Abstract
The crystal structures of the 2-amino-5-halogenopyridines (halogen = Cl (1), Br (2)) and 2,3-diamino-5-halogenopyridines (halogen = Cl (3), Br (4)) were compared with respect to their intermolecular interactions. An ab-initio-based method for evaluating the interaction energies between molecules was employed to estimate the driving forces of crystal formation. As a result, regularities in crystal structure organization were identified. For compounds 1 and 2, a dimeric building unit is formed by two N–H…Npyr hydrogen bonds. These dimers are further connected to neighboring units by C–H…π, C–H…N, N…X (X = Cl, Br), and non-specific interactions. The aforementioned intermolecular interactions give rise to layered structures that are similar but not isotypical. No significant contributions from π–π or N–H…N(H2) interactions are observed in 1 and 2. The structures of 3 and 4 are isotypical and crystallize in the non-centrosymmetric space group P212121. The most important intermolecular interactions are N–H…Npyr, N–H…N(H2), and stacking interactions. These interactions lead to identical columnar-layered structures in both 3 and 4. No significant contributions from halogen bonds of the type N…X (X = Cl, Br) are found in 3 and 4.
1. Introduction
The formation of diverse crystal packing motifs arises from a molecule’s ability to engage in specific and non-specific intermolecular interactions [1], driven by its functional groups. Understanding the interactions of the molecular functional groups and the molecule as a whole is the key to the design of supramolecular synthons for crystal engineering [2,3,4]. According to this concept, molecules containing substituents that may form directional interactions like hydrogen bonds first of all form common motifs in the crystal phase by a standard set of medium-strong to strong intermolecular interactions [5,6]. Hence, modulating the capacity of a molecule to create specific intermolecular interactions represents an effective approach for designing the supramolecular architecture of crystal structures. A class of compounds that has been in the focus of research for many years are aminopyridines [7,8]. Recently we investigated some halogenoanilines by aforementioned methods [9,10]. In general, the establishment of another amino group at benzene/pyridine cores results in some diversification of intermolecular interaction possibilities due to the large variety of functional groups. In detail, the title compounds are able to form more and more complex N–H…π, N–H…N, and C–H…N interactions in the solid state. The bifunctional character of the amino group is well known. It originates from the conjugation degree between the nitrogen lone pair and π-system of the benzene cycle. As a result, the acceptor properties of the amino group are strong enough for N–H…N intermolecular hydrogen bonds. The role of N–H…N interactions in the crystal structure in halogenoaminopyridines and halogenodiaminoanilines strongly depends on the position of the amino and the halogen group in the cycle, respectively. The principles of construction of the corresponding crystal are more complicated to understand if a halogen substituent is present in amino- [11] and diaminobenzene, respectively [12]. The same holds true for protonated aminopyridines that even face tautomerization upon protonation at the N atom of the pyridyl ring [13].
Aminopyridines represent essential structural scaffolds found in a wide range of bioactive natural products, pharmacologically significant compounds, and advanced functional organic materials. Their versatile chemical reactivity and biological relevance have made them valuable targets in synthetic and medicinal chemistry [14,15,16,17]. Given this significance, it is reasonable to anticipate that halogenated derivatives of these diaminopyridines will exhibit similarly promising properties. These derivatives are likely to serve as important intermediates in drug development, materials science, and coordination chemistry, owing to the unique electronic and steric effects introduced by halogen substituents. Thus the study of intermolecular interactions of diaminopyridines with a set of additional substituents like halogen groups is essential for their potential drug development and crystal structure design.
In this contribution, we compare two selected amino-halogenopyridines with two related diamino-halogenopyridines in terms of their energetic profiles and their intermolecular interactions in the solid state.
2. Materials and Methods
The crystal structure analysis presented in this study was performed using methods based on the evaluation of pairwise intermolecular interaction energies within the investigated crystals [18,19,20]. For each independent molecular entity in the asymmetric unit (designated as M0), its first coordination sphere was identified using a standard procedure implemented in the MERCURY program (version 4.2) [21]. Specifically, MERCURY enables the identification of molecules located in the immediate vicinity of M0, based on geometrical criteria for standard intermolecular interactions. The details of this procedure have been previously thoroughly and clearly described [19,20]. Each resulting molecular cluster was divided into dimers consisting of M0 and, in these cases, each neighboring molecule Mi from its coordination sphere. The atomic coordinates of all M0–Mi dimers were extracted from the X-ray diffraction data, except for hydrogen atoms, whose positions were normalized to 1.089 Å for C–H bonds and 1.015 Å for N–H bonds. These values were derived from the geometry optimization of the isolated molecule. Normalization is necessary due to the well-known tendency of X-ray diffraction methods to underestimate X–H bond lengths [22].
The interaction energies within each M0–Mi dimer were calculated using the B97-D3 density functional combined with the Def2-TZVP basis set [23,24,25,26,27]. The basis set superposition error (BSSE) was corrected using the counterpoise method [28]. The B97-D3 functional has been benchmarked as one of the most reliable dispersion-corrected functionals for modeling intermolecular interactions [29]. All calculations were performed using the ORCA 6.0 software package [30].
The analysis of pairwise interaction energies is based on the assumption that these interactions possess vector characteristics [19], with each calculated energy represented as originating from the geometric center of the reference molecule M0 and oriented toward a neighboring molecule Mi. The vector length associated with each interaction was normalized to the strongest pairwise interaction energy in the crystal using the following equation:
Li = (RiEi)/2Estr
Here, Ri denotes the distance between the geometric centers of the interacting molecules M0 and Mi, Ei represents the interaction energy between the two molecules in each pair, and Estr is the energy of the strongest pairwise interaction identified within the crystal.
This normalization procedure ensures that the lengths of the resulting vectors are consistent regardless of the computational approach applied. This method allows the basic molecule to be represented by a set of energy vectors, facilitating the construction of an energy-vector diagram for each crystal structure based on symmetry operations.
3. Results and Discussion
Compounds 1–3 were sourced from the Cambridge Structural Database (CSD) [31] (Table 1, Figure 1). In the case of multiple structures of the same compound, preference was given to those with better experimental data and more recent publication. The structural data of 4 originate from our lab and were published in advance as a structural report [32]. All structures were checked for systematical errors that may corrupt our quantum chemical investigation of the individual intermolecular interactions of each structure as well as the comparison of 1–4. As a first finding we can state that 3 and 4 are isotypical to each other. The shrinking of the unit cell from bromo-substituted 4 to the chloro-substituted 3 is 3.5% at almost the same measuring temperatures. This will affect some intermolecular distances, which need to be investigated in detail.

Table 1.
Relevant crystal data for compounds 1–4.
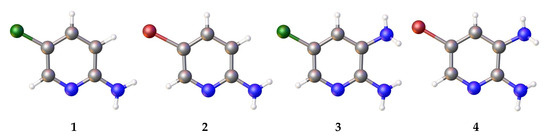
Figure 1.
Investigated compounds 1–4. Green: chlorine, blue: nitrogen, brown: bromine.
3.1. Analysis of the Molecular Structure
The functionality and directionality of an amino group attached to aromatic systems through intermolecular interactions as either a proton donor or acceptor are influenced by the extent of n–π conjugation between the nitrogen lone pair and the aromatic π-system. It is well established that the extent of this conjugation is significantly influenced by the polarizing environment [6,34,35]. Two key structural indicators of conjugation are the C–N bond length and the configuration of the N atom. At maximum conjugation, C–N bond length is minimized to have a partial double bonding, and the nitrogen atom adopts a strict planar configuration.
Investigation of the molecular geometry of compounds 1–4 showed that the C–NH2 bond lengths vary between 1.355 Å and 1.408 Å, while the sum of bond angles around the nitrogen atoms ranges from 333° to 360° (Table 2). These variations indicate a broad spectrum of conjugation degrees between the NH2 group and the pyridine ring, largely dependent on the substituent’s position. In the mono-amino-halogenopyridines 1 and 2, the amino group exhibits the highest degree of conjugation with the π-system of the aromatic ring. This is evidenced by the Car–N bond lengths (Table 2), which approximate the typical value for a planar nitrogen atom (1.355 Å) [36], as well as by the nearly planar geometry at the nitrogen center.

Table 2.
Geometric parameters indicating the degree of conjugation between the amino group and the pyridine π-system in compounds 1–4.
It is noteworthy that the two ortho-amino groups in compounds 3 and 4 are not equivalent (Table 2), likely due to their differing involvement in intermolecular interactions. As a result, in compounds 3 and 4, the NH2 group is expected to be a hydrogen bond donor and acceptor, respectively. Comparable structural characteristics of amino groups have been previously reported in studies on unsubstituted aminopyridines [7].
3.2. Analysis of the Crystal Structures of Compounds 1–4: Focus on Geometrical Parameters of Intermolecular Interactions
In mono-amino-halogenopyridines (compounds 1 and 2), the NH2 group and the heterocyclic nitrogen exhibits dual functionality, serving as a potent proton donor and acceptor. Therefore, the formation of N–H···Npyr hydrogen bonds is expected in the crystal structures of these compounds. Additionally, the presence of a halogen atom suggests the potential for both halogen bonding (X-bonds [37,38]) and hydrogen bonding, where the halogen substituent may act as a proton acceptor.
Investigation of intermolecular interactions revealed that in the crystal of 1, a C–H···Cl hydrogen bond and a Cl···π interaction were observed (Table 3). In the crystal of compound 2, a Br···Br halogen bond was detected (Table 3).

Table 3.
Geometric characteristics of intermolecular interactions in the crystals 1–2.
Depending on the relative orientation of the interacting molecules, the N–H···Npyr hydrogen bond can occur as a pure N–H···N interaction (when the Car–Car–Npyr···H torsion angle exceeds 130°) or as a N–H···π interaction (when the angle is close to 90°) [39]. In many molecular crystals, hydrogen bonds of a mixed N–H···π/N–H···N type are observed when the Car–Car–Npyr···H torsion angle falls within the 85–130° range, involving both the N atom’s lone pair and the π-system of the aromatic cycle.
Detailed analysis of the crystal structure of compound 1 revealed a strong N2–H···N1pyr hydrogen bond (Table 3). The intermolecular N2–H···N1 interaction, characterized by a C–C–N···H’ torsion angle of 113°, can be defined as a mixed N–H···N/N–H···π H-bond (N2–H···N1mix). Its parameters suggest it is significantly weaker than a pure classical N–H···Npyr H-bond (Table 3). Furthermore, two weak C–H···π H-bonds were identified in the crystals of both compounds 1 and 2.
In the crystal of compound 1, centrosymmetric dimers are generated through the strongest N–H···Npyr H-bonds (Figure 2). These dimers are stabilized not only by the mixed-type N–H···N hydrogen bond but also by C–H···π and C–H···Cl interactions. Double layers parallel to the bc plane are formed, which constitute the primary packing motif (Figure 2). It is noteworthy that this type of packing closely resembles that observed in 2-aminopyridine [7].
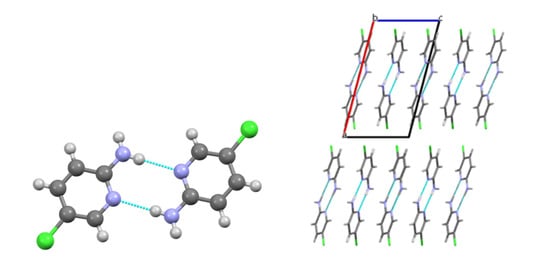
Figure 2.
Molecular packing of 1: a centrosymmetric dimer (left); crystal structure viewed along the b crystallographic axis (right). Green: chlorine, blue: nitrogen.
In the crystal of compound 2, the amino group forms a strong N2–H5···Npyr hydrogen bond, similar to that observed between molecules of compound 1 (Figure 3). However, in this case, the resulting dimers are primarily stabilized by Br···Br halogen bonds, with additional contributions from C–H···π interactions. As a result, the same type of double-layered packing parallel to the bc plane is observed as in the crystal structure of compound 1 (Figure 3).
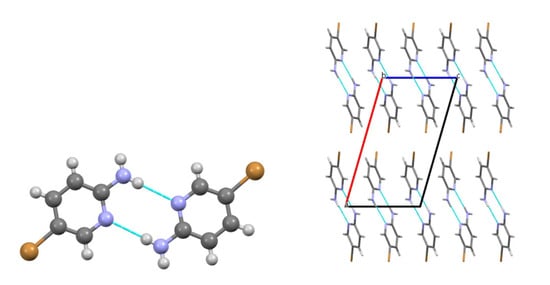
Figure 3.
Molecular packing of 2: a centrosymmetric dimer (left); crystal structure viewed along the b crystallographic axis (right). Blue: nitrogen, brown: bromine.
It is important to highlight that no stacking interactions are observed in mono-amino-halogenopyridines, despite the presence of the aromatic pyridine π-system and the higher conjugation degree of these molecules (Table 3). The same lack of stacking interactions has also been reported in the crystal structures of all isomers of diaminobenzenes and mono-aminopyridines [6,7].
Incorporating a second NH2 group in compounds 3 and 4 enhances the quantity of strong proton donors as well as weak proton acceptors. Consequently, N–H···NLP hydrogen bonds occur, with the amino group serving simultaneously as proton donor and acceptor, together with strong N–H···Npyr hydrogen bonds.
Investigation of intermolecular interactions in crystals 3 and 4 showed that the interactions and their geometric characteristics are very similar. Each amino group creates two hydrogen bonds as a H donor. Only the more pyramidal N3(H2) group functions as a H acceptor, forming a N2–H···N3LP hydrogen bond (Table 2 and Table 4). Consequently, due to the N2–H···N1pyr and N2–H···N3LP hydrogen bonds the zig-zag chains oriented along the b crystallographic axis can be distinguished, similar to those observed in 2,6-diaminopyridine (Figure 4 and Figure 5) [7].

Table 4.
Geometric characteristics of intermolecular interactions in the crystals 3–4.
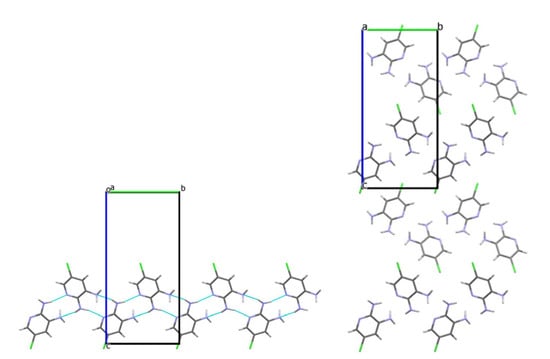
Figure 4.
The zig-zag chain of molecule 3 along the b crystallographic axis (left); crystal structure viewed along the a crystallographic axis (right).
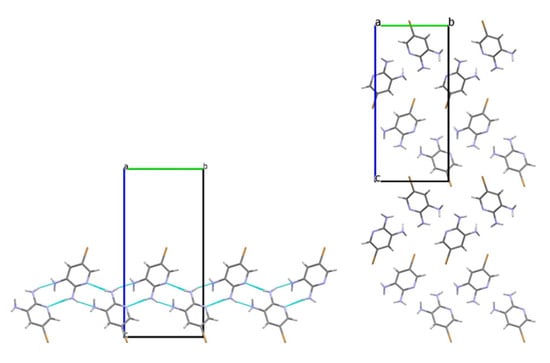
Figure 5.
The zig-zag chain of molecules 4 along the b crystallographic axis (left); crystal structure viewed along the a crystallographic axis (right).
Concurrently, a weaker N3–H···N1 interaction occurs, where the pyridine nitrogen atom serves as the proton acceptor (Table 4). In this interaction, the C–C–N···H′ torsion angle is 107° in compound 3 and more or less identically 106° in compound 4, indicating the involvement of both the lone pair on the pyridine nitrogen atom and the pyridine π-system in proton acceptance. This type of interaction can be described as a hydrogen bond of mixed N–H···π/N–H···N character (N3–H···N1mix), as observed in the crystal of 1 [39].
In addition, “head-to-head” type stacking interactions were identified (Figure 6), along with weaker C/N–H···Hal and C/N–H···π hydrogen bonds (Table 4). It should be noted that, from a general perspective, the crystal structures of compounds 3 and 4 are similar and can be classified as isotypical [40].
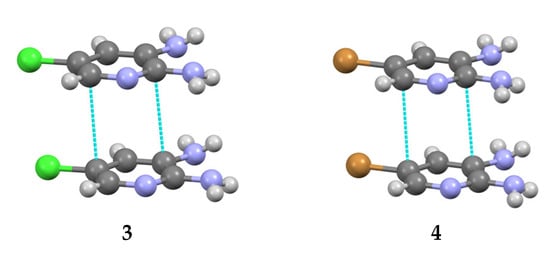
Figure 6.
The stacking dimers in the structures 3–4. Green: chlorine, blue: nitrogen, brown: bromine.
Summarizing the results of the crystal structure analysis from a geometrical perspective, the following conclusions can be drawn:
- (a)
- In mono-amino halogen pyridines 1 and 2, the amino group primarily participates in hydrogen bonding as a H donor;
- (b)
- Due to the presence of two amino groups, compounds 3 and 4 are simultaneously involved in hydrogen bonding interactions, acting as bifunctional proton donors and acceptors;
- (c)
- Stacking interactions are not observed in mono-amino-halogenopyridines;
- (d)
- Although geometric analysis is informative, it does not always allow for unambiguous interpretation of crystal packing motifs or the role of weak or non-specific interactions.
3.3. Energetic Perspective on the Crystal Structures of Compounds 1–4
As noted earlier, the use of geometric criteria alone does not provide a reliable basis for analyzing crystal packing in systems dominated by multiple weak interactions. An improved understanding of crystal packing can be obtained by calculating intermolecular interaction energies with ab initio methods [18,19,20]. The primary advantage of this method is its independence from the specific nature of the interactions, allowing it to comprehensively account for all types present, including hydrogen bonds, halogen bonds, dispersion forces, electrostatic interactions, polarization effects, and more. Importantly, this approach is applicable not only to single molecules as basic building units (BUs) of crystal packing but also to larger aggregates like dimers, trimers, or tetramers [20].
For each building unit (a molecule, dimer, or tetramer) located in the asymmetric unit of the unit cell, the first coordination sphere in all studied crystals contains 12–14 neighboring units. Table 5, Table 6, Table 7 and Table 8 summarize data for dimers whose interaction energies exceed 5% of the total interaction energy between the basic molecule and its first coordination sphere.

Table 5.
Summary of symmetry codes, interaction types, and interaction energies (Eint, kcal/mol) between the basic building unit and neighboring units whose interaction energies exceed 5% of the total, with corresponding relative contributions (ER, %) in the crystal structure of the compound 1.

Table 6.
Summary of symmetry codes, interaction types, and interaction energies (Eint, kcal/mol) between the basic building unit and neighboring units whose interaction energies exceed 5% of the total, with corresponding relative contributions (ER, %) in the crystal structure of the compound 2.

Table 7.
Summary of symmetry codes, interaction types, and interaction energies (Eint, kcal/mol) between the basic building unit and neighboring units whose interaction energies exceed 5% of the total, with corresponding relative contributions (ER, %) in the crystal structure of the compound 3.

Table 8.
Summary of symmetry codes, interaction types, and interaction energies (Eint, kcal/mol) between the basic building unit and neighboring units whose interaction energies exceed 5% of the total, with corresponding relative contributions (ER, %) in the crystal structure of the compound 4.
In the structure of 1, the total interaction energy between the basic molecule and its 14 neighboring molecules is −42.1 kcal/mol. Pairwise interaction analysis reveals one dominant interaction (Table 5, dimer 1-m1) with an energy of −12.02 kcal/mol that is nearly twice as high as the interaction energy with any other neighbor. Consequently, this dimer, stabilized by a N–H···Npyr hydrogen bond, can be considered a complex dimeric building unit in structure 1 (Figure 7a).
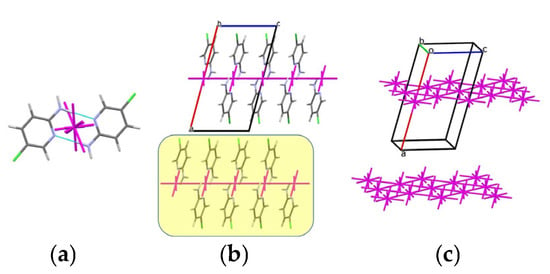
Figure 7.
(a) Centrosymmetric dimer as the building unit in the crystal structure of compound 1; (b) molecular packing and corresponding energy-vector diagrams projected along the b crystallographic axis; (c) layers of dimers represented as energy-vector diagrams, with the layers highlighted in yellow. Green: chlorine, blue: nitrogen.
Further analysis of the first coordination sphere of this dimer shows that it is surrounded by 14 neighboring dimers, with a total interaction energy of −74.2 kcal/mol. The strongest interactions are formed with four of these dimers (Table 5), all of which lie on a common plane. As a result, a layer of dimers can be identified as the primary basic structural motif (BSM) in the crystal of 1 (Figure 7b,c). The interaction energy of the dimeric BU within this layer is −61.9 kcal/mol, with interactions involving mixed N–H···N/N–H···π hydrogen bonds (N–H···Nmix), C–H···π contacts, and non-specific interactions. Weaker interlayer interactions (−12.2 kcal/mol) are mediated by C–H···Cl hydrogen bonds and Cl···Cl halogen bonds. A similar type of crystal packing was previously observed in the structures of 2-aminopyridine and 1,2-diaminobenzene [6,7].
The first coordination sphere of the basic molecule in structure 2 also includes 14 neighbors, with a slightly higher total interaction energy (−50.7 kcal/mol). Similar to structure 1, one strongest pairwise interaction is observed (Table 6, dimer 1-m1), identifying this dimer as the dimeric BU of the structure (Figure 8). The dimer is surrounded by 14 other dimers (total interaction energy −76.1 kcal/mol) and forms four dominant interactions within a layer (Figure 8), resulting in a layer of dimers as the primary BSM. The interaction energy within this layer is −61.4 kcal/mol. Dimers are connected via C–H···π hydrogen bonds and non-specific interactions. The weaker interlayer energy (−14.7 kcal/mol) is due to C–H···Br hydrogen bonds and Br···Br halogen bonds.
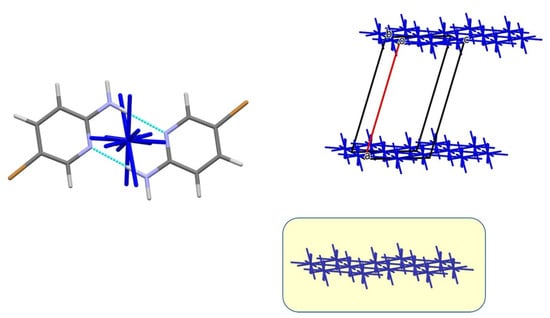
Figure 8.
The centrosymmetric dimer serves as the building unit in the crystal structure of compound 2 (left), layers of dimers are depicted as energy-vector diagrams (right). The layers are highlighted in yellow. Blue: nitrogen, brown: bromine.
In structure 3, the basic molecule is surrounded by 12 neighboring molecules, with a total interaction energy of −53.5 kcal/mol. The pairwise analysis reveals two strongest interactions (dimers 3-m1 and 3-m2), each with an energy of −8.85 kcal/mol, located in opposite directions (Table 7). These dimers form a zig-zag column, which serves as the primary BSM (Figure 9). The total interaction energy of the molecule within this column is −17.7 kcal/mol.
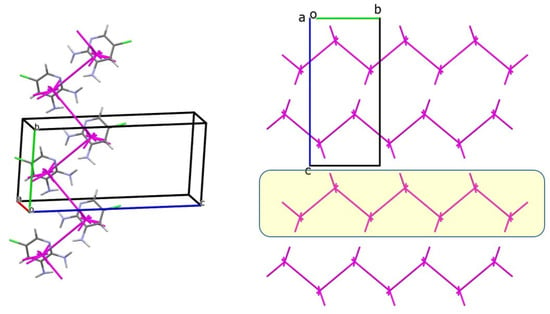
Figure 9.
The primary basic structural motif (BSM) in structure 3 is a zig-zag column extending along the b crystallographic axis (left); energy-vector diagram representation of the packing, projected along the a axis (right). The corresponding layer is highlighted by a yellow box. Green: chlorine, blue: nitrogen.
The anisotropic nature of the interactions between primary BSMs allows for the separation of layers of columns, defining the secondary BSM (BSM2) in structure 3. Within these layers, neighboring columns are connected by stacking interactions. The calculated interaction energy of the basic molecule in the layer amounts to −38.4 kcal/mol. This interaction energy is over three times greater than that observed between adjacent layers (−11.4 kcal/mol). The interlayer contacts involve weaker N–H···Cl hydrogen bonds and Cl···N interactions. Hence, structure 3 exhibits two levels of supramolecular organization (Figure 9). Notably, the N–H···NLP hydrogen bond, in which the amino group acts as a proton acceptor, plays a key role in crystal formation alongside the classical N–H···Npyr hydrogen bond.
In structure 4, the first coordination sphere also includes 12 neighboring molecules, with a total interaction energy of −55.5 kcal/mol. Similar to structure 3, two strongest interactions are observed (Table 8), forming a zig-zag column as the primary BSM (Figure 10). The bond angle defined by the geometric centers of the basic building unit and adjacent molecules measures approximately 90.9° in structure 3 and 91.1° in structure 4. Molecules within the column are linked by N–H…Npyr and N–H…NLP hydrogen bonds. The total interaction energy within the column is −17.9 kcal/mol.
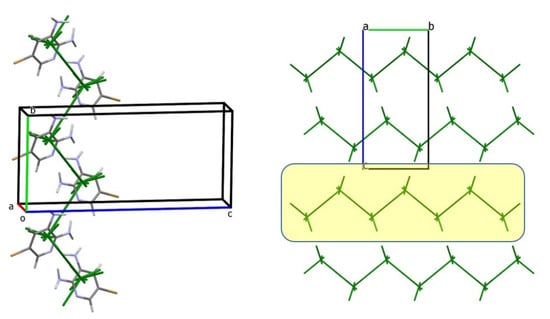
Figure 10.
The primary basic structural motif (BSM) in structure 4 is a zig-zag column extending along the b crystallographic axis (left); energy-vector diagram representation of the packing, projected along the a axis (right). The corresponding layer is highlighted by a yellow box. Blue: nitrogen, brown: bromine.
Likewise, a layer of columns can be identified as the secondary BSM. The interaction energy of the basic molecule with neighbors within this layer is −40.2 kcal/mol, while the interlayer energy is −11.4 kcal/mol. Thus, structure 4 also exhibits columnar-layered packing and can be characterized as isotypical with structure 3.
4. Conclusions
We have analyzed the molecular and crystal structures of mono- and diamino-substituted halogenated pyridines (chloro- and bromo-derivatives). The molecular and crystal structure analysis from a geometric perspective reveals that amino groups participate in hydrogen bonding, acting as bifunctional proton donors and acceptors only in the diamino-substituted compounds. Notably, no stacking interactions were observed in the mono-amino-substituted halogen pyridines.
Energetic analysis of crystal packing demonstrates that the nature of the building unit, basic structural motifs, and overall packing organization differ significantly among crystals 1–4. In the crystal structures of 2-amino-5-chloropyridines (1) and 2-amino-5-bromopyridines (2), layers were identified as the primary structural motif, with C–H···π hydrogen bonds playing a key role in crystal stabilization.
In contrast, the diamino-substituted chloro- and bromopyridines are isotypical, exhibit two levels of organization, and can be classified as columnar-layered. In these structures, N–H···Npyr and N–H···NLP hydrogen bonds, in combination with π–π stacking interactions, are the dominant forces governing crystal formation. Meanwhile, weaker C–H···Hal hydrogen bonds, halogen bonds, and other non-specific interactions play a minor role, mainly in connecting neighboring layers.
Author Contributions
Conceptualization, I.S.K. and G.J.R.; methodology, I.S.K.; validation, I.S.K. and G.J.R.; formal analysis, I.S.K. and G.J.R.; investigation, I.S.K.; data curation, G.J.R.; writing—original draft preparation, I.S.K.; writing—review and editing, G.J.R.; visualization, I.S.K.; project administration, I.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the MSCA4Ukraine project, which is funded by the European Union, grant number 1232296.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Konovalova, I.S.; Shishkina, S.V.; Bani-Khaled, G.; Muzyka, K.; Boyko, A.N. Intermolecular interactions in crystals of benzene and its mono- and dinitro- derivatives: Study from the energetic viewpoint. CrystEngComm 2019, 21, 2908–2919. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Design: Structure and Function Perspectives in Supramolecular Chemistry; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Desiraju, G.R. Chemistry beyond the molecule. Nature 2001, 412, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, S.V.; Konovalova, I.S.; Shishkin, O.V.; Boyko, A.N. Acceptor properties of amino groups in aminobenzene crystals: Study from the energetic viewpoint. CrystEngComm 2017, 19, 6274–6288. [Google Scholar] [CrossRef]
- Shishkina, S.V.; Konovalova, I.S.; Shishkin, O.V.; Boyko, A.N. Influence of substituents on the acceptor properties of the amino groups in the diaminobenzene analogues. CrystEngComm 2017, 19, 7162–7176. [Google Scholar] [CrossRef]
- Konovalova, I.S.; Muzyka, E.N.; Urzhuntseva, V.V.; Shishkina, S.V. Role of intermolecular interactions in formation of mono- and diaminopyridine crystals: Study from the energetic viewpoint. Struct. Chem. 2021, 32, 235–257. [Google Scholar] [CrossRef]
- Betz, R.; Gerber, T.; Hosten, E.; Schalekamp, H. Pyridine-2,3-diamine. Acta Crystallogr. 2011, E67, o2154. [Google Scholar] [CrossRef]
- Konovalova, I.S.; Reiss, G.J. Halogen bonds versus hydrogen bonds in the crystal packing formation of halogen substituted anilines. Z. Kristallogr. Cryst. Mater. 2025, 240, 87–100. [Google Scholar] [CrossRef]
- Konovalova, I.S.; Nöthling, N.; Reiss, G.J. Energetic perspective on the crystal structure organization principles of meta-halogen anilines. J. Mol. Struct. 2025; in print. [Google Scholar]
- Goubitz, K.; Sonneveld, E.J.; Schenk, H. Crystal structure determination of a series of small organic compounds from powder data. Z. Kristallogr. Cryst. Mater. 2001, 216, 176–181. [Google Scholar] [CrossRef]
- Sulovari, A.; Tanski, J.M. Crystallographic and spectroscopic characterization of 5-chloropyridine-2,3-diamine. Acta. Crystallogr. 2017, E73, 1213–1217. [Google Scholar] [CrossRef]
- Reiss, G.J.; Leske, P.B. 2-Amino-pyridin-1-ium triiodide. Acta. Crystallogr. E 2013, 69, o1060–o1061. [Google Scholar] [CrossRef]
- Purushothaman, M.; Loganathan, K.; Sithick, A.K. Synthesis, characterization and biological importance of aminocyanopyridines. Int. J. Chem. Tech. Res. 2012, 4, 479–483. [Google Scholar]
- Huckle, W.R.; Drag, M.D.; Acker, W.R.; Powers, M.; McFall, R.C.; Holder, D.J.; Fujita, T.; Stabilito, I.I.; Kim, D.; Ondeyka, D.L.; et al. Effects of Subtype-Selective and Balanced Angiotensin II Receptor Antagonists in a Porcine Coronary Artery Model of Vascular Restenosis. Circulation 1996, 93, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Rho, M.-C.; Gajulapati, K.; Jung, H.-Y.; Boovanahalli, S.K.; Lee, J.-H.; Song, G.-Y.; Choi, J.-H.; Kim, Y.-K.; Lee, K.; et al. Synthesis of a novel series of imidazo[1,2-α]pyridines as acyl-CoA: Cholesterol acyltransferase (ACAT) inhibitors. Bull. Korean Chem. Soc. 2009, 30, 1297–1304. [Google Scholar]
- Temple, C.J.; Rener, G.A.; Waud, W.R.; Noker, P.E. Antimitotic agents: Structure–activity studies with some pyridine derivatives. J. Med. Chem. 1992, 35, 3686–3691. [Google Scholar] [CrossRef] [PubMed]
- Shishkin, O.V.; Dyakonenko, V.V.; Maleev, A.V. Supramolecular architecture of crystals of fused hydrocarbons based on topology of intermolecular interactions. CrystEngComm 2012, 14, 1795–1804. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Zubatyuk, R.I.; Shishkina, S.V.; Dyakonenko, V.V.; Medviediev, V.V. Role of supramolecular synthons in the formation of the supramolecular architecture of molecular crystals revisited from an energetic viewpoint. Phys. Chem. Chem. Phys. 2014, 16, 6773–6786. [Google Scholar] [CrossRef]
- Shishkina, S.V. Analysis of intermolecular interactions in organic crystals using quantum-chemical methods. Struct. Chem. 2019, 30, 1565–1577. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriquez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Coppens, P. The use of a polarized hydrogen atom in X-ray structure refinement. Acta Crystallogr. Struct. Crystallogr. Cryst. Chem. 1972, B28, 1638–1640. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Goerigk, L.; Grimme, S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys. Chem. Chem. Phys. 2011, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Konovalova, I.S.; Reiss, G.J. Crystal and molecular structure of 5-bromopyridine-2,3-diamine. Z. Kristallogr. New Cryst. Struct. 2024, 239, 891–892. [Google Scholar] [CrossRef]
- Pourayoubi, M.; Ghadimi, S.; Valmoozi, A.A.E. A redetermination of 2-amino-5-chloro-pyridine at 100 K. Acta Cryst. 2007, E63, o4631. [Google Scholar]
- Corozzi, A.; Mennucci, B.; Cammi, R.; Tomasi, J. Structure versus solvent effects on nonlinear optical properties of push–pull systems: A quantum-mechanical study based on a polarizable continuum model. J. Phys. Chem. A 2009, 113, 14774–14784. [Google Scholar] [CrossRef] [PubMed]
- Shishkin, O.V.; Konovalova, I.S.; Zubatyuk, R.I.; Palamarchuk, G.V.; Shishkina, S.V.; Biitseva, A.V.; Rudenko, I.V.; Tkachuk, V.A.; Kornilov, M.Y.; Hordiyenko, O.V.; et al. Remarkably strong polarization of amidine fragment in the crystals of 1-imino-1H-isoindol-3-amine. Struct. Chem. 2013, 24, 1089–1097. [Google Scholar] [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Bürgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994; Volume 2, pp. 741–926. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. A predicted new type of directional noncovalent interaction. Int. J. Quantum Chem. 2007, 107, 2286–2292. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Konovalova, I.S.; Gorb, L.; Leszczynski, J. Novel type of mixed O–H···N/O–H···π hydrogen bonds: Monohydrate of pyridine. Struct Chem. 2009, 20, 37–41. [Google Scholar] [CrossRef]
- Parthe, E. Elements of Inorganic Structural Chemistry, 2nd ed.; K. Sutter Parthe Publisher: Petit-Lancy, Switzerland, 1996. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).