Abstract
BiVO4 photocatalysts were synthesized via a facile surfactant-free method with heat treatment. The heat treatment temperatures influenced the crystal structures and morphologies. The photocatalytic performance is associated with its crystallinity, Brunauer–Emmett–Teller (BET) specific surface area, and band gap energy. The BiVO4 photocatalyst prepared by heat treatment at 700 °C showed the highest photocatalytic activity, promoting 100% degradation of methylene blue (MB) in 60 min under visible-light irradiation. Recycling experiments results indicated that the BiVO4 photocatalysts have excellent photo-stability, and a possible mechanism for the photocatalytic process was proposed by examining the effects of the active species involved in MB degradation. This work could provide new insights into the fabrication of highly efficient and stable BiVO4 photocatalysts for dye degradation.
1. Introduction
In recent years, bismuth vanadate (BiVO4) has attracted increasing attention as a promising photocatalyst owing to its non-toxicity, high stability, and excellent photocatalytic activity in organic dye degradation and water splitting [1,2,3,4,5,6,7,8]. It has three crystalline phases [9,10,11,12,13], monoclinic-scheelite, tetragonal-zircon, and tetragonal-scheelite, and exhibits phase transition under certain conditions. BiVO4 (s-m) is obtained when BiVO4 (z-t) is heated above 350 °C–450 °C, and phase transition between BiVO4 (s-m) and BiVO4 (s-t) occurs reversibly at 255 °C [14].
There are numerous methods reported for the fabrication of BiVO4, including hydrothermal treatment [15,16,17,18], sonochemical method [19], sol-gel method [20,21,22], ionothermal treatment [23], microwave-assisted route [24,25], solution combustion synthesis [26], co-precipitation process [27], molten salt method [28], reverse microemulsion process [29], aqueous method [30,31,32,33], etc. Among all of theses methods, the aqueous method provides a milder environment for the synthesis of monoclinic BiVO4 and allows the reaction parameters as well as the properties of the products to be easily tuned. Akihiko et al. [30] reported an aqueous process for preparation of highly crystalline monoclinic and tetragonal BiVO4 by reaction of the layered potassium vanadates KV3O8 and K3V5O14 with Bi(NO3)3 at 20 °C for 3 d. Kohtani et al. [31] prepared BiVO4 by stirring a equimolar mixture of aqueous Bi(NO3)3·5H2O and NH4VO3 solutions (0.4 mol/L) containing HNO3 (1.84 mol/L) with 7.5 g urea at 90 °C for 8 h. Tokunaga et al. [10] fabricated the BiVO4 by an aqueous process at room temperature by hydrolyzing a nitric acid solution of Bi(NO3)3 and Na3VO4 using bases (Na2CO3 and NaHCO3) to adjust the pH. They found that BiVO4 (s-m) and BiVO4 (s-t) could be selectively prepared by adjusting the preparation time, and that BiVO4 (s-m) obtained using 7.0 g of Na2CO3 showed the highest photocatalytic O2 evolution, while the activity of BiVO4 (s-t) was negligible. Yin et al. [32] synthesized a highly efficient monoclinic BiVO4 photocatalyst by an aqueous method with the assistance of cetyltrimethyl ammonium bromide (CTAB). However, base or surfactants are generally added to the reactions in order to control the crystal structure of the product, causing the production of redundant alkali wastewater containing refractory organics. Therefore, a simple surfactant-free and environmentally-friendly route is highly desirable for the preparation of photocatalysts.
In the present study, a highly efficient monoclinic BiVO4 photocatalyst for the oxidation of methylene blue (MB) under visible light irradiation was synthesized by a surfactant-free aqueous method. The effect of heat treatment temperature on the synthetic BiVO4 photocatalysts has been investigated, and their recycling stabilities were assessed. To understand the mechanism of MB decomposition over the BiVO4 system more completely, we investigated the active species involved in photocatalytic degradation.
2. Results
2.1. Phase Structures
Figure 1 shows the XRD patterns of BiVO4 samples synthesized under different conditions. The sample H0 is a heterophase comprising tetragonal zircon phase (JCPDS card No. 14-0133) and monoclinic scheelite phase (JCPDS card No. 14-0688). According to Vmonoclinic = Imonoclinic(121)/(Imonoclinic(121) + Itetragonal(200)) from XRD patterns [12], the percentage of m-BiVO4 is 27.47%. The samples H400, H500, H600, H700, and H800 can be well indexed to the pure monoclinic phase, from which we can see that z-BiVO4 can transform into m-BiVO4 when it is heated beyond 400 °C. It is worth noting that the relative intensities of the diffraction peaks I040/I121 increase obviously with increasing heat treatment temperature, indicating a preferred (040) surface orientation for the BiVO4 crystal growth.
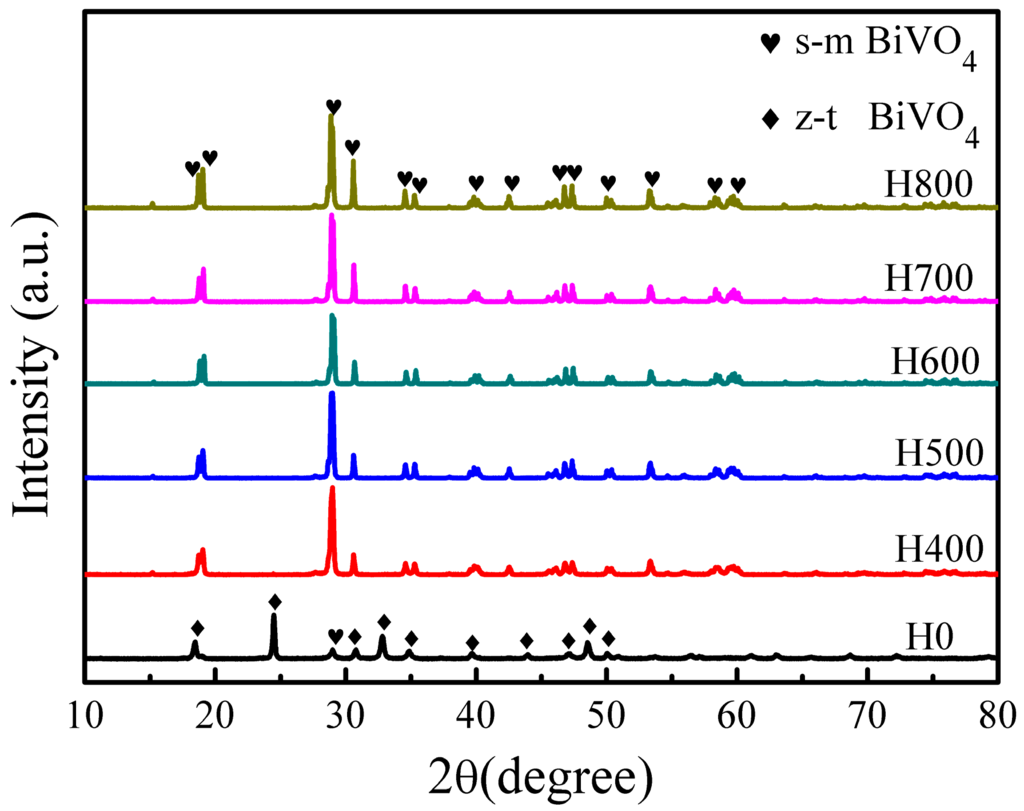
Figure 1.
XRD patterns of the BiVO4 samples synthesized under different conditions.
The average sizes of the crystallites with the (121) peak were calculated using the Scherrer equation (D = Kλ/(βcosθ)), where K is 0.89, λ is the X-ray wavelength (0.154 nm), β is the full width at half maximum, and θ is half of the diffraction angle. The crystal sizes calculated from the (121) peaks increases as heat treatment temperature increases, varying from 55 to 69 nm, as shown in Table 1, indicating that the sample crystallinity improved as heat treatment temperature increases [33].

Table 1.
Some selected properties of BiVO4 samples.
2.2. Raman Spectra
Raman spectroscopy is an appropriate technique for probing the local structure of materials because the bonding states in the coordination polyhedra of a material can be deduced directly from the Raman vibrational spectrum [34]. Thus, Raman spectroscopy was used to elucidate the local structure of the prepared BiVO4 samples, and the results are presented in Figure 2. For the tetragonal zircon type BiVO4 (H0), the most intense Raman band appear at 853 cm−1 is ascribed to the symmetric V-O stretching mode (Ag symmetry), while the weak shoulder at about ca. 759 cm−1 is assigned to the antisymmetric V-O stretching mode (Bg symmetry). The band at 364 cm−1 is attributed to the O-V-O bending mode (Ag symmetry) and the band at 245 cm−1 can be assigned to the Bi-O stretching mode (Eg) [35,36]. For the monoclinic scheelite type BiVO4 samples (H400, H500, H600, H700 and H800), the spectra show a single band at around ca. 827 cm−1 that is normally attributed to the symmetric V-O stretching mode (Ag symmetry), and the bands at around ca. 366 cm−1 and 323 cm−1 are ascribed to the symmetric V-O (Ag symmetry) bending mode and antisymmetric V-O (Bg symmetry) bending mode of VO4 units [34,37], respectively. The external mode (rotation/translation) occurs at 210 cm−1. The V-O bond length can be calculated using the empirical expression: υ [cm−1] = 21349 exp (−1.9176R [Å]) [38], wherein υ is the Raman shift and R is the bond length. The calculated V-O bond length in BiVO4 (s-m) (H700) is 1.696 Å, which is in well agreement with a previous report [34]. The band corresponding to the symmetric V-O (Ag) bending mode in BiVO4 (s-m) appears at 366 cm−1 and is larger than that at 364 cm−1 in BiVO4 (z-t), implying that the bond angle of O-V-O in BiVO4 (s-m) is smaller than that in BiVO4 (z-t) [35]. The Raman spectra reveal that the prepared BiVO4 has two crystal forms, which coincides with the XRD results.
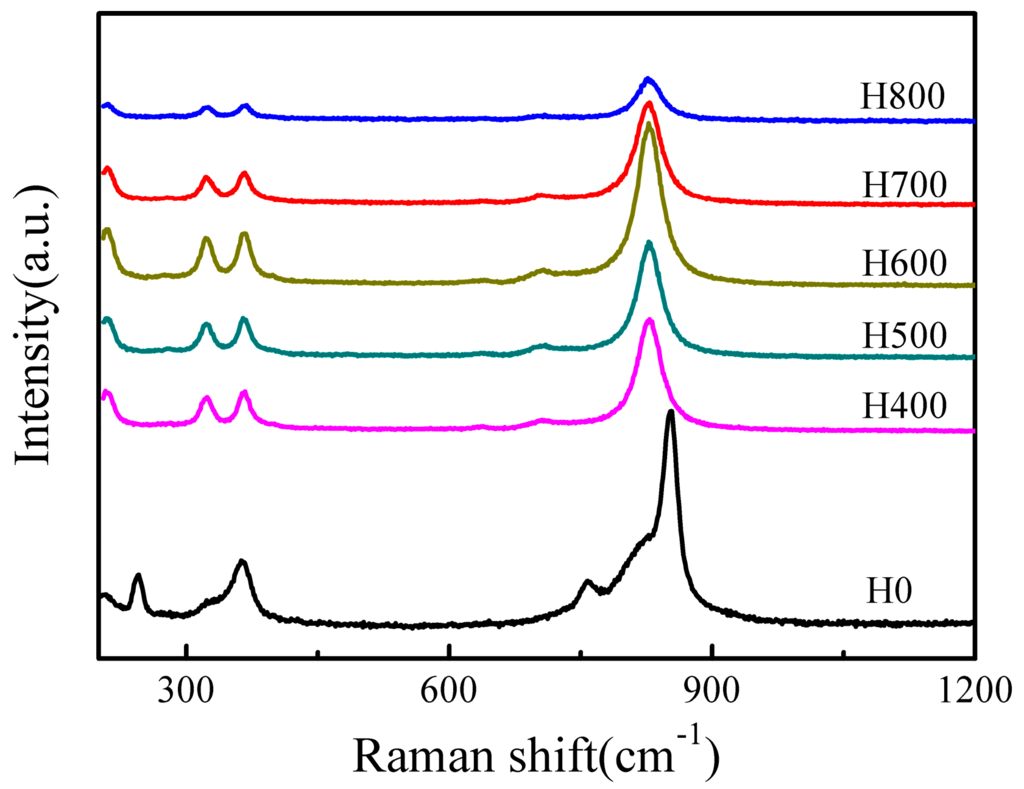
Figure 2.
Raman spectra of the BiVO4 samples synthesized under different conditions.
2.3. Morphologies of BiVO4 Samples
Figure 3 shows the micrographs of the BiVO4 samples observed by SEM, and indicate that heat treatment temperature has a significant influence on the samples morphologies. The sample H0 (Figure 3a) mainly presents a relatively uniform doughnut structure with a diameter of 1.5–2.5 μm formed by small crystals. After heat treatment, the morphology changes drastically. When the heat treatment temperature is 400 °C, the doughnut structure is unchanged compared with H0, but small crystals began to grow (Figure 3b). When the temperature increases further to 500 °C, the doughnut structure is still unchanged, but the crystals grow into larger grains (Figure 3c). Figure 3d shows that when heat treatment temperature is 600 °C, the crystals grow further, and the doughnut structure tends to change to form particles. Large particles with dimensions in the range of 1–3 μm are observed following heat treatment at 700 °C (Figure 3e). It can be seen from the TEM image (Figure 4b) that the sample H700 shows a piece of materials, and the clear lattice fringes (Figure 4c) are indication of high crystallinity. The d-spacing was measured to be 0.308 nm, which agreed well with (121) plane spacing of m-BiVO4. At higher temperature (800 °C), the particles grow larger still with the largest size observed being ca. 6 μm (Figure 3f). The small irregular particles on the surfaces of particles in Figure 3e,f are derived from the grind process.
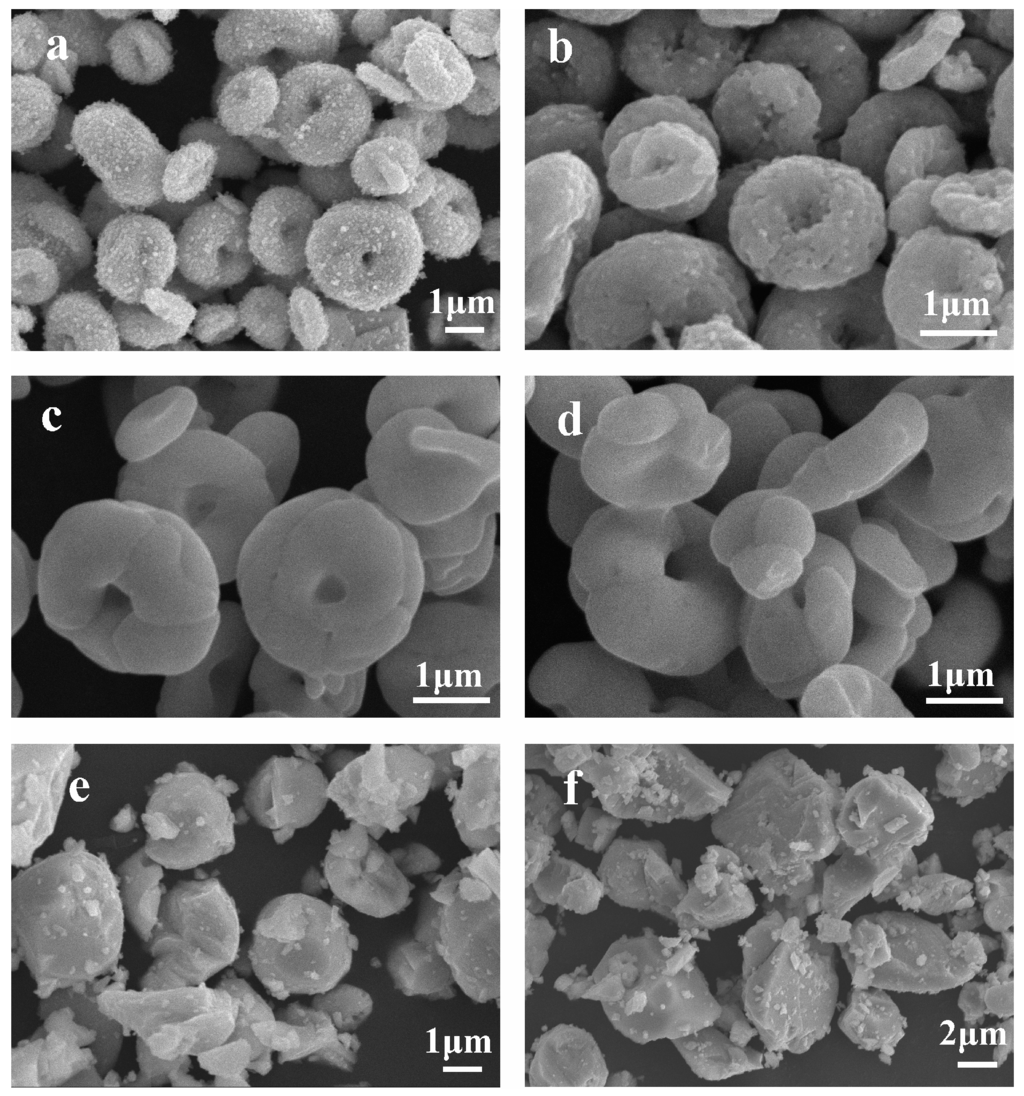
Figure 3.
Typical SEM images of: (a) H0; (b) H400; (c) H500; (d) H600; (e) H700; and (f) H800.
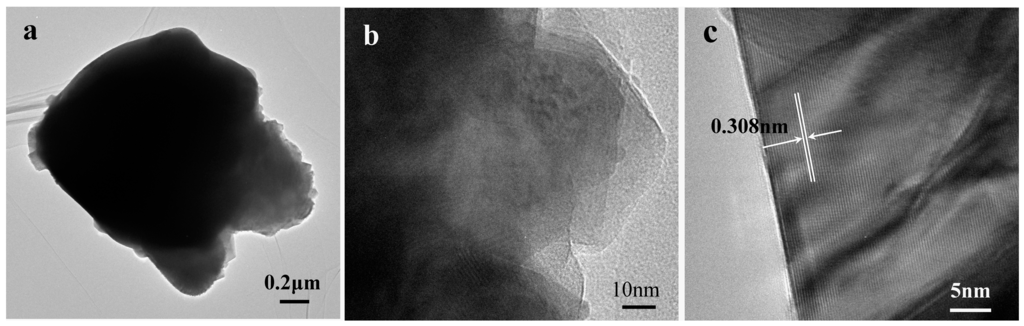
Figure 4.
(a,b) TEM images and (c) HRTEM image of sample H700.
The BET specific surface areas of the BiVO4 samples synthesized under different conditions are summarized in Table 1. The specific surface area varies as heat treatment temperature changes, because the samples morphologies depended on the heat treatment temperature, and the morphology affects the specific surface area [39].
2.4. UV-vis Diffuse Reflectance Spectra (UV-vis DRS)
In order to investigate the light absorption properties of the as-obtained BiVO4, UV-vis diffuse reflectance spectroscopy (UV-vis DRS) was conducted, as shown in Figure 5. All samples exhibit strong absorption in the visible-light region, and the steep shapes of the spectra suggest that the visible or UV-light adsorption can be attributed to the intrinsic transition between the valence band (VB) and the conduction band (CB), rather than to the transition from the impurity to the conduction band [40]. It is worth highlighting that the higher absorption intensity of some samples is due to scattering phenomena linked to the sample morphology [6]. The band gap energy can be calculated by the equation αhυ = A (hυ − Eg)1/2, wherein α is the absorption coefficient, hυ is the photon energy, A is a constant (A = 1), and Eg is the band gap energy. The band gap energy can be estimated from a plot of (αhυ)2 versus hυ, as shown in Figure 5b, and the intercept of the tangent to the x-axis gives a good approximation the band gap energy. Accordingly, the band gap energies of samples H0, H400, H500, H600, H700 and H800 are ca. 2.68, 2.41, 2.38, 2.36, 2.34 and 2.35 eV, respectively, as shown in Table 1, which are all characteristic of the band gap for the monoclinic phase of BiVO4 (except that of H0, because H0 is a heterophase comprising tetragonal zircon phase and monoclinic scheelite phase). The difference in band gap energy demonstrates that the electronic structures of the samples change with heat treatment temperature, and the variations in the electronic structures lead to different degrees for the delocalization of photogenerated carriers, resulting in their different mobility efficiencies.
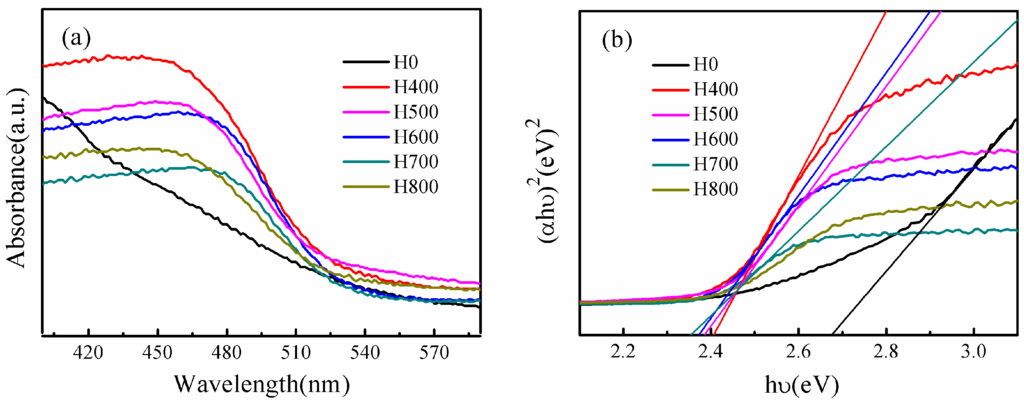
Figure 5.
(a) UV-vis diffuse reflectance spectra, (b) Plots of(αhυ)2 versus photon energy (hυ) of BiVO4 synthesized under different conditions.
2.5. PL Analysis
Photoluminescence (PL) spectra have been widely used to disclose the migration, transfer, and recombination processes of the photogenerated electron–hole pairs in the semiconductor particles [41], PL emission mainly originates from the recombination of excited electrons and holes. Therefore, a low PL intensity implies a low electron–hole recombination rate under light irradiation [42]. Figure 6 shows the room temperature PL spectra of BiVO4 synthesized under different conditions. It is obvious that the normalized PL intensity of these samples decreases in the order of H400 > H500 > H600 > H800 > H700. The PL intensity of the H700 sample is the lowest, indicating that the retarded recombination of the holes formed in the hybrid orbitals of Bi6s and O2p (VB) and the electrons in the V3d orbitals (CB), which can lead to an enhanced photocatalytic activity.
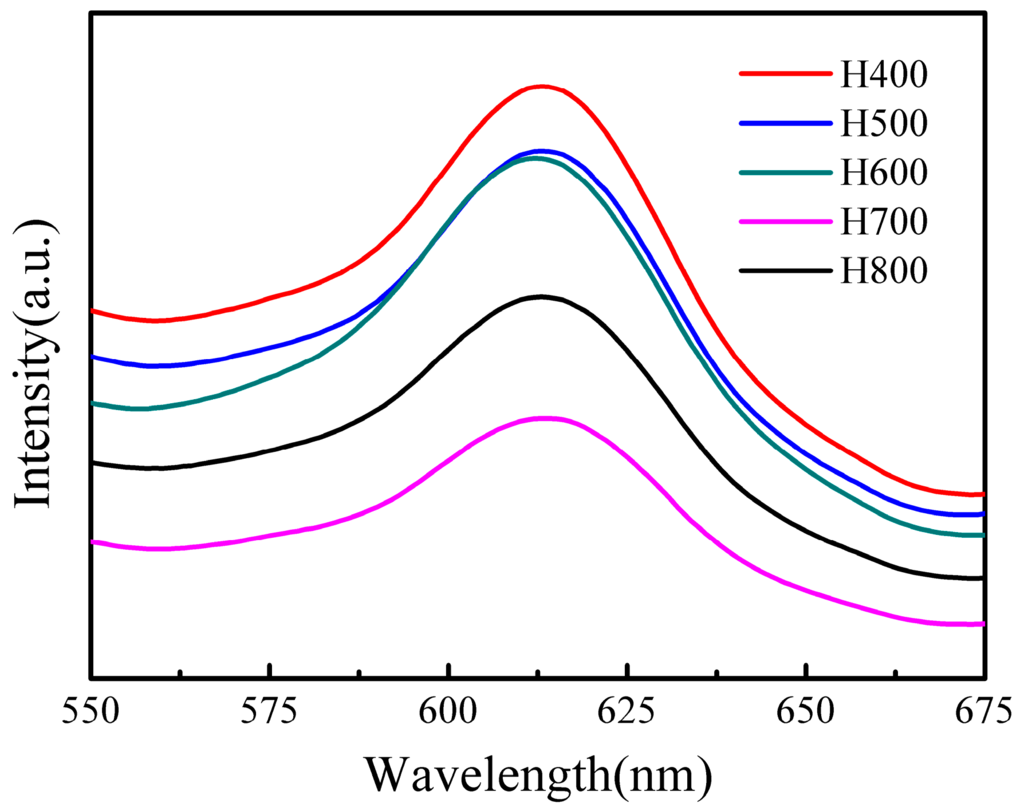
Figure 6.
Room temperature PL spectra of BiVO4 synthesized under different conditions.
2.6. Photocatalytic Activity and Recycling Performance
The photocatalytic performances of the BiVO4 samples were investigated by MB degradation under visible light irradiation, the results of which are shown in Figure 7. Among all of the samples, H700 exhibits the best MB degradation efficiency, and 100% of the MB is degraded after 60 min, while 66.0%, 73.4%, 84.9%, and 97.0% of the MB is degraded after 60 min in the presence of H400, H500, H600, and H800, respectively. All samples exhibit much better photocatalytic activities than that of TiO2 photocatalyst (P25), which degrades only 9.6% of the MB after 60 min. It is worth noting that the adsorption efficiency of H0 is very high (ca. 85.6% in the dark), but its MB degradation efficiency is negligible.
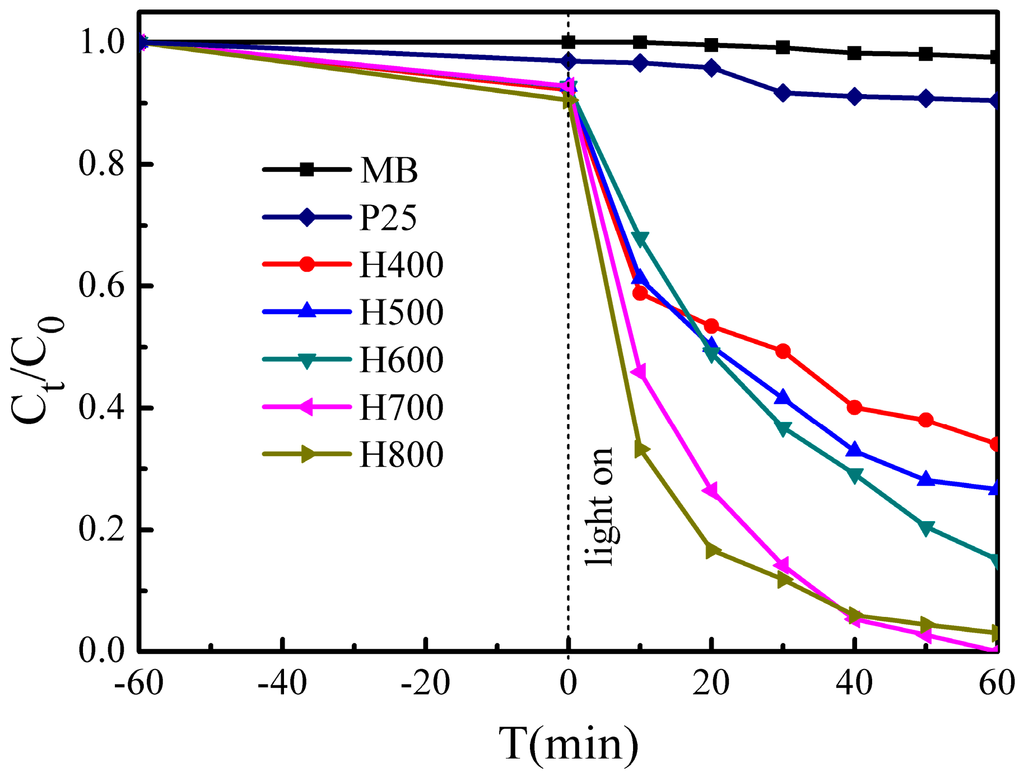
Figure 7.
Photocatalytic activity of samples for MB degradation under visible light irradiation.
We can see that the photocatalytic performance increases with increasing heat treatment temperature until 700 °C, however, when the temperature increased further to 800 °C, the photocatalytic performance decreases. This is because the crystallinity degree increases with increasing the heat treatment temperature, and a highly crystalline structure suppresses the recombination of photogenerated electron–hole pairs [39]; but the surface area of H800 (3.2 m2·g−1) is smaller than that of H700 (4.9 m2·g−1), and the high surface area supplies more active sites for the degradation of MB, and promotes the electron–hole pairs separation effectively [43]. Furthermore, H700 exhibits the lowest band gap energy, and the lower the band gap energy, the higher the photocatalytic performance [44].
These results reflect the discrepancy in the photocatalytic performance of the samples. Therefore, these observations indicate that the crystallinity, BET specific surface area, and band gap energy should be considered as having a combined effect on the photocatalytic performance.
The stability and reusability of a catalyst is important for its practical applications, and thus the photo-stability of the synthesized H700 photocatalyst was evaluated in repeated photocatalytic experiments under the same reaction conditions. Figure 8 shows the MB degradation efficiency during each 60 min cycle. The results show that the photocatalyst exhibits no loss of activity over five cycling runs. The H700 photocatalyst used in the cycling tests was characterized using SEM and XRD before and after the cycling experiments. The corresponding XRD (Figure 9a) and SEM micrograph (Figure 9b) show that the catalysts exhibit no observable changes in crystal structure and morphology. These results suggest that the as-obtained BiVO4 sample does not suffer from photo corrosion, and has excellent stability and reusability for the degradation process.
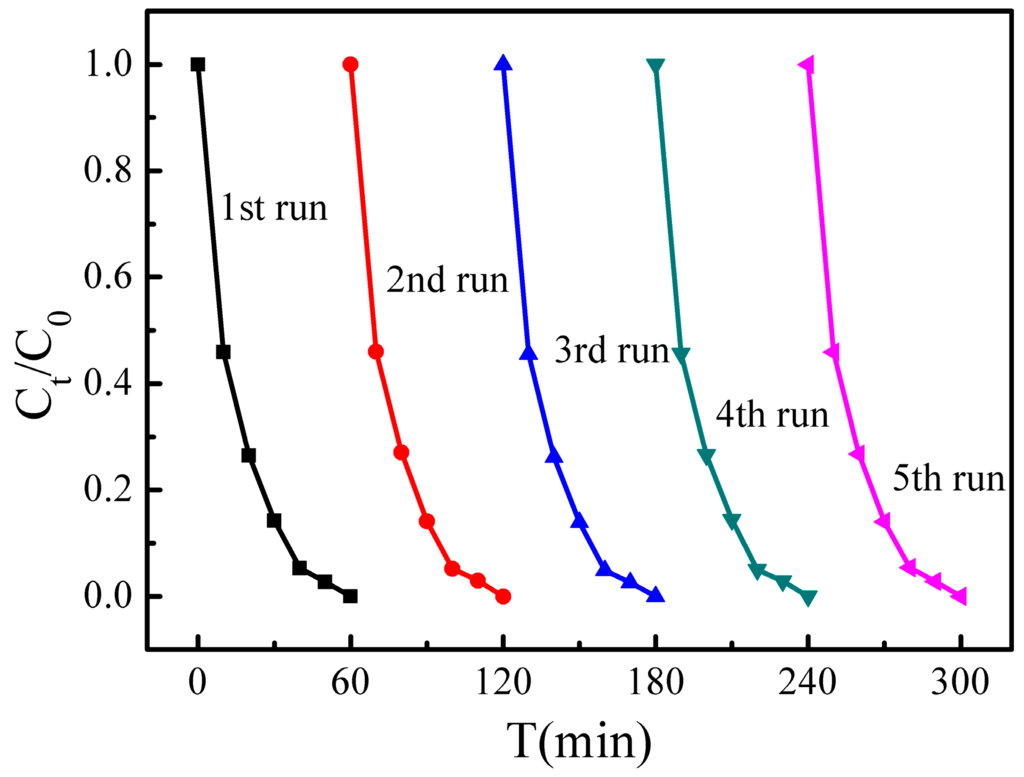
Figure 8.
Cyclic photocatalytic degradation experiments of MB by H700 photocatalyst.
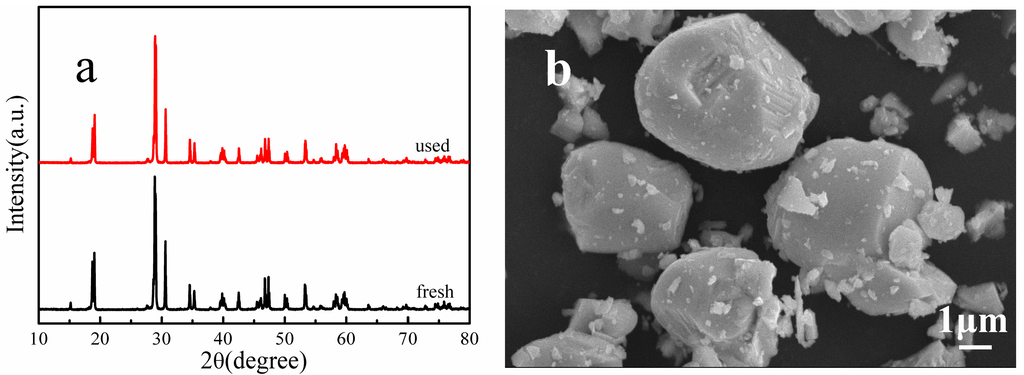
Figure 9.
(a) XRD patterns and (b) SEM micrograph of sample H700 before and after the photo-stability tests.
2.7. Trapping Experiments
We performed reactive species trapping experiments to detect the main oxidative species (h+, •O2− and •OH) in the photocatalytic reaction to further clarify the photocatalytic mechanism for BiVO4. In this study, tert-butanol (t-BuOH, a •OH radical scavenger), p-benzoquinone (BZQ, an •O2− radical scavenger), and disodium ethylene diamine tetra acetate (Na2-EDTA, a hole scavenger) were used [45,46]. As shown in Figure 10, when t-BuOH is added, the photocatalytic activity does not change, implying that •OH radicals are not the main oxidative species in this process. However, the introduction of Na2-EDTA reduces the photocatalytic activity from 100% to 52.3% in 60 min, and the presence of BZQ also causes deactivation of the H700 photocatalyst. These results suggest that h+ and •O2− radicals play a dominant role in the BiVO4 system, and •OH radicals are not involved.
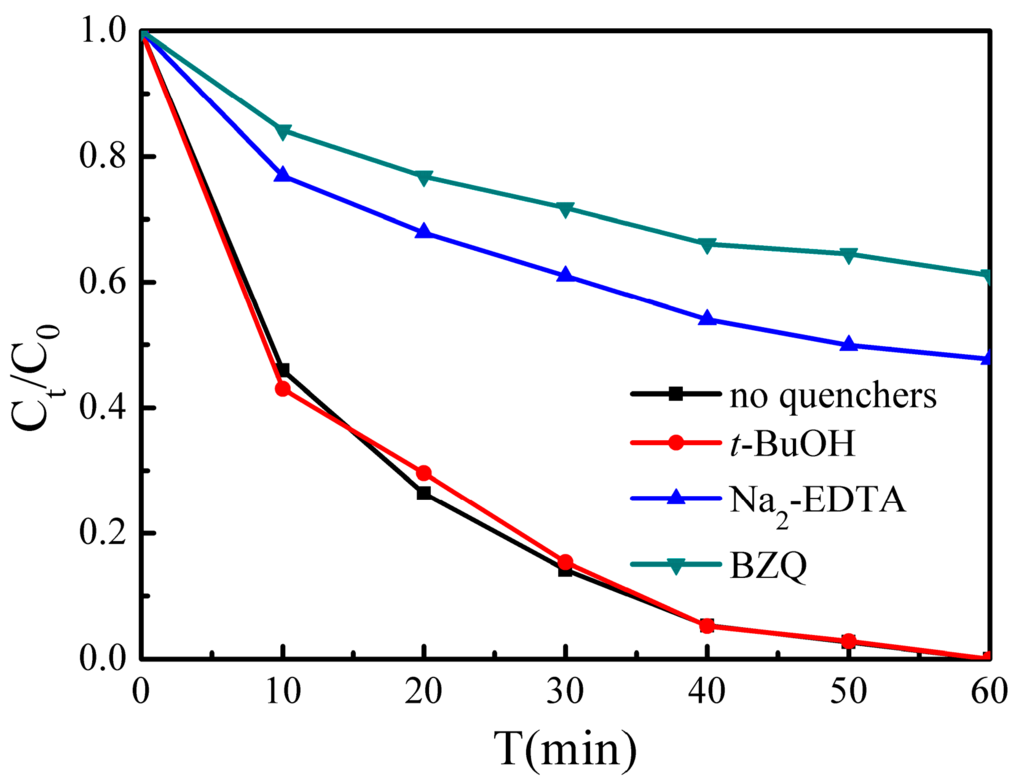
Figure 10.
Reactive species trapping experiments of MB over H700 photocatalyst under visible light irradiation.
Based on the reactive species trapping experiment results, a proposed mechanism for MB photocatalytic degradation by BiVO4 photocatalysts is shown in Figure 11. Under visible light irradiation, photoinduced holes (h+) are generated in the VB by the photoinduced transfer of electrons (e−) from the VB to the CB. The e− are scavenged by oxygen to form •O2− radicals, which can degrade MB effectively. Meanwhile, the h+ in the VB can oxidize MB to its degradation products directly.
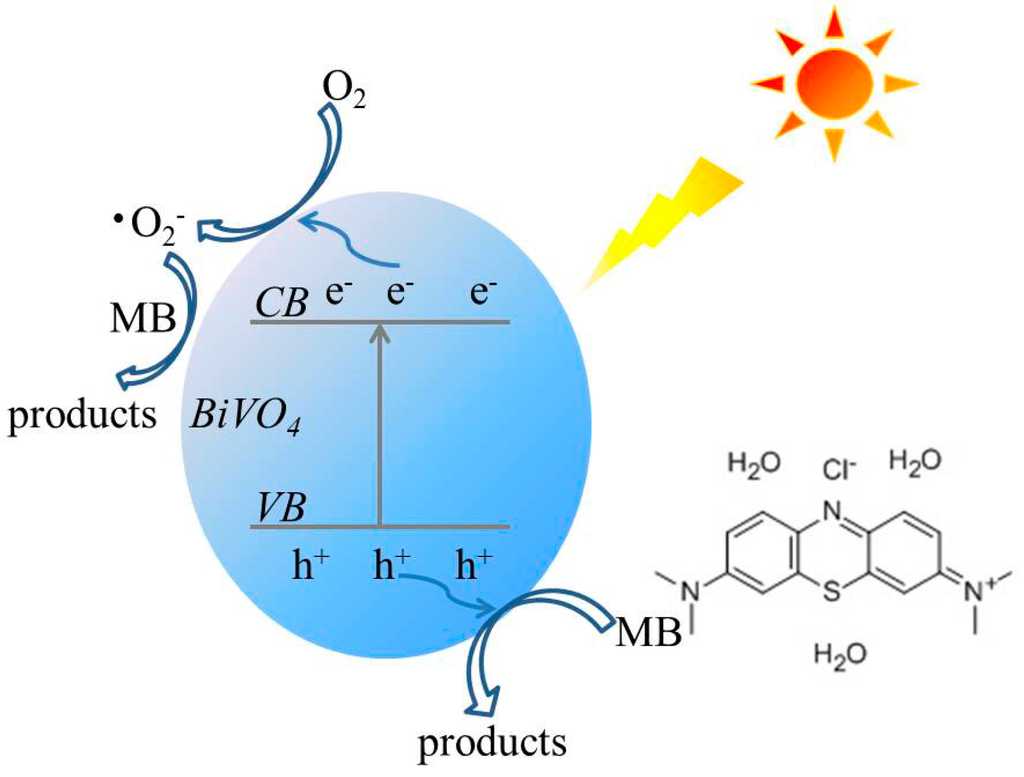
Figure 11.
The schematic diagram of charge separation and photocatalytic process of the BiVO4 photocatalysts under visible light irradiation.
3. Materials and Methods
3.1. Catalyst Preparation
All reagents and solvents were purchased from commercial sources and used without further purification. Bi(NO3)3·5H2O, NH4VO3, and acetic acid (36%) were purchased from Sinopharm Chemical Reagent, China.
In a typical synthesis procedure, 5 mmol Bi(NO3)3·5H2O was dissolved in 25 mL acetic acid (36%) under magnetic stirring for 30 min at room temperature to form solution A; 5 mmol NH4VO3 was dissolved in 25 mL deionized water under magnetic stirring for 30 min at 70 °C to form solution B. Solution B was added dropwise into solution A under stirring. Subsequently, the mixture was put into a water bath and heated at 90 °C for 24 h. After completion of the reaction, the precipitates were filtered, repeatedly washed with deionized water and absolute ethanol, and dried overnight at 60 °C. Then, the obtained sample was heated to 400, 500, 600, 700 and 800 °C for 2 h with a ramp rate of 5 °C·min−1 via muffle furnace, which can be denoted as H400, H500, H600, H700, and H800, respectively, and the sample without heat treatment is named as H0.
3.2. Characterization
The crystal structures of the photocatalysts were determined by X-ray diffraction (XRD, Rigaku Ultima III, Toyko, Japan). The visible light Raman spectra were recorded using a RENISHAW Raman microscope (Raman, INVIA, London, UK) using 633 nm lasers. The morphologies were investigated by scanning electron microscopy (SEM, Hitachi S-3400N II, Toyko, Japan) and transmission electron microscopy (TEM, JEM2100F, Japan). The Brunauer–Emmett–Teller (BET) specific surface areas (SBET) were obtained from N2 adsorption–desorption isotherms determined at liquid N2 temperature on an automatic analyzer (ASAP 2020M, Atlanta, GA, USA). Ultraviolet-visible diffuse reflectance spectra (UV-vis DRS) was performed using a UV-vis spectrometer (Shimadzu UV-2550, Toyko, Japan) with BaSO4 as a reference. The photoluminescence (PL) spectra of photocatalysts were recorded using Fluorescence Spectrophotometer (FP-6500, Toyko, Japan) equipped with a Xe lamp, at an excitation wavelength of 355 nm.
3.3. Photocatalytic Activity
The photocatalytic activities of the samples were evaluated based on MB decolorization using a 350 W Xe-illuminator as the light source. A 420 nm cut-off filter was placed between the Xe-illuminator and the reactor. The powder (0.1 g) was added to 100 mL of MB solution with a concentration of 10 mg/L. The solution was stirred in the dark for 60 min to achieve adsorption–desorption equilibrium between the MB molecules and catalyst particles prior to light irradiation. At various irradiation time intervals, 3 mL suspensions were collected, and centrifuged to remove the photocatalyst particles. The concentrations of the remnant MB were then monitored using a UV-vis spectrometer (Shimadzu UV-2550, Toyko, Japan) at a wavelength of 665 nm.
Cycling experiments were conducted to evaluate the stability of the BiVO4 photocatalyst. After each cycle, the samples were washed with deionized water and absolute ethanol, and then added to fresh MB solution after dried, followed by another cycle.
To explore the decomposition mechanism, reactive species trapping experiments were conducted, in which tert-butanol (t-BuOH, a •OH radical scavenger), p-benzoquinone (BZQ, an •O2− radical scavenger), and disodium ethylene diamine tetra acetate (Na2-EDTA, a hole scavenger) were added, respectively, into the MB solution prior to addition of the photocatalyst.
4. Conclusions
In summary, BiVO4 samples have been successful synthesized by a surfactant free aqueous method with heat treatment, and the temperature of the heat treatment strongly affected the crystal phases, morphology, crystallinity, BET specific surface area, and band gap energy of the samples. The photocatalytic activities of the synthetic BiVO4 samples were influenced by the combined effect of their crystallinity, BET specific surface area, and band gap energy. When the heat treatment temperature was 700 °C, the obtained sample showed the best photocatalytic activity. In addition, the BiVO4 photocatalysts exhibited excellent stability and reusability, and photogenerated holes (h+) and •O2− radicals are the predominant oxidative species in the BiVO4 system.
Acknowledgments
The authors acknowledge the financial support from the National Science Foundation of China (U12301013) and the National Science Foundation of China (51521001).
Author Contributions
Weimin Wang and Minna Guo conceived and designed the experiments; Minna Guo performed the experiments; Minna Guo and Qianglong He analyzed the data; Aiyang Wang and Zhengyi Fu contributed reagents/materials/analysis tools; and Minna Guo wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, X.X.; Wang, T.; Zhang, P.; Zhang, J.J.; Li, A.; Gong, J.L. Enhanced surface reaction kinetics and charge separation of p-n heterojunction Co3O4/BiVO4 photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.M.; Dai, H.X.; Deng, J.G.; Zang, H.J.; Arandiyan, H.; Xie, S.H.; Yang, H.G. 3DOM BiVO4 supported silver bromide and noble metals: High-performance photocatalysts for the visible-light-driven degradation of 4-chlorophenol. Appl. Catal. B Environ. 2015, 168–169, 274–282. [Google Scholar] [CrossRef]
- Li, H.Y.; Sun, Y.J.; Cai, B.; Gan, S.Y.; Han, D.X.; Niu, L.; Wu, T.S. Hierarchically Z-scheme photocatalyst of Ag@AgCl decorated on BiVO4 (040) with enhancing photoelectrochemical and photocatalytic performance. Appl. Catal. B Environ. 2015, 170–171, 206–214. [Google Scholar] [CrossRef]
- Gu, S.N.; Li, W.J.; Wang, F.Z.; Wang, S.Y.; Zhou, H.L.; Li, H.D. Synthesis of buckhorn-like BiVO4 with a shell of CeOx nanodots: Effect of heterojunction structure on the enhancement of photocatalytic activity. Appl. Catal. B Environ. 2015, 170–171, 186–194. [Google Scholar] [CrossRef]
- Hernandez, S.; Barbero, G.; Saracco, G.; Alexe-Ionescu, A.L. Considerations on oxygen bubble formation and evolution on BiVO4 porous anodes used in water splitting photoelectrochemical cells. J. Phys. Chem. C 2015, 119, 9916–9925. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Hernández, S.; Bensaid, S.; Saracco, G.; Russo, N. Green-synthesized W- and Mo-doped BiVO4 oriented along the {040} facet with enhanced activity for the sun-driven water oxidation. Appl. Catal. B Environ. 2016, 180, 630–636. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, P.F.; Zhao, L.; Wu, J.; Qi, X.M.; Yao, W.F. Photocatalytic behavior of BiVO4 immobilized on silica fiber via a combined alcohol-thermal and carbon nanofibers template route. Catal. Commun. 2014, 49, 29–33. [Google Scholar] [CrossRef]
- Guo, M.N.; Wang, Y.; He, Q.L.; Wang, W.J.; Wang, W.M.; Fu, Z.Y.; Wang, H. Enhanced photocatalytic activity of S-doped BiVO4 photocatalysts. RSC Adv. 2015, 5, 58633–58639. [Google Scholar] [CrossRef]
- Fan, H.M.; Jiang, T.F.; Li, H.Y.; Wang, D.J.; Wang, L.L.; Zhai, J.L.; He, D.Q.; Wang, P.; Xie, T.F. Effect of BiVO4 crystalline phases on the photoinduced carriers behavior and photocatalytic activity. J. Phys. Chem. C 2012, 116, 2425–2430. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, M.L.; Huang, Y.; Lin, J.M.; Wu, J.H. The synthesis of bismuth vanadate powders and their photocatalytic properties under visible light irradiation. J. Alloys Compd. 2010, 496, 287–292. [Google Scholar] [CrossRef]
- Ke, D.N.; Peng, T.Y.; Ma, L.; Cai, P.; Dai, K. Effects of hydrothermal temperature on the microstructures of BiVO4 and its Photocatalytic O2 evolution activity under visible light. Inorg. Chem. 2009, 48, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.Z.; Wang, J.; Liang, Y.J. Controlled fabrication and enhanced photocatalystic performance of BiVO4@CeO2 hollow microspheres for the visible-light-driven degradation of rhodamine B. Appl. Surf. Sci. 2015, 349, 529–537. [Google Scholar] [CrossRef]
- Bierlein, J.D.; Sleight, A.W. Ferroelasticity in BiVO4. Solid State Commun. 1975, 16, 69–70. [Google Scholar] [CrossRef]
- Sun, J.X.; Chen, G.; Wu, J.Z.; Dong, H.J.; Xiong, G.H. Bismuth vanadate hollow spheres: Bubble template synthesis and enhanced photocatalytic properties for photodegradation. Appl. Catal. B Environ. 2013, 132–133, 304–314. [Google Scholar] [CrossRef]
- Dong, S.Y.; Feng, J.L.; Li, Y.K.; Hu, L.M.; Liu, M.L.; Wang, Y.F.; Pi, Y.Q.; Sun, J.Y.; Sun, J.H. Shape-controlled synthesis of BiVO4 hierarchical structures with unique natural-sunlight-driven photocatalytic activity. Appl. Catal. B Environ. 2014, 152–153, 413–424. [Google Scholar] [CrossRef]
- Wang, W.Z.; Huang, X.W.; Wu, S.; Zhou, Y.X.; Wang, L.J.; Shi, H.L.; Liang, Y.J.; Zou, B. Preparation of p-n junction Cu2O/BiVO4 heterogeneous nanostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2013, 134–135, 293–301. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. Excellent photocatalytic activity of Yb3+, Er3+ co-doped BiVO4 photocatalyst. Appl. Catal. B Environ. 2014, 152–153, 328–334. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.Z.; Liu, S.W.; Zhang, L.S.; Xu, H.L.; Zhu, W. A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst. J. Mol. Catal. A Chem. 2006, 252, 120–124. [Google Scholar] [CrossRef]
- Shan, L.W.; Wang, G.L.; Suriyaprakash, J.; Li, D.; Liu, L.Z.; Dong, L.M. Solar light driven pure water splitting of B-doped BiVO4 synthesized via a sol-gel method. J. Alloys Comp. 2015, 636, 131–137. [Google Scholar] [CrossRef]
- Ou, M.; Zhong, Q.; Zhang, S.L. Synthesis and characterization of g-C3N4/BiVO4 composite photocatalysts with improved visible-light-driven photocatalytic performance. J. Sol-Gel Sci. Technol. 2014, 72, 443–454. [Google Scholar] [CrossRef]
- Shan, L.W.; Liu, H.G.; Wang, G.L. Preparation of tungsten-doped BiVO4 and enhanced photocatalytic activity. J. Nanopart. Res. 2015, 17, 181. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Y.Q.; Cao, L.X.; Su, G.; Liu, X.Y.; Zhang, L.; Wang, Y.G. Synthesis of monoclinic structured BiVO4 spindly microtubes in deep eutectic solvent and their application for dye degradation. J. Hazard. Mater. 2010, 181, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sun, S.F.; Song, Y.; Yan, X.; Guan, W.S.; Liu, X.L.; Shi, W.D. Microwave-assisted in situ synthesis of reduced graphene oxide-BiVO4 composite photocatalysts and their enhanced photocatalytic performance for the degradation of ciprofloxacin. J. Hazard. Mater. 2013, 250–251, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Obregón, S.; Colón, G. Heterostructured Er3+ doped BiVO4 with exceptional photocatalytic performance by cooperative electronic and luminescence sensitization mechanism. Appl. Catal. B Environ. 2014, 158–159, 242–249. [Google Scholar] [CrossRef]
- Jiang, H.Q.; Endo, H.; Natori, H.; Nagai, M.; Kobayashi, K. Fabrication and photoactivities of spherical-shaped BiVO4 photocatalysts through solution combustion synthesis method. J. Eur. Ceram. Soc. 2008, 28, 2955–2962. [Google Scholar] [CrossRef]
- Yu, J.Q.; Zhang, Y.; Kudo, A. Synthesis and photocatalytic performances of BiVO4 by ammonia co-precipitation process. J. Solid State Chem. 2009, 182, 223–228. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.F.; Liu, Z.S.; Dai, C.H.; Song, Z.W.; Sun, Y.; Fang, J.R.; Zhao, J.G. Low-temperature synthesis of BiVO4 crystallites in molten salt medium and their UV-vis absorption. Ceram. Int. 2010, 36, 2073–2077. [Google Scholar] [CrossRef]
- Chung, C.Y.; Lu, C.H. Reverse-microemulsion preparation of visible-light-driven nano-sized BiVO4. J. Alloys Comp. 2010, 502, L1–L5. [Google Scholar] [CrossRef]
- Akihiko, K.; Keiko, O.; Hideki, K. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar]
- Kohtani, S.; Makino, S.; Kudo, A.; Tokumura, K.; Ishigaki, Y.; Matsunaga, T.; Nikaido, O.; Hayakawa, K.; Nakagaki, R. Photocatalytic degradation of 4-n-Nonylphenol under irradiation from solar simulator: Comparison between BiVO4 and TiO2 photocatalysts. Chem. Lett. 2002, 31, 600–601. [Google Scholar] [CrossRef]
- Yin, W.Z.; Wang, W.Z.; Zhou, L.; Sun, S.M.; Zhang, L. CTAB-assisted synthesis of monoclinic BiVO4 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation. J. Hazard. Mater. 2010, 173, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Kho, Y.K.; Teoh, W.Y.; Iwase, A.; Madler, L.; Kudo, A.; Amal, R. Flame preparation of visible-light-responsive BiVO4 oxygen evolution photocatalysts with subsequent activation via aqueous route. ACS Appl. Mater. Interfaces 2011, 3, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Kudo, A. Effects of structural variation on the photocatalytic performance of hydrothermally synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Li, G.Q.; Bai, Y.; Zhang, W.F. Difference in valence band top of BiVO4 with different crystal structure. Mater. Chem. Phys. 2012, 136, 930–934. [Google Scholar] [CrossRef]
- Yi, J.; Zhao, Z.Y.; Wang, Y.A.; Zhou, D.C.; Ma, C.S.; Cao, Y.C.; Qiu, J.B. Monophasic zircon-type tetragonal Eu1-xBixVO4 solid-solution: Synthesis, characterization, and optical properties. Mater. Res. Bull. 2014, 57, 306–310. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, X.; Liu, H.J.; Qu, J.H.; Huang, C.P. Synthesis of visible-light sensitive M-BiVO4 (M=Ag, Co, and Ni) for the photocatalytic degradation of organic pollutants. Sep. Purif. Tech. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- Hardcastlet, F.D.; Wachs, I.E. Molecular structure of molybdenum oxide in bismuth molybdates by raman spectroscopy. J. Phys. Chem. 1991, 95, 5031. [Google Scholar]
- Zhou, Y.; Vuille, K.; Heel, A.; Probst, B.; Kontic, R.; Patzke, G.R. An inorganic hydrothermal route to photocatalytically active bismuth vanadate. Appl. Catal. A Gen. 2010, 375, 140–148. [Google Scholar] [CrossRef]
- Kudo, A.; Tsuji, I.; Kato, H. AgInZn7S9 solid solution photocatalyst for H2 evolution from aqueous solutions under visible light irradiation. Chem. Commun. 2002, 1958–1959. [Google Scholar] [CrossRef]
- Chang, W.S.; Li, Y.C.M.; Chung, T.W.; Lin, Y.S.; Huang, C.M. Toluene decomposition using silver vanadate/SBA-15 photocatalysts: DRIFTS study of surface chemistry and recyclability. Appl. Catal. A Gen. 2011, 407, 224–230. [Google Scholar] [CrossRef]
- Pan, G.T.; Huang, C.M.; Peng, P.Y.; Yang, T.C.K. Nano-scaled silver vanadates loaded on mesoporous silica: Characterization and photocatalytic activity. Catal. Today 2011, 164, 377–383. [Google Scholar] [CrossRef]
- Li, G.S.; Zhang, D.Q.; Yu, J.C. Ordered mesoporous BiVO4 through nanocasting: A superior visible light-driven photocatalyst. Chem. Mater. 2008, 20, 3983–3992. [Google Scholar] [CrossRef]
- Yao, M.M.; Liu, M.X.; Gan, L.H.; Zhao, F.Q.; Fan, X.Z.; Zhu, D.Z.; Xu, Z.J.; Hao, Z.X.; Chen, L.W. Monoclinic mesoporous BiVO4: Synthesis and visible-light-driven photocatalytic property. Colloids Surf. A Physicochem. Eng. Aspects 2013, 433, 132–138. [Google Scholar] [CrossRef]
- Lei, L.W.; Jin, H.H.; Zhang, Q.; Xu, J.; Gao, D.; Fu, Z.Y. A novel enhanced visible-light-driven photocatalyst via hybridization of nanosized BiOCl and graphitic C3N4. Dalton Trans. 2015, 44, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Yu, Y.; Liu, J.H.; Ma, P.Y.; Wang, Y.C.; Zhang, F.; Fu, Z.Y. Space-confined growth of Ag3PO4 nanoparticles within WS2 sheets: Ag3PO4/WS2 composites as visible-light-driven photocatalysts for decomposing dyes. J. Mater. Chem. A 2015, 3, 19439–19444. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).