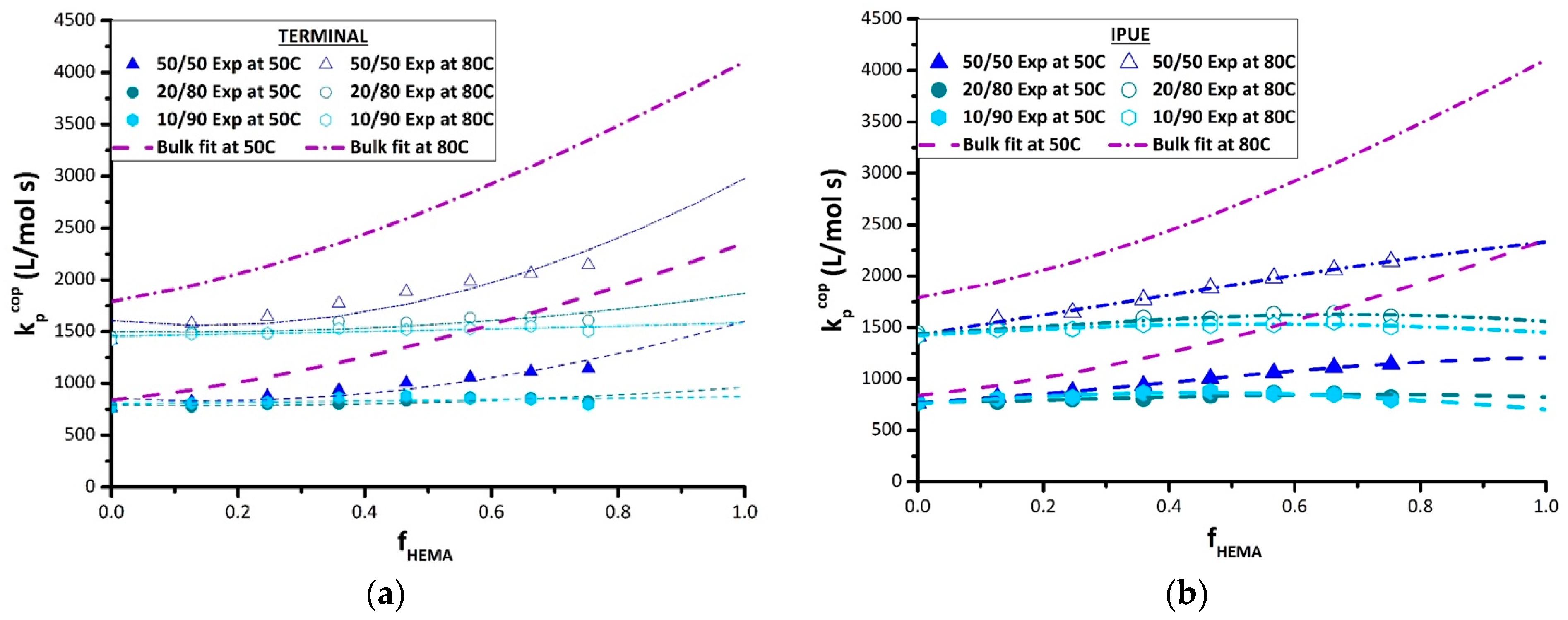
The results from the terminal model fitting of the BMA/HEMA k
p,cop data measured in DMF are also summarized in
Table 4, with the resulting curves plotted in
Figure 8a. The terminal model captures the disrupting effect of DMF on the reactivity of HEMA, with the k
p,HEMA estimates systematically decreasing from the bulk values towards k
p,BMA values as the DMF level is increased from 50 vol% to 90 vol%. As the reactivity ratios are close to unity for the 10 vol% and 20 vol% monomer sets, this flattens out the k
p,cop curves to match well the experimental k
p,cop data. However, the fits to the data obtained with 50 vol% DMF are not as well represented, as seen by comparing the terminal model predictions to the experimental values at 80 °C, and also in the predicted increases in k
p,BMA values that are not seen experimentally.
Thus, the penultimate model was also used to fit the k
p,cop DMF data, with the best-fit parameters summarized in
Table 5 and the fits compared to the experiment in
Figure 8b. With more parameters used, the penultimate model provides a better representation of the experimental data. The prediction method, however, showed high uncertainty in the estimation of the reactivity ratios s
1 and s
2; as discussed in previous literature [
35], precise estimation of these parameters can be quite difficult, especially with an incomplete data set (i.e., no k
p,cop values available at high HEMA content). Nonetheless, the penultimate model provides a better fit to the k
p,BMA endpoint of the curve. Once again, collecting data at higher HEMA levels is required to determine which curve shape—the concave up prediction of the terminal model fits or the concave down prediction of the penultimate model fits—will match the data over the complete composition range. If the IPUE model proves to be more suitable, it could be theorized that the high polarity and H-bonding cause the penultimate unit to exert steric restrictions [
36].