Adsorption, Desorption, Surface Diffusion, Lattice Defect Formation, and Kink Incorporation Processes of Particles on Growth Interfaces of Colloidal Crystals with Attractive Interactions
Abstract
:1. Introduction
2. Results and Discussion
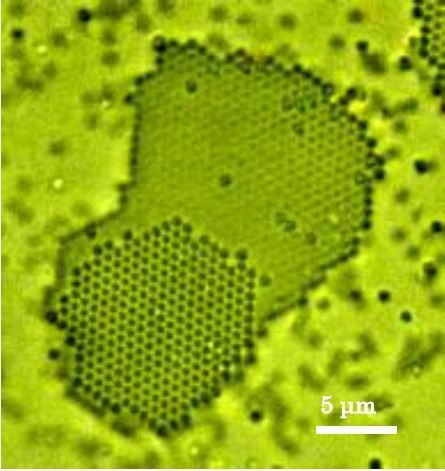
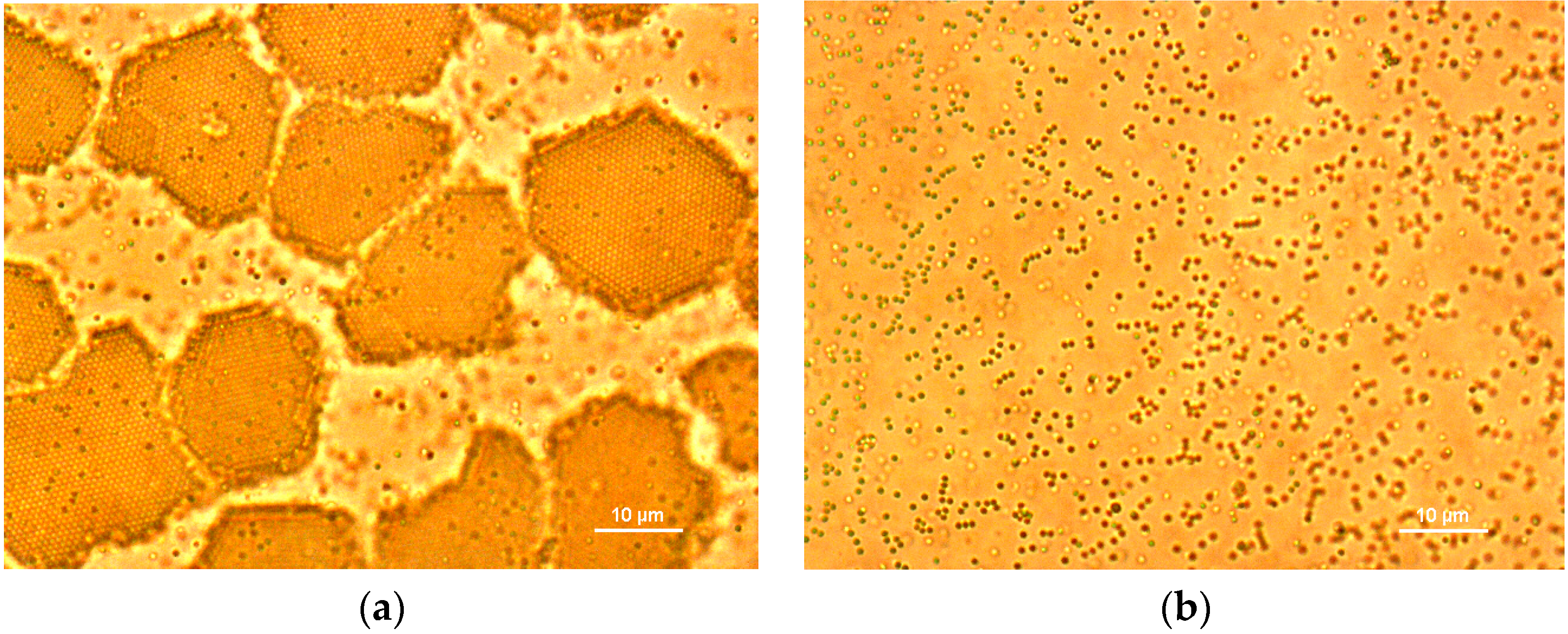
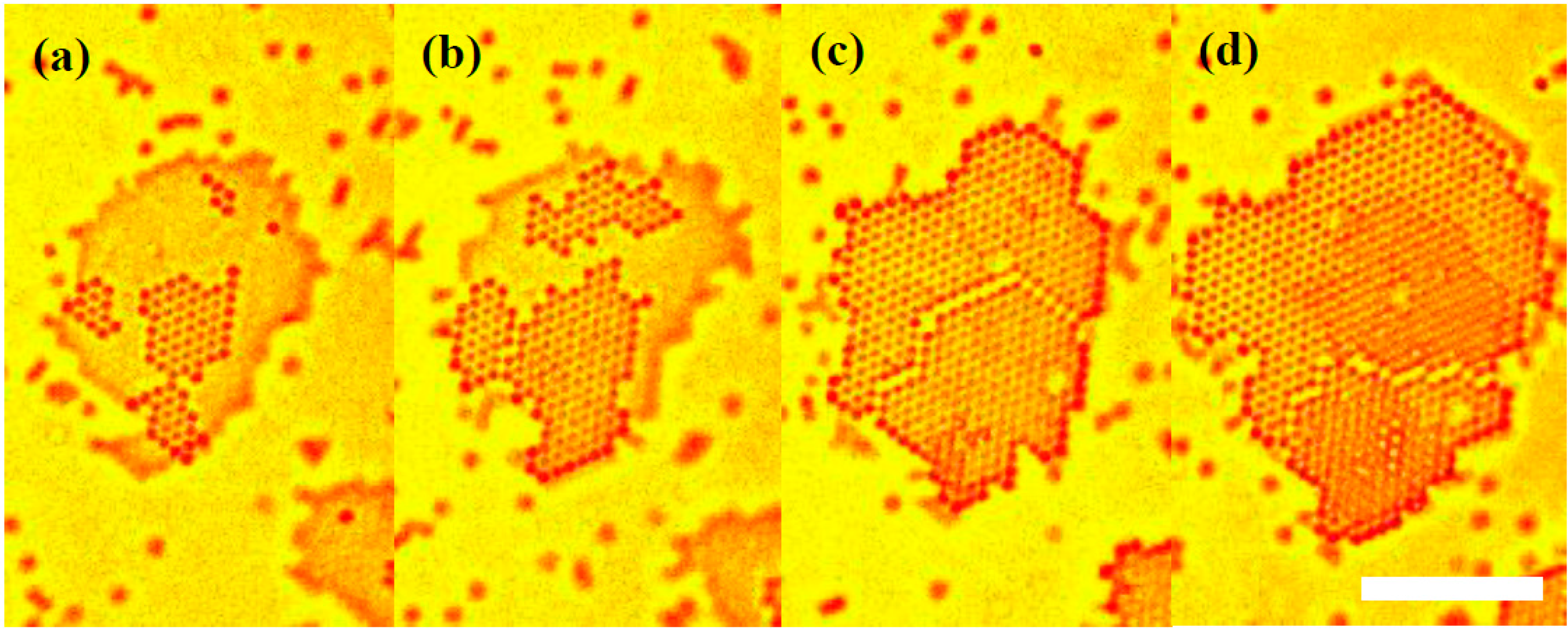
2.1. In Situ Observation of Growth Interface of Crystals
2.2. Equilibrium Conditions
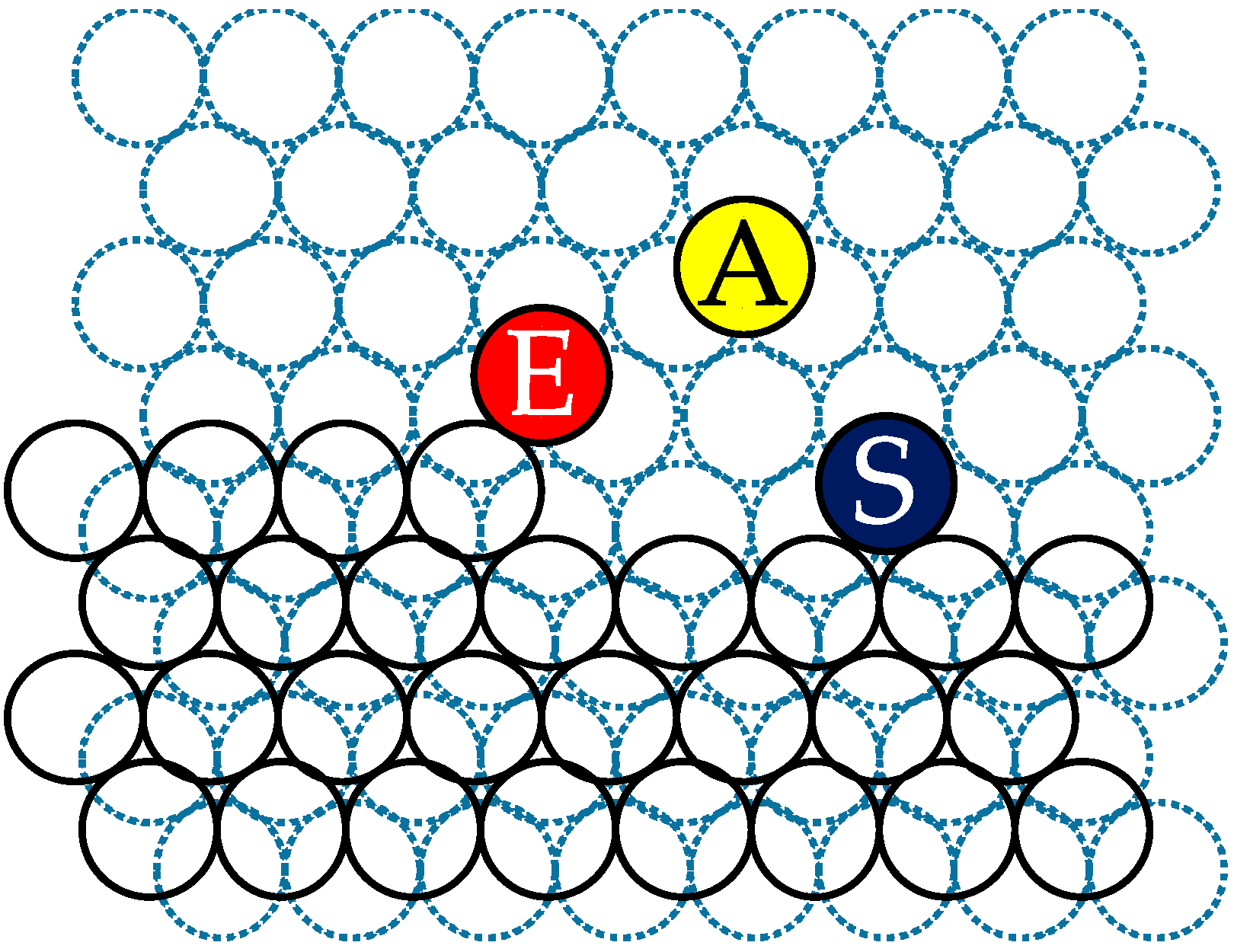
2.3. Surface Diffusion of Particles
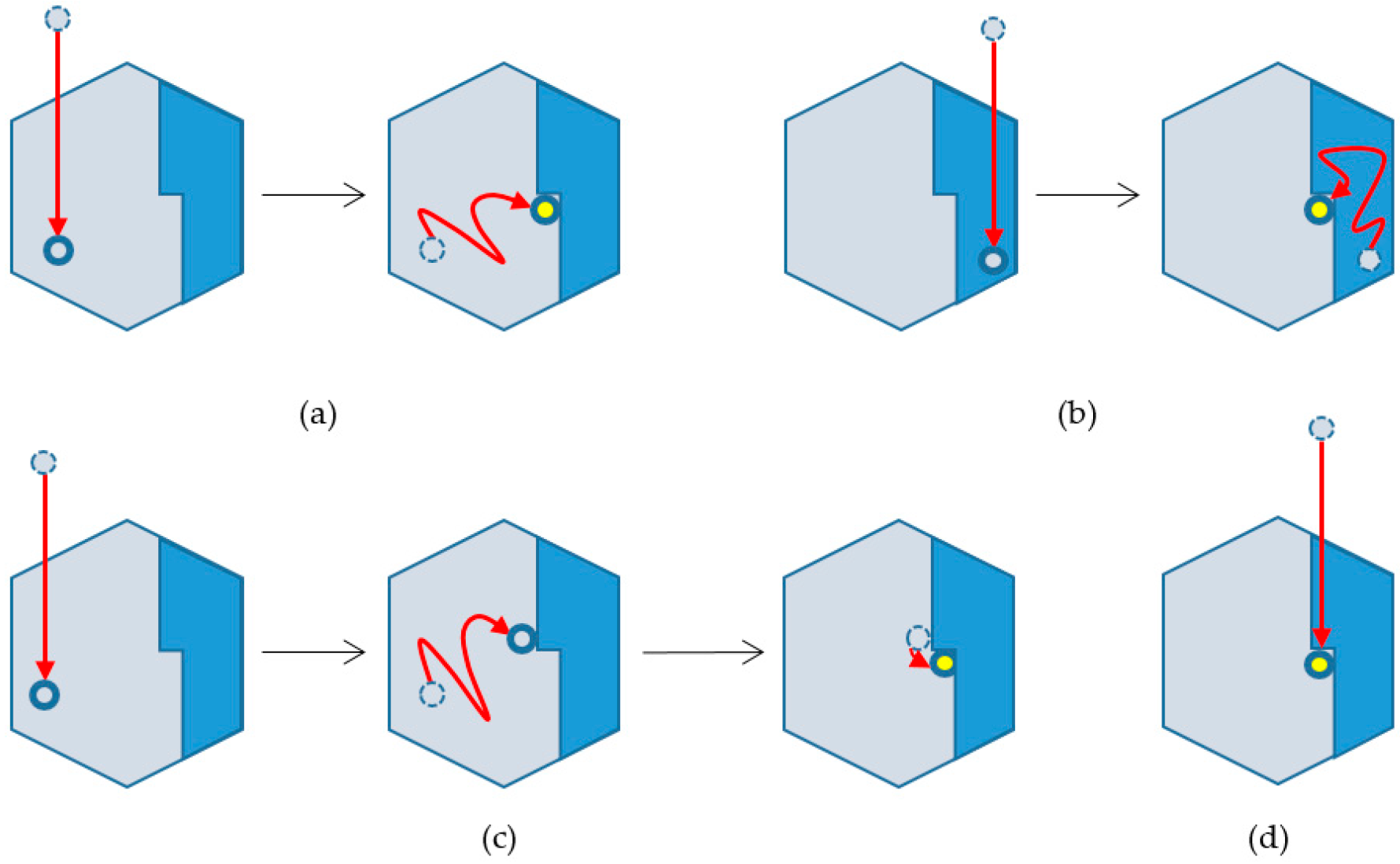
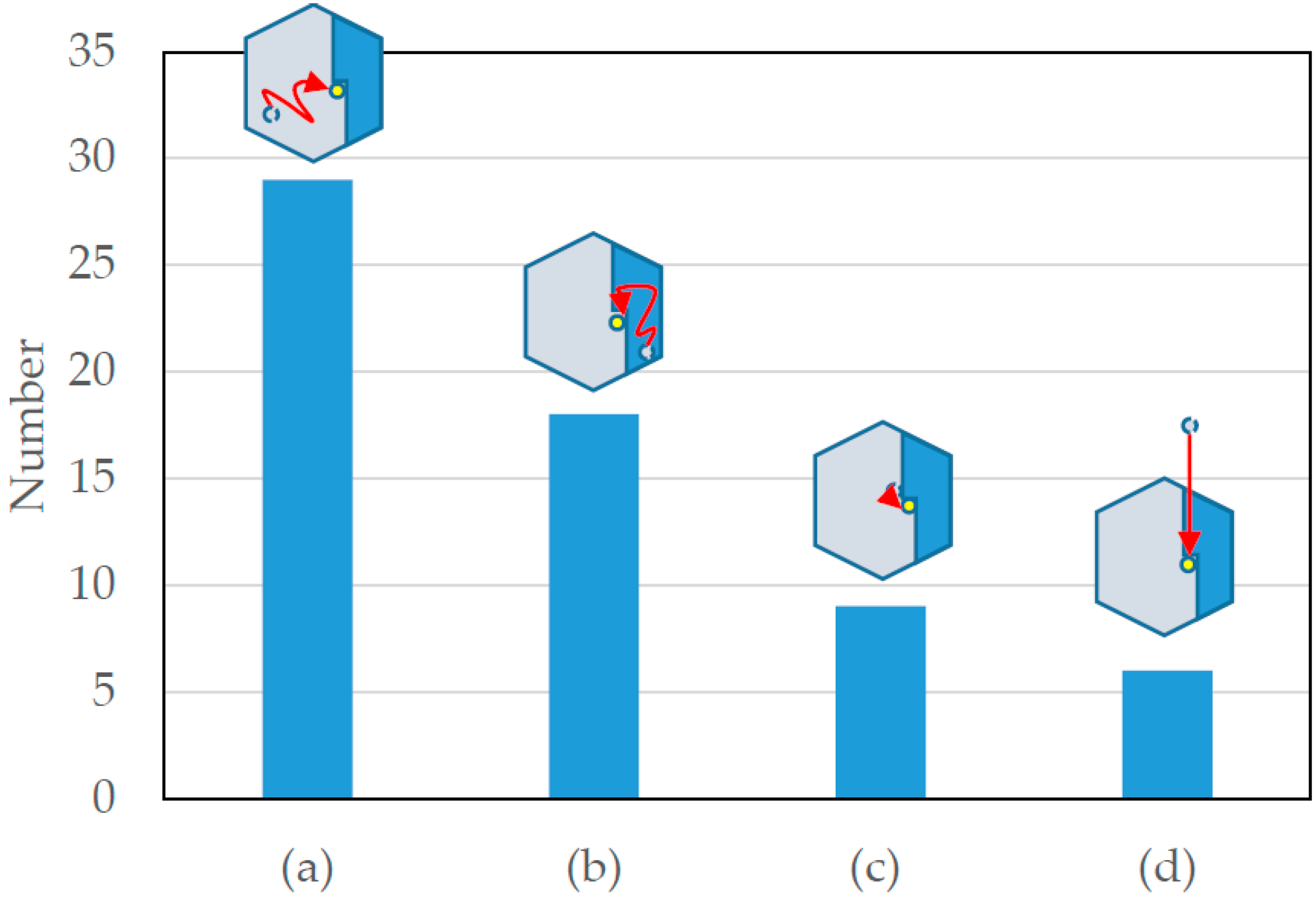
2.4. Classification of Particle Incorporation Processes into Crystals
- Kink incorporation processes via surface diffusion on lower layers
- Those via surface diffusion on upper layers
- Those via surface diffusion on lower layers and adsorption to the particle next to the kink site
- Those via direct incorporation from solution
2.5. Formation of Stacking Disorders
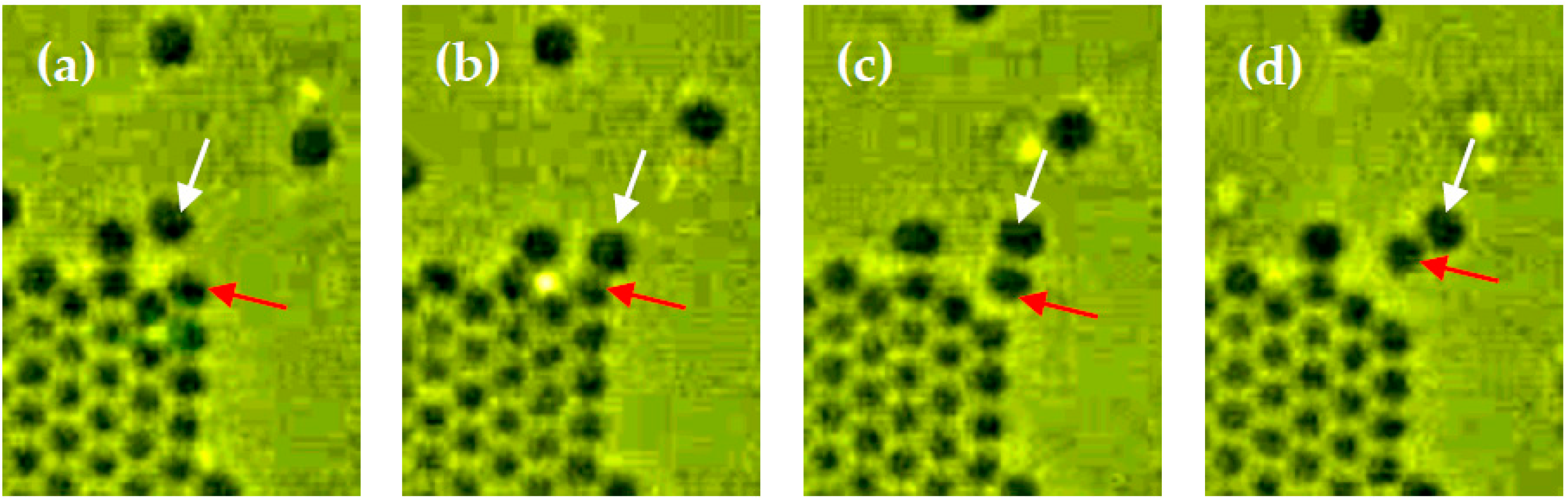
2.6. Particle Desorption from Steps by Attractive Forces from the Other Particles on the Terrace
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
- (1)
- Using a simple optical microscope setup, we observed dynamical behaviors of two-dimensional diffusion of particles on the growth interface of colloidal crystals at the particle level.
- (2)
- First day of the experiment, crystals grew normally, and the growth rates approached zero asymptotically; the system seemed to reach equilibrium. After that, however, the crystals started to dissolve, and in several days the crystals had disappeared completely and quite amazingly. The mechanism of re-dispersion is unclear at the present moment. Thus, we only use the results at the early stages of the experiment in the first day.
- (3)
- Surface diffusion coefficients were directly evaluated using the mean displacement of an adsorbed particle and the mean lifetime of an adsorbed particle before being dissolved again into the surrounding solution . The average coefficient was an order of magnitude smaller than that of bulk diffusion. This is probably due to the high activation barrier for surface diffusion; the activation barrier for surface diffusion is probably higher than that for bulk diffusion in the solution.
- (4)
- Kink incorporation processes were precisely examined. Contrary to the expectation, the process via diffusion along steps were not observed essentially. This is probably attributable to the difference in the number of recovered bonds of adsorbed particles; particles adsorbed on steps recovered the largest number of bonds, and thus, the particles hardly cut the bonds to move along the steps.
- (5)
- Formation of stacking disorders proceeded via two-dimensional nucleation of particle layers at the different lattice sites and growth. The displacement of the boundary between different stacking layers was also observed. The displacement is mainly promoted by the hopping of particles beyond the boundary between the different stacking layers.
- (6)
- Desorption of particles from a step by the adsorption of other particles to the desorbing particle was observed for the first time, as if the adsorbed particles peeled the desorbing particle off the step.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kose, A.; Ozaki, M.; Takano, K.; Kobayashi, Y.; Hachisu, S. Direct observation of ordered latex suspension by metallurgical microscope. J. Colloid Int. Sci. 1973, 44, 330–338. [Google Scholar] [CrossRef]
- Hachisu, S.; Yoshimura, S. Optical demonstration of crystalline superstructures in binary mixtures of latex globules. Nature 1980, 283, 188–189. [Google Scholar] [CrossRef]
- Takano, K.; Hachisu, S. Pressure of Kirkwood—Alder transition in monodisperse latex. J. Chem. Phys. 1977, 67, 2604–2608. [Google Scholar] [CrossRef]
- Pusey, P.N.; van Megen, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 1980, 320, 340–342. [Google Scholar] [CrossRef]
- Davis, K.E.; Russel, W.B.; Glantschnig, W.J. Disorder-to-order transition in settling suspensions of colloidal silica: X-ray measurements. Science 1989, 245, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Monovoukas, Y.; Gast, A.P. Microstructure identification during crystallization of charged colloidal suspensions. Phase Transit. 1990, 21, 183–195. [Google Scholar] [CrossRef]
- Gast, A.P.; Monovoukas, Y. A new growth instability in colloidal crystallization. Nature 1991, 351, 551–555. [Google Scholar] [CrossRef]
- Okubo, T. Giant colloidal single crystals of polystyrene and silica spheres in deionized suspension. Langmuir 1994, 10, 1695–1702. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Wiltzius, P. Real-space structure of colloidal hard-sphere glasses. Science 1995, 270, 1177–1179. [Google Scholar] [CrossRef]
- Weiss, J.A.; Oxtoby, D.W.; Grier, D.G.; Murray, C.A. Martensitic transition in a confined colloidal suspension. J. Chem. Phys. 1995, 103, 1180–1190. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Ruel, R.; Wiltzius, P. Template-directed colloidal crystallization. Nature 1997, 385, 321–324. [Google Scholar] [CrossRef]
- Míguez, H.; Meseguer, F.; López, C.; Mifsud, A.; Moya, J.S.; Vázquez, L. Evidence of FCC crystallization of SiO2 nanospheres. Langmuir 1997, 13, 6009–6011. [Google Scholar] [CrossRef]
- Sawada, T.; Suzuki, Y.; Toyotama, A.; Iyi, N. Quick fabrication of gigantic single-crystalline colloidal crystals for photonic crystal applications. Jpn. J. Appl. Phys. 2001, 40, L1226–L1228. [Google Scholar] [CrossRef]
- Gasser, U.; Weeks, E.R.; Schofield, A.; Pusey, P.N.; Weitz, D.A. Real-space imaging of nucleation and growth in colloidal crystallization. Science 2001, 292, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.; Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. Nature 2001, 409, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, J.P.; Derks, D.; Vergeer, P.; van Blaaderen, A. Stacking faults in colloidal crystals grown by sedimentation. J. Chem. Phys. 2002, 117, 11320–11328. [Google Scholar] [CrossRef]
- Yamanaka, J.; Murai, M.; Iwayama, Y.; Yonese, M.; Ito, K.; Sawada, T. One-directional crystal growth in charged colloidal silica dispersions driven by diffusion of base. J. Am. Chem. Soc. 2004, 126, 7156–7157. [Google Scholar] [CrossRef] [PubMed]
- Schall, P.; Cohen, I.; Weitz, D.A. Visualization of dislocation dynamics in colloidal crystals. Science 2004, 305, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Leunissen, M.E.; Christova, C.G.; Hynninen, A.-P.; Patrick Royall, C.; Campbell, A.I.; Imhof, A.; Dijkstra, M.; van Roij, R.; van Blaaderen, A. Ionic colloidal crystals of oppositely charged particles. Nature 2005, 437, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Yanagiya, S.-I.; Suzuki, Y.; Sawada, T.; Ito, K. Monte Carlo simulation of crystal-fluid coexistence states in the hard-sphere system under gravity with stepwise control. J. Chem. Phys. 2006, 124. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Suzuki, Y.; Yanagiya, S.-I.; Sawada, T.; Ito, K. Shrinking stacking fault through glide of the Shockley partial dislocation in hard-sphere crystal under gravity. Mol. Phys. 2007, 105, 1377–1383. [Google Scholar] [CrossRef]
- Ohtaka, K. Energy band of photons and low-energy photon diffraction. Phys. Rev. B 1979, 19, 5057–5067. [Google Scholar] [CrossRef]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Yang, S.M.; Ozin, G.A. Opal chips: Vectorial growth of colloidal crystal patterns inside silicon wafers. Chem. Commun. 2000, 2507–2508. [Google Scholar] [CrossRef]
- Xia, Y.; Gates, B.; Yin, Y.; Lu, Y. Monodispersed colloidal spheres: Old materials with new applications. Adv. Mater. 2000, 12, 693–713. [Google Scholar] [CrossRef]
- Blanco, A.; Chomski, E.; Grabtchak, S.; Ibisate, M.; John, S.; Leonard, S.W.; Lopez, C.; Meseguer, F.; Miguez, H.; Mondia, J.P.; et al. Large-scale synthesis of a silicon photonic crystal with a complete three-dimensional bandgap near 1.5 micrometres. Nature 2000, 405, 437–440. [Google Scholar] [PubMed]
- Vlasov, Y.A.; Bo, X.-Z.; Sturm, J.C.; Norris, D.J. On-chip natural assembly of silicon photonic bandgap crystals. Nature 2001, 414, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Fujine, T.; Fukuda, K.; Juodkazis, S.; Misawa, H. Formation of free-standing micropyramidal colloidal crystals grown on silicon substrate. Appl. Phys. Lett. 2003, 82, 4283–4285. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.-Y.; Xia, Y. Template-directed growth of (100)-oriented colloidal crystals. Langmuir 2003, 19, 622–631. [Google Scholar] [CrossRef]
- Fudouzi, H.; Sawada, T. Photonic rubber sheets with tunable color by elastic deformation. Langmuir 2006, 22, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sawada, T.; Tamura, K. Colloidal crystallization by a centrifugation method. J. Cryst. Growth 2011, 318, 780–783. [Google Scholar] [CrossRef]
- Hilhorst, J.; Petukhov, A.V. Variable dislocation widths in colloidal crystals of soft thermosensitive spheres. Phys. Rev. Lett. 2011, 107, 095501-1-5. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Mori, A.; Fujiwara, T.; Tamura, K. Precise characterization of grain structures, stacking disorders and lattice disorders of a close-packed colloidal crystal. J. Cryst. Growth 2011, 322, 109–113. [Google Scholar] [CrossRef]
- Suzuki, Y.; Endoh, J.; Mori, A.; Yabutani, T.; Tamura, K. Gravitational annealing of colloidal crystals. Defect Diffus. Forum 2012, 323–325, 555–558. [Google Scholar] [CrossRef]
- Sato, M.; Katsuno, H.; Suzuki, Y. Formation of a crystal of Brownian particles under a uniform external force. Phys. Rev. E 2013, 87, 032403. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sazaki, G.; Hashimoto, K.; Fujiwara, T.; Furukawa, Y. Colloidal crystallization utilizing interfaces of unidirectionally growing ice crystals. J. Cryst. Growth 2013, 383, 67–71. [Google Scholar] [CrossRef]
- Hashimoto, K.; Mori, A.; Tamura, K.; Suzuki, Y. Enlargement of grains of silica colloidal crystals by centrifugation in an inverted-triangle internal-shaped container. Jpn. J. Appl. Phys. 2013, 52, 030201-1-3. [Google Scholar] [CrossRef]
- Hilhorst, J.; de Winter, D.A.M.; Wolters, J.R.; Post, J.A.; Petukhov, A.V. Defect engineering in sedimentary colloidal photonic crystals. Langmuir 2013, 29, 10011–10018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Macfarlane, R.J.; Young, K.L.; Choi, C.H.J.; Hao, L.; Auyeung, E.; Liu, G.; Zhou, X.; Mirkin, C.A. A general approach to DNA-programmable atom equivalents. Nat. Mater. 2013, 12, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Suzuki, Y.; Sato, M. Gravitational tempering in colloidal epitaxy to reduce defects further. Cryst. Growth Des. 2014, 14, 2083–2086. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mori, A.; Sato, M.; Katsuno, H.; Sawada, T. Colloidal crystallization on tilted substrates under gravitational fields. J. Cryst. Growth 2014, 401, 905–909. [Google Scholar] [CrossRef]
- Mori, A.; Suzuki, Y. Identification of triangular-shaped defects often appeared in hard-sphere crystals grown on a square pattern under gravity by Monte Carlo simulations. Phys. B Condens. Matter 2014, 452, 58–65. [Google Scholar] [CrossRef]
- Mahynski, N.A.; Panagiotopoulos, A.Z.; Meng, D.; Kumar, S.K. Stabilizing colloidal crystals by leveraging void distributions. Nat. Commun. 2014, 5, 4472-1-8. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, B.; Dussi, S.; Smallenburg, F.; Meeldijk, J.D.; Groenendijk, D.J.; Filion, L.; Imhof, A.; van Blaaderen, A.; Dijkstra, M. Entropy-driven formation of large icosahedral colloidal clusters by spherical confinement. Nat. Mater. 2015, 14, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, F.; Wang, Z.; Alsayed, A.M.; Zhang, Z.; Yodh, A.G.; Han, Y. Two-step nucleation mechanism in solid–solid phase transitions. Nat. Mater. 2015, 14, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Malik, V.; He, L.; Ern, B.H.; Yin, Y.; Kegel, W.K.; Petukhov, W.K. Tuning the colloidal crystal structure of magnetic particles by external field. Angew. Chem. Int. Ed. 2015, 54, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Statt, A.; Virnau, P.; Binder, K. Finite-size effects on liquid-solid phase coexistence and the estimation of crystal nucleation barriers. Phys. Rev. Lett. 2015, 114, 026101-1-5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, F.; Peng, Y.; Han, Y. Direct observation of liquid nucleus growth in homogeneous melting of colloidal crystals. Nat. Commun. 2015, 6, 6942-1-9. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Yager, K.G.; Zhang, Y.; Xin, H.; Gang, O. Superlattices assembled through shape-induced directional binding. Nat. Commun. 2015, 6, 6912-1-10. [Google Scholar] [CrossRef] [PubMed]
- Petukhov, A.V.; Meijer, J.-M.; Vroege, G.J. Particle shape effects in colloidal crystals and colloidal liquid crystals: Small-angle X-ray scattering studies with microradian resolution. Curr. Opin. Colloid Interface Sci. 2015, 20, 272–281. [Google Scholar] [CrossRef]
- Qi, W.; Peng, Y.; Han, Y.; Bowles, R.K.; Dijkstra, M. Nonclassical nucleation in a solid–solid transition of confined hard spheres. Phys. Rev. Lett. 2015, 115, 185701-1-5. [Google Scholar] [CrossRef] [PubMed]
- Cabane, B.; Li, J.; Artzner, F.; Botet, R.; Labbez, C.; Bareigts, G.; Sztucki, M.; Goehring, L. Hiding in plain view: Colloidal self-assembly from polydisperse populations. Phys. Rev. Lett. 2016, 116, 208001-1-5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Finlayson, C.E.; Snoswell, D.R.E.; Haines, A.; Schäfer, C.; Spahn, P.; Hellmann, G.P.; Petukhov, A.V.; Herrmann, L.; Burdet, P.; et al. Large-scale ordering of nanoparticles using viscoelastic shear processing. Nat. Commun. 2016, 7, 11661-1-10. [Google Scholar] [CrossRef] [PubMed]
- Ise, N.; Okubo, T.; Sugimura, M.; Ito, K.; Nolte, H.J. Ordered structure in dilute solutions of highly charged polymer lattices as studied by microscopy. I. Interparticle distance as a function of latex concentration. J. Chem. Phys. 1983, 78, 536–540. [Google Scholar] [CrossRef]
- Ito, K.; Nakamura, H.; Yoshida, H.; Ise, N. On the dynamic character of “ordered” structure in polymer latex suspensions. J. Am. Chem. Soc. 1988, 110, 6955–6963. [Google Scholar] [CrossRef]
- Larsen, A.E.; Grier, D.G. Like-charge attractions in metastable colloidal crystallites. Nature 1997, 385, 230–233. [Google Scholar] [CrossRef]
- Overbeek, J.T.G. Recent developments in the understanding of colloid stability. J. Colloid Int. Sci. 1977, 58, 408–422. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. Interaction between particles suspended in solutions of macromolecules. J. Polym. Sci. 1958, 33, 183–192. [Google Scholar] [CrossRef]
- Kose, A.; Hachisu, S. Ordered structure in weakly flocculated monodisperse latex. J. Colloid Int. Sci. 1976, 55, 487–498. [Google Scholar] [CrossRef]
- Toyotama, A.; Okuzono, T.; Yamanaka, J. Spontaneous formation of eutectic crystal structures in binary and ternary charged colloids due to depletion attraction. Sci. Rep. 2016, 6, 23292. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.A. Modern Crystallography III Crystal Growth; Springer: Berlin, Germany, 1984; pp. 10–13. [Google Scholar]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. R. Soc. Lond. A 1951, 243, 299–358. [Google Scholar] [CrossRef]
- Dunstan, D.E.; Stokes, J. Diffusing probe measurements of polystyrene latex particles in polyelectrolyte solutions: Deviations from Stokes–Einstein behavior. Macromolecules 2000, 33, 193–198. [Google Scholar] [CrossRef]
- Schwoebel, R.L.; Shipsey, E.J. Step motion on crystal surfaces. J. Appl. Phys. 1966, 37, 3682–3686. [Google Scholar] [CrossRef]


| Particle Number | xs/×10−6 m | τ/s |
|---|---|---|
| 1 | 1.0 | 6.83 |
| 2 | 1.6 | 6.12 |
| 3 | 0.5 | 4.62 |
| 4 | 1.0 | 6.09 |
| 5 | 0.9 | 5.48 |
| 6 | 1.7 | 12.00 |
| 7 | 1.3 | 10.51 |
| 8 | 0.8 | 9.16 |
| 9 | 1.7 | 10.35 |
| 10 | 1.2 | 8.37 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.; Hattori, Y.; Nozawa, J.; Uda, S.; Toyotama, A.; Yamanaka, J. Adsorption, Desorption, Surface Diffusion, Lattice Defect Formation, and Kink Incorporation Processes of Particles on Growth Interfaces of Colloidal Crystals with Attractive Interactions. Crystals 2016, 6, 80. https://doi.org/10.3390/cryst6070080
Suzuki Y, Hattori Y, Nozawa J, Uda S, Toyotama A, Yamanaka J. Adsorption, Desorption, Surface Diffusion, Lattice Defect Formation, and Kink Incorporation Processes of Particles on Growth Interfaces of Colloidal Crystals with Attractive Interactions. Crystals. 2016; 6(7):80. https://doi.org/10.3390/cryst6070080
Chicago/Turabian StyleSuzuki, Yoshihisa, Yoshiaki Hattori, Jun Nozawa, Satoshi Uda, Akiko Toyotama, and Junpei Yamanaka. 2016. "Adsorption, Desorption, Surface Diffusion, Lattice Defect Formation, and Kink Incorporation Processes of Particles on Growth Interfaces of Colloidal Crystals with Attractive Interactions" Crystals 6, no. 7: 80. https://doi.org/10.3390/cryst6070080
APA StyleSuzuki, Y., Hattori, Y., Nozawa, J., Uda, S., Toyotama, A., & Yamanaka, J. (2016). Adsorption, Desorption, Surface Diffusion, Lattice Defect Formation, and Kink Incorporation Processes of Particles on Growth Interfaces of Colloidal Crystals with Attractive Interactions. Crystals, 6(7), 80. https://doi.org/10.3390/cryst6070080