Toxicological Assessment of Cross-Linked Beads of Chitosan-Alginate and Aspergillus australensis Biomass, with Efficiency as Biosorbent for Copper Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitosan Gel Preparation
2.2. Alginate Gel Preparation
2.3. Production of Aspergillus australensis Biomass
2.4. Synthesis of Cross-Linked Composites Beads
2.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
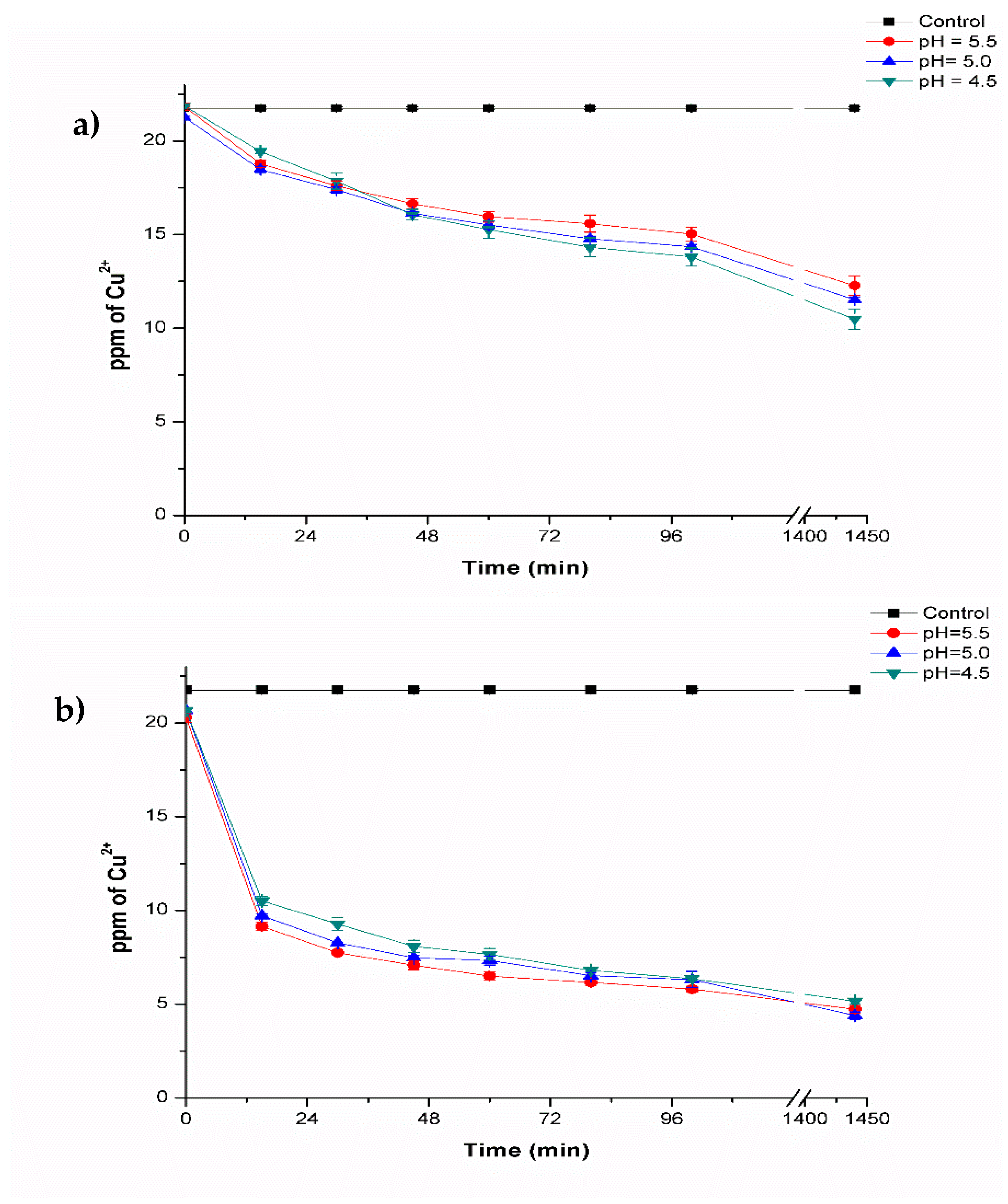
2.6. Biosorption of Copper Using Composites Beads
2.6.1. Biosorption of Copper Using Fresh Composites Beads
2.6.2. Biosorption of Copper Using Lyophilized Composites Beads
2.7. Quantification of Copper and Biosorption Capacity of Composites Beads
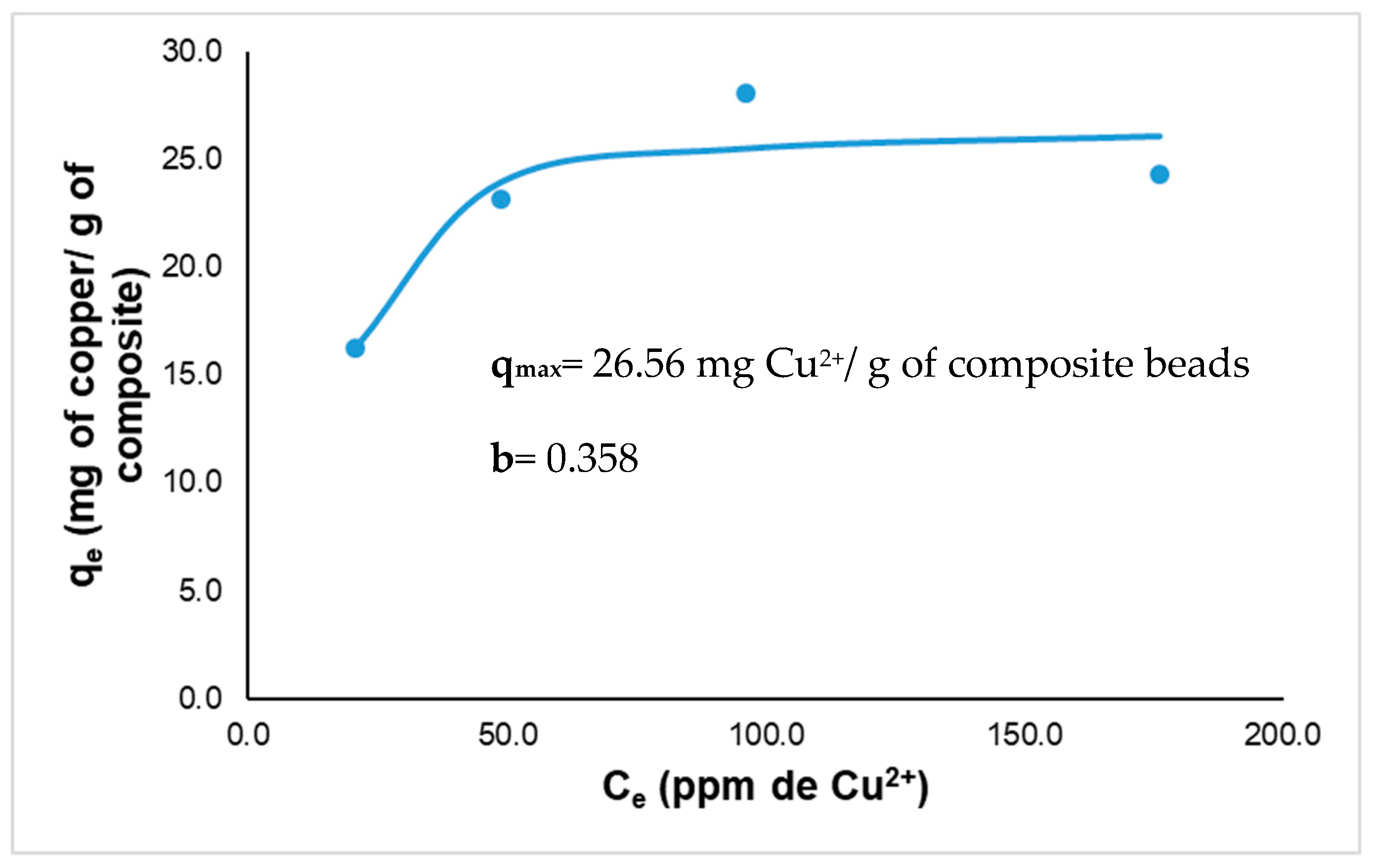
2.8. Langmuir Isotherm Studies
2.9. Toxicological Assays
2.9.1. Acute Toxicity on Brine Shrimp Artemia salina Nauplii
2.9.2. Acute phytotoxicity on lettuce (Lactuca sativa) and chili pepper (Capsicum annuum) seeds
3. Results and Discussion
3.1. Synthesis of Composites Beads
3.2. FT-IR Analysis of the Composite Beads
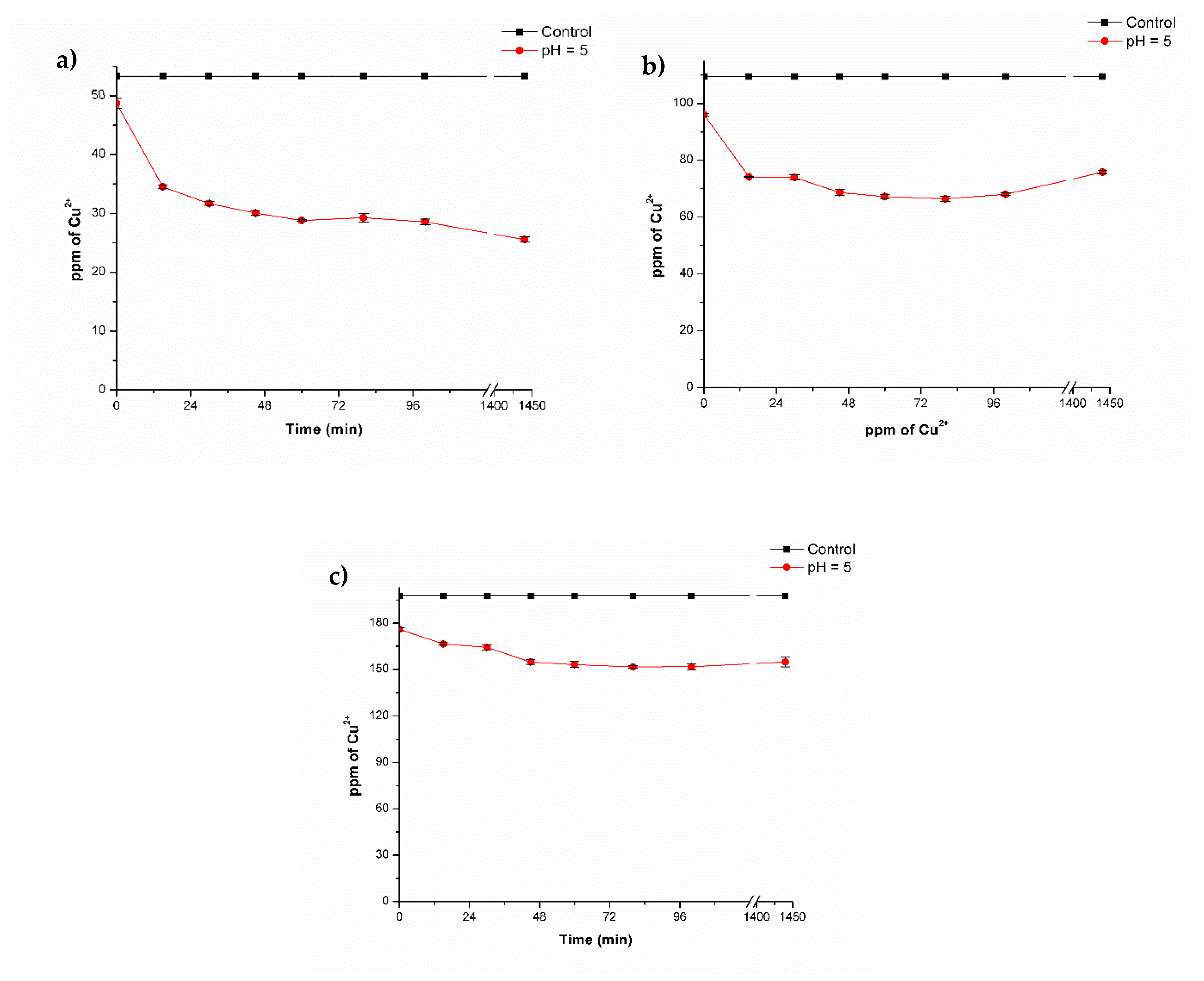
3.3. Biosorption of Copper by Biocomposites Beads
Effect of Copper Concentration
3.4. Toxicological Assays
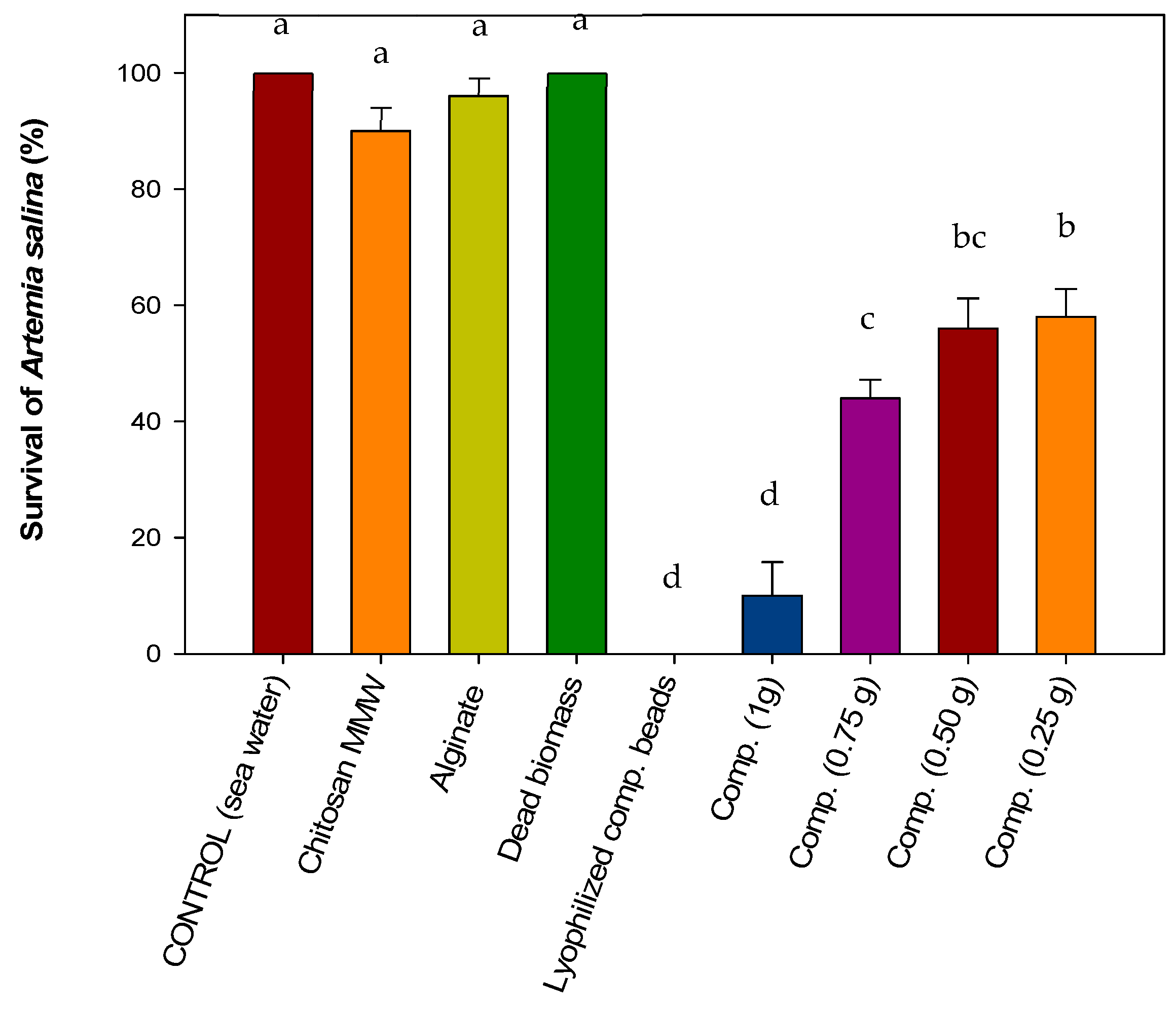
3.4.1. Acute Toxicity on Brine Shrimp Artemia salina
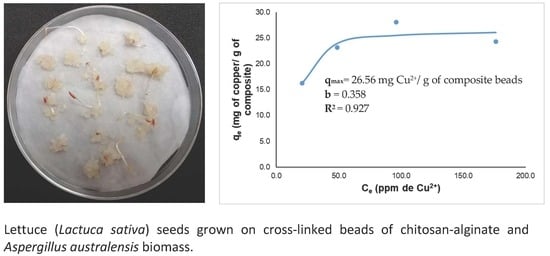
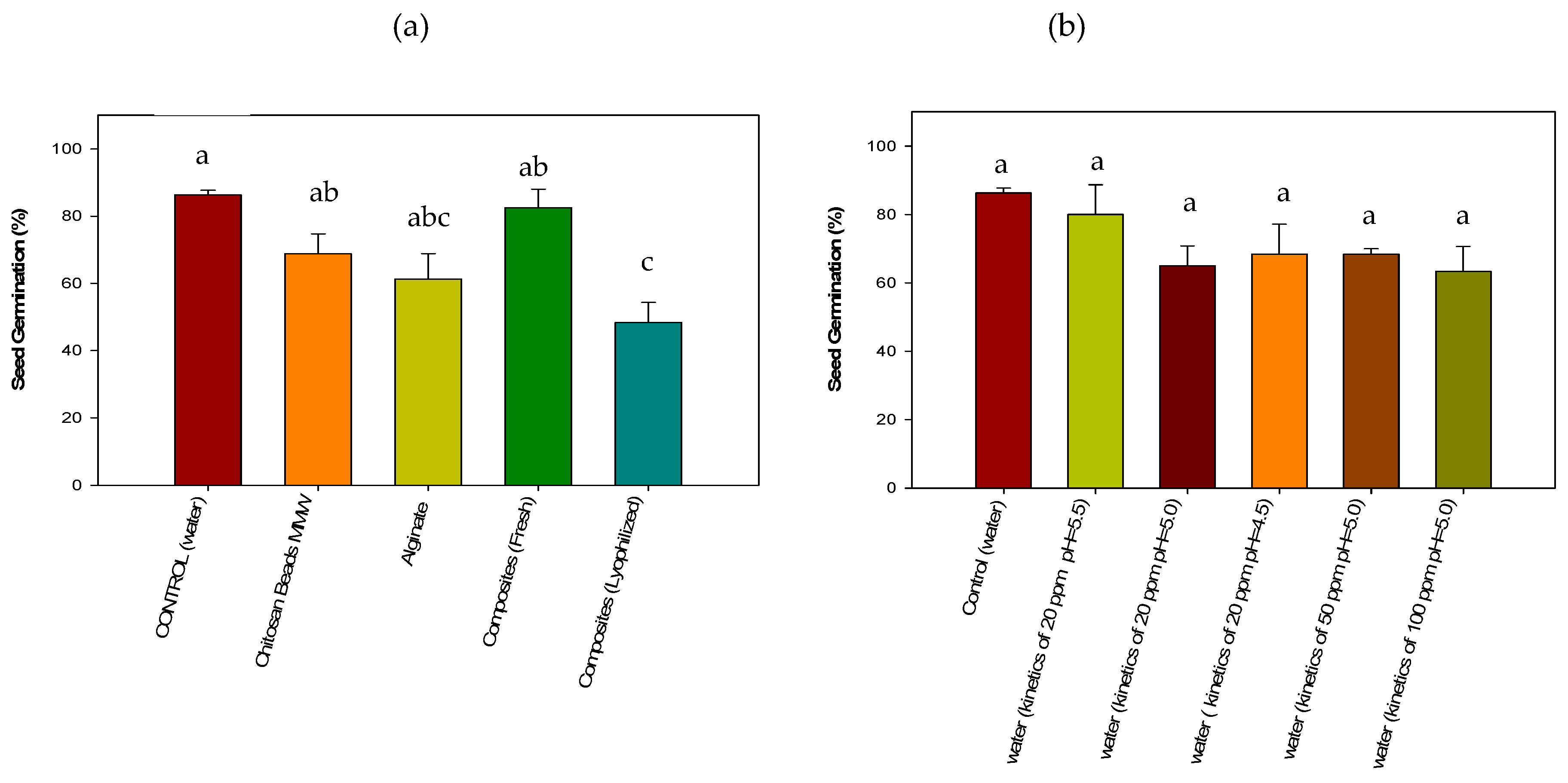
3.4.2. Acute Phytotoxicity on Lactuca sativa Seeds
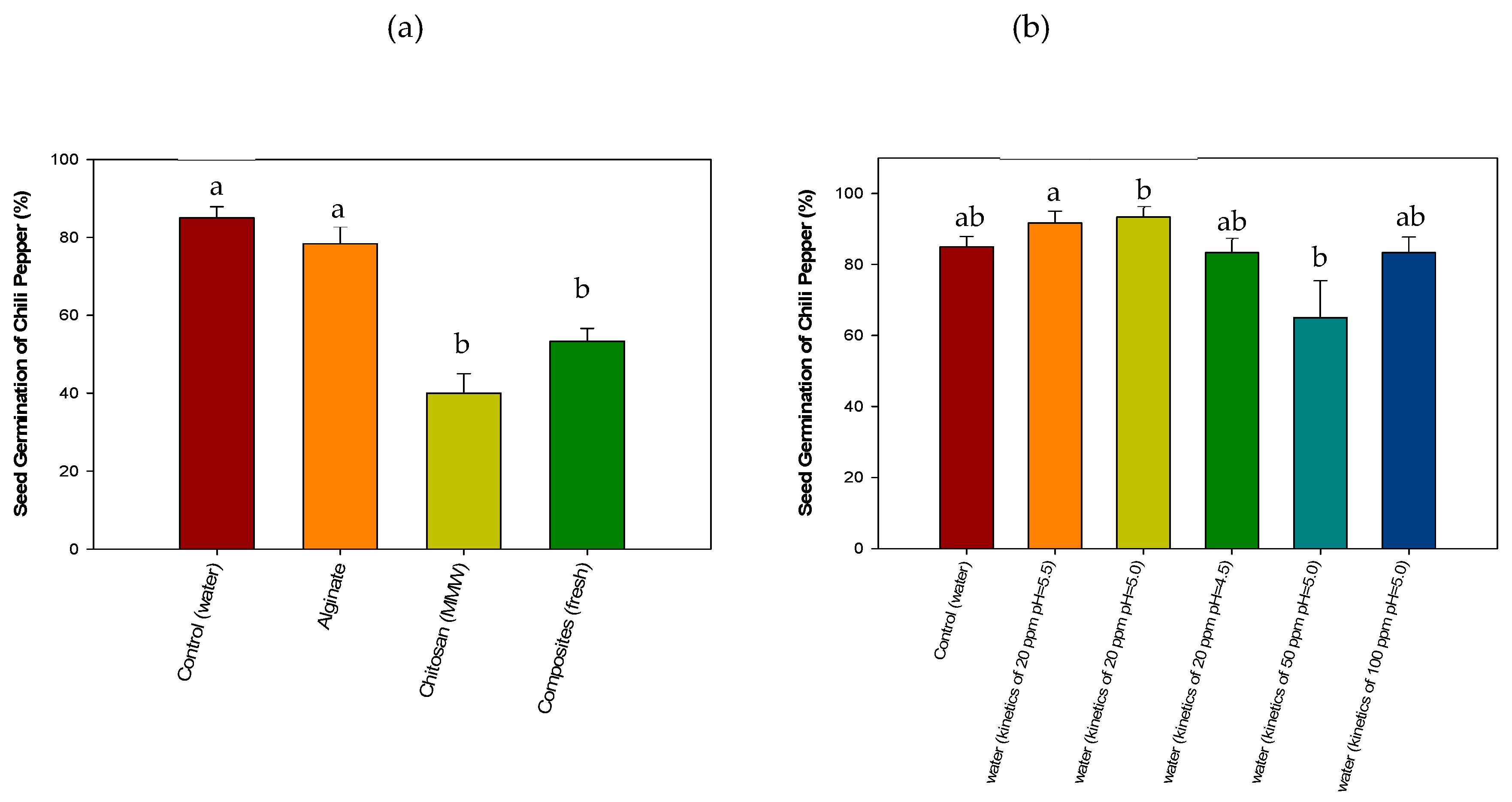
3.4.3. Acute Phytotoxicity on Chili Pepper (Capsicum annuum) Seeds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sen Gupta, S.; Bhattacharyya, K.G. Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J. Environ. Manag. 2008, 87, 46–58. [Google Scholar] [CrossRef]
- Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Luckey, T.D.; Venugopal, B. Introduction to heavy metal toxicity in mammals. In Physiologic and Chemical Basis for Metal Toxicity; Springer: Boston, MA, USA, 1977; pp. 1–37. [Google Scholar]
- Ngah, W.S.W.; Fatinathan, S. Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2010, 91, 958–969. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Arulkumar, M.; Palvannan, T. Utilization of agro-industrial waste Jatropha curcas pods as an activated carbon for the adsorption of reactive dye remazol brilliant blue R (RBBR). J. Clean. Prod. 2012, 22, 67–75. [Google Scholar] [CrossRef]
- Moe, S.T.; Skjaak-Braek, G.; Elgsaeter, A.; Smidsroed, O. Swelling of covalently crosslinked alginate gels: Influence of ionic solutes and nonpolar solvents. Macromolecules 1993, 26, 3589–3597. [Google Scholar] [CrossRef]
- Chauhan, S. Modification of chitosan for sorption of metal ions. J. Chem. Pharm. Res. 2015, 7, 49–55. [Google Scholar]
- Alikutty, P.; Abdul Mujeeb, V.M.; Zubair, M.A.; Muraleedharan, K.; Mujeeb Rahman, P. Studies on the sorption capacity for Pb(II) and Hg(II) of citralidene chitosan. Polym. Bull. 2014, 71, 1919–1932. [Google Scholar] [CrossRef]
- Laus, R.; de Fávere, V.T. Competitive adsorption of Cu(II) and Cd(II) ions by chitosan crosslinked with epichlorohydrin–triphosphate. Bioresour. Technol. 2011, 102, 8769–8776. [Google Scholar] [CrossRef]
- Mladenovska, K.; Cruaud, O.; Richomme, P.; Belamie, E.; Raicki, R.S.; Venier-Julienne, M.-C.; Popovski, E.; Benoit, J.P.; Goracinova, K. 5-ASA loaded chitosan–Ca–alginate microparticles: Preparation and physicochemical characterization. Int. J. Pharm. 2007, 345, 59–69. [Google Scholar] [CrossRef]
- Escudero, C.; Fiol, N.; Villaescusa, I.; Bollinger, J.-C. Arsenic removal by a waste metal (hydr)oxide entrapped into calcium alginate beads. J. Hazard. Mater. 2009, 164, 533–541. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crespo-Alonso, M.; Biesuz, R.; Alberti, G.; Pilo, M.I.; Spano, N.; Sanna, G. Sorption of chrysoidine by row cork and cork entrapped in calcium alginate beads. Arab. J. Chem. 2014, 7, 133–138. [Google Scholar] [CrossRef]
- Samuel, J.; Pulimi, M.; Paul, M.L.; Maurya, A.; Chandrasekaran, N.; Mukherjee, A. Batch and continuous flow studies of adsorptive removal of Cr(VI) by adapted bacterial consortia immobilized in alginate beads. Bioresour. Technol. 2013, 128, 423–430. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Bennett, R.M.; Cordero, P.R.F.; Bautista, G.S.; Dedeles, G.R. Reduction of hexavalent chromium using fungi and bacteria isolated from contaminated soil and water samples. Chem. Ecol. 2013, 29, 320–328. [Google Scholar] [CrossRef]
- Baik, W.; Bae, J.; Cho, K.; Hartmeier, W. Biosorption of heavy metals using whole mold mycelia and parts thereof. Bioresour. Technol. 2002, 81, 167–170. [Google Scholar] [CrossRef]
- Wan Ngah, W.; Endud, C.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Šillerová, H.; Komárek, M.; Liu, C.; Poch, J.; Villaescusa, I. Biosorbent encapsulation in calcium alginate: Effects of process variables on Cr(VI) removal from solutions. Int. J. Biol. Macromol. 2015, 80, 260–270. [Google Scholar] [CrossRef]
- Gomes, P.F.; Lennartsson, P.R.; Persson, N.-K.; Taherzadeh, M.J. Heavy metal biosorption by Rhizopus Sp. biomass immobilized on textiles. Water Air Soil Pollut. 2014, 225, 1834. [Google Scholar] [CrossRef]
- Iram, S.; Shabbir, R.; Zafar, H.; Javaid, M. Biosorption and Bioaccumulation of copper and lead by heavy metal-resistant fungal isolates. Arab. J. Sci. Eng. 2015, 40, 1867–1873. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Said-Fernández, S. A modified microplate cytotoxicity assay with brine shrimp larve (Artemia salina). Pharmacologyonline 2006, 3, 633–638. [Google Scholar]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Hakimi Ibrahim, M.; Zuhairi Abdullah, A.; Salamatinia, B.; Gholami, Z. Preparation of chitosan beads for the adsorption of reactive blue 4 from aqueous solutions. Iran. J. Energy Environ. 2016, 7, 124–128. [Google Scholar]
- Guibal, E.; Milot, C.; Tobin, J.M. Metal-anion sorption by chitosan beads: Equilibrium and kinetic studies. Ind. Eng. Chem. Res. 1998, 37, 1454–1463. [Google Scholar] [CrossRef]
- Adina, C.; Florinela, F.; Abdelmoumen, T.; Carmen, S. Application of FTIR spectroscopy for a rapid determination of some hydrolytic enzymes activity on Sea buckthorn substrate. Roman. Biotechnol. Lett. 2010, 15, 5738–5744. [Google Scholar]
- Lichawska, M.E.; Bodek, K.H.; Jezierska, J.; Kufelnicki, A. Coordinative interaction of microcrystalline chitosan with oxovanadium (IV) ions in aqueous solution. Chem. Cent. J. 2014, 8, 50. [Google Scholar] [CrossRef]
- Wanule, D.; Balkhande, J.V.; Ratnakar, P.U.; Kulkarni, A.N.; Bhowate, C.S. Extraction and FTIR analysis of chitosan from american cockroach, Periplaneta americana. Int. J. Eng. Sci. Innov. Technol. 2008, 3, 299–304. [Google Scholar]
- Dai, Y.-N.; Li, P.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. A novel pH sensitive N-succinyl chitosan/alginate hydrogel bead for nifedipine delivery. Biopharm. Drug Dispos. 2008, 29, 173–184. [Google Scholar] [CrossRef]
- Loukidou, M.X.; Matis, K.A.; Zouboulis, A.I.; Liakopoulou-Kyriakidou, M. Removal of As(V) from wastewaters by chemically modified fungal biomass. Water Res. 2003, 37, 4544–4552. [Google Scholar] [CrossRef]
- Morteza, H.K.; Mohammad, K.; Mobina, K.; Sahar, K. Reinforcement of chitosan nanoparticles obtained by an ionic cross-linking process. Iran. Polym. J. 2011, 20, 445–456. [Google Scholar]
- Goycoolea, F.M.; Lollo, G.; Remuñán-López, C.; Quaglia, F.; Alonso, M.J. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Shi, S.; Zheng, X.; Guo, G.; Wei, Y.; Qian, Z. Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol. 2008, 8, 89. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef]
- Bai, R.S.; Abraham, T.E. Studies on enhancement of Cr(VI) biosorption by chemically modified biomass of Rhizopus nigricans. Water Res. 2002, 36, 1224–1236. [Google Scholar] [CrossRef]
- Yu, K.; Ho, J.; McCandlish, E.; Buckley, B.; Patel, R.; Li, Z.; Shapley, N.C. Copper ion adsorption by chitosan nanoparticles and alginate microparticles for water purification applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 31–41. [Google Scholar] [CrossRef]
- Sánchez-Duarte, R.G.; del Rosario Martínez-Macías, M.; Correa-Murrieta, M.A.; Saldívar-Cabrales, J.; Sanchez-Machado, D.I.; López-Cervantes, J. Síntesis de hidrogeles de quitosano a partir de cáscara de camarón para ensayos de adsorción de cobre. Rev. Int. Contam. Ambient. 2017, 33, 93–98. [Google Scholar]
- Giraldo, J.D.; Rivas, B.L.; Elgueta, E.; Mancisidor, A. Metal ion sorption by chitosan-tripolyphosphate beads. J. Appl. Polym. Sci. 2017, 134, 45511. [Google Scholar] [CrossRef]
- Deng, X.; Wang, P. Isolation of marine bacteria highly resistant to mercury and their bioaccumulation process. Bioresour. Technol. 2012, 121, 342–347. [Google Scholar] [CrossRef]
- Rosaria Panuccio, M.; Crea, F.; Sorgonà, A.; Cacco, G. Adsorption of nutrients and cadmium by different minerals: Experimental studies and modelling. J. Environ. Manag. 2008, 88, 890–898. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Ibarra-Buscano, S.; Kan, C.-C.; Futalan, C.M.; Dalida, M.L.P.; Wan, M.-W. Removal of copper, nickel, lead, and zinc using chitosan-coated montmorillonite beads in single- and multi-metal system. Desalin. Water Treat. 2016, 57, 9799–9812. [Google Scholar] [CrossRef]
- Mokhter, M.; Magnenet, C.; Lakard, S.; Euvrard, M.; Aden, M.; Clément, S.; Mehdi, A.; Lakard, B.; Mokhter, M.A.; Magnenet, C. Use of modified colloids and membranes to remove metal ions from contaminated solutions. Colloids Interfaces 2018, 2, 19. [Google Scholar] [CrossRef]
- Shim, J.-W.; Park, S.-J.; Ryu, S.-K. Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers. Carbon N. Y. 2001, 39, 1635–1642. [Google Scholar] [CrossRef]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Lavtizar, V.; Kimura, D.; Asaoka, S.; Okamura, H. The influence of seawater properties on toxicity of copper pyrithione and its degradation product to brine shrimp Artemia salina. Ecotoxicol. Environ. Saf. 2018, 147, 132–138. [Google Scholar] [CrossRef]
- FAO. Manual Para el Cultivo y uso de Artemia en Acuicultura. Available online: http://www.fao.org/docrep/field/003/AB474S/AB474S00.htm#TOC (accessed on 9 December 2018).
- Toi, H.T.; Boeckx, P.; Sorgeloos, P.; Bossier, P.; Van Stappen, G. Bacteria contribute to Artemia nutrition in algae-limited conditions: A laboratory study. Aquaculture 2013, 388–391, 1–7. [Google Scholar] [CrossRef]
- Fernández, R.G. Artemia bioencapsulation I. Effect of particle sizes on the filtering behavior of Artemia franciscana. J. Crustac. Biol. 2001, 21, 435–442. [Google Scholar] [CrossRef]
- Foteinis, S.; Chatzisymeon, E. Life cycle assessment of organic versus conventional agriculture. A case study of lettuce cultivation in Greece. J. Clean. Prod. 2016, 112, 2462–2471. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, Y. Effect of various salt–alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ. Exp. Bot. 2005, 54, 8–21. [Google Scholar] [CrossRef]
- Draget, K.I.; Østgaard, K.; Smidsrød, O. Alginate-based solid media for plant tissue culture. Appl. Microbiol. Biotechnol. 1989, 31, 79–83. [Google Scholar] [CrossRef]
- Smidsrød, O.; SkjåkBræk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Draget, K.I.; Myhre, S.; SkjåkBræk, G.; Østgaard, K. Regeneration, cultivation and differentiation of plant protoplasts immobilized in Ca-alginate beads. J. Plant Physiol. 1988, 132, 552–556. [Google Scholar] [CrossRef]
- Inoue, Y.; Yamaoka, K.; Kimura, K.; Sawai, K.; Arai, T. Effects of low pH on the induction of root hair formation in young lettuce (Lactuca sativa L. cv. grand rapids) seedlings. J. Plant Res. 2000, 113, 39–44. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Alaoui-Sossé, B.; Genet, P.; Vinit-Dunand, F.; Toussaint, M.-L.; Epron, D.; Badot, P.-M. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004, 166, 1213–1218. [Google Scholar] [CrossRef]
- Romic, D.; Romic, M.; Borosic, J.; Poljak, M. Mulching decreases nitrate leaching in bell pepper (Capsicum annuum L.) cultivation. Agric. Water Manag. 2003, 60, 87–97. [Google Scholar] [CrossRef]


| pH | Residual Concentration (ppm of Cu2+) | Removal Efficiency (%) | |
|---|---|---|---|
| Control | 5.5 | 21.765 ± 0.079 a | - |
| Fresh Composite | |||
| F1 | 5.5 | 12.26 ± 0.51 b | 43.62 ± 2.36 |
| F2 | 5.0 | 11.52 ± 0.15 bc | 47.04 ± 0.69 |
| F3 | 4.5 | 10.48 ± 0.54 c | 51.84 ± 2.49 |
| Lyophilized Composite | |||
| L1 | 5.5 | 4.72 ± 0.20 de | 78.29 ± 0.94 |
| L2 | 5.0 | 4.39 ± 0.19 e | 79.80 ± 0.87 |
| L3 | 4.5 | 5.15 ± 0.15 d | 76.29 ± 0.71 |
| Initial Concentration (Co) | Equilibrium Concentration (Ec) | Removal Efficiency (%) | q (mg of Cu2+/g of beads) |
|---|---|---|---|
| 20.7 ± 0.08 | 4.39 ± 0.19 | 79.80 ± 0.87 | 16.2 |
| 48.7 ± 0.82 | 25.6 ± 0.40 | 52.07 ± 0.76 | 23.9 |
| 96.1 ± 0.46 | 66.39 ± 0.87 | 39.34 ± 0.80 | 25.5 |
| 176.0 ± 0.91 | 151.61 ± 0.74 | 23.22 ± 0.37 | 26.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Cortés, A.G.; Almendariz-Tapia, F.J.; Gómez-Álvarez, A.; Burgos-Hernández, A.; Luque-Alcaraz, A.G.; Rodríguez-Félix, F.; Quevedo-López, M.Á.; Plascencia-Jatomea, M. Toxicological Assessment of Cross-Linked Beads of Chitosan-Alginate and Aspergillus australensis Biomass, with Efficiency as Biosorbent for Copper Removal. Polymers 2019, 11, 222. https://doi.org/10.3390/polym11020222
Contreras-Cortés AG, Almendariz-Tapia FJ, Gómez-Álvarez A, Burgos-Hernández A, Luque-Alcaraz AG, Rodríguez-Félix F, Quevedo-López MÁ, Plascencia-Jatomea M. Toxicological Assessment of Cross-Linked Beads of Chitosan-Alginate and Aspergillus australensis Biomass, with Efficiency as Biosorbent for Copper Removal. Polymers. 2019; 11(2):222. https://doi.org/10.3390/polym11020222
Chicago/Turabian StyleContreras-Cortés, Ana Gabriela, Francisco Javier Almendariz-Tapia, Agustín Gómez-Álvarez, Armando Burgos-Hernández, Ana Guadalupe Luque-Alcaraz, Francisco Rodríguez-Félix, Manuel Ángel Quevedo-López, and Maribel Plascencia-Jatomea. 2019. "Toxicological Assessment of Cross-Linked Beads of Chitosan-Alginate and Aspergillus australensis Biomass, with Efficiency as Biosorbent for Copper Removal" Polymers 11, no. 2: 222. https://doi.org/10.3390/polym11020222
APA StyleContreras-Cortés, A. G., Almendariz-Tapia, F. J., Gómez-Álvarez, A., Burgos-Hernández, A., Luque-Alcaraz, A. G., Rodríguez-Félix, F., Quevedo-López, M. Á., & Plascencia-Jatomea, M. (2019). Toxicological Assessment of Cross-Linked Beads of Chitosan-Alginate and Aspergillus australensis Biomass, with Efficiency as Biosorbent for Copper Removal. Polymers, 11(2), 222. https://doi.org/10.3390/polym11020222