Abstract
We report here an in-depth study concerning the synthesis, NMR, and X-ray structure determination of two new polymorphs of 2-chloro-3′,4′-diacetoxy-acetophenone. A new, ecologically friendly method of synthesis in the solid phase, as well as a suitable method for protecting hydroxyl functionality, is presented. The 1H- and 13C-NMR spectra as well as the single crystal X-ray diffraction studies proved unambiguously the structure of the compounds: the two polymorphs of 2-chloro-3′,4′-diacetoxy-acetophenone and 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone. The polymorph I crystalizes in the monoclinic P21/c space group, while polymorph II crystalizes in the Sohnke P212121 space group of the orthorhombic system, with no interstitial solvate molecules. Significant differences were observed in the supramolecular interactions in the crystal structure of the two polymorphs. Polymorph I is characterized as a parallel packing of weakly interacting supramolecular layers oriented in the 1 1 0 plane. The crystal structure of polymorph II is much more complex: each molecule is interconnected through 12 (twelve) hydrogen bonds with 9 (nine) adjacent symmetry-related molecules. The monoacetoxy derivative 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 crystallizes in the monoclinic P21/c space group, with one molecule in the asymmetric unit.
1. Introduction
Crystal polymorphism is an important phenomenon of solid-state chemistry that consists of the ability of a substance to crystallize in at least two crystalline forms, differing by molecular conformation, crystal packing, or both [1,2]. Polymorph compounds display different physical, chemical, and biological properties, such as melting point, color, solubility, dissolution rate, density, hardness, morphology, stability, and bioavailability, including efficacy and toxicity [1,2,3,4,5,6]. Therefore, crystal polymorphism has numerous and fascinating applications in various fields of activity, including the pharmaceutical industry, agriculture, materials science (dyes and pigments, batteries, optoelectronic devices), etc. [1,2,7,8,9,10,11,12,13,14]. Polyphenols are natural and/or synthetic compounds with phenyl rings substituted by more than one hydroxyl group (-OH). There are many ways in which polyphenols are classified, the most general one being in three main categories: phenolic acids, flavonoids, and non-flavonoids [15]. Naturally occurring polyphenols are considered secondary bioactive compounds derived from plant sources [16,17], while synthetic polyphenols are obtained by synthesis [17,18,19]. Whether they are natural or synthetic compounds, polyphenols possess a wide range of beneficial actions for human health, including high antioxidative properties, antimicrobial, anticancer, and antiviral activity, as well as antihypertensive, neuroprotective, and immunomodulatory properties [20,21,22,23,24,25,26,27,28,29]. This is why polyphenols are of great interest in the pharmaceutical industry, beauty products, dietary supplements, and food industry (almost in all segments, from processing to food preservation) [17,24,28,30]. In continuation of our research in the field of biologically active natural and functionalized polyphenols [31,32,33,34] and their polymorphism properties [35], and keeping in mind protecting the hydroxyl functionality for further transformation, we present herein a new case of polymorphism for the 2-chloro-3′,4′-diacetoxy-acetophenone molecule.
2. Materials and Methods
2.1. Materials
2-Chloro-3′,4′-dihydroxy-acetophenone, acetic anhydride, and solvents were purchased from Merck and Sigma-Aldrich and used as received without any further purification processes. The uncorrected melting points were obtained using an A. Krüss Optronic Melting Point Meter KSPI. Commercial silica gel plates 60 F254 (Merck Darmstadt, Germany) were used for analytical thin-layer chromatography, which was then observed under UV light (max = 254 or 365 nm). The NMR spectra were recorded using an Avance III 500 MHz spectrometer from Bruker, Vienna, Austria, which operates at 125 MHz for 13C and 500 MHz for 1H. Chemical shifts were given as parts per million (ppm), coupling constants (J) in Hz, and delta (δ) units. Single crystal X-ray diffraction data for compound 3 were collected on an Oxford-Diffraction XtaLAB Synergy, Dualflex, HyPix diffractometer using Cu Kα radiation, while, for polymorph I and polymorph II, the data were collected on an XCALIBUR Eos CCD diffractometer equipped with graphite-monochromated Mo Kα radiation. Unit cell determination and data integration were carried out using the CrysAlisPro package from Oxford Diffraction [36]. The structures were solved with the SHELXT program using the intrinsic phasing method and refined by the full-matrix least-squares method on F2 with SHELXL [37,38]. Olex2 was used as an interface to the SHELX programs [39]. Non-hydrogen atoms were refined anisotropically. Hydrogen atoms were added in idealized positions and refined using a riding model. Selected crystallographic data and structure refinement details are provided in Table 1, along with the corresponding CIF-files (see also Supplementary Materials, CCDC no. 11491, 2481078, and 2481079). The supplementary crystallographic data can be obtained free of charge via www.ccdc.cam.ac.uk (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223–336-033; or deposit@ccdc.ca.ac.uk).

Table 1.
Crystal data and details of structure refinement.
2.2. Methods
The acetylation of hydroxyl groups from 2-chloro-3′,4′-dihydroxy-acetophenone with acetic anhydride took place as follows: in a 25 mL round-bottom flask, 1 mmol of 2-chloro-3′,4′-dihydroxy-acetophenone and 3 mmol of acetic anhydride were added under solvent-free conditions. This mixture was homogeneously mixed by stirring (magnetic stirrer) and maintained at 60 °C using an oil bath. After 3 h, the reaction was complete. The liquid reaction mixture was then cooled down to room temperature, and a white precipitate formed. According to the TLC, the white precipitate is a mixture of 2 compounds.
a) If the precipitate is washed twice with diethyl ether, than filtered off and the compounds are separated by flash chromatography column on silica (CH2Cl2–MeOH = 99:1), after crystallization from methylene chloride (the crystals were grown up by slow evaporation at 20 degrees), the two polymorphous compounds are obtained (they were fully characterized by NMR and X-ray).
b) If the precipitate is dissolved in a small amount of methylene chloride, than filtered off and the compounds are separated by flash chromatography column on silica (CH2Cl2–MeOH = 99:1), after crystallization from methylene chloride (the crystals were grown up by slow evaporation at 20 degrees), the diacylated 2-chloro-3′,4′-diacetoxy-acetophenone 2 and the monoacetoxy derivative 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 are obtained (they were fully characterized by NMR and X-ray).
Scheme 1 presents the chemical structure with atom numbering for compounds 2 and 3.
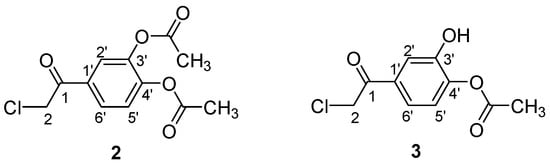
Scheme 1.
Atom numbering for 2-chloro-3′,4′-diacetoxy-acetophenone 2 and 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3.
2-chloro-3′,4′-diacetoxy-acetophenone 2.
Polymorph I was obtained as white prism crystals, ɳ = 63%, m.p. = 103–104 °C.
Polymorph II was obtained as white plate crystals, ɳ = 12%, m.p. = 132–133 °C. 1H NMR (500 MHz, CDCl3, δ, ppm): 2.31 (3H, s, methyl protons of acetoxy from 3′ position), 2.32 (3H, s, methyl protons of acetoxy from 4′ position), 4.66 (2H, s, methylene protons from 2 position), 7.35 (1H, d, J = 8.5 Hz, H-6′), 7.81 (1H, d, J = 0.2 Hz, H-2′), 7.87 (1H, dd, J = 8.5 Hz, J = 0.2 Hz, H-5′); 13C NMR (125 MHz, CDCl3, δ, ppm): 20.68 (carbon from methyl group of acetoxy from 4′ position), 20.79 (carbon from methyl group of acetoxy from 3′ position), 45.82 (carbon from methylene group from 2 position), 124.15 (C-5′), 124.20 (C-2′), 127.17 (C-6′), 132.65 (C-1′), 142.60 (C-3′), 146.83 (C-4′), 167.67 (C from ester C=O from 3′ position), 168.06 (C from ester C=O from 4′ position), 189.33 (C from ketone C=O from 1 position).
2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 was obtained as white plate crystals, ɳ = 21%, m.p. = 135–136 °C. 1H NMR (500 MHz, CDCl3, δ, ppm): 2.40 (3H, s, methyl protons of acetoxy from 4′ position), 4.63 (2H, s, methylene protons from 2 position), 6.07 (1H, s, proton from OH from 3′ position), 7.08 (1H, d, J = 6 Hz, H-6′), 7.77 (2H, m, H-2′ and H-5′); 13C NMR (125 MHz, CDCl3, δ, ppm): 21.09 (carbon from methyl group of acetoxy from 4′ position), 45.69 (carbon from methylene group from 2 position), 117.71 (C-6′), 123.78 (C-2′), 127.69 (C-4′), 128.34 (C-5′), 138.53 (C-1′), 152.46 (C-3′ from 3′ position of aromatic ring), 169.05 (C from ester C=O from 4′ position), 189.24 (C from ketone C=O from 1 position).
3. Results and Discussions
Initially, we tried to obtain our new compound 2-chloro-3′,4′-diacetoxy-acetophenone 2 using a literature procedure applied in related cases [40], by treating 2-chloro-3′,4′-dihydroxy-acetophenone 1 with acetic anhydride (AcAc) and using sodium hydroxide as the base, but we were unsatisfied with the reaction yield (less than 50%) and reaction conditions (use of toxic sodium hydroxide, long reaction time). This is why we tried different catalysts and reaction conditions, and, finally, we found an excellent setup procedure under solvent-free conditions, as we described above in the Materials and Methods Section, Scheme 2.
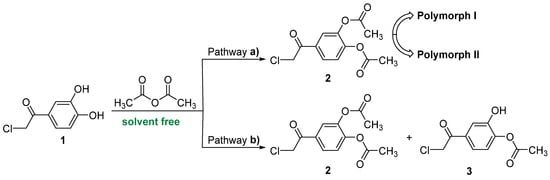
Scheme 2.
Reaction pathway between 2-chloro-3′,4′-dihydroxy-acetophenone and acetic anhydride under solvent-free conditions.
We wish to point out that our method of synthesis has some undeniable advantages: solvent-free conditions, no catalyst, higher conversions of substrates in short reaction time, mild reaction temperature, no side reactions, high atom economy (86.47%), and a good environmental factor (Ef = 775 kg waste / kg product).
Our surprise came in the separation and purification process of the compounds when, after the flash chromatography, we obtained 2 (two) compounds. Initially, the separation and purification took place after procedure b). After crystallization from methylene chloride, the diacylated 2-chloro-3′,4′-diacetoxy-acetophenone 2 (m.p. = 103–104 °C, major, yield 63%) and the monoacetoxy derivative 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 (m.p. = 135–136 °C, minor, yield 21%) were obtained. The 1H- and 13C-NMR spectra confirm the structures and are presented in Figure 1 and Figure 2 and Table 2.
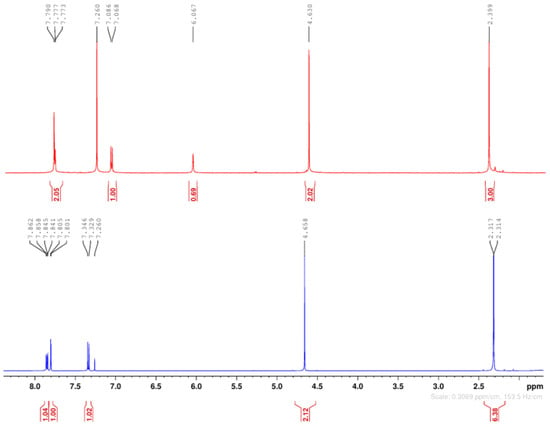
Figure 1.
Superposed 1H-NMR spectra of 2-chloro-3′,4′-diacetoxy-acetophenone (down) and 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 (up).
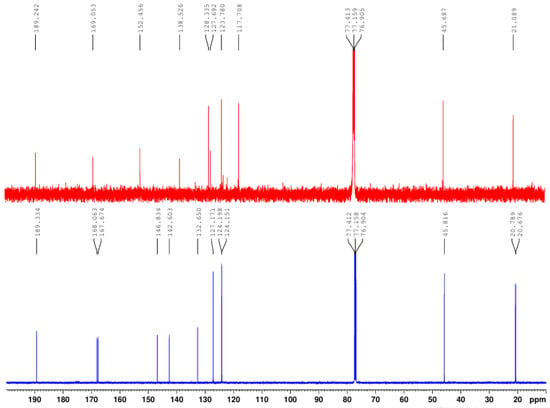
Figure 2.
Superposed 13C-NMR spectra of 2-chloro-3′,4′-diacetoxy-acetophenone (down) and 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 (up).

Table 2.
The main 1H- and 13C-NMR data of compounds 2 and 3.
As we can see in Figure 1 and Table 2, in the 1H-NMR spectra, the relevant signals for the structure are the two methyl groups from the acetoxy moiety and the methylene groups between the carbonyl ketone group and the chlorine atom.
In compound 2’s spectra (Figure 1, down), the two methyl groups from the acetoxy moiety appear at 2.31 ppm (protons of CH3 from position 3′) and 2.32 ppm (protons of CH3 from position 4′), respectively, as 6 (six) equivalent protons. The methylene protons appear at 4.65 ppm as a singlet. These findings are in accordance with the structure of 2-chloro-3′,4′-diacetoxy-acetophenone 2. In compound 3’s spectra (Figure 1, up), the situation changes completely: we found only a methyl group from the acetoxy moiety at 2.40 ppm and 3 (three) equivalent protons. The methylene protons appear at 4.63 ppm (as a singlet), and at 6.07 ppm, a supplementary broad singlet appears, which could be assigned to the proton from the hydroxyl moiety. These findings are in accordance with the structure of the monoacetoxy derivative 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 from Scheme 1.
The 13C-NMR spectra are also in accordance with the considerations found above, and the differences in the spectra are even more profound. As Figure 2 and Table 2 reveal, in the 13C-NMR spectra, the relevant signals for the structure are the two carbons from the methyl groups and the two carbons from the carbonyl ester groups of the acetoxy moiety. In compound 2’s spectra (Figure 2, down), the two carbons from the methyl groups appear at 20.79 ppm (carbon of CH3 from position 3′) and 20.68 ppm (carbon of CH3 from position 4′), respectively. The two carbons from the carbonyl ester groups of the acetoxy moiety appear at 168.06 ppm (C from ester C=O from 4′ position) and 167.67 ppm (C from ester C=O from 3′ position), respectively. These findings are also in accordance with the structure of 2-chloro-3′,4′-diacetoxy-acetophenone 2. In compound 3’s spectra (Figure 2, up), the situation changes again completely: we found only a carbon from the methyl group of the acetoxy moiety at 21.09 ppm (carbon of CH3 from position 4′) and a single carbon from the carbonyl ester group of the acetoxy moiety at 169.05 ppm (C from ester C=O from 4′ position). These findings are also in accordance with the structure of the monoacetoxy derivative 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 from Scheme 1. The remaining signals are also in accordance with the proposed structures.
If the separation and purification took place after procedure a), after crystallization from methylene chloride, polymorph I (m.p. = 103–104 °C) and polymorph II (m.p. = 132–133 °C) can be obtained (according to the 1H-, 13C-NMR-, and X-ray spectra).
The X-ray spectral analysis of the monocrystal also proved the structure of the compounds: the two polymorphic structures of 2-chloro-3′,4′-diacetoxy-acetophenone 2 [polymorph I (with the m.p. = 103–104 °C) and polymorph II (with the m.p. = 132–133 °C)] and 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 (with the m.p. = 135–136 °C).
The results of the single crystal X-ray diffraction study for polymorphs I and II are illustrated in Figure 3, while the geometric parameters are summarized in Table S1 (see Supplementary Materials). According to X-ray crystallography, the reported structures represent two polymorphs of 2-chloro-3′,4′-diacetoxy-acetophenone. Polymorph I crystalizes in the monoclinic P21/c space group (ρcalcd = 1.411 g/cm3), while polymorph II crystalizes in the Sohnke P212121 space group of the orthorhombic system (ρcalcd = 1.406 g/cm3). In both structures, the asymmetric part of the unit cell comprises one molecule without interstitial solvate molecules.
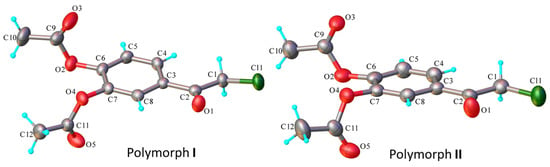
Figure 3.
X-ray molecular structure of polymorphs I and II with atom labeling and thermal ellipsoids at 40% probability level.
As can be seen from the overlay diagram presented in Figure 4, the conformation of the two molecules is very close except for the chloroacetyl fragments, which exhibit insignificant but opposite deviation from the mean plane of the aromatic ring. The acetoxy groups in both polymorphs, due to steric overcrowding, are almost perpendicular with respect to the aromatic rings. The values of the dihedral angles between the two planar fragments are in the range of 86.7(4) ÷ 89.2(2)° and 81.23(1) ÷ 89.22(1)° for I and II, respectively.
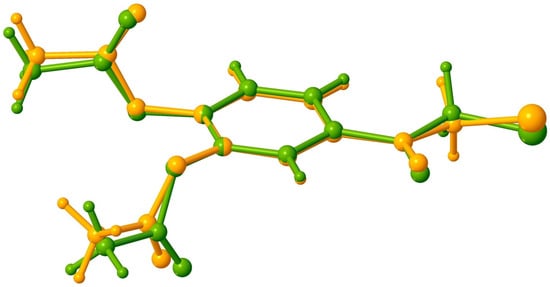
Figure 4.
Overlay of polymorph I (yellow) and polymorph II (green).
In order to reveal the structural distinctions of the two polymorphs, apart from the geometric characteristics of individual molecules, analyzing the supramolecular interactions in the crystal is also crucial. Therefore, the short intermolecular contacts in crystals I and II were thoroughly analyzed. Seeing that there are no conditions for classical hydrogen bonding, only C-H···O and C-H···Cl interactions are expected to determine the crystal packing motifs. The geometric parameters of the H-bonds are summarized in Table 3. It is noteworthy noting that no π-π stacking arrangements were revealed in the polymorph structures of I and II. This arises from the steric hindrance caused by the opposite orientation of two acetoxy groups relative to the aromatic ring.

Table 3.
Hydrogen bond parameters.
As indicated by the data from Table 3, in the crystal structure of polymorph I, there are two short intermolecular contacts C-H···O, involving carbonyl O1 or acetoxy O5 atoms as acceptors, which are responsible for assembling the molecules into one-dimensional supramolecular arrays (Figure 5).
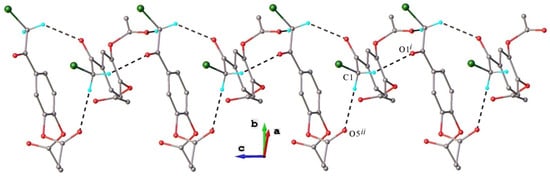
Figure 5.
View of 1D supramolecular assembly in crystal structure of polymorph I showing role of C-H···O hydrogen bonding. Non-relevant H atoms are not shown for clarity.
The third short intermolecular contacts C-H···Cl (Table 3) occur within the 2D supramolecular layer (Figure 6 and Figure 7), which represents the main structural modules formed in crystal I. Thus, the crystal structure of polymorph I can be characterized as a parallel packing of weakly interacting supramolecular layers oriented in the 1 1 0 plane.
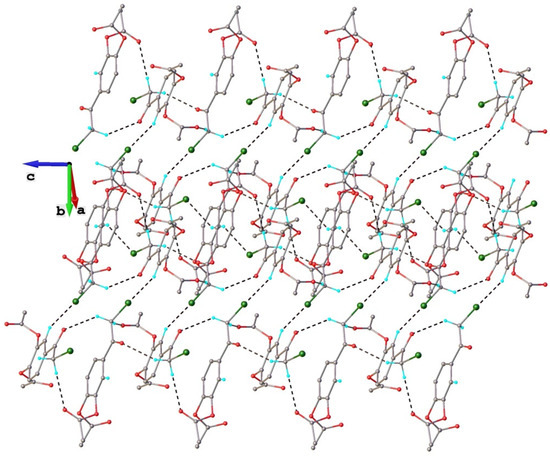
Figure 6.
View of two-dimensional supramolecular layer in crystal structure of polymorph I.
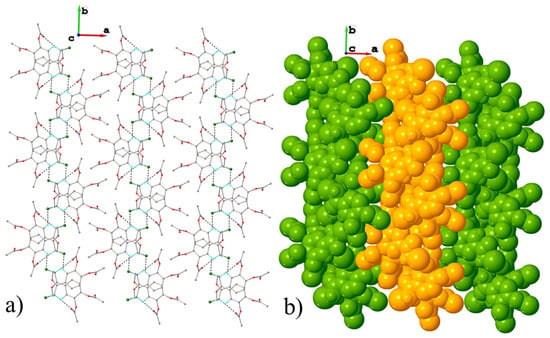
Figure 7.
(a) Projection of crystal structure along a axis showing parallel packing of 2D layers in polymorph I; (b) sphere packing representation in polymorph I.
When comparing the crystal structures of polymorphs I and II, it can be seen that in the latter case, the number of short intermolecular contacts is much higher (Table 3). Further analysis of the crystal packing did not reveal the presence of common structural modules specific to both crystals I and II. Indeed, as can be observed from Figure 8 illustrating the multitude intermolecular interactions exhibited by the isolated molecule in the crystal structure of polymorph II, each molecule is interconnected through twelve hydrogen bonds with nine adjacent symmetry-related molecules.
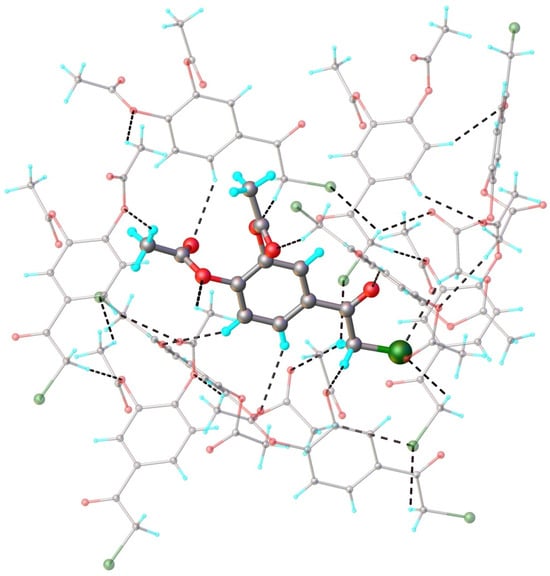
Figure 8.
Fragment of crystal structure showing role of C-H···O and C-H···Cl hydrogen bonding in crystal structure of polymorph II. Symmetry generated molecules are drawn with faded colors.
This specific structural module influences crystal packing, which determines the assembly of a quite dense and complex three-dimensional supramolecular network, as illustrated in Figure 9.
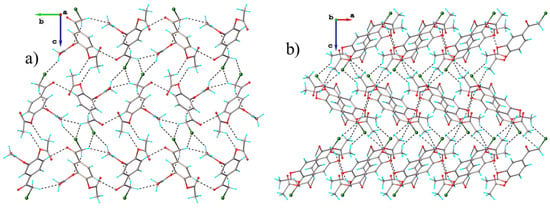
Figure 9.
Projection of crystal structure in crystal structure of polymorph II: (a) along a; (b) along b.
The monoacetoxy derivative 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 crystallizes in the monoclinic P21/c space group, with one molecule in the asymmetric unit. There are no interstitial solvent molecules in the crystal. The molecular structure of compound 3 is illustrated in Figure 10. As can be observed, the acetoxy group, due to steric overcrowding, is in nearly perpendicular orientation with respect to the main part of the molecule, which adopts essentially planar configuration. The absolute value of the C6-C7-O1-C9 torsion angle is 95.4(2)°. There are two contacts between the molecules in the adjacent stacks that are shorter than the sum of the van der Waals radii. In the absence of π-π stacking interactions, the crystal packing of compound 3 is mostly driven by the system of O-H···O and C-H···O contacts that are shorter than the sum of van der Waals radii, which organize the adjacent molecules into two-dimensional supramolecular layers in parallel arrangement to the 0 1 0 plane, as shown in Figure 11. The geometric parameters of the hydrogen bonds are listed in Table 3. The shortest contacts, C10-H10b···Cl1i (H···Cl1 = 3.110 Å, C···Cl = 3.647 Å, ∠CHCl = 116.9°, symmetry code (i) −x, y − 0.5, 1.5 − z) and Cl1···Cl1i (Cl···Cl = 3.737 Å, symmetry code (i) −x, 2 − y, 1 − z), between the molecules in the adjacent layers, which exceed the sum of van der Waals radii, indicate that the crystal structure is built from the parallel packing of the discrete 2D supramolecular network, as shown in Figure 12.
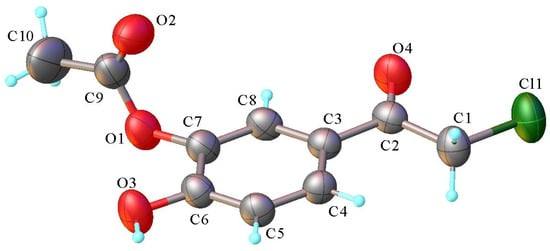
Figure 10.
X-ray molecular structure of compound 3 with atom labeling and thermal ellipsoids at 50% probability level.
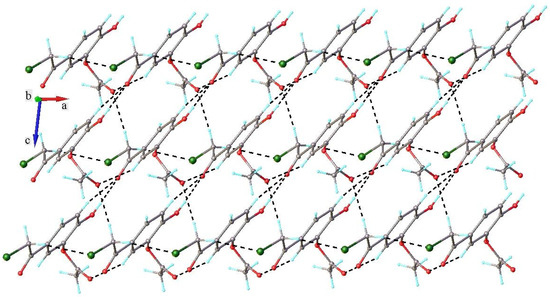
Figure 11.
Crystal packing in 3 viewed along b axis showing role of H-bonding in formation of 2D supramolecular layer.
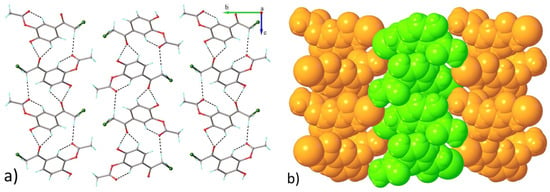
Figure 12.
Projection of crystal structure 3 along a axis showing parallel packing of 2D layers. (a) Projection of crystal structure 3 along a axis showing (b) parallel packing of 2D layers.
A possible explication for the polymorph II formation could be the in situ conversion of the monoacetoxy derivative to the diacetoxy during the crystallization process from methylene chloride.
4. Conclusions
This paper presents an in-depth study concerning the synthesis, NMR, and X-ray structure determination of two new polymorphs of 2-chloro-3′,4′-diacetoxy-acetophenone. A new method of synthesis, in solid phase, for 2-chloro-3′,4′-diacetoxy-acetophenone is presented. Our method has some undeniable advantages: less energetic consumption, shorter reaction time, no side reactions, high yield, no organic solvent used (reaction being in solid phase), high atom economy, and a good environmental factor. Keeping in mind the 12th principles of green chemistry, our method of synthesis could be considered ecologically friendly. This method of synthesis also has the advantage of being a facile and suitable pathway for protecting hydroxyl functionality for further chemical transformations.
According to the conditions of separation and purification, the method allows for obtaining either the two polymorphs of 2-chloro-3′,4′-diacetoxy-acetophenone or the diacetoxy and the monoacetoxy derivatives of 2-chloro-3′,4′-dihydroxy-acetophenone. The 1H- and 13C- NMR spectra as well as the single crystal X-ray diffraction studies proved unambiguously the structure of the compounds: the two polymorphs of 2-chloro-3′,4′-diacetoxy-acetophenone and 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone. The X-ray studies for polymorphs I and II reveal that the conformation of the two molecules is very close, except for the chloroacetyl fragment, which exhibits a slight but opposite deviation from the mean plane of the aromatic ring. Polymorph I crystalizes in the monoclinic P21/c space group, while polymorph II crystalizes in the Sohnke P212121 space group of the orthorhombic system, with no interstitial solvate molecules. Dramatic differences were observed in the supramolecular interactions in the crystal structure of the two polymorphs. The crystal structure of polymorph I is characterized as a parallel packing of weakly interacting supramolecular layers oriented in the 1 1 0 plane. In the crystal structure of polymorph I, there are two short intermolecular contacts C-H···O, involving carbonyl O1 or acetoxy O5 atoms as acceptors, which are responsible for assembling the molecules into one-dimensional supramolecular arrays. The third short intermolecular contacts C-H···Cl occur within the 2D supramolecular layer, which represents the main structural modules formed in crystal I. The crystal structure of polymorph II is much more complex: each molecule is interconnected through twelve hydrogen bonds with nine adjacent symmetry-related molecules. This specific structural module influences crystal packing, which determines the assembly of a quite dense and complex three-dimensional supramolecular network. The monoacetoxy derivative 2-chloro-3′-hydroxy-4′-acetoxy-acetophenone 3 crystallizes in the monoclinic P21/c space group, with one molecule in the asymmetric unit.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15090780/s1.
Author Contributions
Conceptualization and formal analysis, I.I.M. and V.M.; Investigation, R.A.T. and S.S.; Writing—original draft, review and editing, R.A.T., S.S., V.M., and I.I.M.; Supervision, I.I.M.; Project administration, R.A.T. and I.I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC 2481078 and 2481079 contains the full crystallographic data for this article. These are available free of charge at www.ccdc.cam.ac.uk/data_request/cif (accessed on 18 August 2025), by emailing data_request@ccdc.cam.ac.uk or by contacting the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: +44-1223-336033.
Acknowledgments
The authors are thankful to the Alexandru Ioan Cuza University of Iasi for financial support, and to the Institute of Interdisciplinary Research, CERNESIM, and RECENRAIR centers for the infrastructure used.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bernstein, J. Polymorphism in Molecular Crystals, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2020; ISBN 978-0-19-965544-1. [Google Scholar]
- Byrn, S.R.; Zografi, G.; Chen, X. Solid State Properties of Pharmaceutical Materials; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-118-14530-2. [Google Scholar]
- Chaudhary, S.; Losus, R.M.; Dobrzańska, L. Polymorphism and the Phenomenon of Whole-Molecule Disorder Revealed in a Novel Dipodal Thiopyridine Ligand. Crystals 2025, 15, 289. [Google Scholar] [CrossRef]
- Nogueira, B.A.; Castiglioni, C.; Fausto, R. Color polymorphism in organic crystals. Commun. Chem. 2020, 3, 34. [Google Scholar] [CrossRef]
- Sengupta, B.; Beasley, M.; Mason, B. Conformational Polymorphism in Organic Crystals: Structural and Functional Aspects—A Review. Curr. Res. Mater. Chem. 2019, 1, 104. [Google Scholar]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef]
- Zhou, D.; Bier, I.; Santra, B.; Jacobson, L.D.; Wu, C.; Garaizar Suarez, A.; Ramirez Almaguer, B.; Yu, H.; Abel, R.; Friesner, R.A.; et al. A robust crystal structure prediction method to support small molecule drug development with large scale validation and blind study. Nat. Commun. 2025, 16, 2210. [Google Scholar] [CrossRef] [PubMed]
- Gavale, R.; Khana, F.; Misra, R. Polymorphism in mechanochromic luminogens: Recent advances and perspectives. J. Mater. Chem. C 2025, 13, 1063–1129. [Google Scholar] [CrossRef]
- Nagaraju, V.; Jange, C.; Wassgren, C.; Ambrose, K. Understanding urea polymorphism and cocrystallization to develop enhanced fertilizers: A review. J. Environ. Chem. Eng. 2024, 12, 114308. [Google Scholar] [CrossRef]
- Artusio, F.; Contreras-Montoya, R.; Gavira, J.A. Advances in Pharmaceutical Crystals: Control over Nucleation and Polymorphism. Crystals 2024, 14, 805. [Google Scholar] [CrossRef]
- Barman, D.; Annadhasan, M.; Bidkar, A.P.; Rajamalli, P.; Barman, D.; Ghosh, S.S.; Chandrasekar, R.; Krishnan Iyer, P. Highly efficient color-tunable organic co-crystals unveiling polymorphism, isomerism, delayed fluorescence for optical waveguides and cell-imaging. Nat. Commun. 2023, 14, 6648. [Google Scholar] [CrossRef]
- Kim, Y.; Allain, C.; Guillot, R.; Audebert, P. s-Tetrazine derivative exhibiting unprecedented polymorphism-dependent emission properties. J. Photochem. Photobiol. A Chem. 2023, 439, 114629. [Google Scholar] [CrossRef]
- Huang, W.; Tang, Y.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Nitrogen-Rich Tetrazolo[1,5-b]pyridazine: Promising Building Block for Advanced Energetic Materials. J. Am. Chem. Soc. 2020, 142, 3652–3657. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H. A Practical Guide to Pharmaceutical Polymorph Screening & Selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar] [CrossRef]
- Haslam, E. Practical Polyphenols: From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1998; ISBN 0521465133. [Google Scholar]
- Dolzhko, D.; Melnyk, N.; Kruk, A.; Granica, S.; Piwowarski, J. Traditional use of polar extracts from lavender flowers—systematic review of literature data. Prospect. Pharm. Sci. 2024, 22, 92–101. [Google Scholar] [CrossRef]
- Czerwińska, M.; Kuśnierek, M. Lycium barbarum fruits—Phytochemistry and activity of goji berries—From tradition to clinical studies. Prospect. Pharm. Sci. 2024, 22, 35–57. [Google Scholar] [CrossRef]
- Colomer, R.; Sarrats, A.; Lupu, R.; Puig, T. Natural Polyphenols and their Synthetic Analogs as Emerging Anticancer Agents. Curr. Drug Targets 2017, 18, 147–159. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentao, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Mandal, M.K.; Gan, W.; Domb, A.J. Phenolate-based bioactive compounds: Design, delivery and biomedical applications. Coord. Chem. Rev. 2025, 544, 216941. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. [Google Scholar] [CrossRef]
- Kataki, C. Unveiling the Anti-Inflammatory and Immunomodulatory Effects of Secondary Metabolites. In Secondary Metabolites and Their Applications in Various Diseases; Athanasios, A., Saurabh, K.J., Roma, P., Eds.; IGI Global Scientific Publishing: London, UK, 2025; pp. 193–222. ISBN 9798369391129. [Google Scholar]
- Mandal, M.K.; Domb, A.J. Antimicrobial Activities of Natural Bioactive Polyphenols. Pharmaceutics 2024, 16, 718. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Sepodes, B.; Fernandes, P.C.; Ribeiro, M.H. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent developments in polyphenol applications on human health: A review with current knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador†, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Bechelany, M.; Karav, S. Polyphenols in Foods and Their Use in the Food Industry: Enhancing the Quality and Nutritional Value of Functional Foods. Int. J. Mol. Sci. 2025, 26, 5803. [Google Scholar] [CrossRef] [PubMed]
- Sardaru, M.C.; Al Matarneh, C.M.; Simionescu, N.; Mangalagiu, I.I.; Pinteala, M.; Danac, R. New Monoquarternary Salts of N-Heterocycles: Synthesis and Antitumor Assessment. Rev. Roum. Chim. 2024, 69, 63–74. [Google Scholar] [CrossRef]
- Lungu, C.N.; Bratanovici, B.I.; Grigore, M.M.; Antoci, V.; Mangalagiu, I.I. Hybrid imidazole-pyridine derivatives: Computational approach to novel anticancer DNA intercalators. Curr. Med. Chem. 2020, 27, 154–169. [Google Scholar] [CrossRef]
- Balan, A.M.; Miron, A.; Tuchilus, C.; Rotinberg, P.; Mihai, C.T.; Mangalagiu, I.I.; Zbancioc, G. Syntheses, Antimicrobial and Antitumoral Activity of Some Novel Dihydroxyacetophenone Derivatives. Med. Chem. 2014, 10, 476–483. [Google Scholar]
- Luca, M.C.; Tura, V.; Mangalagiu, I.I. Considerations concerning design and mechanism of action of a new class of dual DNA intercalators. Med. Hyp. 2010, 75, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Mangalagiu, I.I. Concominant polymorphism and conformational isomerism in 4-acetylresorcinol. Acta Cryst. C 2009, C65, o300–o302. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis Pro Software System; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Mingoia, M.; Conte, C.; Di Rienzo, A.; Dimmito, M.P.; Marinucci, L.; Magi, G.; Turkez, H.; Cufaro, M.C.; Del Boccio, P.; Di Stefano, A.; et al. Synthesis and Biological Evaluation of Novel Cinnamic Acid-Based Antimicrobials. Pharmaceuticals 2022, 15, 228. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).