Abstract
The chlorine (Cl) and bromine (Br) co-doped lithium nickel manganese cobalt oxide (LiNi1/3Co1/3Mn1/3O2) was successfully synthesized by the molten salt method. The synthesized LiNi1/3Co1/3Mn1/3O2 compound demonstrates spherical morphology, which is formed by aggregated spherical-like or polygon primary particles. Halogen substitution would contribute to the growth of the primary particles. The LiNi1/3Co1/3Mn1/3O2 compound has the typical hexagonal layered structure, and no impurity phase is detected. The surface oxidation state of the compound is improved after Cl and Br substitution. Moreover, the Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 compound exhibits both improved rate capacity and cycle stability at a high voltage (4.6 V) compared with the pristine LiNi1/3Co1/3Mn1/3O2. The initial discharge capacities of Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 are 208.9 mAh g−1, 200.6 mAh g−1, 188.2 mAh g−1, 173.3 mAh g−1, and 157.1 mAh g−1 at the corresponding rates of 0.1C, 0.2C, 0.5C, 1C, and 3C respectively. The capacity retention at 1C after 50 cycles is increased from 81.1% to 93.2% by co-doping. The better contact between the electroactive particles of the electrode and the smaller resistance enhance the electric conductivity of the Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 cathode. The synthesized LiNi1/3Co1/3Mn1/3O2 is a promising cathode material for a high-power and large-capacity lithium-ion battery.
1. Introduction
The tremendous demand for a high-power and large-capacity lithium-ion battery is still ongoing, which is suitable for the energy storage systems of electric vehicles and power stations supplied by wind and solar energy [,,]. Among the various components of a lithium-ion battery, the cathode material is a key factor in optimizing the overall performance of a battery. In recent years, LiNi1/3Co1/3Mn1/3O2 proposed by Ohzuku and Makimura [] has been investigated extensively as a promising cathode material due to its higher performance and lower cost compared with commercial LiCoO2. In the pristine LiNi1/3Co1/3Mn1/3O2 structure, the Co, Ni, and Mn exist in the oxidation states of +3, +2/+3 and +3/+4, respectively. The redox process at 2.5~4.4 V corresponds to the Ni2+/Ni4+ couple, and that at 4.4~4.6 V corresponds to the Co3+/Co4+ couple. Much attention has been given to the improvements of its structural stability and electrochemical behavior below 4.5 V [,,,,]. In our previous work, LiNi1/3Co1/3Mn1/3O2 cathode materials were synthesized by the molten salt method, and the electrochemical performance between 2.8–4.4 V was investigated [,]. Although the optimized LiNi1/3Co1/3Mn1/3O2 compounds exhibit an improved electrochemical performance, the cycling behavior at high voltage (especially above 4.4 V) should be further enhanced [,,]. It was presumed that the mechanism of capacity fading for LiNi1/3Co1/3Mn1/3O2 at the high voltage was mainly attributed to the structure transformation and the gradual dissolution of Co and Mn [,]. It was also reported that the capacity fading of LiNi1/3Co1/3Mn1/3O2 in the potential region of 2.5–4.6 V was larger than that of 2.5–4.4 V because of the larger charge transfer resistance due to the low electric conductivity []. The research on the mechanism of capacity fading of LiNi1/3Co1/3Mn1/3O2 at the high voltage is still in progress. In order to suppress the structure transformation and the dissolution and improve the electrical conductivity of the compounds, the metal oxides were coated onto or substituted for LiNi1/3Co1/3Mn1/3O2, such as Li2O-B2O3-Li2SO4, ZrO2, CeO2, ALD, TiO2, FePO4, and Li2MnO3 [,,,,,,]. Meanwhile, several research groups have paid attention to the anion substitution for oxygen in order to improve the cyclic performances of the cathode material at the high voltage. The F substitution for oxygen in the LiNi1/3Co1/3Mn1/3O2 cathode material improved the thermal stability and the electrochemical property of LiNi1/3Co1/3Mn1/3O2 at 4.6 V [,]. The improved low voltage (4.3 V) performance of Cl-doped and Br-doped LiNi1/3Co1/3Mn1/3O2 was reported []. The enhanced lithium ion diffusions of F, Cl, and Br-substituted spinel LiMn2O4 were demonstrated in our previous works [,]. In brief, the substitution of halogen ions for the O anion has a significant influence on the physical and chemical performance of the cathode materials.
In this paper, the Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 was synthesized by the molten salt method. The morphological, structural, surface oxidation state, and high-voltage electrochemical properties of the Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 were compared with the pristine LiNi1/3Co1/3Mn1/3O2.
2. Materials and Methods
LiNi1/3Co1/3Mn1/3O2 compounds were synthesized by the molten salt method using Ni1/3Co1/3Mn1/3(OH)2 precursors (made in Kelong, Henan, China, Ni 21.63 wt%, Co 21.44 wt%, Mn 19.66 wt%), LiX (X = Cl, Br) and a stoichiometric amount of eutectic molten salts (0.76LiOH·H2O-0.24Li2CO3). Cl and Br were used as the substitution for oxygen. The molar ratios of Cl and Br for the compound were both 0.05. According to the previous work [], the stoichiometric Ni1/3Co1/3Mn1/3(OH)2, molten salts, and LiX were ground with a mortar and pestle. The mixture was put into the alumina crucible, heated up to 480 °C, and kept for 2 h; subsequently, it was heated to 850 °C with the heating rate of 3 °C min−1 and kept for another 5 h again in the muffle furnace. Then, the synthesized LiNi1/3Co1/3Mn1/3O2 compounds (marked as the pristine LNCM and the Cl&Br co-doped LNCM here) were spontaneously cooled down to the ambient temperature. Finally, the compounds were washed with distilled water to remove the excess molten salts and dried at 100 °C.
The morphology and energy dispersive spectroscopy (EDS) analysis of the synthesized compounds were observed with scanning electron microscopy (SEM, JSM5600LV, JEOL USA, Inc., 11 Dearborn Road, Peabody, MA 01960). The phase purity and crystal structure were characterized by X-ray diffractometry (XRD, D/max-2000, Rigaku, Tokyo, Japan) with Cu-Kα radiation (λ = 1.54056 Å) operating at 30 kV and 30 mA. The scan range was from 10° to 80°, and a step of 0.01° was used. The lattice parameters a and c were calculated after full profile fitting using the MDI Jade 5.0 software. The surface properties of the LNCM compounds were investigated by X-ray photoelectron spectroscopy (XPS, K-Alpha 1063, Thermo Fisher Scientific, 168 Third Avenue, Waltham, MA, USA 02451) using a monochromatic X-ray generated from Al Kα (1486.6 eV).
The positive electrode consisted of 85% as-prepared composites, 10% acetylene black and 5% polyvinylidene fluoride (PVDF) as a binder, and metal Al foil was used as a collector. Celgard 2400 was used as separator, which was soaked in 1.0 mol L−1 LiPF6/EC + DMC (EC:DMC = 1:1 in volume ratio) electrolyte. Lithium metal foil was used as the counter electrode during the electrochemical measurements. All of the cells were assembled in an argon-filled glove box. The charge–discharge tests were carried out using a Land BT2001A automatic battery test system. The charge–discharge tests were completed in the voltage range of 2.5–4.6 V. AC impedance measurements were carried out with the scanning range of 1 mHz to 100 kHz using a CHI660B electrochemical workstation system (CH Instrument, Shanghai, China). All of the tests were performed at room temperature.
3. Results and Discussions
The SEM images of the precursor and the synthesized LiNi1/3Co1/3Mn1/3O2 compounds are illustrated in Figure 1. As can be observed from Figure 1a,b, the spherical Ni1/3Co1/3Mn1/3(OH)2 precursor compounds consist of flake-shaped primary particles, with an average diameter of ~10 μm. The pristine and halogen Cl and Br co-doped LNCM compounds keep the spherical morphology and consist of spherical-like or polygon primary particles, with an average diameter bigger than 10 μm, as shown in Figure 1c–f. The average primary particle diameter of the Cl and Br co-doped LNCM (~1.5 μm) is larger than that of the pristine LNCM (~1 μm). Cl and Br substitution would contribute to the growth of the primary particles of the LiNi1/3Co1/3Mn1/3O2 compound, which is consistent with the foregoing research on the fluorine-doped spinel LiMn2O4 []. With Cl and Br co-doping, the size of the primary particle becomes bigger, and the space between the primary particles increases, as shown in Figure 1f, which may allow the electrolyte solution to penetrate into the surface of these primary particles quickly.

Figure 1.
SEM images of the Ni1/3Co1/3Mn1/3(OH)2 precursor and the synthesized LiNi1/3Co1/3Mn1/3O2 compounds: (a,b) Ni1/3Co1/3Mn1/3(OH)2 precursor; (c,d) Pristine LNCM; (e,f) Cl&Br co-doped LNCM.
The X-ray diffraction patterns of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds are shown in Figure 2. The results reveal that the LiNi1/3Co1/3Mn1/3O2 compounds have the typical hexagonal layered structure of a-NaFeO2 type with the space group R-3m. No obvious impurity phase is detected. In the typical XRD patterns of the LiNi1/3Co1/3Mn1/3O2 cathode material, the peak splits of (006)/(102) and (108)/(110) are known to be indicators of a layered structure []. The clear peak splits of (006)/(102) and (108)/(110) are observed from Figure 2, which indicate the highly ordered layered structure of the prepared LiNi1/3Co1/3Mn1/3O2 compounds. With Cl and Br co-doped, the positions of the (006)/(102) and (108)/(110) peaks move to higher angles, and the degrees of splitting increase. From Figure 2, it is shown that the Cl and Br co-doped sample shows a kind of reflection splitting that is most clearly visible for the (108) reflection, but also for the (110) and (102) reflections shown within the insets. Generally, this points to a multi-phase composition of the sample, i.e., probably two R-3m layered phases with slightly different lattice constants due to a different content of either Cl&Br or alternatively Li, which also has a strong influence on the lattice parameters. The integrated intensity ratio of the (I006 + I102)/I101 and I003/I104 has been recognized as the criterion of the layered LiNi1/3Co1/3Mn1/3O2 crystal structure [,,]. A value of I003/I104 less than 1.2 has been recognized as a criterion for the existence of undesirable cation mixing in the layered LiNi1/3Co1/3Mn1/3O2 compound. Furthermore, the intensity ratio of (I006 + I102)/I101 is an indicator of the hexagonal ordering; the lower the value, the better the hexagonal ordering. In our experiments, the higher I003/I104 and the lower (I006 + I102)/I101 values indicate a well-defined layered structure and less cation disordering, helping to exhibit better electrochemical performance. This result is proven in the following electrochemical measurements. It can be speculated that halogen ions doping enhanced the structural stability. Our results are accordance with those obtained for the chlorine doping [].
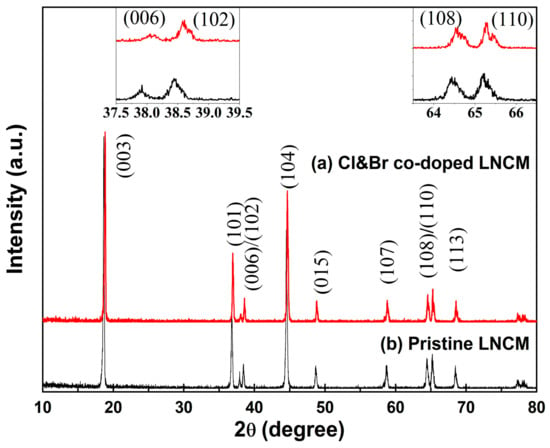
Figure 2.
XRD patterns of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds: (a) Cl&Br co-doped LNCM; (b) Pristine LNCM.
The core-level XPS spectra of Ni 2p, Co 2p, Mn 2p, and O 1s for the synthesized LiNi1/3Co1/3Mn1/3O2 compounds are shown in Figure 3. All of the spectra were energy calibrated with the C 1s standard using the energy value of 284 eV, and then fitted using the Thermo Avantage software. It was reported that the Ni 2p3/2 binding energy observed at 854.0 eV was consistent with that of NiO [], while the Ni 2p3/2 binding energy observed at 855.1 eV was aroused with the folding of Ni(OH)2 and Ni2O3 []. As shown in Figure 3, the Ni 2p3/2 binding energy peak is observed at 854.5 eV. Therefore, the Ni cations in the pristine sample may consist of Ni2+ and Ni3+ oxidation states. The relative amounts of Ni2+ and Ni3+ are estimated to be 75.31% and 24.69% using the software. The Co 2p3/2 binding energy observed at 779.66 eV is consistent with that of Co2O3 []. The Co of the pristine LNCM is the Co3+ oxidation state in a pristine LNCM compound. The Mn 2p3/2 binding energies of Mn3+/Mn4+ should be observed at 641.4/642.4 eV, according to reports in the literature [,,]. The Mn 2p3/2 XPS of the pristine LNCM centers at 641.7 eV, which indicates that the oxidation state of Mn of the pristine LNCM is a mixed oxidation state of Mn3+ and Mn4+. In the case of Cl and Br co-doped LNCM, the spectra of Ni2p3/2, Co2p3/2, and Mn2p3/2 shift to higher binding energy, indicating that the valences of the transition metals increase. The relative amounts of Ni2+ and Ni3+ are estimated to 70.06% and 29.94%, respectively. The binding energy for Mn2p3/2 of the Cl and Br co-doped LNCM is 642.24 eV, which agrees well with that of Mn4+ reported in the literature []. After being ion-etched for 30 s, the Ni, Mn, and Co XPS core level spectra of the Cl and Br co-doped LNCM shift back to almost the same binding energy of the pristine LNCM. This indicates that the doping mainly affected the surface oxidation state of LNCM, and only happened to the surface near the regions of the material. This is probably because the synthesized Ni1/3Co1/3Mn1/3(OH)2 precursors were used to prepare the LNCM compound, and it is hard for Cl and Br to diffuse into the bulk material uniformly.
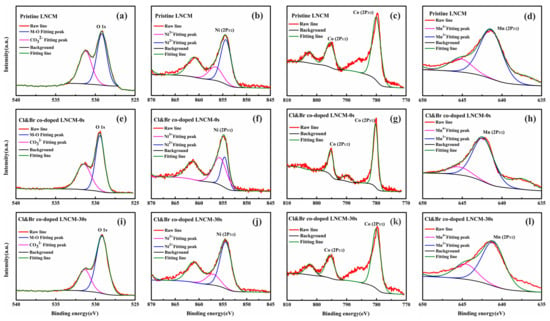
Figure 3.
XPS spectra of (a) O 1s, (b) Ni 2p3/2, (c) Co 2 p3/2, (d) Mn 2p3/2 for Pristine LNCM; (e) O 1s, (f) Ni 2p3/2, (g) Co 2p3/2, (h) Mn 2p3/2 for Cl&Br co-doped LNCM; (i) O 1s, (j) Ni 2p3/2, (k) Co 2p3/2, (l) Mn 2p3/2 for Cl&Br co-doped LNCM being ion-etched for 30 s.
The initial charge/discharge capacities and the cyclic performances of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds were evaluated at different rates in 2.5~4.6 V at room temperature. Figure 4 shows the initial charge/discharge curves of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds at 0.1C, 0.2C, 0.5C, 1C, and 3C respectively. The pristine LNCM delivers discharge capacities of 203.1 mAh g−1, 191.8 mAh g−1, 170.2 mAh g−1, 152.6 mAh g−1, and 135.8 mAh g−1, while the Cl and Br co-doped LNCM delivers 208.9 mAh g−1, 200.6 mAh g−1, 188.2 mAh g−1, 173.3 mAh g−1, and 157.1 mAh g−1 at the corresponding rates of 0.1C, 0.2C, 0.5C, 1C, and 3C, respectively. The initial discharge capacities of Cl and Br co-doped LNCM are 5.8 mAh g−1, 8.8 mAh g−1, 18 mAh g−1, 20.7 mAh g−1, and 21.3 mAh g−1 higher than the pristine one at the corresponding rates, respectively. It is indicated that the initial electrochemical capacity has been improved by Cl and Br co-doping. As shown in SEM analysis, the pores on the surface of co-doped LNCM can increase the contact area of the electrode/electrolyte, and also provide channels for the intercalation/deintercalation of lithium ions. More Ni2+ exists in the pristine LNCM, which can cause more cation disordering, as shown in XPS analysis, and a larger R(sei+ct) value, which is discussed in the following electrochemical impedance spectra measurement (EIS) analysis, may lead to a lower initial coulomb efficiency of pristine LNCM than the Cl and Br co-doped LNCM.
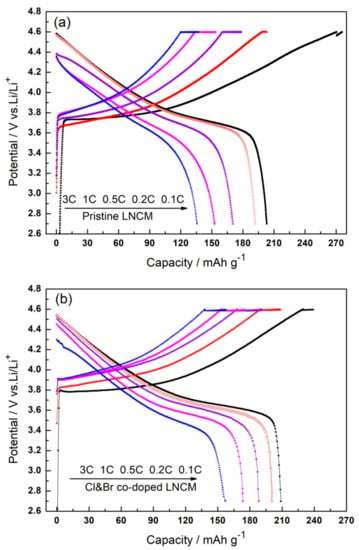
Figure 4.
Initial charge and discharge capacities of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds at 0.1~3C: (a) Pristine LNCM; (b) Cl&Br co-doped LNCM.
The cycle performances of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds at different rates are shown in Figure 5. It demonstrates that the Cl and Br co-doped LNCM has an excellent cycle stability, and it can restore to the initial capacity of 0.1C after two cycles at 0.1C, 10 cycles at 0.2C, 0.5C, 1C, 3C, and then back to 0.2C, respectively, while the capacities of the pristine LNCM decrease rapidly. The cyclic curves of the pristine LNCM and the Cl and Br co-doped LNCM at 1C for 50 cycles show that the initial and 50th discharge capacities, and the capacity retention of the Cl and Br co-doped LNCM are 173.3 mAh g−1, 161.5 mAh g−1, and 93.2% respectively, which are obviously higher than the pristine LNCM (152.6 mAh g−1, 123.7 mAh g−1, and 81.1% for comparison). We presume that the good cycle performance of the Cl and Br co-doped LNCM compounds benefits from the enhanced structure stability after doping and the Mn4+ in the surface layer of the LiNi1/3Co1/3Mn1/3O2 compounds, which can avoid the dissolution and the John-Teller effect of Mn3+. Also, the observed differences in rate capability should rather be attributed to the different morphology, which is a side effect of doping, or rather to the surface doping on an atomic level, probably by the change of electronic structure or diffusion.

Figure 5.
Rate capabilities (a) and cycling performance (b) of the synthesized LiNi1/3Co1/3Mn1/3O2 compounds.
After cycling for 100 cycles, the cells were disassembled and washed with dimethylcarbonate in the argon-filled glove box to completely remove the residual lithium salts. The SEM images of the synthesized LiNi1/3Co1/3Mn1/3O2 electrodes are shown in Figure 6. It can be seen that the surface morphology of the pristine LiNi1/3Co1/3Mn1/3O2 electrode changes a lot. The obvious cracks appear between the LiNi1/3Co1/3Mn1/3O2 composites and acetylene black particles, which result in inferior contact between the electroactive LiNi1/3Co1/3Mn1/3O2 particles during the charge–discharge process. As to the Cl and Br co-doped LNCM electrode, the surface morphology almost doesn’t change, and the acetylene black still covers the LiNi1/3Co1/3Mn1/3O2 particles completely, which may keep the effective conductive behavior during cycling. It may be the contribution of the space between primary particles, as shown in Figure 1, that stably adhere the acetylene black onto the surface of the LiNi1/3Co1/3Mn1/3O2 particles. Chemical composition (wt%) of the electrodes determined by energy dispersive spectroscopy (EDS) with the particles in Figure 6b,d,f,g are listed in Table 1. The molar ratio of Ni:Co:Mn has a small variation after being cycled. It indicates that Mn dissolution is depressed by halogen doping, which would keep the structure stability.

Figure 6.
SEM images of the synthesized LiNi1/3Co1/3Mn1/3O2 electrodes after cycling (100 cycles): (a,b) Pristine LNCM before cycling; (c,d) Pristine LNCM after cycling; (e,f) Cl&Br co-doped LNCM before cycling; (g,h) Cl&Br co-doped LNCM after cycling.

Table 1.
Chemical composition (wt%) of the electrodes determined by energy dispersive spectroscopy (EDS) with the particles in Figure 6b,d,f,g.
XRD patterns of the synthesized LiNi1/3Co1/3Mn1/3O2 electrodes after 100 cycles at 1C are shown in Figure 7. Except for the peaks at 65° and 77°, which represent the metal Al foil, there are no impurity peaks. The (I006 + I102)/I101 values increase, and the I003/I104 values decrease after cycling. These results demonstrate that the two samples keep the layered structure, but the cation ordering in the layered hexagonal structure was changed in the pristine LNCM compound. The Cl and Br co-doped LNCM keeps a better crystallinity than the pristine one, as the densities of diffraction peaks are higher than the latter.
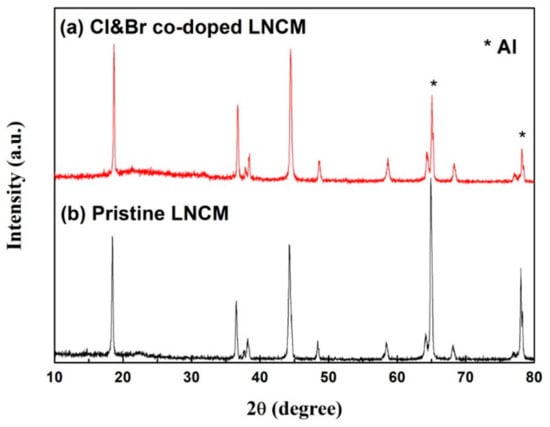
Figure 7.
XRD patterns of the synthesized LiNi1/3Co1/3Mn1/3O2 electrodes after 100 cycles: (a) Cl&Br co-doped LNCM; (b) Pristine LNCM.
Electrochemical impedance spectra measurements were carried out for the pristine LNCM and Cl and Br co-doped LNCM electrodes after the first and 100th cycle. The obtained results are shown in Figure 8. The impedance plots show a high-frequency intercept at the Z’ axis, a broad depressed semicircle, and a straight line in the low-frequency region. The high-frequency intercept is due to the ohmic resistance (Rs). The depressed semicircle is related to the formation of a surface layer on the active material and the intercalation/deintercalation of lithium ions into/from the electrodes. An equivalent circuit used to fit the spectra is shown in the inset of Figure 8a []. The spectra are fitted using a combination of surface film and charge transfer resistance, R(sei+ct), and the corresponding constant phase element (CPE). The R(sei+ct) values of the pristine LNCM and Cl and Br co-doped LNCM after the first/100th cycle are calculated as 142 Ω/308 Ω and 59 Ω/104 Ω, respectively. Cl and Br co-doped LNCM has a smaller R (sei+ct) value than the pristine one. The reduced R (sei+ct) value favored the electrochemical reaction upon lithium ion intercalation/deintercalation, which implied the enhanced the cyclic stability. These results are in accordance with the electrochemical charge–discharge test.
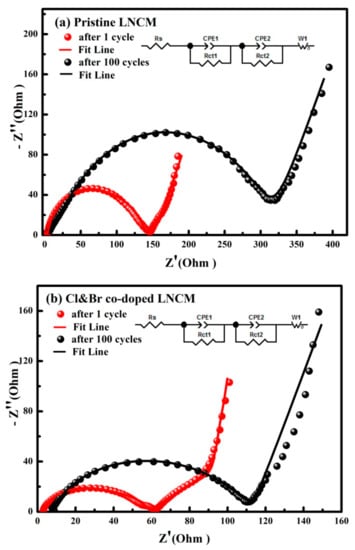
Figure 8.
Electrochemical impedance spectra of the synthesized LiNi1/3Co1/3Mn1/3O2 electrodes after different cycles: (a) Pristine LNCM; (b) Cl&Br co-doped LNCM.
4. Conclusions
A Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 compound was synthesized by the molten salt method. It was characterized by scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), galvanostatic charge–discharge measurements and electrochemical impedance spectra measurement (EIS). SEM results show that the pristine and halogen co-doped LiNi1/3Co1/3Mn1/3O2 compounds keep the spherical morphology consisting with spherical-like or polygon primary particles. Cl and Br substitution would contribute to the growth of the primary particles. The XRD results demonstrate that the LiNi1/3Co1/3Mn1/3O2 compound has the typical hexagonal layered structure, and no impurity phase is detected. XPS results indicated that the surface oxidation state of the compound is improved with Cl and Br substitution. The Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 compound exhibits both an improved rate capacity and cycle stability at a high voltage (4.6 V) compared with the pristine LiNi1/3Co1/3Mn1/3O2. The initial discharge capacities of Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 are 208.9 mAh g−1, 200.6 mAh g−1, 188.2 mAh g−1, 173.3 mAh g−1, and 157.1 mAh g−1 at the corresponding rates of 0.1C, 0.2C, 0.5C, 1C, and 3C, respectively. The capacity retention after 50 cycles at 1C is increased from 81.1% to 93.2% after doping. The better contact between the electroactive particles of the electrode and the smaller resistance would enhance the electric conductivity of the Cl and Br co-doped LiNi1/3Co1/3Mn1/3O2 cathode.
Author Contributions
Formal analysis, K.C.; Investigation, X.G.; Methodology, Q.L.; Writing—original draft, H.Z.; Writing—review & editing, Z.C.
Funding
This research was funded by the National Natural Science Foundation of China with grants [51604042, 51874048] and the Natural Science Foundation of Hunan Province of China with grant [2016JJ3008].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.-K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Ohzuku, T.; Makimura, Y. Layered Lithium insertion material of LiNi1/2Mn1/2O2: A possible alternative to LiCoO2 for advanced lithium-ion batteries. Chem. Lett. 2001, 30, 744–745. [Google Scholar] [CrossRef]
- Roberts, M.; Owen, J. High-throughput method to study the effect of precursors and temperature, applied to the synthesis of LiNi1/3Co1/3Mn1/3O2 for lithium batteries. ACS Comb. Sci. 2011, 13, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Huang, M.; Huang, J.; Fang, Z. Preparation and Rate Capability of Carbon Coated LiNi1/3Co1/3Mn1/3O2 as Cathode Material in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 12408–12415. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, Y.; Liu, H.; Pinto, J.; Jiang, X.; Yang, G. Improved high rate capacity and lithium diffusion ability of LiNi1/3Co1/3Mn1/3O2 with ordered crystal structure. J. Electrochem. Soc. 2012, 159, A506–A513. [Google Scholar] [CrossRef]
- Jiang, K.-C.; Xin, S.; Lee, J.-S.; Kim, J.; Xiao, X.-L.; Guo, Y.-G. Improved kinetics of LiNi1/3Mn1/3Co1/3O2 cathode material through reduced graphene oxide networks. Phys. Chem. Chem. Phys. 2012, 14, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, D.; Tang, Y.; Huang, Y.; Pang, W.; Guo, Z.; Zhou, Z. In situ chelating synthesis of hierarchical LiNi1/3Co1/3Mn1/3O2 polyhedron assemblies with ultralong cycle life for Li-ion batteries. Small 2018, 14, 1704354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.; Chen, Z.; Li, Q.; Xie, T.; Li, L.; Lai, Y. Molten salt synthesis and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathode materials. Synth. Met. 2014, 187, 123–129. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Xie, T.; Li, L.J.; Xu, M.; Zhu, H.L.; Wang, W.H. Characterization of Na substituted LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium ion battery. Ionics 2014, 20, 629–634. [Google Scholar] [CrossRef]
- Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Periasamy, P.; Kalaiselvi, N.; Kim, H.S. High voltage and high capacity characteristics of LiNi1/3Co1/3Mn1/3O2 cathode for lithium battery applications. Int. J. Electrochem. Sci. 2007, 2, 689–699. [Google Scholar]
- Kim, G.-H.; Kim, J.-H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Improvement of high-voltage cycling behavior of surface-modified Li[Ni1/3Co1/3Mn1/3]O2 cathodes by fluorine substitution for li-ion batteries. J. Electrochem. Soc. 2005, 152, A1707–A1713. [Google Scholar] [CrossRef]
- Zeng, Y.W. Investigation of LiNi1/3Co1/3Mn1/3O2 cathode particles after 300 discharge/charge cycling in a lithium-ion battery by analytical TEM. J. Power Sources 2008, 183, 316–324. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.J.; Ehrenberg, H.; Du, F.; Wang, C.Z.; Chen, G. Characterizations on the structural and electrochemical properties of LiNi1/3Mn1/3Co1/3O2 prepared by a wet-chemical process. Solid State Ionics 2008, 178, 1969–1974. [Google Scholar] [CrossRef]
- Lv, D.; Wang, L.; Hu, P.; Sun, Z.; Chen, Z.; Zhang, Q.; Cheng, W.; Ren, W.; Bian, L.; Xu, J.; et al. Li2O-B2O3-Li2SO4 modified LiNi1/3Co1/3Mn1/3O2 cathode material for enhanced electrochemical performance. Electrochim. Acta 2017, 247, 803–811. [Google Scholar] [CrossRef]
- Li, X.; Peng, H.; Wang, M.-S.; Zhao, X.; Huang, P.-X.; Yang, W.; Xu, J.; Wang, Z.-Q.; Qu, M.-Z.; Yu, Z.-L. Enhanced electrochemical performance of Zr-modified layered LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion batteries. ChemElectroChem 2015, 3, 130–137. [Google Scholar] [CrossRef]
- Wu, F.; Wang, M.; Su, Y.; Bao, L.; Chen, S. Surface of LiCo1/3Ni1/3Mn1/3O2 modified by CeO2-coating. Electrochim. Acta 2009, 54, 6803–6807. [Google Scholar] [CrossRef]
- Riley, L.A.; Atta, S.V.; Cavanagh, A.S.; Yan, Y.; George, S.M.; Liu, P.; Dillon, A.C.; Lee, S.-H. Electrochemical effects of ALD surface modification on combustion synthesized LiNi1/3Mn1/3Co1/3O2 as a layered-cathode material. J. Power Sources 2011, 196, 3317–3324. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; He, X.; Zhao, R.; Jiang, C.; Wan, C. TiO2 coating of LiNi1/3Co1/3Mn1/3O2 cathode materials for Li-ion batteries. Ionics 2006, 12, 215–218. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Yoo, E.; Ishida, M.; Zhou, H. Fabrication of FePO4 layer coated LiNi1/3Co1/3Mn1/3O2: Towards high-performance cathode materials for lithium ion batteries. Electrochim. Acta 2012, 83, 253–258. [Google Scholar] [CrossRef]
- Son, M.Y.; Hong, Y.J.; Choi, S.H.; Kang, Y.C. Effects of ratios of Li2MnO3 and Li(Ni1/3Mn1/3Co1/3)O2 phases on the properties of composite cathode powders in spray pyrolysis. Electrochim. Acta 2013, 103, 110–118. [Google Scholar] [CrossRef]
- Li, D.; Sasaki, Y.; Kobayakawa, K.; Noguchi, H.; Sato, Y. Preparation, morphology and electrochemical characteristics of LiNi1/3Mn1/3Co1/3O2 with LiF addition. Electrochim. Acta 2006, 52, 643–648. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, Q.; Wang, L.; Hu, Y.; Sun, N.; Shen, Y.; Wang, Y. Synthesis and characterization of Li1.05Co1/3Ni1/3Mn1/3O1.95X0.05 (X = Cl, Br) cathode materials for lithium-ion battery. C. R. Chim. 2013, 16, 845–849. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhu, H.L.; Hu, G.R.; Xiao, J.; Peng, Z.D.; Liu, Y.X. Electrochemical performances and structure characteristic of LiMn2O4−xYx(Y = F, Cl, Br) compounds. Trans. Nonferrous Met. Soc. China 2004, 14, 1151–1155. [Google Scholar]
- Chen, Z.-Y.; Gao, L.Z.; Liu, X.Q.; Yu, Z.L. Properties and structure of spinel Li-Mn-O-F compounds for cathode materials of secondary lithium-ion battery. Chin. J. Chem. 2011, 19, 347–351. [Google Scholar] [CrossRef]
- Gao, Y.; Yakovleva, M.V.; Ebner, W.B. Novel LiNi1−xTix/2Mgx/2O2 compounds as cathode materials for safer lithium-ion batteries. Electrochem. Solid-State Lett. 1998, 1, 117–119. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Lu, Q.; Gendrond, F.; Julien, C.M. Minimization of the cation mixing in Li1+x(NMC)1-xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Reimers, J.N.; Rossen, E.; Jones, C.D.; Dahn, J.R. Structure and electrochemistry of LixFeyNi1−yO2. Solid State Ionics 1993, 61, 335–344. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Oswald, S.; Brückner, W. XPS depth profile analysis of non-stoichiometric NiO films. Surf. Interface Anal. 2004, 36, 17–22. [Google Scholar] [CrossRef]
- Kageyama, M.; Li, D.; Kobayakawa, K.; Sato, Y.; Lee, Y.-S. Structural and electrochemical properties of LiNi1/3Mn1/3Co1/3O2−xFx prepared by solid state reaction. J. Power Sources 2006, 157, 494–500. [Google Scholar] [CrossRef]
- Iwanowski, R.J.; Heinonen, M.H.; Janik, E. X-ray photoelectron spectra of zinc-blende MnTe. Chem. Phys. Lett. 2004, 387, 110–115. [Google Scholar] [CrossRef]
- Zhu, H.L.; Xie, T.; Chen, Z.Y.; Li, L.J.; Xu, M.; Wang, W.H.; Lai, Y.Q.; Li, J. The impact of vanadium substitution on the structure and electrochemical performance of LiNi0.5Co0.2Mn0.3O2. Electrochim. Acta 2014, 135, 77–85. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).