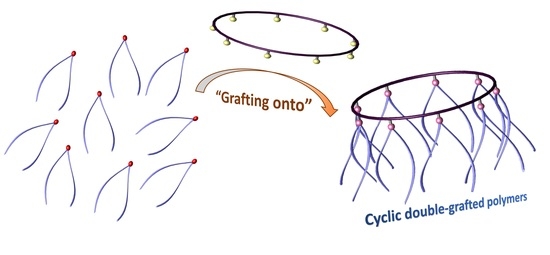
Design and Synthesis of a Cyclic Double-Grafted Polymer Using Active Ester Chemistry and Click Chemistry via A “Grafting onto” Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
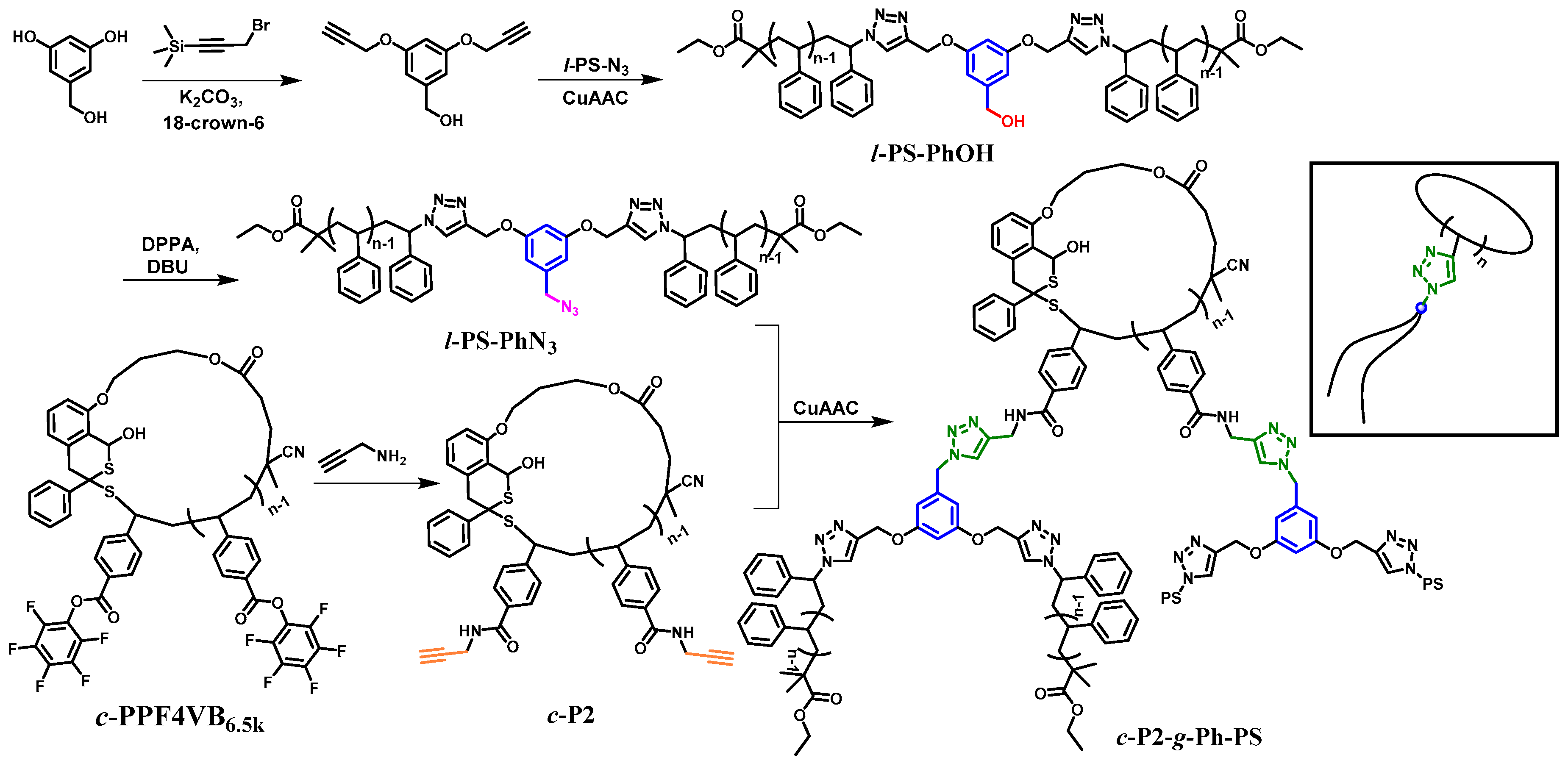
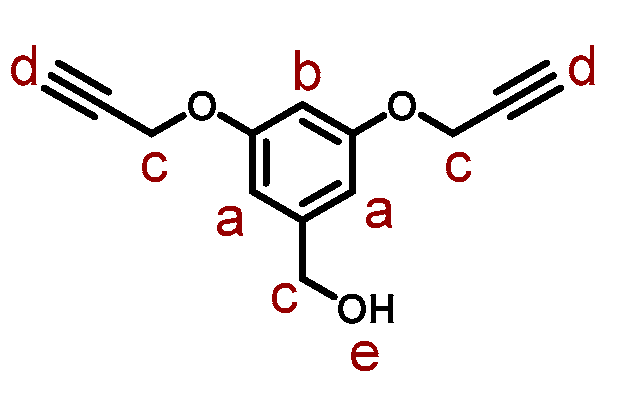
2.3. Synthesis of 3,5-bis(propargyloxy)benzyl Alcohol
2.4. Synthesis of Hydroxyl-Containing Polymer Double-Chain (l-PS-PhOH)
2.5. Synthesis of Azide-Containing Polymer Double-Chain (l-PS-PhN3)
2.6. Synthesis of Cyclic Double-Grafted Polymer (c-P2-g-Ph-PS)
3. Results and Discussion
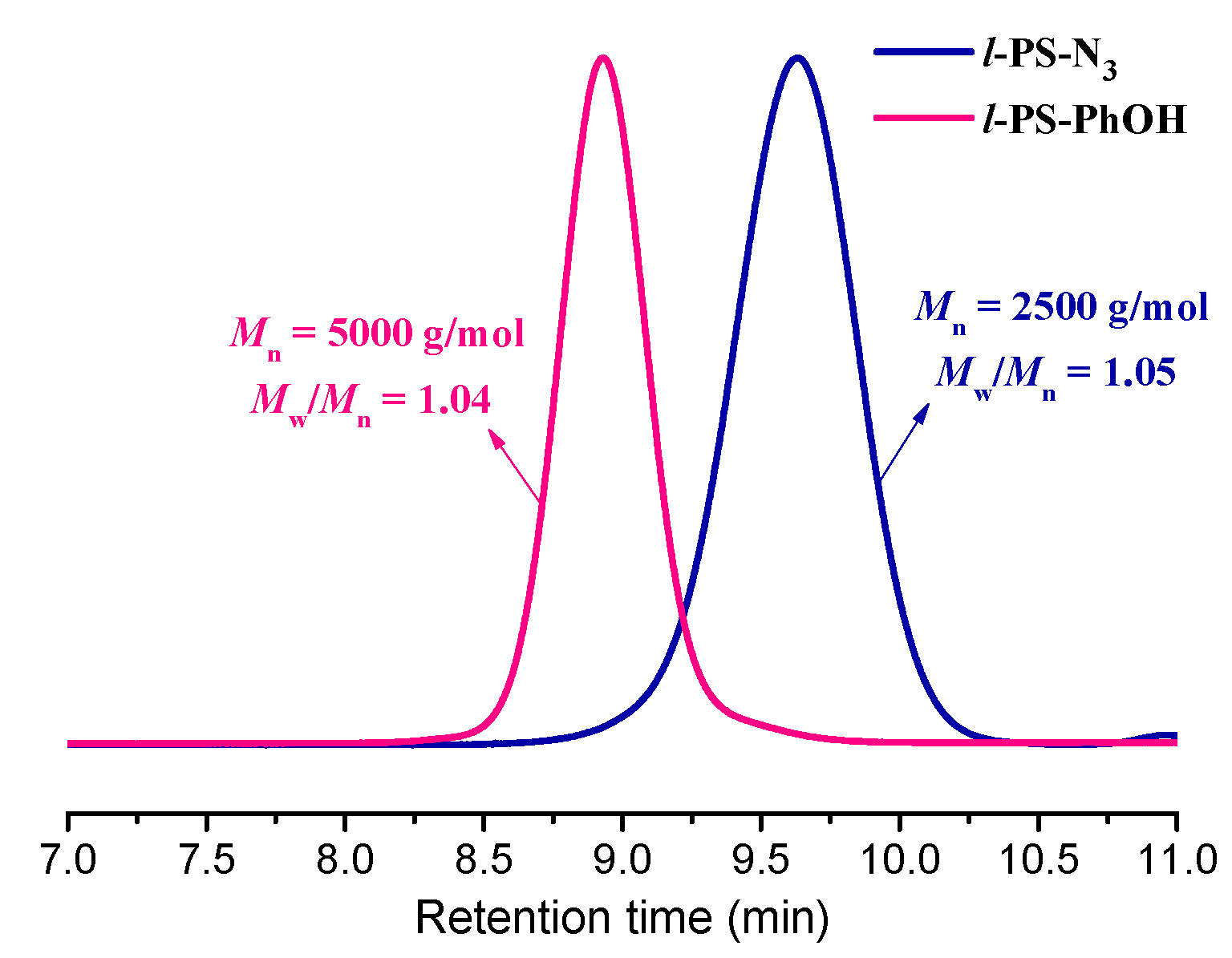
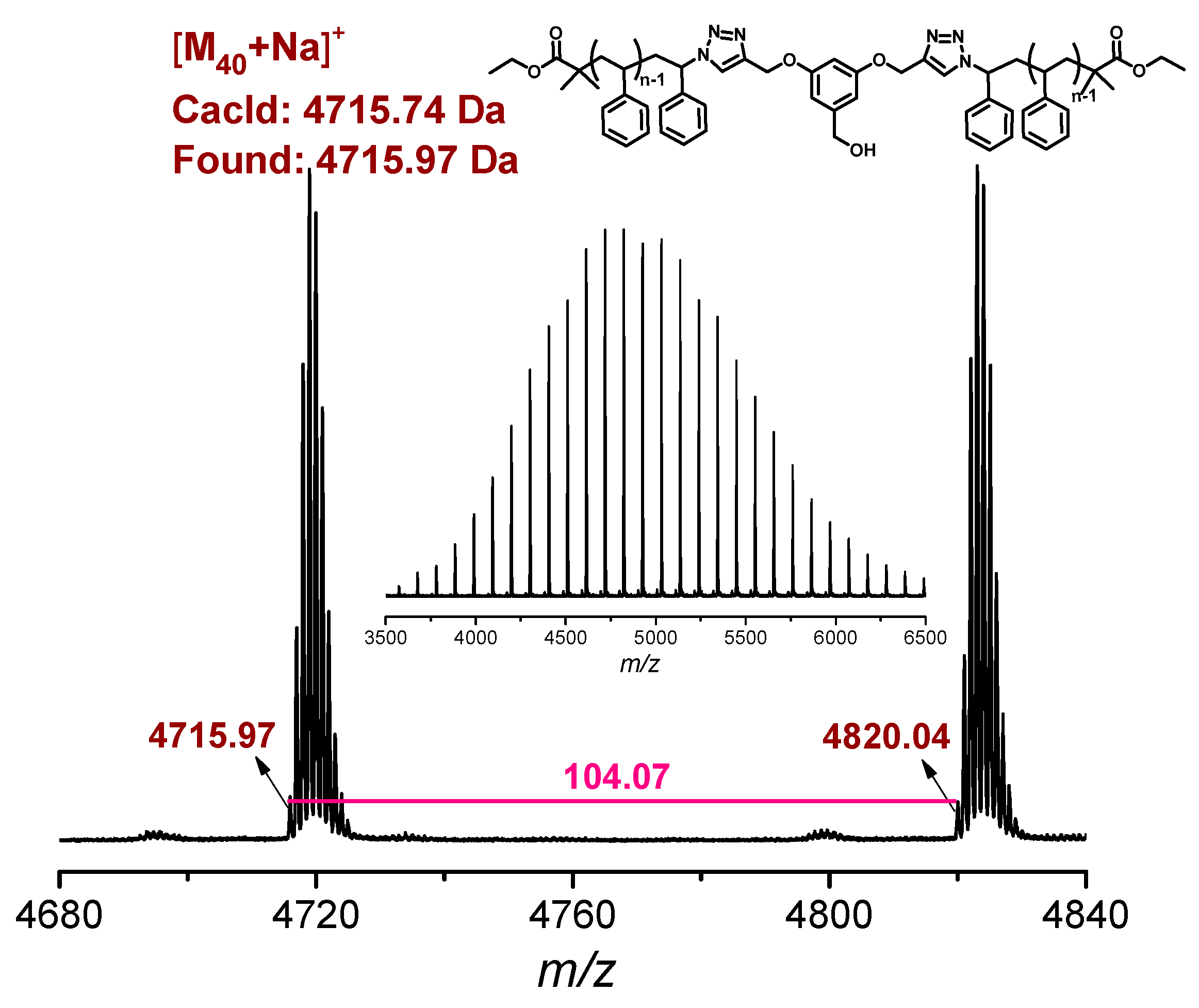
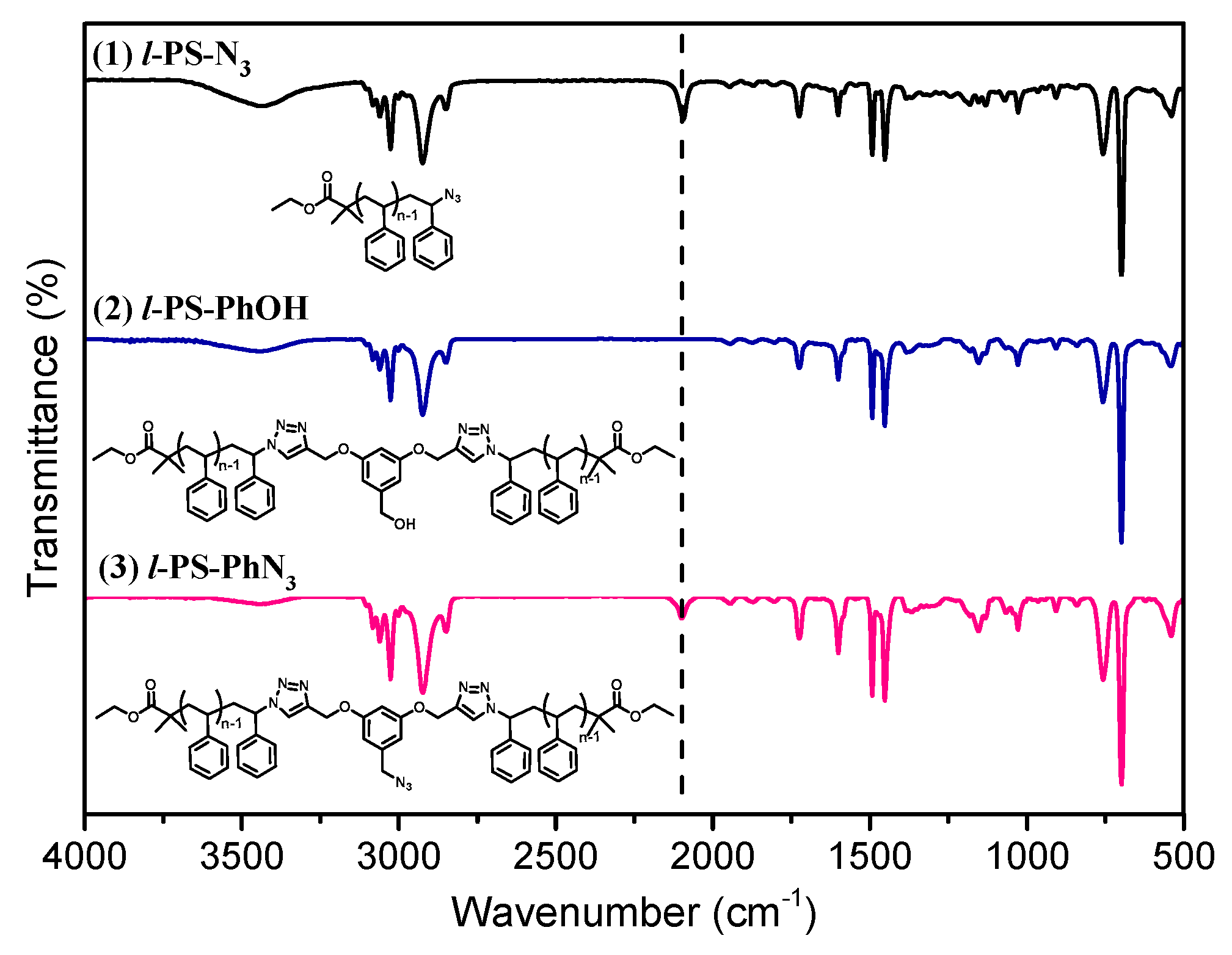
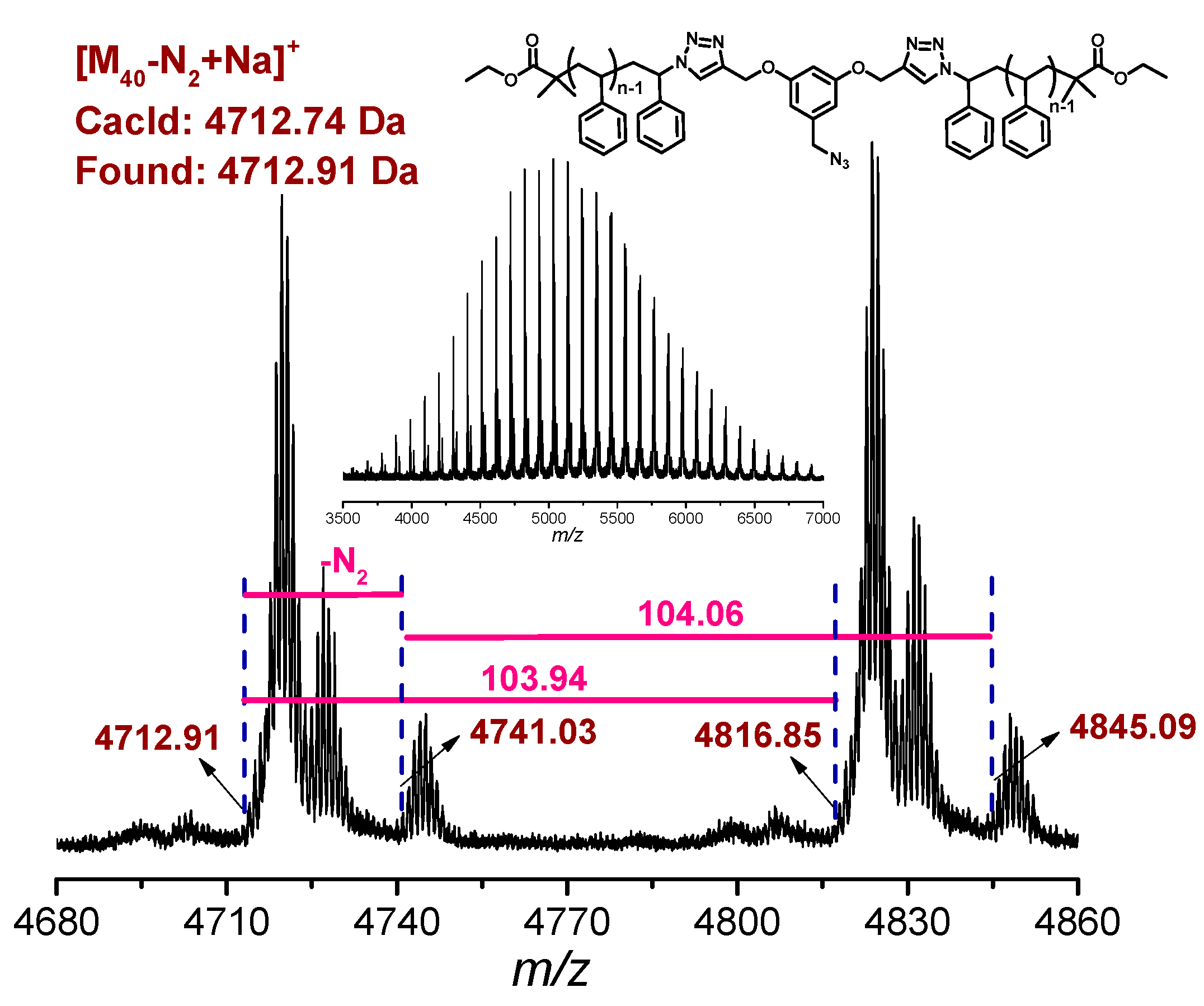
3.1. Synthesis of l-PS-PhOH and l-PS-PhN3
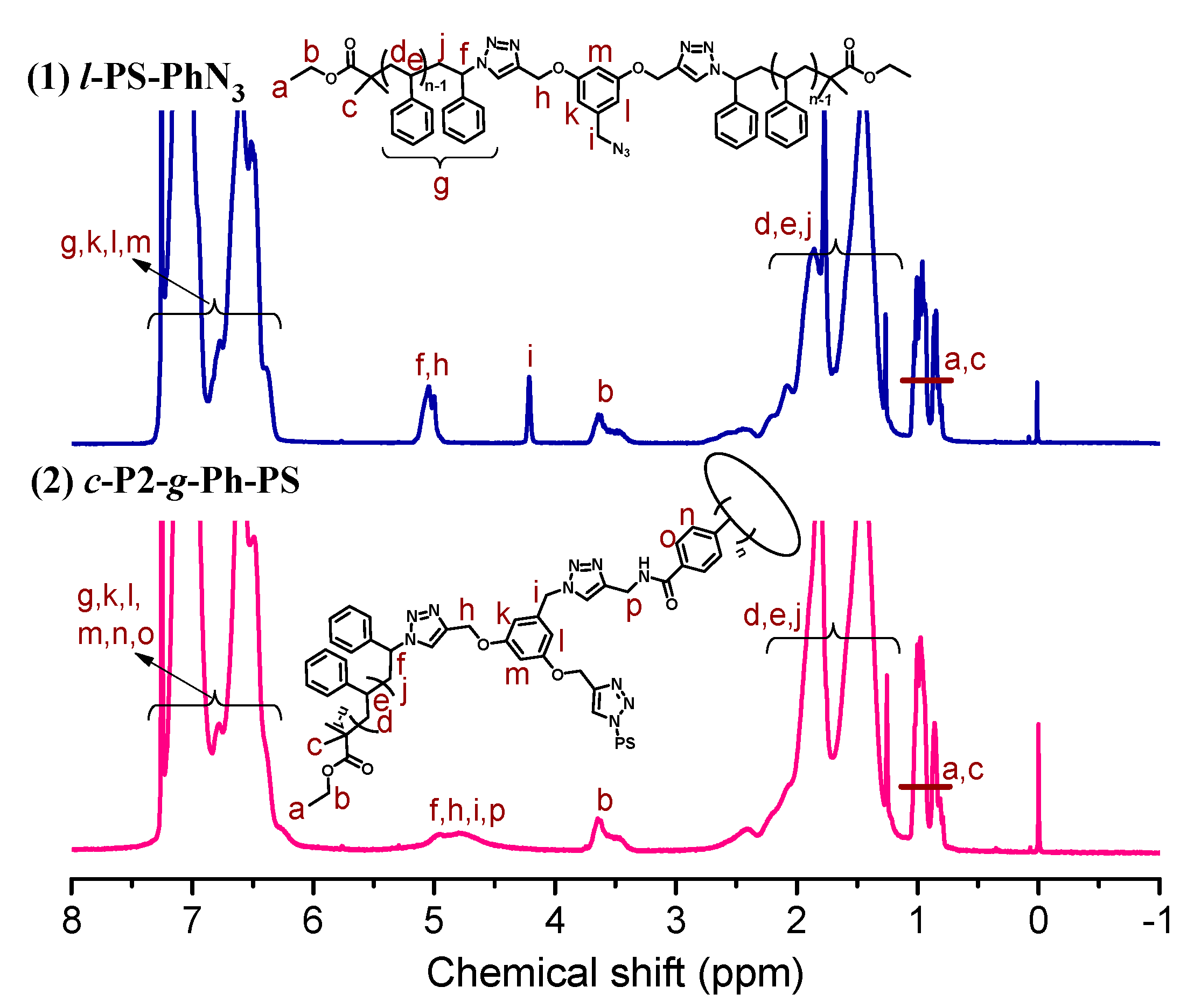
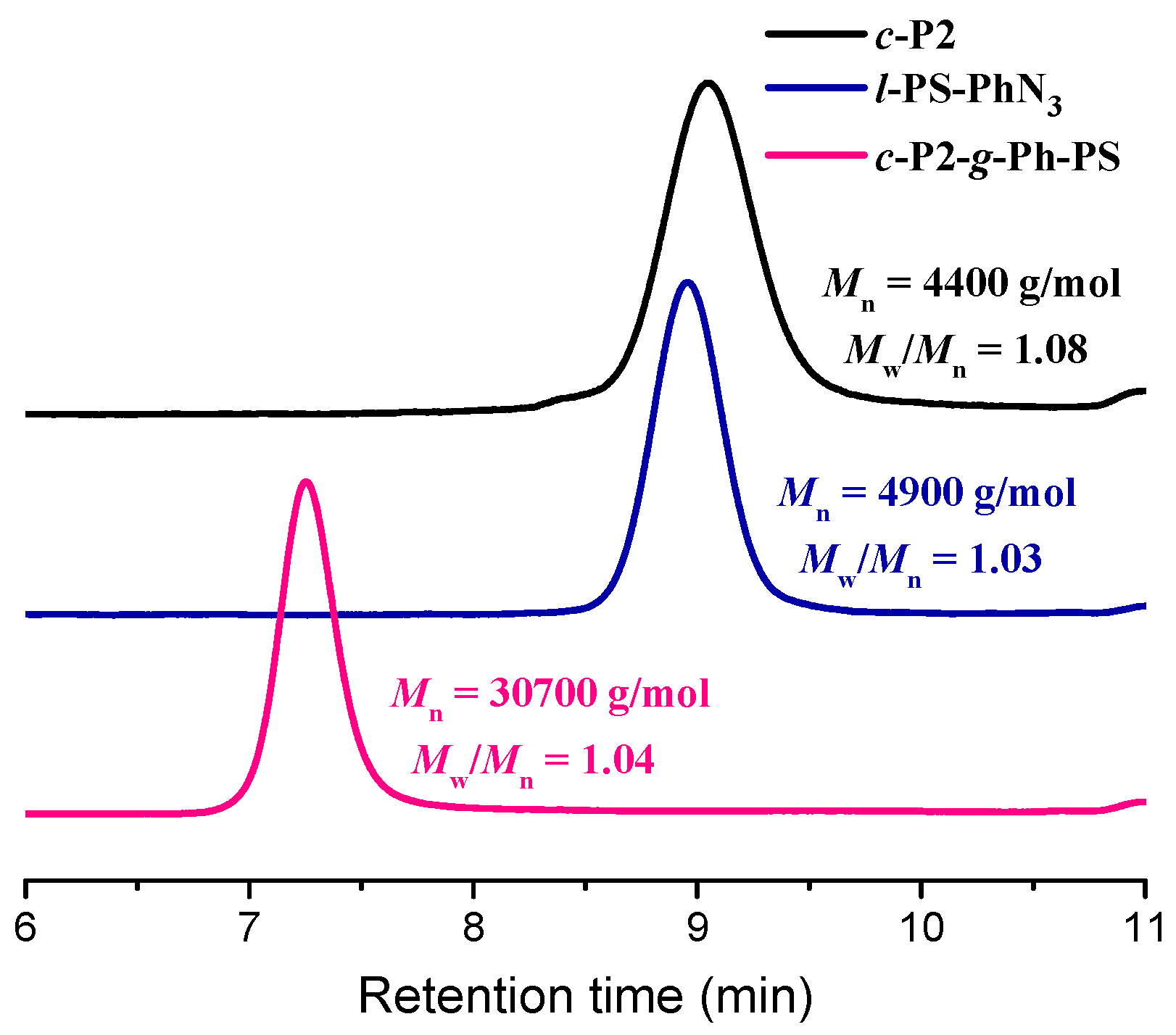
3.2. Synthesis of l-PS-PhN3 and c-P2-g-Ph-PS
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Feng, C.; Li, Y.; Yang, D.; Hu, J.; Zhang, X.; Huang, X. Well-defined graft copolymers: From controlled synthesis to multipurpose applications. Chem. Soc. Rev. 2011, 40, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Johnson, J.A.; Lu, Y.Y.; Burts, A.O.; Xia, Y.; Durrell, A.C.; Tirrell, D.A.; Grubbs, R.H. Drug-loaded, bivalent-bottle-brush polymers by graft-through ROMP. Macromolecules 2010, 43, 10326–10335. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C.D.; Thelakkat, M. Poly-(3-hexylthiophene) bottlebrush copolymers with tailored side-chain lengths and high charge carrier mobilities. J. Mater. Chem. C 2016, 4, 5370–5378. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, Y.; Walther, A.; Bolisetty, S.; Schumacher, M.; Schmalz, H.; Ballauff, M.; Müller, A.H. Water-soluble organo-silica hybrid nanowires. Nat. Mater. 2008, 7, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Rzayev, J. Well-defined organic nanotubes from multicomponent bottlebrush copolymers. J. Am. Chem. Soc. 2009, 131, 6880–6885. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Schmidt, M. Solvent-Induced Length Variation of Cylindrical Brushes. Macromol. Rapid Commun. 2001, 22, 787–791. [Google Scholar] [CrossRef]
- Gu, L.; Shen, Z.; Feng, C.; Li, Y.; Lu, G.; Huang, X.; Wang, G.; Huang, J. Synthesis of PPEGMEA-g-PMAA densely grafted double hydrophilic copolymer and its use as a template for the preparation of size-controlled superparamagnetic Fe3O4/polymer nano-composites. J. Mater. Chem. 2008, 18, 4332–4340. [Google Scholar] [CrossRef]
- Xu, Y.; Bolisetty, S.; Ballauff, M.; Muller, A.H.E. Switching the morphologies of cylindrical polycation brushes by ionic and supramolecular inclusion complexes. J. Am. Chem. Soc. 2009, 131, 1640–1641. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Liu, H.; Gao, H.; Feng, C.; Li, Y.; Huang, X. Construction of semi-fluorinated amphiphilic graft copolymer bearing a poly(2-methyl-1,4-bistrifluorovinyloxybenzene) backbone and poly(ethylene glycol) side chains via the grafting-onto strategy. RSC Adv. 2015, 5, 39668–39676. [Google Scholar] [CrossRef]
- Xu, B.; Gu, G.; Feng, C.; Jiang, X.; Hu, J.; Lu, G.; Zhang, S.; Huang, X. (PAA-g-PS)-co-PPEGMEMA asymmetric polymer brushes: Synthesis, self-assembly, and encapsulating capacity for both hydrophobic and hydrophilic agents. Polym. Chem. 2016, 7, 613–624. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Sun, X.; Hu, J.; Shi, P.; Huang, X. Semifluorinated Synergistic Nonfouling/Fouling-Release Surface. ACS Appl. Mater. Interfaces 2017, 9, 16517–16523. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Huang, X. Polymer brushes: Efficient synthesis and applications. Acc. Chem. Res. 2018, 51, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Lohn, X.J. Thixotropic supramolecular pectin-poly(ethylene glycol) methacrylate (PEGMA) hydrogels. Polymers 2016, 8, 404. [Google Scholar] [CrossRef]
- Wang, Y.; Baily, T.S.; Hong, M.; Chen, E.Y.-X. Stereoregular brush polymers and graft copolymers by chiral zirconocene-mediated coordination polymerization of P3HT macromers. Polymers 2017, 9, 139. [Google Scholar] [CrossRef]
- Shin, E.J.; Jeong, W.; Brown, H.A.; Koo, B.J.; Hedrick, J.L.; Waymouth, R.M. Crystallization of cyclic polymers: Synthesis and crystallization behavior of high molecular weight cyclic poly(ε-caprolactone)s. Macromolecules 2011, 44, 2773–2779. [Google Scholar] [CrossRef]
- Lee, C.-U.; Li, A.; Ghale, K.; Zhang, D. Crystallization and melting behaviors of cyclic and linear polypeptoids with alkyl side chains. Macromolecules 2013, 46, 8213–8223. [Google Scholar] [CrossRef]
- Clarson, S.J.; Semlyen, J.A. Cyclic polysiloxanes: 1. Preparation and characterization of poly(phenylmethylsiloxane). Polymer 1986, 27, 1633–1636. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, D. Cyclic poly(α-peptoid)s and their block copolymers from N-heterocyclic carbene-mediated ring-opening polymerizations of N-substituted N-carboxylanhydrides. J. Am. Chem. Soc. 2009, 131, 18072–18074. [Google Scholar] [CrossRef]
- Culkin, D.A.; Jeong, W.; Csihony, S.; Gomez, E.D.; Balsara, N.P.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic Polymerization of Lactide to Cyclic Poly(Lactide) by Using N-Heterocyclic Carbene Organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2627–2630. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Benitez, D.; Grubbs, R.H. An “endless” route to cyclic polymers. Science 2002, 297, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-P.; Tanaka, F.; Winnik, F.M. Temperature-induced phase transition of well-defined cyclic poly(N-isopropylacrylamide)s in aqueous solution. Macromolecules 2007, 40, 7069–7071. [Google Scholar] [CrossRef]
- Laurent, B.A.; Grayson, S.M. Synthetic approaches for the preparation of cyclic polymers. Chem. Soc. Rev. 2009, 38, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Monteiro, M.J. Cyclic polymers: Methods and strategies. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2085–2097. [Google Scholar] [CrossRef]
- Tezuka, Y. Topological polymer chemistry for designing multicyclic macromolecular architectures. Polym. J. 2012, 44, 1159–1169. [Google Scholar] [CrossRef]
- Hoskins, J.N.; Grayson, S.M. Cyclic polyesters: Synthetic approaches and potential applications. Polym. Chem. 2011, 2, 289–299. [Google Scholar] [CrossRef]
- Josse, T.; De Winter, J.; Gerbaux, P.; Coulembier, O. Cyclic Polymers by Ring-Closure Strategies. Angew. Chem. Int. Ed. 2016, 55, 13944–13958. [Google Scholar] [CrossRef]
- Wei, H.; Chu, D.S.H.; Zhao, J.; Pahang, J.A.; Pun, S.H. Synthesis and evaluation of cyclic cationic polymers for nucleic acid delivery. ACS Macro Lett. 2013, 2, 1047–1050. [Google Scholar] [CrossRef]
- Cortez, M.A.; Godbey, W.T.; Fang, Y.; Payne, M.E.; Cafferty, B.J.; Kosakowska, K.A.; Grayson, S.M. The synthesis of cyclic poly(ethylene imine) and exact linear analogues: An evaluation of gene delivery comparing polymer architectures. J. Am. Chem. Soc. 2015, 137, 6541–6549. [Google Scholar] [CrossRef]
- Wan, X.; Liu, T.; Liu, S. Synthesis of amphiphilic tadpole-shaped linear-cyclic diblock copolymers via ring-opening polymerization directly initiating from cyclic precursors and their application as drug nanocarriers. Biomacromolecules 2011, 12, 1146–1154. [Google Scholar] [CrossRef]
- Chen, B.; Jerger, K.; Fréchet, J.M.J.; Szoka, F.C., Jr. The influence of polymer topology on pharmacokinetics: Differences between cyclic and linear PEGylated poly(acrylic acid) comb polymers. J. Controll. Release 2009, 140, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, C.E.; Tan, N.; Boydston, A.J.; Pun, S.H. ATRP synthesis of sunflower polymers using cyclic multimacroinitiators. ACS Macro Lett. 2015, 4, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.E.; Wei, H.; Tan, N.; Boydston, A.J.; Pun, S.H. Sunflower polymers for folate-mediated drug delivery. Biomacromolecules 2015, 17, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tezuka, Y.; Zhang, Z.; Li, N.; Zhang, W.; Zhu, X. Recent advances in the construction of cyclic grafted polymers and their potential applications. Polym. Chem. 2018, 9, 677–686. [Google Scholar] [CrossRef]
- Xia, Y.; Boydston, A.J.; Grubbs, R.H. Synthesis and direct imaging of ultrahigh molecular weight cyclic brush polymers. Angew. Chem. Int. Ed. 2011, 50, 5882–5885. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Fu, Q.; Huang, J. Synthesis of amphiphilic macrocyclic graft copolymer consisting of a poly(ethylene oxide) ring and multi-polystyrene lateral chains. Macromolecules 2006, 39, 5190–5193. [Google Scholar] [CrossRef]
- Pang, X.; Wang, G.; Jia, Z.; Liu, C.; Huang, J. Preparation of the amphiphilic macro-rings of poly(ethylene oxide) with multi-polystyrene lateral chains and their extraction for dyes. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5824–5837. [Google Scholar] [CrossRef]
- Fan, X.; Wang, G.; Huang, J. Synthesis of macrocyclic molecular brushes with amphiphilic block copolymers as side chains. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1361–1367. [Google Scholar] [CrossRef]
- Zhang, K.; Tew, G.N. Cyclic brush polymers by combining ring-expansion metathesis polymerization and the “grafting from” technique. ACS Macro Lett. 2012, 1, 574–579. [Google Scholar] [CrossRef]
- Yao, W.; Li, Y.; Feng, C.; Lu, G.; Huang, X. Synthesis of a sun-shaped amphiphilic copolymer consisting of a cyclic perfluorocyclobutyl aryl ether-based backbone and lateral PMAA side chains. RSC Adv. 2014, 4, 52105–52116. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, L.; Zhang, W.; Zhang, Z.; Zhu, X. Synthesis of diverse cyclic-brush polymers with cyclic polystyrene as a universal template via a grafting-from approach. Polym. Chem. 2016, 7, 2112–2120. [Google Scholar] [CrossRef]
- Tu, X.; Meng, C.; Liu, Z.; Sun, L.; Zhang, X.; Zhang, M.; Sun, M.; Ma, L.; Liu, M.; Wei, H. Synthesis and phase transition of poly(N-isopropylacrylamide)-based thermo-sensitive cyclic brush bolymer. Polymers 2017, 9, 301. [Google Scholar] [CrossRef]
- Li, H.; Jérôme, R.; Lecomte, P. Amphiphilic sun-shaped polymers by grafting macrocyclic copolyesters with PEO. Macromolecules 2008, 41, 650–654. [Google Scholar] [CrossRef]
- Zhang, K.; Lackey, M.A.; Wu, Y.; Tew, G.N. Universal cyclic polymer templates. J. Am. Chem. Soc. 2011, 133, 6906–6909. [Google Scholar] [CrossRef] [PubMed]
- Lahasky, S.H.; Serem, W.K.; Guo, L.; Garno, J.C.; Zhang, D. Synthesis and characterization of cyclic brush-like polymers by N-heterocyclic carbene-mediated zwitterionic polymerization of N-propargyl N-carboxyanhydride and the grafting-to approach. Macromolecules 2011, 44, 9063–9074. [Google Scholar] [CrossRef]
- Li, A.; Lu, L.; Li, X.; He, L.; Do, C.; Garno, J.C.; Zhang, D. Amidine-mediated zwitterionic ring-opening polymerization of N-alkyl N-carboxyanhydride: Mechanism, kinetics, and architecture elucidation. Macromolecules 2016, 49, 1163–1171. [Google Scholar] [CrossRef]
- Du, P.; Li, A.; Li, X.; Zhang, Y.; Do, C.; He, L.; Rick, S.W.; John, V.T.; Kumar, R.; Zhang, D. Aggregation of cyclic polypeptoids bearing zwitterionic end-groups with attractive dipole–dipole and solvophobic interactions: A study by small-angle neutron scattering and molecular dynamics simulation. Phys. Chem. Chem. Phys. 2017, 19, 14388–14400. [Google Scholar]
- Cai, T.; Yang, W.J.; Neoh, K.-G.; Kang, E.-T. Preparation of jellyfish-shaped amphiphilic block-graft copolymers consisting of a poly(ε-caprolactone)-block-poly(pentafluorostyrene) ring and poly (ethylene glycol) lateral brushes. Polym. Chem. 2012, 3, 1061–1068. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, L.; Zhang, X.; Zhang, K.; Wang, Y. Emulsion templating cyclic polymers as microscopic particles with tunable porous morphology. Langmuir 2016, 32, 1460–1467. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, X.; Wang, J.; Zhang, Z.; Zhang, W.; Zhu, X. Synthesis of a cyclic-brush polymer with a high grafting density using active ester chemistry via the “grafting onto” approach. Polym. Chem. 2018, 9, 5155–5163. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, L.; Zhang, W.; Zhou, N.; Zhang, Z.; Zhu, X. The Suzuki coupling reaction as a post-polymerization modification: A promising protocol for construction of cyclic-brush and more complex polymers. Polym. Chem. 2015, 6, 4669–4677. [Google Scholar] [CrossRef]
- Zhang, K.; Zha, Y.; Peng, B.; Chen, Y.; Tew, G.N. Metallo-supramolecular cyclic polymers. J. Am. Chem. Soc. 2013, 135, 15994–15997. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Theato, P. Activated ester containing polymers: Opportunities and challenges for the design of functional macromolecules. Chem. Rev. 2016, 116, 1434–1495. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Yin, L.; Zhang, S.; Zhang, Z.; Zhang, W.; Zhu, X. Design and Synthesis of a Cyclic Double-Grafted Polymer Using Active Ester Chemistry and Click Chemistry via A “Grafting onto” Method. Polymers 2019, 11, 240. https://doi.org/10.3390/polym11020240
Liu M, Yin L, Zhang S, Zhang Z, Zhang W, Zhu X. Design and Synthesis of a Cyclic Double-Grafted Polymer Using Active Ester Chemistry and Click Chemistry via A “Grafting onto” Method. Polymers. 2019; 11(2):240. https://doi.org/10.3390/polym11020240
Chicago/Turabian StyleLiu, Meng, Lu Yin, Shuangshuang Zhang, Zhengbiao Zhang, Wei Zhang, and Xiulin Zhu. 2019. "Design and Synthesis of a Cyclic Double-Grafted Polymer Using Active Ester Chemistry and Click Chemistry via A “Grafting onto” Method" Polymers 11, no. 2: 240. https://doi.org/10.3390/polym11020240
APA StyleLiu, M., Yin, L., Zhang, S., Zhang, Z., Zhang, W., & Zhu, X. (2019). Design and Synthesis of a Cyclic Double-Grafted Polymer Using Active Ester Chemistry and Click Chemistry via A “Grafting onto” Method. Polymers, 11(2), 240. https://doi.org/10.3390/polym11020240