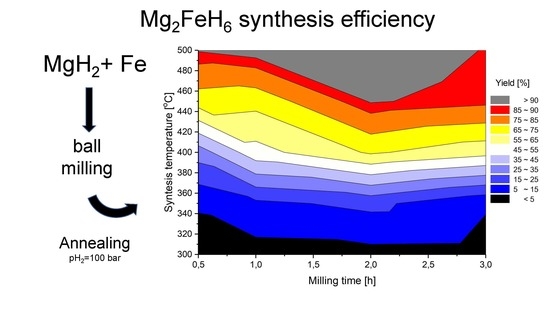
Mg2FeH6 Synthesis Efficiency Map
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
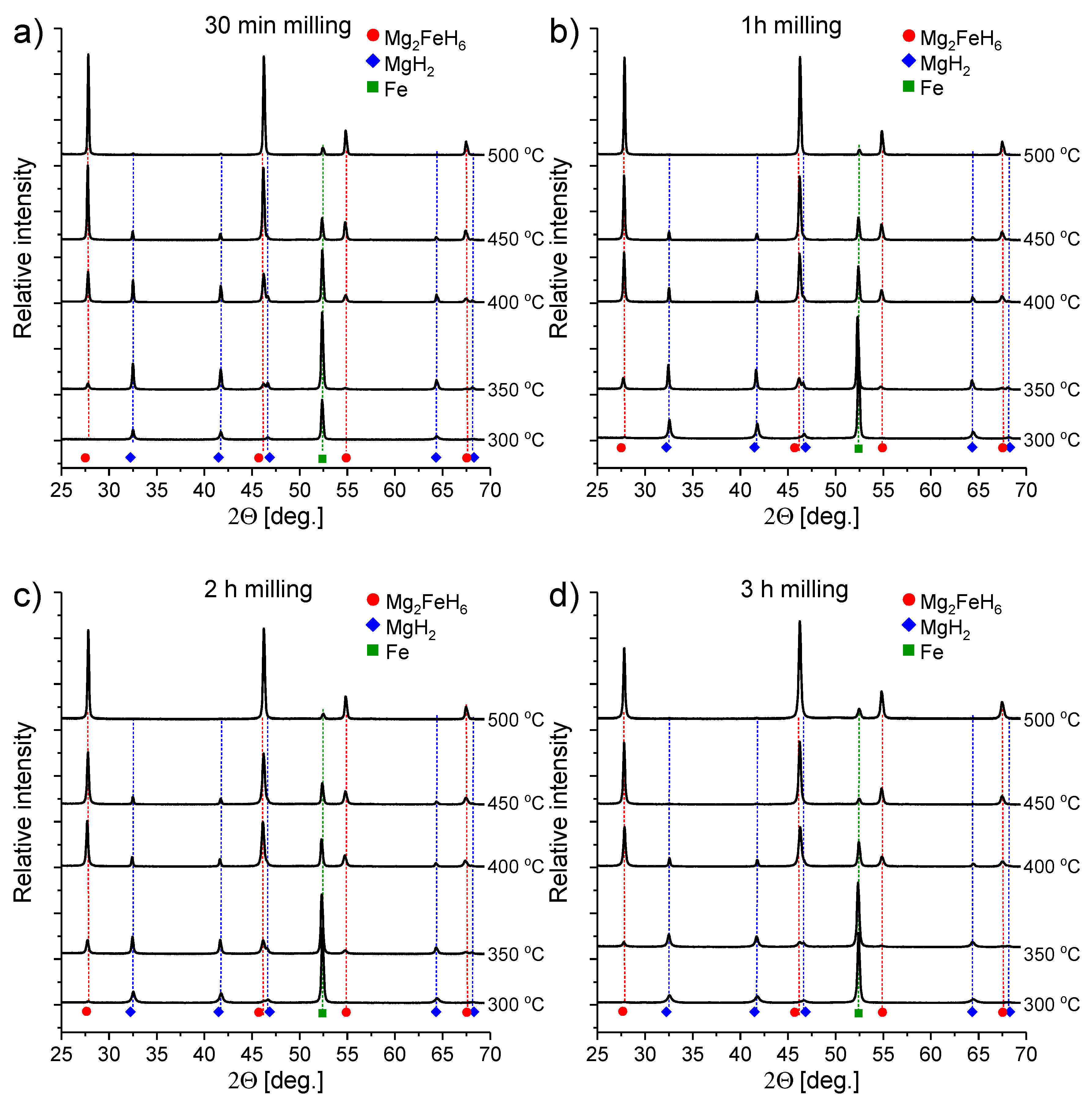
3.1. Phase Composition
3.2. Microstructure
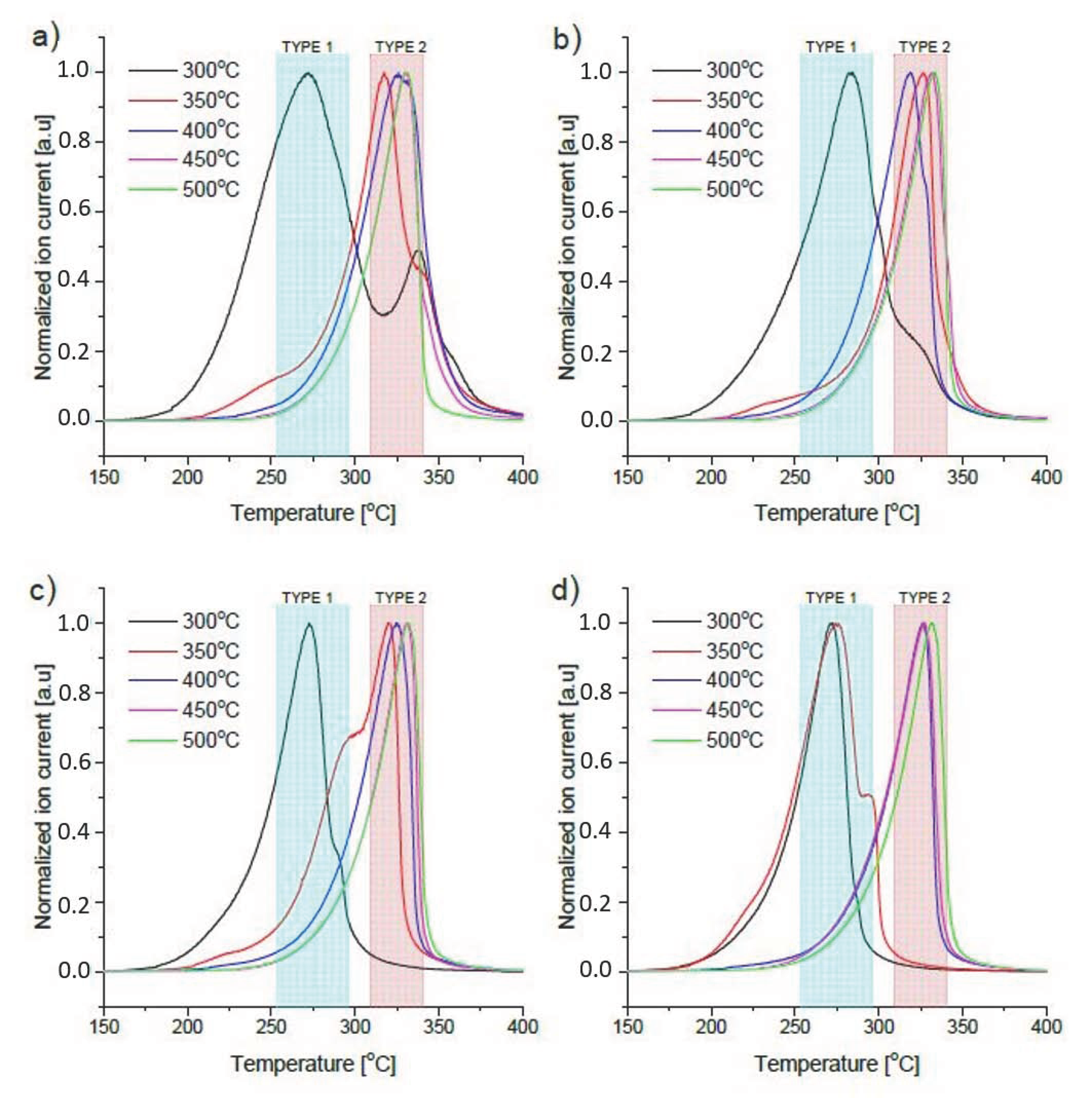
3.3. Decomposition Properties
3.4. Synthesis Efficiency Map and Conclusions
4. Summary
- A two-step Mg2FeH6 synthesis method based on high-energy milling and sintering under high temperature and hydrogen pressure was more efficient compared to conventional routes, resulting in a yield up to 97%.
- Both the milling time and sintering temperature of the precursor powders had a decisive influence on the Mg2FeH6 synthesis efficiency.
- Increasing the milling time of the powders up to a certain length improves the process yield. For the experimental setup used, the highest reaction yield was found with a milling time of 2 h.
- Increasing the sintering temperature improves the process efficiency.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
References
- Bogdanovic, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and dynamics of the Mg-Fe-H system and its potential for thermochemical thermal energy storage. J. Alloys Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Peinecke, K.; Peil, S.; Felderhoff, M. Development of a heat storage demonstration unit on the basis of Mg2FeH6 as heat storage material and molten salt as heat transfer media. Int. J. Hydrog. Energy 2017, 42, 13818–13826. [Google Scholar] [CrossRef]
- D’Entremont, A.; Corgnale, C.; Sulic, M.; Hardy, B.; Zidan, R.; Motyka, T. Modeling of a thermal energy storage system based on coupled metal hydrides (magnesium iron-sodium alanate) for concentrating solar power plants. Int. J. Hydrog. Energy 2017, 42, 22518–22529. [Google Scholar] [CrossRef]
- Didisheim, J.J.; Zolliker, P.; Yvon, K.; Fischer, P.; Schefer, J.; Gubelmann, M.; Williams, A.F. Dimagnesium iron(II) hydride, Mg2FeH6, containing octahedral FeH64- anions. Inorg. Chem. 1984, 23, 1953–1957. [Google Scholar]
- Raman, S.S.S.; Davidson, D.J.; Bobet, J.L.; Srivastava, O.N. Investigations on the synthesis, structural and microstructural characterizations of Mg-based K2PtCl6 type (Mg2FeH6) hydrogen storage material prepared by mechanical alloying. J. Alloys Compd. 2002, 333, 282–290. [Google Scholar] [CrossRef]
- Herrich, M.; Ismail, N.; Handstein, A.; Pratt, A.; Gutfleisch, O. Synthesis and decomposition of Mg2FeH6 prepared by reactive milling. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2004, 108, 28–32. [Google Scholar] [CrossRef]
- Li, S.L.; Varin, R.A.; Morozova, O.; Khomenko, T. Controlled mechano-chemical synthesis of nanostructured ternary complex hydride Mg2FeH6 under low-energy impact mode with and without pre-milling. J. Alloys Compd. 2004, 384, 231–248. [Google Scholar] [CrossRef]
- Varin, R.A.; Li, S.; Calka, A.; Wexler, D. Formation and environmental stability of nanocrystalline and amorphous hydrides in the 2Mg-Fe mixture processed by controlled reactive mechanical alloying (CRMA). J. Alloys Compd. 2004, 373, 270–286. [Google Scholar] [CrossRef]
- Polanski, M.; Płociński, T.; Kunce, I.; Bystrzycki, J. Dynamic synthesis of ternary Mg2FeH6. Int. J. Hydrog. Energy 2010, 35, 1257–1266. [Google Scholar]
- Polanski, M.; Nielsen, T.K.; Cerenius, Y.; Bystrzycki, J.; Jensen, T.R. Synthesis and decomposition mechanisms of Mg2FeH6 studied by in-situ synchrotron X-ray diffraction and high-pressure DSC. Int. J. Hydrog. Energy 2010, 35, 3578–3582. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Varin, R.A.; Plocinski, T. Rapid hydrogenation at 30 °C of magnesium (Mg) and iron (Fe) nanocomposite obtained through a decomposition of Mg2FeH6 precursor. Int. J. Hydrog. Energy 2011, 36, 1059–1065. [Google Scholar] [CrossRef]
- Polanski, M.; Witek, K.; Nielsen, T.K.; Jaroszewicz, L.; Bystrzycki, J. The influence of the milling time on the yield of Mg2FeH6 from a two-step synthesis conducted in a custom-made reactor. Int. J. Hydrog. Energy 2013, 38, 2785–2789. [Google Scholar] [CrossRef]
- Puszkiel, J.; Gennari, F.; Larochette, P.A.; Karimi, F.; Pistidda, C.; Gosalawit-Utke, R.; Jepsen, J.; Jensen, T.R.; Gundlach, C.; von Colbe, J.B.; et al. Sorption behavior of the MgH2-Mg2FeH6 hydride storage system synthesized by mechanical milling followed by sintering. Int. J. Hydrog. Energy 2013, 38, 14618–14630. [Google Scholar] [CrossRef]
- De Lima, G.F.; Garroni, S.; Baró, M.D.; Suriñach, S.; Kiminami, C.S.; Botta, W.J.; Peres, M.M.; Jorge Junior, A.M. 2Mg-Fe alloys processed by hot-extrusion: Influence of processing temperature and the presence of MgO and MgH2 on hydrogenation sorption properties. J. Alloys Compd. 2011, 509, S460–S463. [Google Scholar] [CrossRef]
- Lima, G.F.; Triques, M.R.M.; Kiminami, C.S.; Botta, W.J.; Jorge, A.M. Hydrogen storage properties of 2Mg-Fe after the combined processes of hot extrusion and cold rolling. J. Alloys Compd. 2014, 586, S409–S412. [Google Scholar] [CrossRef]
- De Lima Andreani, G.F.; Miglioli, M.M.; Triques, M.R.M.; Roche, V.; Kiminami, C.S.; Botta, W.J.; Jorge, A.M. Hydrogen storage properties of 2Mg-Fe mixtures processed by hot extrusion at different temperatures. Int. J. Hydrog. Energy 2017, 42, 11493–11500. [Google Scholar] [CrossRef]
- Gorsse, S.; Prakasam, M.; Couillaud, S.; Bouaziz, O.; Bobet, J.L.; Bellanger, P. Light iron and hard magnesium nanocomposites. Mater. Sci. Eng. A 2016, 651, 987–990. [Google Scholar] [CrossRef]
- Zaïdi, W.; Bonnet, J.P.; Zhang, J.; Cuevas, F.; Latroche, M.; Couillaud, S.; Bobet, J.L.; Sougrati, M.T.; Jumas, J.C.; Aymard, L. Reactivity of complex hydrides Mg2FeH6, Mg2CoH5 and Mg2NiH4 with lithium ion: Far from equilibrium electrochemically driven conversion reactions. Int. J. Hydrog. Energy 2013, 38, 4798–4808. [Google Scholar] [CrossRef]
- Retuerto, M.; Alonso, J.A.; Martínez, R.; Jiménez-Villacorta, F.; Sánchez-Benítez, J.; Fernández-Díaz, M.T.; Garcia-Ramos, C.A.; Ruskov, T. Neutron Powder Diffraction, X-ray absorption and Mössbauer spectroscopy on Mg2FeH6. Int. J. Hydrog. Energy 2015, 40, 9306–9313. [Google Scholar] [CrossRef]
- Chaudhary, A.L.; Dietzel, S.; Li, H.W.; Akiba, E.; Bergemann, N.; Pistidda, C.; Klassen, T.; Dornheim, M. Synthesis of Mg2FeD6 under low pressure conditions for Mg2FeH6 hydrogen storage studies. Int. J. Hydrog. Energy 2017, 42, 11422–11428. [Google Scholar] [CrossRef]
- Farina, L.; Brutti, S.; Trequattrini, F.; Palumbo, O.; Gatto, S.; Reale, P.; Silvestri, L.; Panero, S.; Paolone, A. An extensive study of the Mg Fe H material obtained by reactive ball milling of MgH2 and Fe in a molar ratio 3:1. Int. J. Hydrog. Energy 2017, 42, 22333–22341. [Google Scholar] [CrossRef]
- Lang, J.; Fritzche, H.; Asselli, A.A.C.; Huot, J. In-situ neutron diffraction investigation of Mg2FeH6 dehydrogenation. Int. J. Hydrog. Energy 2017, 42, 3087–3096. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Larochette, P.A.; Gennari, F.C. Thermodynamic and kinetic studies of Mg-Fe-H after mechanical milling followed by sintering. J. Alloys Compd. 2008, 463, 134–142. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Larochette, P.A.; Gennari, F.C. Thermodynamic-kinetic characterization of the synthesized Mg2FeH6-MgH2 hydrides mixture. Int. J. Hydrog. Energy 2008, 33, 3555–3560. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Larochette, P.A.; Baruj, A.; Meyer, G.; Gennari, F.C. Hydrogen cycling properties of xMg-Fe materials (x: 2, 3 and 15) produced by reactive ball milling. Int. J. Hydrog. Energy 2016, 41, 1688–1698. [Google Scholar] [CrossRef]
- Ul Haq, B.; Kanoun, M.B.; Ahmed, R.; Bououdina, M.; Goumri-Said, S. Hybrid functional calculations of potential hydrogen storage material: Complex dimagnesium iron hydride. Int. J. Hydrog. Energy 2014, 39, 9709–9717. [Google Scholar] [CrossRef]
- Deng, S.; Xiao, X.; Han, L.; Li, Y.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Hydrogen storage performance of 5LiBH4 + Mg2FeH6 composite system. Int. J. Hydrog. Energy 2012, 37, 6733–6740. [Google Scholar] [CrossRef]
- Langmi, H.W.; McGrady, G.S.; Newhouse, R.; Rönnebro, E. Mg2FeH6-LiBH4 and Mg2FeH6-LiNH2 composite materials for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 6694–6699. [Google Scholar] [CrossRef]
- Gosselin, C.; Deledda, S.; Hauback, B.C.; Huot, J. Effect of synthesis route on the hydrogen storage properties of 2MgH2-Fe compound doped with LiBH4. J. Alloys Compd. 2015, 645, S304–S307. [Google Scholar] [CrossRef]
- Chen, X.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage in Mg2Fe(Ni)H6 nanowires synthesized from coarse-grained Mg and nano sized γ-Fe(Ni) precursors. Int. J. Hydrog. Energy 2016, 41, 14795–14806. [Google Scholar] [CrossRef]
- De Lima Andreani, G.F.; Triques, M.R.M.; Kiminami, C.S.; Botta, W.J.; Roche, V.; Jorge, A.M. Characterization of hydrogen storage properties of Mg-Fe-CNT composites prepared by ball milling, hot-extrusion and severe plastic deformation methods. Int. J. Hydrog. Energy 2016, 41, 23092–23098. [Google Scholar] [CrossRef]
- Xu, C.C.; Xiao, X.Z.; Shao, J.; Liu, L.X.; Qin, T.; Chen, L.X. Effects of Ti-based additives on Mg2FeH6 dehydrogenation properties. Trans. Nonferr. Met. Soc. China 2016, 26, 791–798. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Jung, J.Y.; Suh, J.Y.; Lee, Y.S.; Shim, J.H.; Cho, Y.W. Low temperature formation of Mg2FeH6 by hydrogenation of ball-milled nano-crystalline powder mixture of Mg and Fe. Mater. Des. 2017, 135, 239–245. [Google Scholar] [CrossRef]
- Panas, A.J.; Fikus, B.; Płatek, P.; Kunce, I.; Witek, K.; Kuziora, P.; Olejarczyk, A.; Dyjak, S.; Michalska-Domańska, M.; Jaroszewicz, L.; et al. Pressurised-cell test stand with oscillating heating for investigation heat transfer phenomena in metal hydride beds. Int. J. Hydrog. Energy 2016, 41, 16974–16983. [Google Scholar] [CrossRef]
- Castro, F.J.; Gennari, F.C. Effect of the nature of the starting materials on the formation of Mg2FeH6. J. Alloys Compd. 2004, 375, 292–296. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2-Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Shahi, R.R.; Tiwari, A.P.; Shaz, M.A.; Srivastava, O.N. Studies on de/rehydrogenation characteristics of nanocrystalline MgH2 co-catalyzed with Ti, Fe and Ni. Int. J. Hydrog. Energy 2013, 38, 2778–2784. [Google Scholar] [CrossRef]
- Gennari, F.C.; Castro, F.J.; Andrade Gamboa, J.J. Synthesis of Mg2FeH6 by reactive mechanical alloying: Formation and decomposition properties. J. Alloys Compd. 2002, 339, 261–267. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Larochette, R.A.; Gennari, F.C. Hydrogen storage properties of Mg(x)Fe (x: 2, 3 and 15) compounds produced by reactive ball milling. J. Power Sources 2009, 186, 185–193. [Google Scholar] [CrossRef]
- Asselli, A.A.C.; Leiva, D.R.; Jorge, A.M.; Ishikawa, T.T.; Botta, W.J. Synthesis and hydrogen sorption properties of Mg2FeH6-MgH0 nanocomposite prepared by reactive milling. J. Alloys Compd. 2012, 536, S250–S254. [Google Scholar] [CrossRef]
- Kuziora, P.; Wyszyńska, M.; Polanski, M.; Bystrzycki, J. Why the ball to powder ratio (BPR) is insufficient for describing the mechanical ball milling process. Int. J. Hydrog. Energy 2014, 39, 9883–9887. [Google Scholar] [CrossRef]
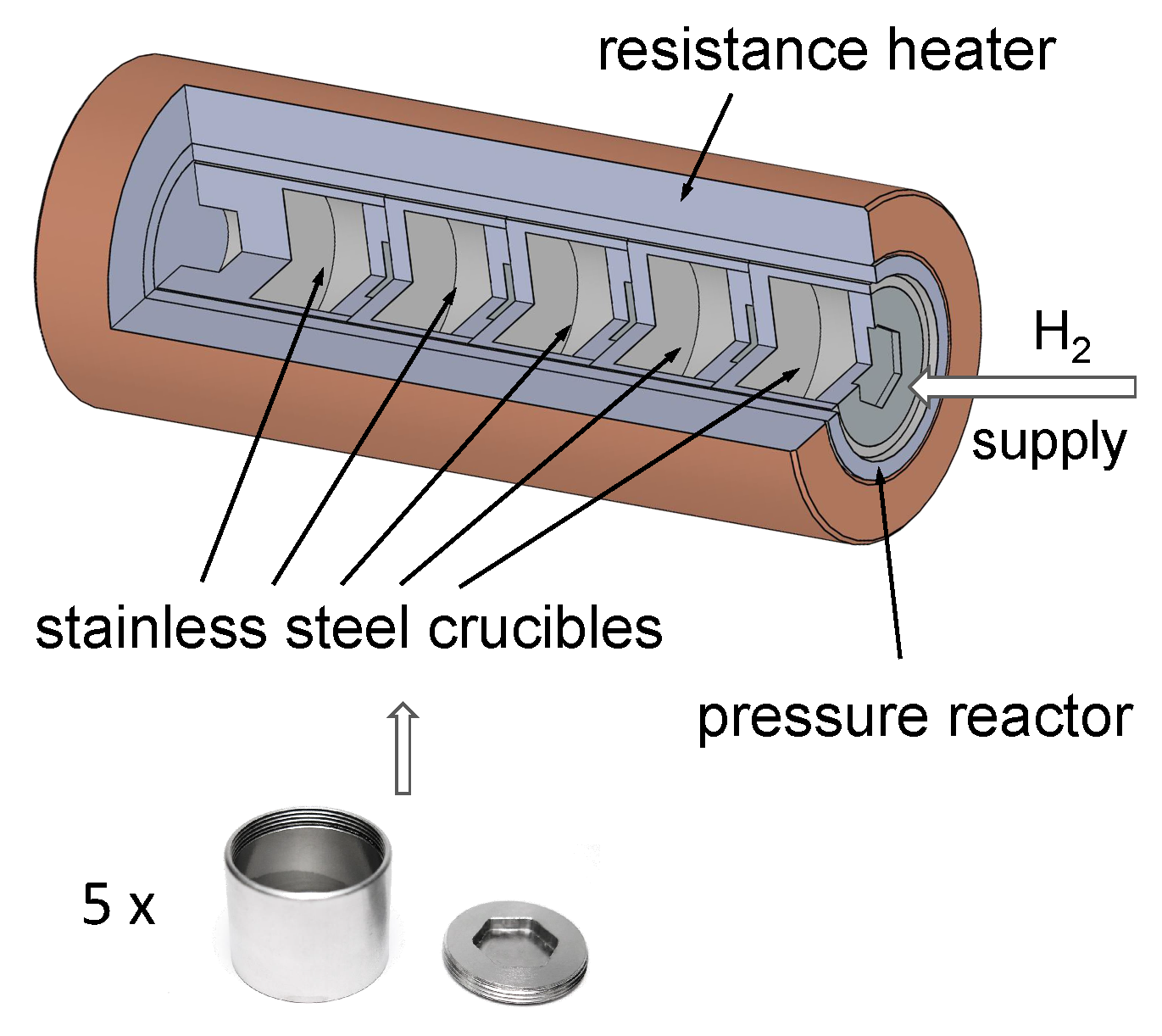
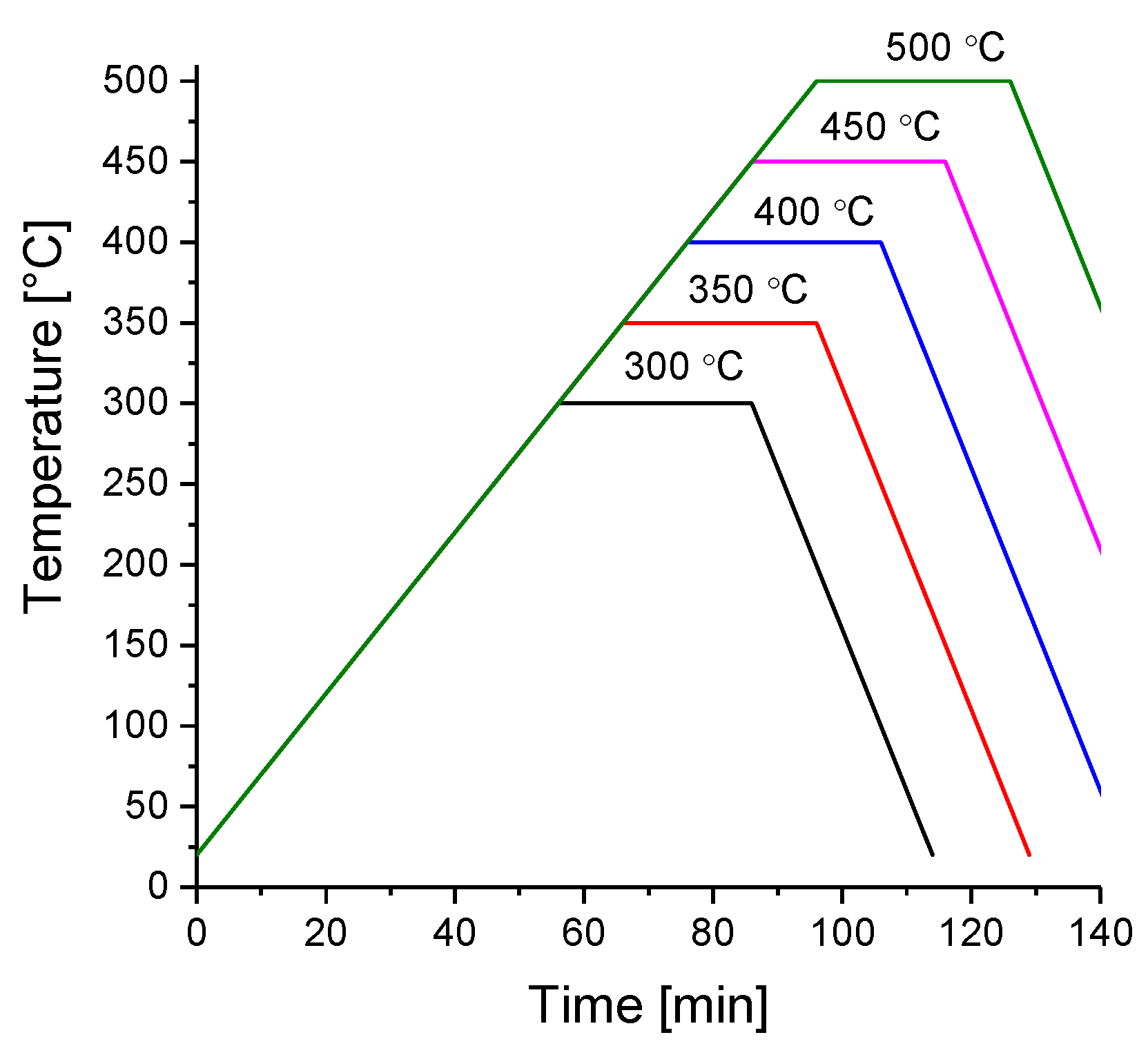


| Parameter (mm) | Value (unit) |
|---|---|
| Revolution speed | 650 (rpm) |
| Milling time per cycle | 10 (min) |
| Balls diameter | 10 (mm) |
| Number of balls | 30 (pcs.) |
| Number of cycles | 3, 6, 12, 18 (0.5, 1, 2, 3 (h)) |
| Ball to powder ratio | 7:1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witek, K.; Karczewski, K.; Karpowicz, M.; Polanski, M. Mg2FeH6 Synthesis Efficiency Map. Crystals 2018, 8, 94. https://doi.org/10.3390/cryst8020094
Witek K, Karczewski K, Karpowicz M, Polanski M. Mg2FeH6 Synthesis Efficiency Map. Crystals. 2018; 8(2):94. https://doi.org/10.3390/cryst8020094
Chicago/Turabian StyleWitek, Katarzyna, Krzysztof Karczewski, Magdalena Karpowicz, and Marek Polanski. 2018. "Mg2FeH6 Synthesis Efficiency Map" Crystals 8, no. 2: 94. https://doi.org/10.3390/cryst8020094
APA StyleWitek, K., Karczewski, K., Karpowicz, M., & Polanski, M. (2018). Mg2FeH6 Synthesis Efficiency Map. Crystals, 8(2), 94. https://doi.org/10.3390/cryst8020094