Abstract
Y5F3[AsO3]4 crystallizes needle-shaped in the tetragonal space group P4/ncc with the lattice parameters a = 1143.80(8) pm, c = 1078.41(7) pm and c/a = 0.9428 for Z = 4. The yttrium-fluoride substructure linked via secondary contacts forms a three-dimensional network {[Y5F3]12+} and the remaining part consists of ψ1-tetrahedral [AsO3]3− units, which leave lone-pair channels along [001]. In contrast, platelet-shaped Y5Cl3[AsO3]4 crystals adopt the monoclinic space group C2/c with the lattice parameters a = 1860.56(9) pm, b = 536.27(3) pm, c = 1639.04(8) pm and β = 105.739(3)° for Z = 4. Condensation of [(Y1,2)O8]13− polyhedra via four common edges each leads to fluorite-like {[(Y1,2)O
]5−} layers spreading out parallel to the (100) plane. Their three-dimensional linkage occurs via the (Y3)3+ cations with their Cl− ligands on the one hand and the As3+ cations with their lone-pairs of electrons on the other, which also form within [AsO3]3− anions lone-pair channels along [010]. Both colorless compounds can be obtained by solid-state reactions from corresponding mixtures of the binaries (Y2O3, As2O3 and YX3 with X = F and Cl) at elevated temperatures of 825 °C, most advantageously under halide-flux assistance (CsBr for Y5F3[AsO3]4 and ZnCl2 for Y5Cl3[AsO3]4). By replacing a few percent of YX3 with EuX3 or TbX3, Eu3+- or Tb3+-doped samples are accessible, which show red or green luminescence upon excitation with ultraviolet radiation.
1. Introduction
As a consequence of the lanthanoid contraction, yttrium displays a cationic radius (ri(Y3+) = 101.9 pm, Z(Y) = 39) close to the one of holmium (ri(Ho3+) = 101.5 pm, Z(Ho) = 67), despite having only half the atomic mass [1]. Owing to the electronic closed-shell situation of Y3+ ([Kr]), yttrium(III) compounds serve as perfect hosts for getting doped with same-size luminescent cations of the lanthanoid series (e.g., Eu3+ or Tb3+), especially when they contain hard anions (O2− or F−) to avoid quenching effects. But not only Y2O3 [2] and YF3 [3] are suitable for this purpose, also mixed anionic compounds like YOF [4] are promising candidates, even when they bear soft anions (e.g., YOCl [5] or Y2O2S [6]). Moreover, the necessary energy-transfer processes for luminescence can be eased, when inorganic antennae are present in more complex systems, such as fluoride salts with oxoanions. These serve as energy reservoirs for incoming radiation and act with charge-transfer or electronic lone-pair activities. Most prominent examples are the oxomolybdates(VI) and oxotungstates(VI) YF[MoO4] [7] and YF[WO4] [8] for the first, or the oxoselenates(IV) YF[SeO3] [9,10] and Y3F[SeO3]4 [11] for the second case. Even here, the softer Cl− anions can be tolerated, as the chloride oxomolybdate(VI) YCl[MoO4] [7], the chloride oxotungstate(VI) YCl[WO4] [12,13] and the chloride oxoantimonate(III) YClSb2O4 [14] might demonstrate. The idea of combining most of these prerequisites leads to investigations of the yttrium(III) halide oxoarsenates(III) Y5X3[AsO3]4 (X = F and Cl) as host materials for doping with small amounts of Eu3+ or Tb3+ in order to harvest red or green light from a colorless compound upon UV irradiation.
2. Experimental
The new yttrium(III) fluoride oxoarsenate(III) Y5F3[AsO3]4 and its doped samples could be prepared via partial metallothermic reduction. For this purpose, elemental yttrium (Y: ChemPur, 99.9%, 89.5 mg), yttrium trifluoride (YF3: Heraeus, 99.9%, 35.6 mg) and arsenic sesquioxide (As2O3: Aldrich, 99.99%, 199.2 mg) were reacted within a flux of cesium bromide (CsBr: Merck, 99.9%, 800 mg) in evacuated glassy silica ampoules (Equation (1)). Europium trifluoride (EuF3: ChemPur, 99.9%, 1.6 mg) and terbium trifluoride (TbF3: ChemPur, 99.9%, 1.6 mg) were used as dopants, replacing aliquots of YF3.
The ampoules were sealed under inert gas (argon), treated with a temperature program at 825 °C and brought to room temperature over several days. In this way, phase-pure powders could be obtained and only the monolithically grown single crystal of arsenic per batch had to be removed. As an example, Figure 1 shows the Rietveld refinement of an undoped powder sample of Y5F3[AsO3]4 measured with a STADI-P diffractometer (Stoe & Cie, Darmstadt, Germany) using Cu-Kα radiation (λ = 154.06 pm).
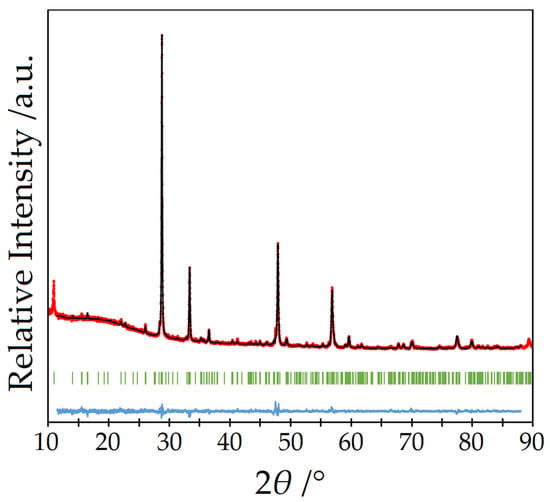
Figure 1.
Rietveld refinement (black) of a powder diffractogram of undoped Y5F3[AsO3]4 (red) with the Bragg positions (green) of Y5F3[AsO3]4 and the Yobs–Ycal difference curve (blue).
The new yttrium(III) chloride oxoarsenate(III) Y5Cl3[AsO3]4 and its doped samples were able to be prepared by mixing yttrium sesquioxide (Y2O3: Merck, 99.99%, 108.3 mg), yttrium trichloride (YCl3: ChemPur, 99.9%, 45.4 mg) and arsenic sesquioxide (As2O3: Aldrich, 99.99%, 94.9 mg) with a flux of zinc chloride (ZnCl2: Merck, 99.9%, 800 mg) in evacuated glassy silica ampoules (Equation (2)). Europium trichloride (EuCl3: Aldrich, 99.9%, 1.9 mg) and terbium trichloride (TbCl3: ChemPur, 99.9%, 1.9 mg) were used as dopants to replace the corresponding amounts of YCl3.
The ampoules were sealed under inert gas (argon), treated with a temperature program at 825 °C and brought to room temperature over several days. In this way, also phase-pure powder samples could be obtained. As an example, Figure 2 shows the Rietveld refinement of an undoped powder of Y5Cl3[AsO3]4 measured with a Rigaku SmartLab X-ray powder diffractometer (Rigaku, Tokyo, Japan) with Cu-Kα radiation (λ = 154.06 pm).
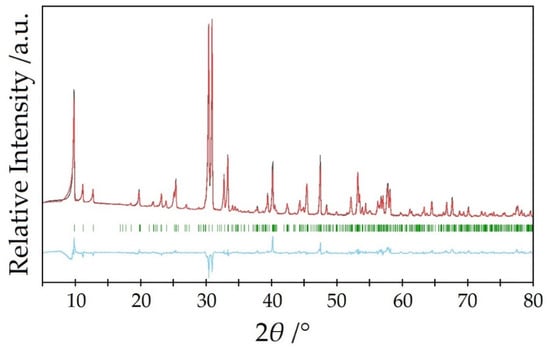
Figure 2.
Rietveld refinement (black) of a powder diffractogram of undoped Y5Cl3[AsO3]4 (red) with the Bragg positions (green) of Y5Cl3[AsO3]4 and the Yobs–Ycal difference curve (blue).
Suitable single crystals of needle-shaped Y5F3[AsO3]4 and platelet-shaped Y5Cl3[AsO3]4 (Figure 3) were transferred into glass capillaries (Hilgenberg, Malsfeld, Germany) and fixed with grease, then measured with Mo-Kα radiation (λ = 71.07 pm) on a κ-CCD single-crystal diffractometer (Bruker-Nonius, Karlsruhe, Germany). After numerical absorption correction with the program HABITUS, included in the X-SHAPE [15] suite, using the SHELX-97 program package [16], the resulting data sets could be solved and successfully refined with direct methods in the tetragonal space group P4/ncc for Y5F3[AsO3]4 and in the monoclinic space group C2/c for Y5Cl3[AsO3]4.
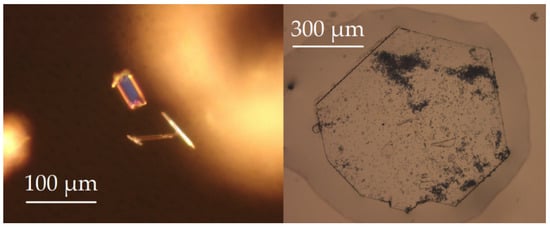
Figure 3.
Brick- or needle-shaped crystals of Y5F3[AsO3]4 (left) and platelet-shaped crystal of Y5Cl3[AsO3]4 (right).
The samples were recorded on an FS920 fluorescence spectrometer (Edinburgh Instruments, Livingston, UK) at room temperature with a Xe900 continuous xenon lamp. Three scans were recorded with respect to the lamp intensity and the average is displayed in the spectra. They were plotted and standardized with respect to their intensity with Origin 2019b.
3. Results and Discussion
3.1. Crystal-Structure Description of Y5F3[AsO3]4
The new yttrium(III) fluoride oxoarsenate(III) Y5F3[AsO3]4 crystallizes needle-shaped in the tetragonal space group P4/ncc with the lattice parameters a = 1143.80(8) pm, c = 1078.41(7) pm and c/a = 0.943 for Z = 4. As expected and as can be seen from the Shannon radii [1], it should assume a unit-cell size between those of Dy5F3[AsO3]4 (a = 1148.34(8) pm, c = 1082.69(7) pm, c/a = 0.943) [17] and Ho5F3[AsO3]4 (a = 1143.26(8) pm, c = 1078.14(7) pm, c/a = 0.943) [17] in terms of the unit-cell dimensions.
There are three different cationic sites present in this structure, two of them belong to yttrium (4c for Y1 and 16g for Y2) and one to arsenic (16g). In contrast, the anions have five different sites, three of which belong to oxygen (all at 16g) and two to fluorine (4c for F1 and 8f for F2). The (Y1)3+ cation centers a square prism of oxygen (d(Y1–O) = 243–249 pm), which is capped by two fluoride anions [(Y1)O8(F1)(F1′)]15−. One F− anion comes close to (Y1)3+ (d(Y1–F1) = 240 pm), while the second one is much further away (d(Y1⋯F1′) = 300 pm) and can therefore only be regarded as a secondary-contact ligand (Figure 4, left). In contrast, the (Y2)3+ cation forms a bicapped trigonal prism [(Y2)O6F2]11− with six oxygen atoms and two fluoride anions without secondary contacts (Figure 4, mid). However, the range of bond lengths is significantly wider (d(Y2–F) = 221–261 pm, d(Y2–O) = 227–250 pm). These distances are still within the expected range, however, especially when compared with Y7O6F9 [18,19], where yttrium-fluorine distances from 218 to 260 pm (plus 300 pm) are present and secondary contacts occur here as well. The yttrium-oxygen distances of 220–268 pm also fall into a similar interval. The As3+ cation forms an isolated ψ1-tetrahedron [AsO3]3− with three oxygen atoms (Figure 4, right), showing arsenic-oxygen distances in the range from 176 to 181 pm, which are quite typical, especially when compared with the crystalline As2O3 modifications (claudetite-I: 172–181 pm [20], claudetite-II: 177–182 pm [21], arsenolite: 179 pm [22]).
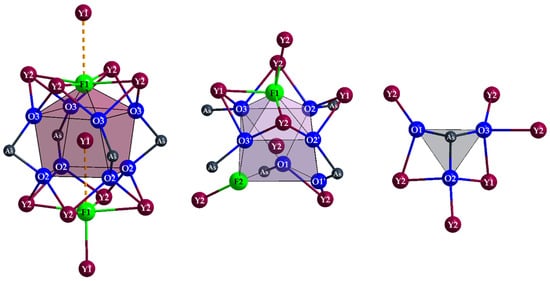
Figure 4.
Capped square prism [(Y1)O8(F1)(F1′)]15− (left), bicapped trigonal prism [(Y2)O6F2]11− (mid) and ψ1-tetrahedron [AsO3]3− (right) with complete cationic decoration in the tetragonal crystal structure of Y5F3[AsO3]4.
The structure becomes easier to understand, if it is divided into two units, firstly the ψ1-tetrahedron [AsO3]3− as just presented, and secondly the substructure of fluoride and yttrium. Both fluoride anions have clearly very different coordination environments. While (F1)− is surrounded by five plus one Y3+ cations in the shape of an elongated octahedron [(F1)(Y2)4(Y1)(Y1′)]17+, (F2)− forms a chevron [(F2)(Y2)2]5+ (∡(Y2–F2–Y2) = 147°) with just two Y3+ cations (Figure 5).
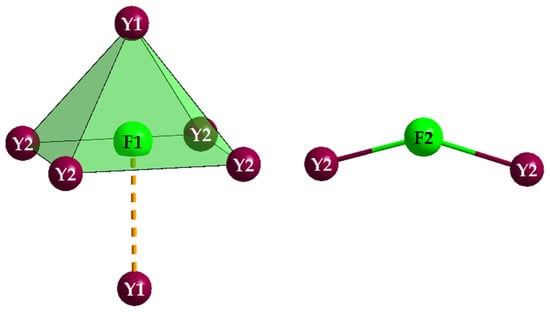
Figure 5.
Capped square pyramid [(F1)(Y2)4(Y1)(Y1′)]17+ (left) and chevron [(F2)(Y2)2]5+ (right) in the crystal structure of Y5F3[AsO3]4.
The [(F1)(Y2)4(Y1)(Y1′)]17+ pseudo-octahedra form chains according to {[(F1)(Y2)(Y1)]14+} (t = terminal, v = vertex-connecting) through corner-linkage via Y1, which run parallel to the c-axis. These chains are connected to the [(F2)(Y2)2]5+ chevrons via Y2 to create a three-dimensional network (Figure 6, top).
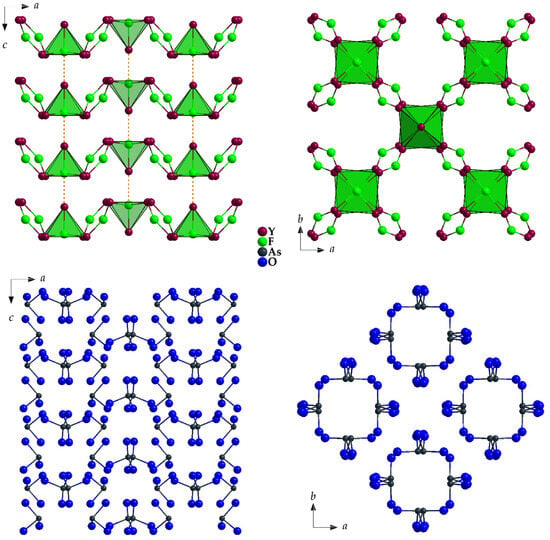
Figure 6.
The yttrium-fluoride substructure linked via secondary Y⋯F’ contacts to form a three-dimensional network {[Y5F3]12+} (top) and the substructure of ψ1-tetrahedral [AsO3]3− anions, which leave channels along [001] due to their lone pairs (bottom) in the crystal structure of Y5F3[AsO3]4 as viewed along [010] (left) and [001] (right).
Figure 7 shows the combination of both substructures ({[Y5F3]12+} + 4 [AsO3]3−) with a view along [001] and the unit-cell edges marked.
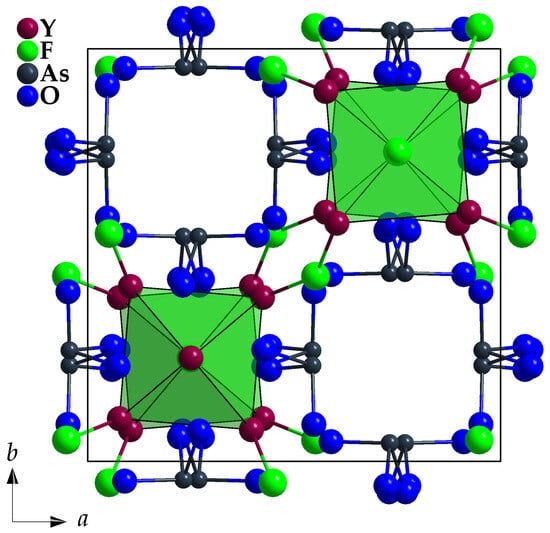
Figure 7.
View at the unit-cell content of Y5F3[AsO3]4 along [001] with emphasized vertex-connected [FY5+1]17+ polyhedra and discrete ψ1-tetrahedral [AsO3]3− units.
Table 1 summarizes the crystallographic data for Y5F3[AsO3]4, while Table 2 lists the atomic positions, Table 3 offers selected interatomic distances and Table 4 contains the motifs of mutual adjunction for its crystal structure.

Table 1.
Crystallographic data of Y5F3[AsO3]4 and their determination.

Table 2.
Fractional atomic coordinates, Wyckoff sites and symmetries as well as Ueq values for Y5F3[AsO3]4.

Table 3.
Selected interatomic distances (d/pm) in the crystal structure of Y5F3[AsO3]4.

Table 4.
Motifs of mutual adjunction for the Y5F3[AsO3]4 structure.
3.2. Crystal-Structure Description of Y5Cl3[AsO3]4
In spite of their identical crystallographic prerequisites (crystal system: monoclinic, space group: C2/c, Pearson symbol: mC96, Wyckoff sequence: f11e1c1), the crystal structure of Y5Cl3[AsO3]4 differs considerably from the one of La5Cl3[AsO3]4 [23]. This is clearly reflected by their lattice parameters (Y5Cl3[AsO3]4: a = 1860.56(9) pm, b = 536.27(3) pm, c = 1639.04(8) pm, β = 105.739(3)° versus La5Cl3[AsO3]4: a = 1803.65(9) pm, b = 548.39(3) pm, c = 1723.16(9) pm, β = 107.432(3)° [23]) already, displaying a larger a-axis and a smaller c-axis, while the b-axis and the β-angle develop as expected by replacing the bigger La3+ (ri = 116 pm) with the smaller Y3+ cation (ri = 102 pm). The molar volumes of 237.0 cm3∙mol−1 for the yttrium juxtaposed to 244.8 cm3∙mol−1 for the lanthanum compound establish the anticipated trend, however.
Common structural features are three crystallographically different RE3+ cations (RE = Y and La), two of them decorated with only eight oxygen atoms [(RE1,2)O8]13−, the third one with four oxygen atoms and four chloride anions [(RE3)O4Cl4]9−, providing square hemi- or antiprismatical coordination spheres (Figure 8, top). All independent oxygen atoms (O1–O6) belong as ligands to As3+ cations (As1 and As2) to constitute two different, but very similar ψ1-tetrahedral [AsO3]3− anions (Figure 9) with pronounced stereochemical lone-pair activity.
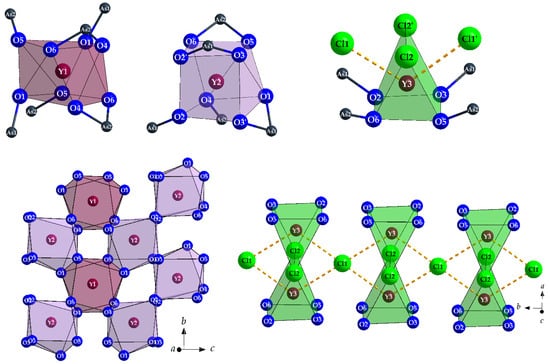
Figure 8.
Square [(Y1,2)O8]13− antiprism or prism and bicapped trigonal [(Y3)O4Cl2Cl’2]9− prism in the crystal structure of Y5Cl3[AsO3]4 with their complete As3+ decoration (top) and their linkage via common oxygen edges to {[(Y1,2)O]5−} layers parallel to the (100) plane (bottom left) and {[(Y3)O(Cl2)(Cl1)]6.5−} strands propagating along [010] (bottom right).
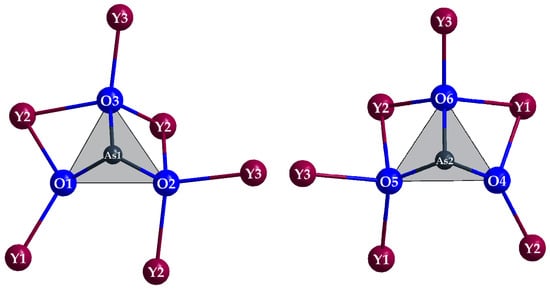
Figure 9.
The two discrete ψ1-tetrahedral [AsO3]3− anions in the crystal structure of Y5Cl3[AsO3]4 with their complete Y3+ decoration.
Remarkably different is the function of the two independent Cl− anions, however. While Cl2 has two short contacts to Y3, Cl1 shows a peculiar coordination sphere of eight almost equidistant trivalent cations (four Y3+ and four As3+) arranged as an idealized cube (Figure 10). The other way around, (Y3)3+ carries two close (Cl2)− and two far away (Cl1)− anions (Figure 8, right) as chloride ligands.
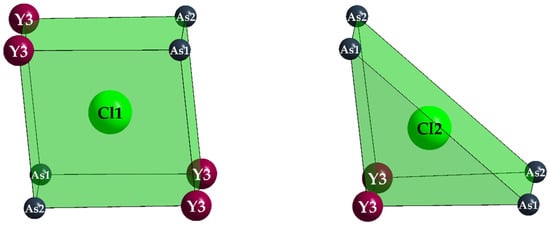
Figure 10.
Square [(Cl1)As4Y4]23+ (left) and trigonal [(Cl2)As4Y2]17+ prism (right) in the crystal structure of Y5Cl3[AsO3]4.
Condensation of the [(Y1)O8]13− and [(Y2)O8]13− polyhedra via four common edges each leads to fluorite-like {[(Y1,2)O]5−} (e = edge-connecting) layers spreading out parallel to the (100) plane (Figure 8, bottom left). Their three-dimensional linkage occurs via (Y3)3+ cations with their Cl− ligands as {[(Y3)O(Cl2)(Cl1)]6.5−} (t = terminal, v = vertex-connecting) strands along [010] on the one hand (Figure 8, bottom right) and As3+ cations with their lone-pairs of electrons on the other (Figure 11).
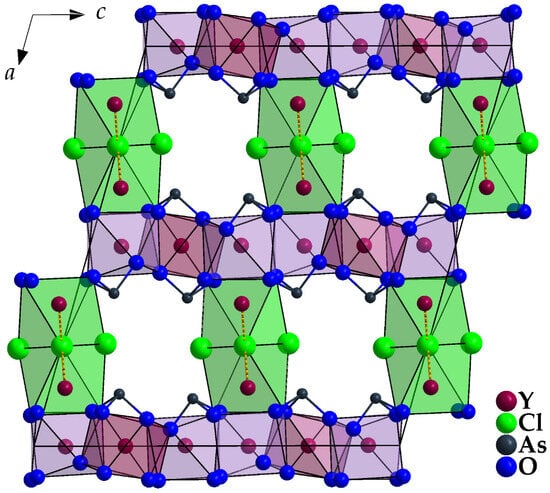
Figure 11.
View at the unit-cell content of monoclinic Y5Cl3[AsO3]4 along [010] with emphasized [(Y1,2)O8]13− and [(Y3)O4Cl2Cl’2]9− polyhedra and discrete ψ1-tetrahedral [AsO3]3− units.
Table 5 summarizes the crystallographic data for Y5Cl3[AsO3]4, while Table 6 lists the atomic positions, Table 7 offers selected interatomic distances and Table 8 contains the motifs of mutual adjunction for its crystal structure.

Table 5.
Crystallographic data of Y5Cl3[AsO3]4 and their determination.

Table 6.
Fractional atomic coordinates, Wyckoff sites and symmetries as well as Ueq values for Y5Cl3[AsO3]4.

Table 7.
Selected interatomic distances (d/pm) in the crystal structure of Y5Cl3[AsO3]4.

Table 8.
Motifs of mutual adjunction for the Y5Cl3[AsO3]4 structure.
3.3. Luminescence of Eu3+- and Tb3+-Doped Samples
None of the samples exhibits a luminescence under a UV lamp of 254 and 366 nm visible by the naked eye. This is presumably due to the low doping degree of about 0.6%. The effect of the low doping concentration can especially be seen in the spectra of Y5X3[AsO3]4:Tb3+ (Figure 12b,d). Besides the normally recorded 5D4 → 7FJ (J = 3–6) transitions in the range from 480 to 630 nm, also the transitions 5D3 → 7FJ (J = 3–6) between 370 and 470 nm can be observed, adding a blue hue to the otherwise green emission. These energetically higher transitions are more intense the further away the Tb3+ cations are from adjacent ones, since the cross relaxation 5D3 → 5D4 with simultaneous excitation of a neighbouring Tb3+ particle from 7F6 to excited 7FJ levels is less probable then [24]. A certain difference can be observed for the terbium-doped influence into the yttrium fluoride and chloride hosts. For X = Cl the transitions from the 5D3 state are significantly stronger than for the samples with X = F, which leads to the assumption that the doped cations are somewhat closer in the latter case.
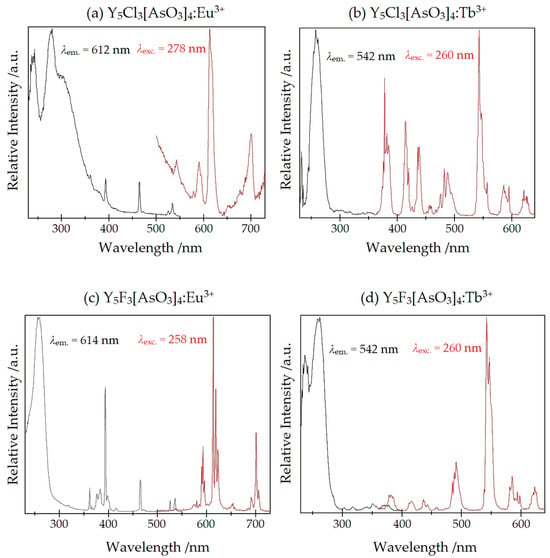
Figure 12.
The luminescence spectra of (a) Y5Cl3[AsO3]4:Eu3+, (b) Y5Cl3[AsO3]4:Tb3+, (c) Y5F3[AsO3]4:Eu3+ and (d) Y5F3[AsO3]4:Tb3+. The emission spectra are displayed in red and those for excitation in black.
The excitation spectra of the terbium-doped compounds display very weak f–f transitions from the 7F6 ground state between 300 and 380 nm and an intense broad band at about 235 and 260 nm originating from f–d transitions. The energetic position of these transitions hint at a rather weak coordination of the surrounding halide and oxide anions [25]. The exact position of all transitions and those of a suitable doped reference are listed in Table 9 and Table 10.

Table 9.
The excitation wavelengths and the assigned transitions of Y5X3[AsO3]4:Tb3+ (X = Cl and F) and Tb2[B2(SO4)6] as reference from [26].

Table 10.
The emission wavelengths and the assigned transitions of Y5X3[AsO3]4:Tb (X = Cl and F) and Y1.998Tb0.002[B2(SO4)6] as reference from [26].
The spectra of the europium-doped compounds (Figure 12a,c) exhibit similar features as the previously discussed terbium spectra. The transitions from the energetically higher 5D1 state to 7FJ (J = 1, 2) in the regime from 530 to 560 nm are weakly present as well as the 5D0 → 7FJ (J = 1–4) transitions between 580 and 710 nm. The hypersensitive 5D0 → 7F2 electric dipolar transition is stronger than the 5D0 → 7F1 magnetic dipolar transition, which is caused by the absence of inversion symmetry at the doped sites. This is in accordance with the site symmetry of the yttrium atoms’ Wyckoff positions (Table 2 and Table 6).
The excitation spectra display f–f transitions from the 7F0 ground state between 350 and 530 nm. The excitation at 535 nm is due to the 7F1 → 5D1 transition. Additionally, a broad charge-transfer band (CT) of europium around 260 nm occurs. For X = Cl this charge transfer is split up into three peaks, possibly because of the varied surrounding at the different yttrium sites. For [EuCl6]3− in acetonitril the charge-transfer band is reported at 301 nm and for [Eu(SO4)]+ at 240 nm [27], which falls into the same range as the measured ones. A compilation of the exact positions for all transitions and these of a reference can be found in Table 11 and Table 12.

Table 11.
The excitation wavelengths and the assigned transitions of Y5X3[AsO3]4:Eu3+ (X = Cl and F) and Eu2[B2(SO4)6] as reference from [26].

Table 12.
The emission wavelengths and the assigned transitions of Y5X3[AsO3]4:Eu3+ (X = Cl and F) and Eu2[B2(SO4)6] as reference from [26].
In contrast to the antimony compounds YSb2O4X (X = Cl and Br) [14], where the lone pair at the Sb3+ cations contributes to the relevant energy-transfer processes, no such participation of the lone pair at the As3+ cations could be detected for the here investigated Y5X3[AsO3]4:Eu3+ or Y5X3[AsO3]4:Tb3+ samples with X = F and Cl.
3.4. Conclusions and Outlook
With Y5F3[AsO3]4 and Y5Cl3[AsO4]4, two new compounds were obtained that have absolutely the same composition apart from the halogen, but very different crystal structures. It would be very interesting to find out, whether the structures with the heavy halides bromide (Y5Br3[AsO3]4) and iodide (Y5I3[AsO3]4) are also different or if they follow the new structure type of the chloride Y5Cl3[AsO3]4 or the lanthanoid(III) bromide derivatives Ln5Br3[AsO3]4 [28,29]. Moreover, luminescence measurements were carried out for undoped samples and those doped with Eu3+ and Tb3+ for both Y5F3[AsO3]4 and Y5Cl3[AsO3]4.
Author Contributions
Conceptualization, R.J.C.L. and T.S.; methodology, R.J.C.L., M.M., F.L., F.C.Z., H.A.H. and T.S.; software, R.J.C.L. and M.M.; validation, R.J.C.L. and M.M.; formal analysis, R.J.C.L. and M.M.; investigation, R.J.C.L. and M.M.; resources, H.A.H. and T.S.; data curation, R.J.C.L. and M.M.; writing—original draft preparation, R.J.C.L., M.M., T.S. and H.A.H.; writing—review and editing, T.S. and H.A.H.; visualization, R.J.C.L. and M.M.; supervision, T.S. and H.A.H.; project administration, T.S. and H.A.H.; funding acquisition, T.S. and H.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
The authors thank Falk Lissner for the single-crystal X-ray diffraction measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to resolve typographical errors. This change does not affect the scientific content of the article.
References
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1975, A32, 751–767. [Google Scholar] [CrossRef]
- Brauer, G.; Gradinger, H. Über heterotype Mischphasen bei Seltenerdoxyden I. Z. Anorg. Allg. Chem. 1954, 276, 209–226. [Google Scholar] [CrossRef]
- Zalkin, A.; Templeton, D.H. The crystal structure of YF3 and related compounds. J. Am. Chem. Soc. 1953, 75, 2453–2458. [Google Scholar] [CrossRef]
- Hund, F. Yttriumoxyfluorid. Z. Anorg. Allg. Chem. 1951, 265, 62–66. [Google Scholar] [CrossRef]
- Meyer, G.; Staffel, T. Die Tieftemperatur-Synthese von Oxidhalogeniden, YOX (X = Cl, Br, I), als Quelle der Verunreinigung von Yttriumtrihalogeniden, YX3, bei der Gewinnung nach der Ammoniumhalogenid-Methode. Die Analogie von YOCl und YSCl. Z. Anorg. Allg. Chem. 1986, 532, 31–36. [Google Scholar] [CrossRef]
- Drafall, L.E.; McCarthy, G.J.; Sipe, C.A.; White, W.B. On the preparation and X-ray powder data of rare earth sulfides and oxysulfides. Proc. Rare-Earth Res. Conf. 1974, 2, 954–959. [Google Scholar]
- Schleid, T.; Strobel, S.; Dorhout, P.K.; Nockemann, P.; Binnemans, K.; Hartenbach, I. YF[MoO4] and YCl[MoO4]: Two halide derivatives of yttrium ortho-oxomolybdate: Syntheses, structures, and luminescence properties. Inorg. Chem. 2008, 47, 3728–3735. [Google Scholar] [CrossRef][Green Version]
- Schustereit, T. Synthese und Charakterisierung Anionischer Derivate von Seltenerdmetall-Oxidomolybdaten und -Wolframaten mit Hilfe Festkörper- und Nasschemischer Methoden. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2015. [Google Scholar]
- Lipp, C.; Schleid, T. A new rare-earth metall(III) fluoride oxoselenate(IV): YF[SeO3]. Z. Anorg. Allg. Chem. 2008, 634, 657–661. [Google Scholar] [CrossRef]
- Chou, S.-C.; Greiner, S.; Magdysyuk, O.V.; Dinnebier, R.E.; Schleid, T. Theoretical and experimental analysis of structural phase transitions for ScF[SeO3] and YF[SeO3]. Z. Anorg. Allg. Chem. 2014, 640, 3203–3211. [Google Scholar] [CrossRef]
- Li, P.-F.; Hu, C.-L.; Kong, F.; Mao, J.-G. The first UV nonlinear optical selenite material. Fluorination control in CaYF(SeO3)2 and Y3F(SeO3)4. Angew. Chem. Int. Ed. 2023, 62, e202301420. [Google Scholar] [CrossRef]
- Schleid, T.; Hartenbach, I. On halide derivatives of rare-earth metal(III) oxidomolybdates(VI) and -tungstates(VI). Z. Kristallogr. 2016, 231, 449–466. [Google Scholar] [CrossRef]
- Schustereit, T.; Netzsch, P.; Höppe, H.A.; Hartenbach, I. Green light. On YCl[WO4] as host material for luminescence active Tb3+ cations. Z. Anorg. Allg. Chem. 2018, 644, 1749–1753. [Google Scholar] [CrossRef]
- Locke, R.J.C.; Goerigk, F.C.; Schäfer, M.J.; Höppe, H.A.; Schleid, T. Synthesis, crystal structures and spectroscopic properties of pure YSb2O4Br and YSb2O4Cl as well as Eu3+- and Tb3+-doped samples. Roy. Soc. Chem. Adv. 2022, 12, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Herrendorf, W.; Bärnighausen, H. HABITUS: Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE, version 1.06; Fa. Stoe: Darmstadt, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97: Programs for Solution and Refinement of Crystal Structures from X-Ray Diffraction Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Locke, R.J.C.; Ledderboge, F.; Goerigk, F.C.; Zimmer, F.C.; Schleid, T. The isotypic series of tetragonal lanthanoid(III) fluoride oxoarsenates(III) Ln5F3[AsO3]4 (Ln = Eu–Lu). Z. Naturforsch. 2024, 79b, 357–367. [Google Scholar] [CrossRef]
- Bevan, D.J.M.; Mann, A.W. The crystal structure of Y7O6F9. Acta Crystallogr. 1975, B31, 1406–1411. [Google Scholar] [CrossRef]
- Bevan, D.J.M.; Mohyla, J.; Hoskins, B.F.; Steen, R.J. The crystal structures of some Vernier phases in the yttrium oxide-fluoride system. Eur. J. Solid State Inorg. Chem. 1990, 27, 451–465. [Google Scholar]
- Pertlik, F. Verfeinerung der Kristallstruktur des Minerals Claudetit, As2O3 (Claudetit I). Monatsh. Chem.–Chem. Monthly 1978, 109, 277–282. [Google Scholar] [CrossRef]
- Pertlik, F. Die Kristallstruktur der monoklinen Form von As2O3 (Claudetit II). Monatsh. Chem.–Chem. Monthly 1975, 106, 755–762. [Google Scholar] [CrossRef]
- Pertlik, F. Structure refinement of cubic As2O3 (arsenolite) with single crystal data. Czech. J. Phys. 1978, B28, 170–176. [Google Scholar] [CrossRef]
- Goerigk, F.C.; Schander, S.; Ben Hamida, M.; Kang, D.-H.; Ledderboge, F.; Wickleder, M.S.; Schleid, T. Die monoklinen Seltenerdmetall(III)-Chlorid-Oxidoarsenate(III) mit der Zusammensetzung SE5Cl3[AsO3]4 (SE = La − Nd, Sm). Z. Naturforsch. 2019, 74b, 497–506. [Google Scholar] [CrossRef]
- Höppe, H.A. Rare-Earth Elements, 1st ed.; de Gruyter: Berlin, Germany, 2024. [Google Scholar]
- Netzsch, P.; Bariss, H.; Bayarjargal, L.; Höppe, H.A. Tb(HSO4)(SO4)—A green emitting hydrogensulfate sulfate with second harmonic generation response. Dalton Trans. 2019, 48, 16377–16383. [Google Scholar] [CrossRef] [PubMed]
- Netsch, P.; Hämmer, M.; Gross, P.; Bariss, H.; Block, T.; Heletta, L.; Pöttgen, R.; Bruns, J.; Huppertz, H.; Höppe, H.A. RE2[B2(SO4)6] (RE = Y, La–Nd, Sm, Eu, Tb–Lu): A silicate-analogous host structure with weak coordination behaviour. Dalton Trans. 2019, 48, 4387–4397. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Locke, R.J.C.; Ledderboge, F.; Goerigk, F.C.; Zimmer, F.C.; Schleid, T. About the Rare-Earth Metal(III) Bromide Oxoarsenates(III) RE5Br3[AsO3]4 with A- (RE = La and Ce) or B-Type Structure (RE = Pr, Nd, Sm–Tb) and RE3Br2[AsO3][As2O5] (RE = Y, Dy–Yb). Solids 2025, 6, 4. [Google Scholar] [CrossRef]
- Locke, R.J.C. Synthese und Charakterisierung von Seltenerdmetall-Halogenid-Oxoarsenaten(III) und -Oxoantimonaten(III) mit Ausläufern zu den entsprechenden -Oxobismutaten(III). Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2025. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).