Abstract
Pazufloxacin is a fluoroquinolone antibiotic synthesized by Toyama Chemical Co., Ltd. (Tokyo, Japan) in the 1990s. Up until now, the X-ray crystal structure of its mesylate salt had not been determined. The dissolution and recrystallization of pazufloxacin mesylate from different solvents afforded the salts pazufloxacinium mesylate (1), pazufloxacinium mesylate dihydrate (2), pazufloxacinium mesylate hydrate (3) and pazufloxacinium mesylate bis(peroxosolvate) (4), which were all crystallographically characterized. Molecular and crystal structures of these compounds, as well as their thermal behavior, were studied. For all compounds, single-crystal X-ray diffraction confirmed that a proton migrates from methanesulfonic acid to the amino group of pazufloxacin to form a salt. Dehydration of two hydrates occurs as a two-step single-crystal-to-powder process, leading to the formation of two metastable polymorphs of the anhydrous salt. In the solid state, the peroxosolvate compound is stable under ambient conditions for several months, thus making this drug–drug solid suitable for topical application.
1. Introduction
The phenomenon of hydrate/solvate formation in organic crystals has aroused much interest both from theoretical and practical viewpoints [1,2,3]. For crystallographers, the factors governing co-crystallization with solvent molecules are of interest. Desiraju in 1991 proposed that the probability of formation of organic hydrates increases when the number of acceptor groups in the molecule exceeds the number of donor groups [4]. A comparison of the donor/acceptor ratios in both hydrate and anhydrous solids revealed that the greater the difference between the number of donors and acceptors, the higher the probability of hydrate formation [5]. This comes from the fact that water molecules typically act as donors of two hydrogen bonds but acceptors of only one H-bond [5,6]. Large molecular volume leaving voids for solvent molecules, high polarity and presence of functional groups that readily form H-bonds also favor the formation of solvated solids [5].
Interest of pharmacists, biochemists and material chemists in different solid forms of a substance including solvates comes from the great impact of crystal packing and H-bonding on solid-state properties. Bioavailability, stability, tabletability and solubility speed are among the properties affected by the presence of solvent molecules in a crystal structure [7,8,9]. Since both water and other solvents can be used in different steps of active pharmaceutical ingredient (API) production, hydrate–solvate interconversions are worthy of discussion [10,11,12]. In addition, unexpected hydrate formation can result in patent disputes between pharmaceutical companies [13,14]. Thus, much effort has been devoted to analyzing structural motifs of compounds used as APIs [11,15,16,17,18,19,20].
Herein, we report the crystal structures of anhydrous pazufloxacin mesylate (Scheme 1) used as an antibiotic in Japan since the 2000s [21], two of its hydrates and a peroxosolvate. Although the Cambridge Structural Database contains hundreds of crystal structures of fluoroquinolone antibiotics (floxacines), the crystal structures of neither the free base pazufloxacin nor its salts have been reported to date.
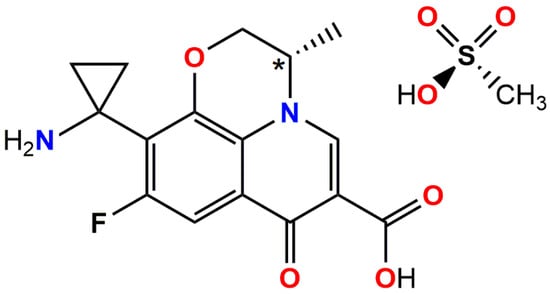
Scheme 1.
Schematic representation of pazufloxacin mesylate (PzfMes; Pzf+ = pazufloxacinium, Mes− = mesylate). Stereocenter C10 is marked with an asterisk.
Pazufloxacin is an amphoteric molecule. Similarly to other fluoroquinolones, it can form a zwitterion, a cation or a carboxylate anion depending on the pH of the medium [22] and is thus able to take part in numerous hydrogen bonds. Particularly, norfloxacin sesquihydrate [23] (Refcode {ROMDUT}) and ciprofloxacin hexahydrate [24] (Refcode {COVPIN}) exist as zwitterions. In neutral and positively charged fluoroquinolones, the carboxylic group is involved in intramolecular O-H···O bonding with the carbonyl oxygen atom of the quinoline ring. In cations, the additional proton is located on nitrogen atoms within heterocyclic rings outside the main API core. These include nitrogen atoms in pyrrolo[3,4-b]pyridine, piperazine and diazabicyclo[2.2.1]heptane, which are able to act as donors of up to two hydrogen atoms for H-bonding. Only sitafloxacinium, which contains an aminium group, can participate in three hydrogen bonds as a donor (Refcode {LUVDIR} [25]). Thus, pazufloxacinium is another example of a positively charged fluoroquinolone expected to form up to three H-bonds as a donor. Strong acceptors of H-bonding in this cation include the oxygen atoms of the carboxylic and ketone groups; however, the mesylate anion is also expected to act as a hydrogen bond acceptor. Given that in pazufloxacinium mesylate the cation is bulky, polar and contains multiple H-bonding functional groups, with the number of acceptors exceeding the number of donors, one can expect the salt to co-crystallize with water or hydrogen peroxide to balance the number of donors and acceptors of H-bonding [4,5]. Although stable peroxosolvates accept five to six hydrogen bonds in addition to providing two donor H-bonds [26], the formation of extended H2O2 or mixed H2O:H2O2 clusters could also improve the balance between donor and acceptor groups. Previously, an attempt to obtain peroxosolvates of quinoline antibiotics resulted in multidrug solid forms of nalidixic acid and ciprofloxacin; however, both compounds were unstable under ambient conditions, which precludes their application in medical practice [27].
2. Materials and Methods
Commercially available pazufloxacin mesylate was used (CAS 163680-77-1). Recrystallization was performed in air using acetonitrile, acetone, butyl acetate and ethanol (“chemically pure”) and distilled water. IR spectra were measured by using a Perkin Elmer Spectrum 65 instrument spectrophotometer (Waltham, MA, USA) by the attenuated total reflection (ATR) method in the range 4000–400 cm−1. The thermal stability of the samples was determined with a BAXIT synchronous thermal analyzer BXT-DSC-TGA1250 (Baxit, Shanghai, China). The sample was heated in an open ceramic vessel at the rate of 5 °C/min from 25 to 350 °C with an airflow of 250 mL/min. A D2 Phaser diffractometer (Bruker AXS, Karlsruhe, Germany) equipped with a copper X-ray source (CuKα radiation, λ = 1.5406 Å) and a LYNXEYE XE-T high-resolution position-sensitive detector was applied to acquire the laboratory powder XRD data. Bragg–Brentano geometry (reflection mode) and ambient conditions were applied. The samples were loaded onto the plate sample holders and rotated while the data were collected at a rate of 15 rpm. The PXRD patterns were recorded in the 4–30° 2θ range with a step size of 0.02° and a timestep of 1 s.
2.1. Preparation and Crystallization of the Salts
Pazufloxacinium mesylate, (C16H16FN2O4)(CH3O3S) (PzfMes, 1a): Pazufloxacin mesylate (0.03 g, 0.07 mmol) was dissolved in 10 mL of ethanol. After 3 h, colorless plate crystals of 1a formed (yield: 0.025 g, 83%). 1a is the stable polymorph of PzfMes. Heating of pazufloxacinium mesylate hydrates 2 and 3 (see below) above 150 °C resulted in formation of two metastable polymorphs of PzfMes, designed as 1b and 1c, respectively.
Pazufloxacinium mesylate dihydrate, (C16H16FN2O4)(CH3O3S)·2H2O (PzfMes·2H2O, 2): Pazufloxacin mesylate (0.03 g, 0.07 mmol) was dissolved in 10 mL of acetonitrile with several drops of water under heating (yield: 0.025 g, 77%). The solution was cooled to room temperature. After 12 h, colorless plate crystals of 2 formed.
Pazufloxacinium mesylate hydrate, (C16H16FN2O4)(CH3O3S)·1.667H2O (PzfMes·1.667H2O, 3): Pazufloxacin mesylate (0.03 g, 0.07 mmol) was dissolved in 10 mL of acetone with several drops of water under heating (yield: 0.023 g, 71%). The solution was cooled to room temperature. After 48 h, colorless needle crystals of 3 formed.
Pazufloxacinium mesylate bis(peroxosolvate), (C16H16FN2O4)(CH3O3S)·2H2O2 (PzfMes·2H2O2, 4): Pazufloxacin mesylate (0.04 g, 0.097 mmol) was dissolved in 0.5 mL of hydrogen peroxide (30%) and left at 10 °C. After a day, colorless plate crystals of 4 formed.
2.2. Single-Crystal X-Ray Diffraction
Single crystals of 1a–4 were obtained from reaction mixtures. The intensities of reflections for 2 and 3 were measured using synchrotron radiation (λ = 0.7517 Å, graphite monochromator) and a Rayonix SX165 detector of “Belok/XDS” station of NRC “Kurchatov Institute” [28,29]. Indexing and integration of data were performed using the XDS program complex [30]. Data collection for 1a at 120.0(2) and 293.0(2) K and for 4 at 120.0(2) K was performed on a Bruker APEX II diffractometer (Bruker AXS, Inc., Madison, WI, USA) equipped with a CCD area detector, a graphite monochromator for MoKα radiation (λ = 0.71073 Å) and Oxford Cryosystem (Oxford, UK).
The structures were solved with a dual-space method with SHELXT ver. 18/3 [31] program and refined by the full-matrix least-squares technique against F2(hkl) in anisotropic approximation for ordered non-hydrogen atoms with SHELXL ver. 18/3 [32] and the Olex2 ver. 1.5 [33] software package. The positions of H(C) atoms were calculated, and those of H(N) and H(O) atoms were taken from Fourier maps. All H atoms were refined in a riding model with Uiso(H) = 1.5Ueq(Xi) for water molecules and methyl groups, and 1.2Ueq(Xi) for other atoms. Detailed crystallographic information is given in Table 1.

Table 1.
Crystallographic data and experimental details for 1a–4.
CCDC 2454777, 2454778, 2454779, 2,454,780 and 2,454,781 contain the supplementary crystallographic data for compounds 1a (T = 120 K), 1a (T = 293 K), 2, 3 and 4, respectively. These data can be obtained free of charge via www.ccdc.cam.ac.uk/structures (accessed on 11 September 2025) or by e-mailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; fax: +44-1223-336033.
Topological analysis of underlying nets was performed using the ToposPro 5.4.1.0 package [34].
3. Results and Discussion
3.1. Preparation, TGA and IR Spectroscopy
Pure pazufloxacin mesylate was recrystallized from different solvents. Recrystallization from EtOH afforded monoclinic P phase 1a, which corresponds to the initial substance based on its powder X-ray diffraction pattern (Figure S1, Electronic Supporting Information, ESI) and contains no solvent in accordance with single-crystal X-ray diffraction (see next section). Recrystallization from MeCN:H2O and butyl acetate:H2O mixtures yielded monoclinic C dihydrate 2. Orthorhombic P hydrate 3 was obtained from acetone:H2O mixtures. Monoclinic bis(hydrogen peroxide) solvate 4 precipitated from 30% hydrogen peroxide solution. Powder XRD patterns for these substances are shown in Figure 1, and their Rietveld refinement profiles are provided in Figures S1–S4 (ESI).
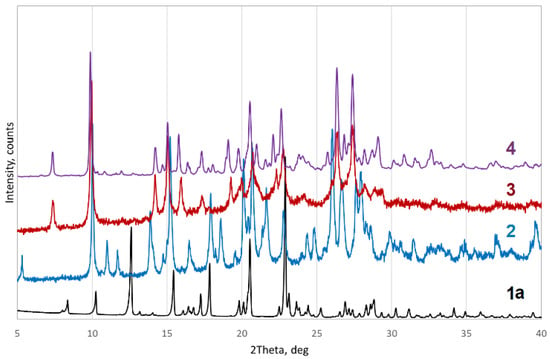
Figure 1.
Powder XRD patterns for 1a–4.
TGA curves for 1a–3 solids were measured over a temperature range of 25 to 350 °C. Dihydrate 2 undergoes a continuous weight loss from 80 to 160 °C, with weight loss corresponding to two water molecules per cation. A two-step dehydration is observed at 82–104 °C for 3 (two out of five molecules are lost to obtain pazufloxacin mesylate monohydrate), and the second step occurs above 200 °C. Finally, the solids decompose above 255 °C. No peaks corresponding to phase transitions were observed on the DSC curves (Figure S5, ESI).
Compounds 1a and 2 were further characterized by infrared (IR) spectroscopy (Figure 2). The spectra show the absence of water molecules in compound 1a. Bands corresponding to ν(C=O) vibrations of the COOH and C=O groups for 1a and 2 are found at 1690–1699 cm−1; due to the presence of water molecules and additional H-bonds in 2, the band is shifted to a lower frequency. Broad peaks in the region of 2800–3000 cm−1 can be attributed to aliphatic C–H stretching vibrations, while aromatic C–H stretching vibrations manifest themselves at 3050–3057 cm−1. The band at 1039 cm−1 corresponds to stretching vibrations of the C–F bond. Peaks at 1450–1550 cm−1 are related to C=C vibrations of the aromatic ring. The spectrum of compound 2 is distinguished by the presence of bands at 3363, 3450 and 3550 cm−1 expected for O–H vibrations.
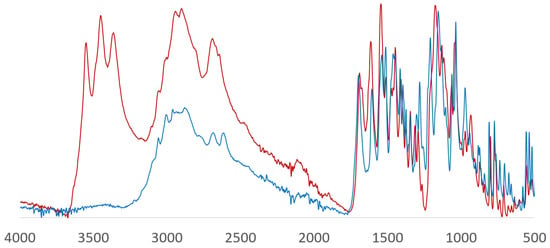
Figure 2.
IR spectra of 1a (blue) and 2 (red).
3.2. Molecular Structures 1a–4
Molecular and crystal structures of 1a–4 were investigated by means of single-crystal X-ray diffraction. Crystallographic data and refinement details are provided in the Experimental Section. A molecular view of the asymmetric unit is presented in Figure 3. The pazufloxacin contains a chiral center (Scheme 1); thus, all the solids crystallize in chiral space groups with Flack parameters close to zero (Table 1). The latter is known to be sensitive to incorrect space group assignment, twinning and pseudo-symmetry [35]. The anhydrous pazufloxacin mesylate 1a crystallizes in space group P21. Its asymmetric unit contains two cations and two anions. That of compound 2 (space group C2) contains two cations, two anions and four water molecules, while in the asymmetric unit of 3 (space group P212121), three cations, three anions and five water molecules were found. Salt 4 (space group P21) contains two cations, two anions and four H2O2 molecules. For all cations, the S configuration at the C10 atom was confirmed. It is worth noting that 1a and 2 are pseudo-centrosymmetric structures which emulate the P21/n and I2/c packings, respectively, broken by positions of C11 and C13 atoms connected with the stereocenter C10. No phase transitions were found for a single crystal of 1a upon cooling from room temperature to 120.0(2) K.
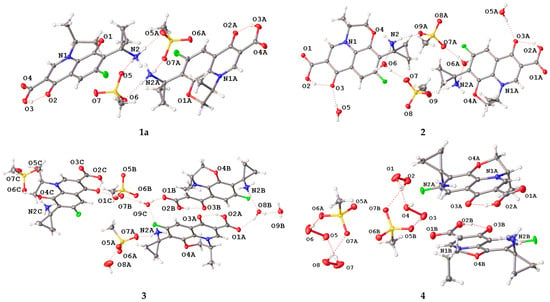
Figure 3.
Asymmetric unit of 1a–4 in representation of atoms with thermal ellipsoids (p = 50%).
The Mogul analysis of bond lengths by means of the Mercury 4.0 package [36] indicates that all bond lengths in the compounds are standard, except for the unusually short C7–C14 bonds (1.490(4)–1.502(3) Å) between the cyclopropyl and the aromatic cycles (Table 2). The Mogul database reports an average value of 1.530(12) Å for the C(sp3)–C(NH3+) bond in analogous compounds. Deviations from the expected values were also observed for the C14–C7–C6 and C14–C7–C8 bond angles involving the carbon atom of the cyclopropyl fragment. Since the S–O bond lengths are all similar, it was established that the compounds are salts containing mesylate anions and pazofloxacinium cations. The data quality allowed us to locate position of the hydrogen atoms on Fourier difference maps. These reveal that the amino groups of pazufloxacin are protonated to NH3+, and the OH group of the COOH fragment participates in an intramolecular bond with the carbonyl oxygen atom.

Table 2.
Selected bond distances (Å) and angles (°).
Overall, the molecular conformation of pazufloxacinium is rather rigid (Figure 4, Table 2). In almost all cases, the six-membered O4-C8-C9-N1-C10-C11 ring adopts a “half-chair” conformation: the stereocenter C10 lies below the plane formed by the N1–C9(sp2)–C8(sp2)–O4 atoms with a deviation of 0.138(6)–0.268(5) Å, and C11H2 is above the plane with a deviation of 0.497(5)–0.574(3) Å. An exception is found in the B molecule of cation 3, where the O4-C8-C9-N1-C10-C11 cycle adopts an envelope conformation, with the C10B atom deviating from the mean plane of the other atoms by 0.667(4) Å. The most prominent differences in molecular conformations correspond to mutual position of the cyclopropyl and amino groups in respect to the tricycle due to rotation about the single C7–C14 bond.
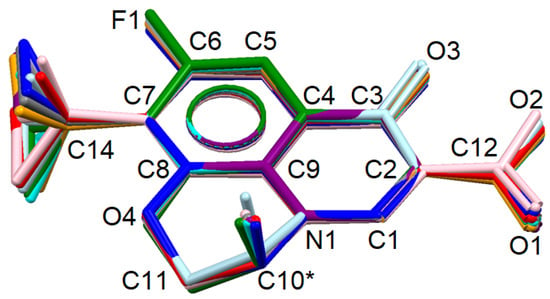
Figure 4.
Comparison of pazufloxacinium conformations in solid 1a (purple and pink), 2 (red and orange), 3 (green, cyan and blue) and 4 (grey and light blue). H atoms are omitted. Stereocenter C10* is marked with an asterisk sign.
3.3. Crystal Packing in Solid 1a–4
As we mentioned above, all pazufloxacinium cations contain an intramolecular O–H···O bond between the carboxylic and carbonyl groups. The geometric parameters of all intra- and intermolecular H-bonds in 1a–3 are listed in Table S1 (ESI). Thus, the cation is able to act as a donor for only three intermolecular H-bonds. At the same time, each mesylate possesses three acceptor oxygen atoms, and the cation itself also has acceptor carboxylate, carbonyl and oxo groups. As a result, in the solvent-free 1a, each cation is involved in two N–H···O=S bonds with an anion and one N–H···O=C bond with the carboxylic group of a cation. Overall, the H-bonding network in 1a has a layered structure, where the layers are separated by hydrophobic functional groups (Figure 5). Since the mesylate anions act as linkers in the H-bonding net, it can be simplified. Each pazufloxacinium cation is connected to three other cations, forming a honeycomb net.
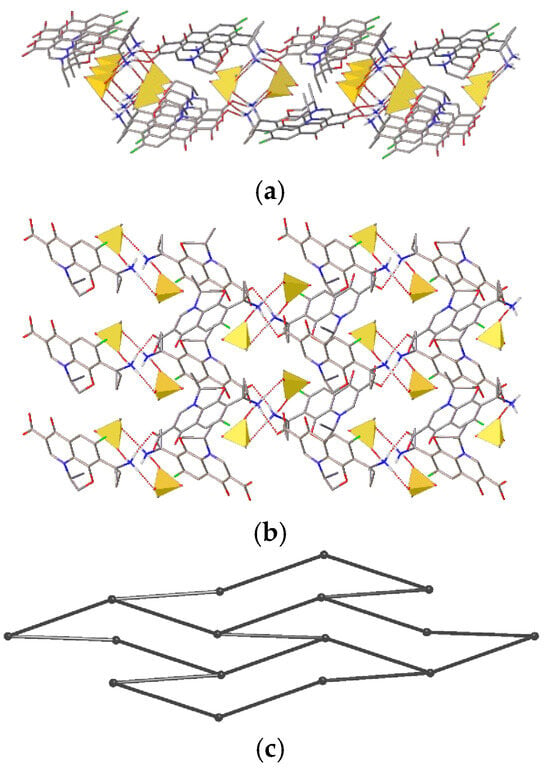
Figure 5.
Fragment of the H-bonded net in 1a: (a) side view, (b) top view and (c) underlying net. H(C) and H(O) atoms are omitted. Mesylate anions are shown as yellow tetrahedra.
Although a water molecule can potentially form two H-bonds as a donor and two as an acceptor with unchanged balance of donors and acceptors, it typically acts as a three-connected node in a H-bonded net, utilizing only one acceptor site per two donor connections [5,6]. In 2, both mesylate anions take part in three H-bonds with two cations and a water molecule; amino groups of both cations interact with two anions and a water molecule. Two water molecules act as three-connected nodes taking part in O–H···O=S, O–H···O=C and N–H···O bonds, and two molecules bridge a keto and a carbonyl group through two O–H···O bonds. Thus, presence of water molecules results in the formation of additional H-bonds, causing the anions and cations to act as three- and six-connected (with four water molecules and two anions) nodes, respectively. The resulting H-bonded network is a novel 3,3,6-c three-periodic net (Figure 6). In terms of the notation described in Ref. [37], its point symbol is {4.62}2{42.610.83}. The H-bonding nets can alternatively be described as layers of the {Pzf +·2H2O} composition parallel to the (001) plane connected by hydrogen bonds with anions. The layers after removing bridge water molecules also form a honeycomb net.
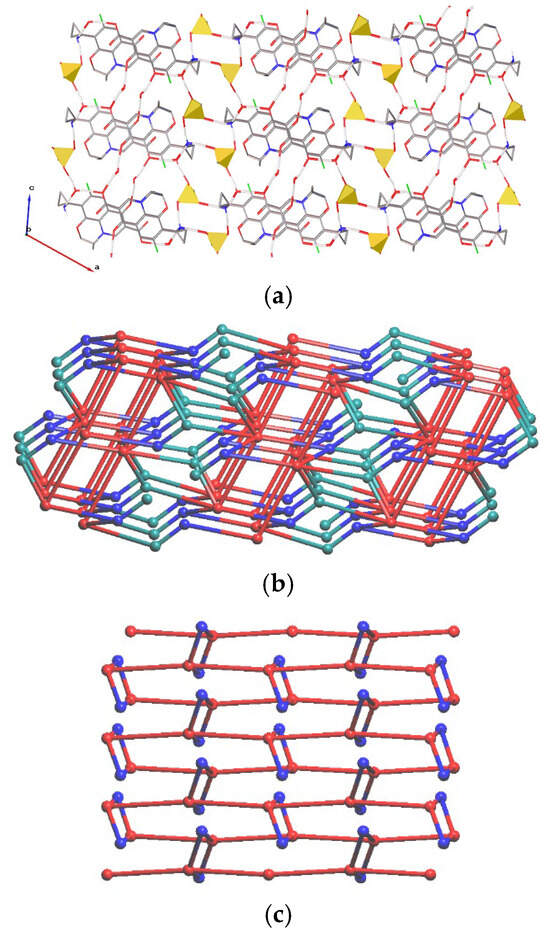
Figure 6.
Fragment of the (a) H-bonded net in 2. H(C) and H(O) atoms are omitted. Mesylate anions are shown as yellow tetrahedra. Underlying net of (b) 2 (view along axis b) and (c) the {Pzf+·2H2O} layer (view along axis a). Red, teal and blue spheres correspond to centers of gravity of cations, anions and water molecules.
The presence of three symmetrically independent cations and anions, and five water molecules in 3 results in variation in their connectivity. Similarly, with 2, all cations are involved in two H-bonds with anions and a N–H···O bond with a water molecule. Although the anions act as three-coordinated nodes, these realize different coordination: with three cations, with two cations and a water molecule, or with a cation and two water molecules. Among water molecules, two-coordinated (bridge between two anions), three-coordinated and four-coordinated ones are present. Three-coordinated include (i) an acceptor for a N–H···O and a donor of two O–H···O bonds with a water and a carboxyl oxygen, (ii) an acceptor of an O–H···O bond with a water molecule and a donor of two O–H···O=S bonds, and (iii) an acceptor for a water molecule and donor of O–H···O bonds with an anion and a keto group of a cation. Finally, one molecule acts as an acceptor of two N–H···O bonds and a donor of a H-bond with a water molecule and a carboxyl group. Overall, the H-bonding network in 3 is to the three-periodic net with a point symbol {4.102}{4.7.9}{4.72}2{42.6.102.12}{42.6}{43.62.7.8.92.10}{43.62.8}3. Water molecules containing atoms act as linkers between layers situated in the (010) direction (Figure 7).
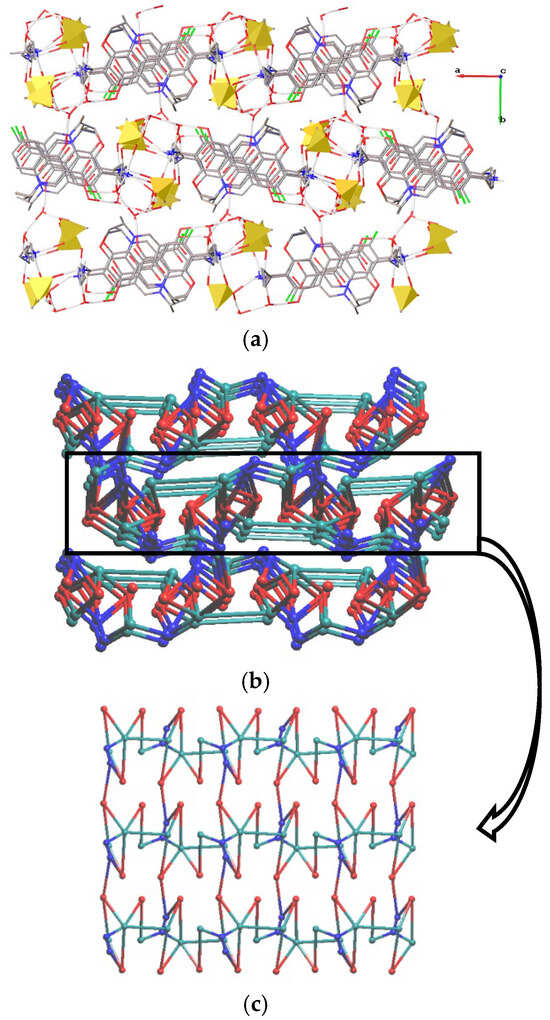
Figure 7.
Fragment of the (a) H-bonded net in 3. H(C) and H(O) atoms are omitted. Mesylate anions are shown as yellow tetrahedra. Underlying net of (b) 3 (view along axis a) and (c) the layer (view along axis b). Red, teal and blue spheres correspond to centers of gravity of cations, anions and water molecules.
Each NH3 group in the two cations of 4 is involved in two H-bonds with anions and one with a solvent. In addition, an oxygen atom of the carboxylic group acts as an acceptor for an HO–OH···O bond. Both anions bridge two cations and two solvent molecules. Free rotations along the HO–OH bond of the hydrogen peroxide is characterized by HO–OH torsion angles ranging from 84 to 178 °. Although H2O2 in stable peroxosolvates typically accepts five to six H-bonds [26], in 4, one H2O2 molecule is only a donor for two protons, and the other three molecules act as donors for two and acceptors for only two H-bonds. The reason is imbalance between strong donors and acceptors of H-bonding, which is compensated in 4 by weak C–H···O interactions with r(C···O) = 3.142(4)–3.519(4) Å (Table S1). Overall structural H-bonded motif in 4 is a chain parallel with crystallographic axis b of the {3.42.52.7}{32.42.5.6}2{3} topology (Figure 8).
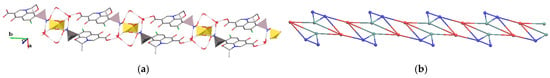
Figure 8.
Fragment of the (a) H-bonded net and (b) underlying net in 4. H(C) atoms are omitted. Mesylate anions are shown as tetrahedra. Red, teal and blue spheres correspond to centers of gravity of cations, anions and H2O2 molecules.
All salts realize numerous C–H···O, C–H···F and C–H···π interactions, and π···π stacking between two cations was also found in all salts. The presence of polar groups allows the formation of different types of stacking interactions, such as those between delocalized π-electrons of condensed cycles or between oxygen atoms and the π-system (Figure 9). The angles between mean planes of condensed cycles do not exceed 9.3(1)°. The dimers depicted in Figure 9, left, were found in crystal structures of 2, 3 and 4. These are characterized by atom–mean plane distances shorter than 3.4 Å (the shortest distance of 3.186(3) Å was found in 2). This may be indicative of “Coulombic compression” between similarly charged disk-shaped ions held together by the surrounding environment of oppositely charged ions [38].
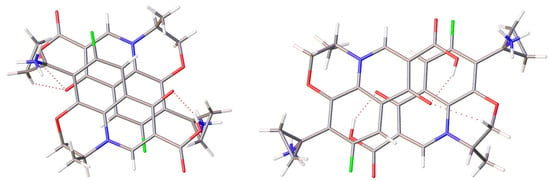
Figure 9.
Selected stacking dimers found in (left) 1a and (right) 3.
3.4. Multitemperature Study
Based on XRD data, heating of 2 and 3 above 200 °C results in amorphous phases. XRD patterns of 2 and 3 heated to 200 °C are expected to correspond to anhydrous phases; however these differ from both each other and 1a (Figure 10), indicating formation of three different polymorphs of anhydrous pazufloxacin mesylate.
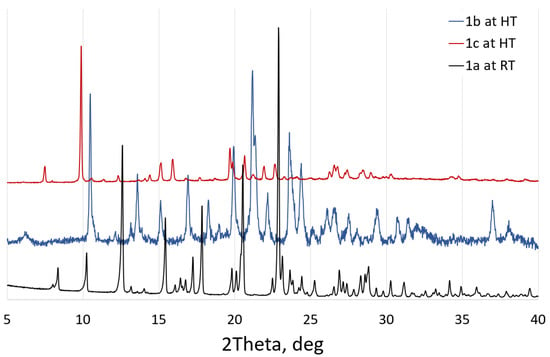
Figure 10.
Polymorphs of anhydrous pazufloxacin mesylate as obtained from recrystallization from EtOH, heating of 2 to 200 °C (to obtain polymorph 1b) or heating of 3 to 200 °C (resulted in 1c).
We performed a multitemperature XRD analysis of two hydrates in order to reveal the peculiarities of the thermal behavior of these solids. Unfortunately, heating of single-crystals of 2 or 3 to 50 °C resulted in their decomposition into a powder, which did not allow us to perform a multitemperature single-crystal XRD study. However, powder XRD patterns indicated that the samples remained crystalline phases within the 25–200 °C range. As it follows from the powder XRD data, dehydration occurs between 40 and 50 and at 125 °C for 2, and between 40 and 50 and between 150 and 175 °C for salts 3 (Figure 11). The temperatures of phase formation are notably lower than those observed on the TGA curves, probably due to different heating rate and crystallite size.
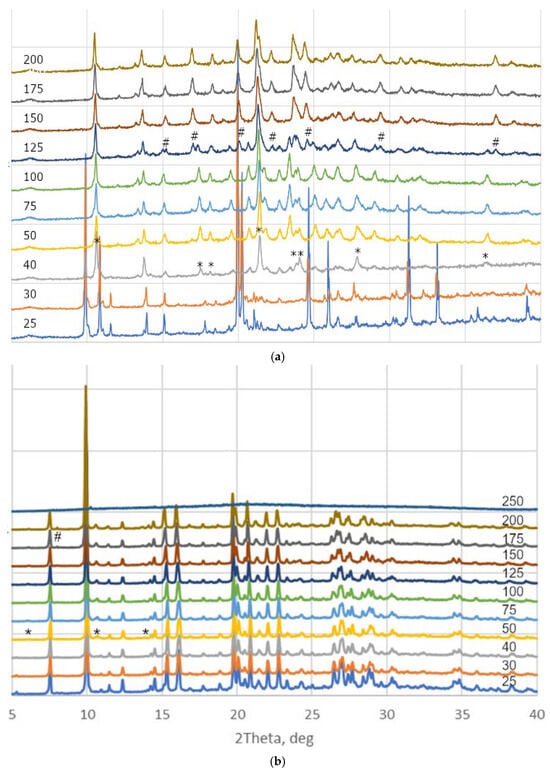
Figure 11.
Powder XRD patterns of (a) 2 and (b) 3 under heating from 25 to 250 °C. Appearance of new phases is marked with “*” and “#” signs.
X-ray powder diffraction is a vital technique used to determine crystal structures of pharmaceutical substances including cocrystal and polymorphic forms [39]. We indexed novel phases using Topas 5.4.1.0 software [40], taking initial crystal parameters, space groups and pazufloxacin chirality into account, and created Pawley refinement plots of the proposed phases. The list of different phases with Rbragg and Rwp values as well as Pawley fit plots for the best Rwp are given in ESI (Tables S2 and S3 and Figures S7–S10). For monoclinic 2 heated to 75 °C, the best Rwp corresponded to the P1 space group with crystal parameters a = 9.246(5), b = 7.108(3), c = 15.719(8) Å, α = 101.98(2), β = 110.73(2) and γ = 70.96(2)°. Further heating to 200 °C results in conversion to the P2 or P21 space group with crystal parameters a = 17.533(8), b = 7.298(3), c = 15.141(7) Å and β = 106.51(2)° (Table S2). It is worth noting that molecular volume, which goes to one formula unit for anhydrous PzfMes at room temperature and 2 heated to 75 and 200 °C, is 452, 454 and 464 Å3. This confirms at least partial dehydration of 2 at 75 °C. For orthorhombic 3 heated to 75 °C, prominent change in β unit cell parameter resulted in formation of a monoclinic phase with the P21 space group and crystal parameters a = 16.150(2), b = 17.706(2), c = 20.168(3) Å and β = 104.81(1)°. The decrease in the unit cell volume from 5793(2) to 5576(1) Å3 under heating probably refers to dehydration of the sample. The phase obtained at heating of 3 to 200 °C, indexed in the P2 or P21 space group with crystal parameters a = 15.837(2), b = 17.877(2), c = 13.578(1) Å and β = 98.85(1)° (Table S3). The volume per formula unit for 3 heated to 75 and 200 °C is 474 and 475 Å3, which can be both due to anomalous thermal behavior or opposite effects of dehydration and thermal expansion. Unfortunately, the phases obtained at high temperature were found to be unstable after standing in air for a few hours, and structure solution and refinement against low-angle high-temperature data was not possible.
4. Conclusions
In continuation of our studies devoted to crystallographic analysis of active pharmaceutical ingredients [41,42,43,44,45], the structure and composition of the solid phases of pazufloxacin mesylate crystallized from different solvents were studied. It was found that this compound can be obtained either as an anhydrous salt or as hydrates (solvates), depending on the solvent. The first crystal structures of pazufloxacin were obtained, which reveal a relatively rigid molecular conformation, the presence of an intramolecular COOH···O=C hydrogen bond and protonation of the amino group. It was demonstrated that the amino group readily forms polyfurcated hydrogen bonds as a donor, while the acceptor capacity is limited to the carboxylic and carbonyl oxygen atoms, which form no more than two and one intermolecular H-bond(s), respectively. Upon heating, both hydrates are dehydrated in a single-crystal-to-powder manner, resulting in two different metastable phases of the anhydrous salt. The solid peroxosolvate remained stable in air at room temperature for several months, making this phase a prospective candidate for dermal, otorhinolaryngologic or dental application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15090812/s1. Figures S1–S4; Rietveld refinement of powder XRD data for 1a–4. Figure S5; TGA curves for 1a–3. Figure S6; Fragment of π···π stacking in 1a–4. Figures S7–S10; Pawley fits of hkl phases for dehydrated 2 and 3. Table S1; Parameters of H-bonds in 1a–4. Tables S2, S3; Indexing of powder XRD patterns for dehydrated 2 and 3.
Author Contributions
Conceptualization, A.V.V.; formal analysis, P.A.B. and A.S.G.; investigation, E.D.T., A.S.G., P.A.B. and P.V.D.; writing—original draft preparation, E.D.T. and A.V.V.; writing—review and editing, A.V.V.; visualization, A.V.V.; funding acquisition, A.V.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-73-00027.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
X-ray diffraction studies for 2 and 3 were performed using the unique scientific facility “Kurchatov synchrotron radiation source “KISI-Kurchatov” of the National Research Center “Kurchatov Institute”. TGA and IR spectra were obtained employing the equipment of the Center for Molecular Composition Studies of INEOS RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Takieddin, K.; Khimyak, Y.Z.; Fábián, L. Prediction of Hydrate and Solvate Formation Using Statistical Models. Cryst. Growth Des. 2016, 16, 70–81. [Google Scholar] [CrossRef]
- Braga, D.; Casali, L.; Grepioni, F. The Relevance of Crystal Forms in the Pharmaceutical Field: Sword of Damocles or Innovation Tools? Int. J. Mol. Sci. 2022, 23, 9013. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.; Yusop, S.N.; Roberts, K.J. Crystallisation of Organic Materials from the Solution Phase: A Molecular, Synthonic and Crystallographic Perspective. Crystallogr. Rev. 2022, 28, 97–215. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydration in Organic Crystals: Prediction from Molecular Structure. J. Chem. Soc. Chem. Commun. 1991, 426–428. [Google Scholar] [CrossRef]
- Infantes, L.; Fábián, L.; Sam Motherwell, W.D. Organic Crystal Hydrates: What Are the Important Factors for Formation. CrystEngComm 2007, 9, 65–71. [Google Scholar] [CrossRef]
- Gillon, A.L.; Feeder, N.; Davey, R.J.; Storey, R. Hydration in Molecular CrystalsA Cambridge Structural Database Analysis. Cryst. Growth Des. 2003, 3, 663–673. [Google Scholar] [CrossRef]
- Griesser, U.J. The Importance of Solvates. In Polymorphism: In the Pharmaceutical Industry; Wiley-VCH: Weinheim, Germany, 2006; pp. 211–233. ISBN 3-527-31146-7. [Google Scholar]
- Dhaval, M.; Dudhat, K.; Gadoya, A.; Shah, S.; Pethani, T.; Jambukiya, N.; Patel, A.; Kalsariya, C.; Ansari, J.; Borkhataria, C. Pharmaceutical Salts: Comprehensive Insights from Fundamental Chemistry to FDA Approvals (2019–2023). AAPS PharmSciTech 2025, 26, 36. [Google Scholar] [CrossRef]
- Tao, Y.; Gao, Y.; Zhang, B.; Hu, K.; Xie, Y.; Zhang, L.; Yang, S.; Lu, Y. Advances in Quantitative Analytical Methods for Solid Drugs. Crystals 2025, 15, 38. [Google Scholar] [CrossRef]
- Surov, A.O.; Drozd, K.V.; Ramazanova, A.G.; Churakov, A.V.; Vologzhanina, A.V.; Kulikova, E.S.; Perlovich, G.L. Polymorphism of Carbamazepine Pharmaceutical Cocrystal: Structural Analysis and Solubility Performance. Pharmaceutics 2023, 15, 1747. [Google Scholar] [CrossRef]
- Li, W.; Zhou, L.; Tian, B.; Chen, K.; Feng, Y.; Wang, T.; Wang, N.; Huang, X.; Hao, H. Polymorphism of Pradofloxacin: Crystal Structure Analysis, Stability Study, and Phase Transformation Behavior. Pharm. Res. 2023, 40, 999–1012. [Google Scholar] [CrossRef]
- D’Abbrunzo, I.; Voinovich, D.; Perissutti, B. Mechanochemical Synthesis of Praziquantel Hemihydrate in the Presence of Five Solvents with Different Water Miscibility. Crystals 2024, 14, 374. [Google Scholar] [CrossRef]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: Oxford, England, 2020; ISBN 978-0-19-965544-1. [Google Scholar]
- Bučar, D.-K.; Lancaster, R.W.; Bernstein, J. Disappearing Polymorphs Revisited. Angew. Chem. Int. Ed. 2015, 54, 6972–6993. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Azim, Y. Challenges and Opportunities of Pharmaceutical Cocrystals: A Focused Review on Non-Steroidal Anti-Inflammatory Drugs. RSC Med. Chem. 2021, 12, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Batisai, E. Multicomponent Crystals of Anti-Tuberculosis Drugs: A Mini-Review. RSC Adv. 2020, 10, 37134–37141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, R.; Wang, L.; Chen, C.; Zhao, Y.; Guo, W.; Zhang, Z. Ciprofloxacin Salts of Benzene Mono/Di-Carboxylate: Crystal Structures and the Improvement of Solubility. CrystEngComm 2024, 26, 2662–2672. [Google Scholar] [CrossRef]
- Costa, W.S.; de Oliveira, Y.S.; Ayala, A.P. Polymorphism in Cocrystals of Metronidazole Benzoate. CrystEngComm 2023, 25, 4716–4728. [Google Scholar] [CrossRef]
- Prashanth, J.; Surov, A.O.; Drozd, K.V.; Perlovich, G.L.; Balasubramanian, S. Polymorphs, Cocrystal and Hydrate of Nilutamide. CrystEngComm 2023, 25, 3501–3513. [Google Scholar] [CrossRef]
- Baranowska, J.; Szeleszczuk, Ł. Exploring Various Crystal and Molecular Structures of Gabapentin—A Review. Crystals 2024, 14, 257. [Google Scholar] [CrossRef]
- Muratani, T.; Inoue, M.; Mitsuhashi, S. In Vitro Activity of T-3761, a New Fluoroquinolone. Antimicrob. Agents Chemother. 1992, 36, 2293–2303. [Google Scholar] [CrossRef][Green Version]
- Bryskier, A. Fluoroquinolones. In Antimicrobial Agents: Antibacterials and Antifungals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 668–788. ISBN 978-1-119-73921-0. [Google Scholar][Green Version]
- Ravindra, N.V.; Panpalia, G.M.; Jagarlapudi, A.R.P.S. Norfloxacin Sesquihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o303. [Google Scholar] [CrossRef]
- Turel, I.; Bukovec, P.; Quirós, M. Crystal Structure of Ciprofloxacin Hexahydrate and Its Characterization. Int. J. Pharm. 1997, 152, 59–65. [Google Scholar] [CrossRef]
- Sun, H.-Y.; She, N.-F. Synthesis and Crystal Structure of 7-((7S)-7-Aminospiro [2.4]Heptan-5-Yl)-8-Chloro-6-Fluoro-1-((1R,2S)-Cis-2-Fluorocyclopropyl)-4-Oxo-1,4-Dihydroqun-Oline-3-Carboxylic Acid Fumaric Acid Monohydrate. Jiegou Huaxue 2016, 35, 1054. [Google Scholar] [CrossRef]
- Chernyshov, I.Y.; Vener, M.V.; Prikhodchenko, P.V.; Medvedev, A.G.; Lev, O.; Churakov, A.V. Peroxosolvates: Formation Criteria, H2O2 Hydrogen Bonding, and Isomorphism with the Corresponding Hydrates. Cryst. Growth Des. 2017, 17, 214–220. [Google Scholar] [CrossRef]
- Kiseleva, M.A.; Prikhodchenko, P.V.; Churakov, A.V. Novel Peroxosolvates of Quinolone Antibiotics Containing Large Hydrogen Peroxide Clusters. Mend. Commun. 2024, 34, 25–27. [Google Scholar] [CrossRef]
- Lazarenko, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Burlov, A.S.; Koshchienko, Y.V.; Vlasenko, V.G.; Khrustalev, V.N. High-Throughput Small-Molecule Crystallography at the ‘Belok’ Beamline of the Kurchatov Synchrotron Radiation Source: Transition Metal Complexes with Azomethine Ligands as a Case Study. Crystals 2017, 7, 325. [Google Scholar] [CrossRef]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G.; Clemente, D.A.; Linden, A.; Spek, A.L. Centrosymmetric and Pseudo-Centrosymmetric Structures Refined as Non-Centrosymmetric. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; O’KEeffe, M.; Proserpio, D.M. Vertex-, Face-, Point-, Schläfli-, and Delaney-Symbols in Nets, Polyhedra and Tilings: Recommended Terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Gavezzotti, A.; Rizzato, S. “Coulombic Compression”, a Pervasive Force in Ionic Solids. A Study of Anion Stacking in Croconate Salts. Cryst. Growth Des. 2014, 14, 357–366. [Google Scholar] [CrossRef]
- Chernyshev, V.V. Structural Characterization of Pharmaceutical Cocrystals with the Use of Laboratory X-Ray Powder Diffraction Patterns. Crystals 2023, 13, 640. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An Optimization Program Integrating Computer Algebra and Crystallographic Objects Written in C++. J. Appl. Cryst. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Goloveshkin, A.S.; Korlyukov, A.A.; Vologzhanina, A.V. Novel Polymorph of Favipiravir—An Antiviral Medication. Pharmaceutics 2021, 13, 139. [Google Scholar] [CrossRef]
- Buikin, P.; Korlyukov, A.; Kulikova, E.; Novikov, R.; Vologzhanina, A. Crystal Structure of Rilpivirine Hydrochloride, N6H19C22Cl. Powder Diffr. 2024, 39, 151–158. [Google Scholar] [CrossRef]
- Buikin, P.; Korlyukov, A.; Ushakov, I.; Goloveshkin, A.; Kulikova, E.; Vologzhanina, A. Crystal Structure of Palbociclib Form A, C24H29N7O2. Powder Diffr. 2024, 39, 270–274. [Google Scholar] [CrossRef]
- Buikin, P.; Vologzhanina, A.V.; Svetogorov, R.D.; Bakuleva, N.; Novikov, R.A.; Aysin, R.R.; Bukalov, S.S.; Korlyukov, A.A. Polymorphs of the Antiviral Drug 6-[2-(1H-Imidazol-4-Yl)Ethylamino]-5-Oxopentanoic Acid (C10H15N3O3, IPA): Crystal Structures, DFT Studies, NMR, and Vibrational Spectra. Cryst. Growth Des. 2024, 24, 8589–8597. [Google Scholar] [CrossRef]
- Goloveshkin, A.S.; Kulikova, E.S.; Novikov, R.A.; Vologzhanina, A.V.; Korlyukov, A.A. Crystal Structure of Nilotinib Hydrochloride Monohydrate According to Powder X-Ray Diffraction Data. J. Struct. Chem. 2024, 65, 585–595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).