Y5F3[AsO3]4 and Y5Cl3[AsO3]4: Two Non-Isostructural Yttrium Halide Oxoarsenates(III) and Their Potential as Hosts for Luminescent Eu3+- and Tb3+-Doping
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
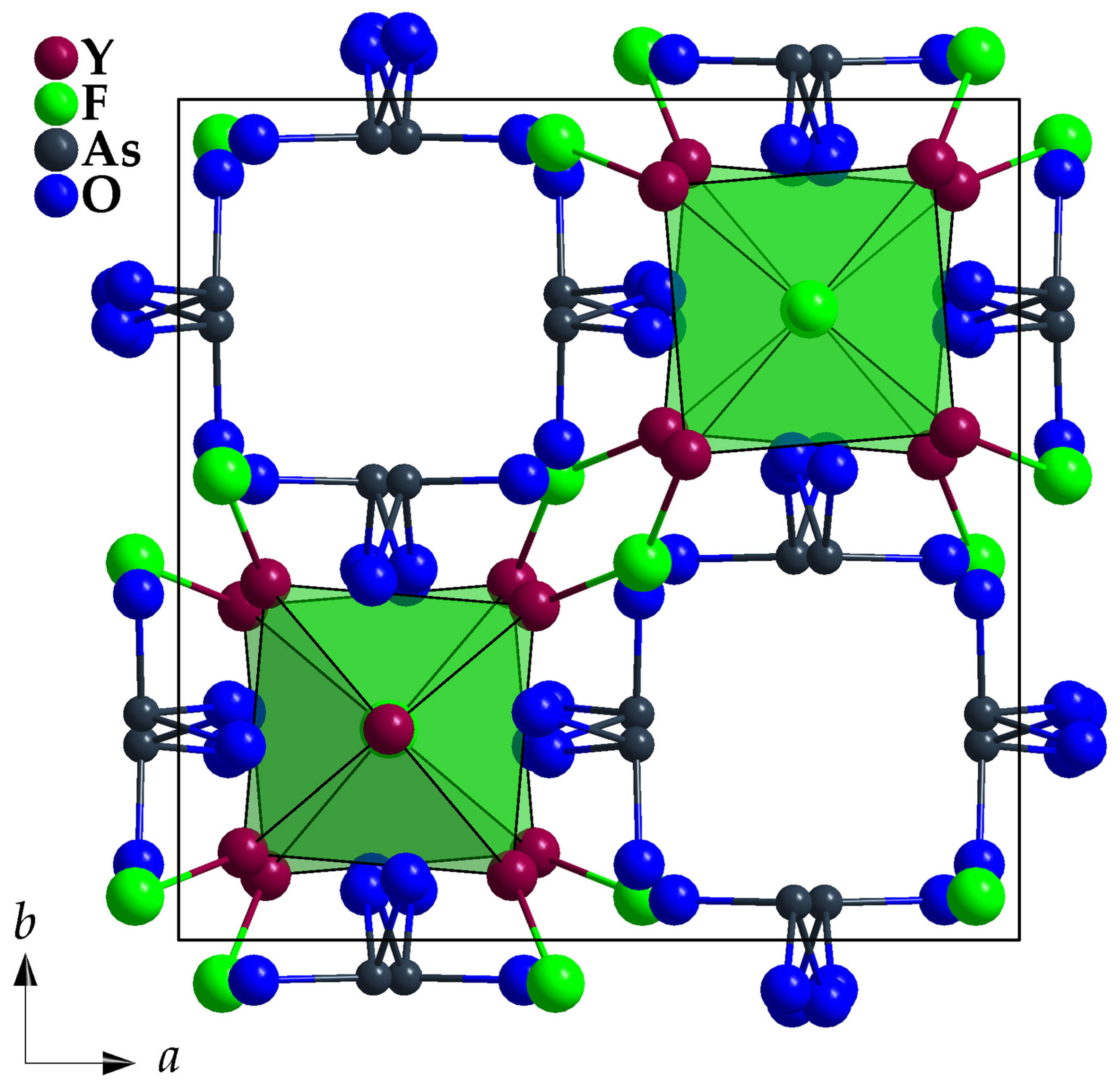
3.1. Crystal-Structure Description of Y5F3[AsO3]4
3.2. Crystal-Structure Description of Y5Cl3[AsO3]4
3.3. Luminescence of Eu3+- and Tb3+-Doped Samples
3.4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1975, A32, 751–767. [Google Scholar] [CrossRef]
- Brauer, G.; Gradinger, H. Über heterotype Mischphasen bei Seltenerdoxyden I. Z. Anorg. Allg. Chem. 1954, 276, 209–226. [Google Scholar] [CrossRef]
- Zalkin, A.; Templeton, D.H. The crystal structure of YF3 and related compounds. J. Am. Chem. Soc. 1953, 75, 2453–2458. [Google Scholar] [CrossRef]
- Hund, F. Yttriumoxyfluorid. Z. Anorg. Allg. Chem. 1951, 265, 62–66. [Google Scholar] [CrossRef]
- Meyer, G.; Staffel, T. Die Tieftemperatur-Synthese von Oxidhalogeniden, YOX (X = Cl, Br, I), als Quelle der Verunreinigung von Yttriumtrihalogeniden, YX3, bei der Gewinnung nach der Ammoniumhalogenid-Methode. Die Analogie von YOCl und YSCl. Z. Anorg. Allg. Chem. 1986, 532, 31–36. [Google Scholar] [CrossRef]
- Drafall, L.E.; McCarthy, G.J.; Sipe, C.A.; White, W.B. On the preparation and X-ray powder data of rare earth sulfides and oxysulfides. Proc. Rare-Earth Res. Conf. 1974, 2, 954–959. [Google Scholar]
- Schleid, T.; Strobel, S.; Dorhout, P.K.; Nockemann, P.; Binnemans, K.; Hartenbach, I. YF[MoO4] and YCl[MoO4]: Two halide derivatives of yttrium ortho-oxomolybdate: Syntheses, structures, and luminescence properties. Inorg. Chem. 2008, 47, 3728–3735. [Google Scholar] [CrossRef][Green Version]
- Schustereit, T. Synthese und Charakterisierung Anionischer Derivate von Seltenerdmetall-Oxidomolybdaten und -Wolframaten mit Hilfe Festkörper- und Nasschemischer Methoden. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2015. [Google Scholar]
- Lipp, C.; Schleid, T. A new rare-earth metall(III) fluoride oxoselenate(IV): YF[SeO3]. Z. Anorg. Allg. Chem. 2008, 634, 657–661. [Google Scholar] [CrossRef]
- Chou, S.-C.; Greiner, S.; Magdysyuk, O.V.; Dinnebier, R.E.; Schleid, T. Theoretical and experimental analysis of structural phase transitions for ScF[SeO3] and YF[SeO3]. Z. Anorg. Allg. Chem. 2014, 640, 3203–3211. [Google Scholar] [CrossRef]
- Li, P.-F.; Hu, C.-L.; Kong, F.; Mao, J.-G. The first UV nonlinear optical selenite material. Fluorination control in CaYF(SeO3)2 and Y3F(SeO3)4. Angew. Chem. Int. Ed. 2023, 62, e202301420. [Google Scholar] [CrossRef]
- Schleid, T.; Hartenbach, I. On halide derivatives of rare-earth metal(III) oxidomolybdates(VI) and -tungstates(VI). Z. Kristallogr. 2016, 231, 449–466. [Google Scholar] [CrossRef]
- Schustereit, T.; Netzsch, P.; Höppe, H.A.; Hartenbach, I. Green light. On YCl[WO4] as host material for luminescence active Tb3+ cations. Z. Anorg. Allg. Chem. 2018, 644, 1749–1753. [Google Scholar] [CrossRef]
- Locke, R.J.C.; Goerigk, F.C.; Schäfer, M.J.; Höppe, H.A.; Schleid, T. Synthesis, crystal structures and spectroscopic properties of pure YSb2O4Br and YSb2O4Cl as well as Eu3+- and Tb3+-doped samples. Roy. Soc. Chem. Adv. 2022, 12, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Herrendorf, W.; Bärnighausen, H. HABITUS: Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE, version 1.06; Fa. Stoe: Darmstadt, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97: Programs for Solution and Refinement of Crystal Structures from X-Ray Diffraction Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Locke, R.J.C.; Ledderboge, F.; Goerigk, F.C.; Zimmer, F.C.; Schleid, T. The isotypic series of tetragonal lanthanoid(III) fluoride oxoarsenates(III) Ln5F3[AsO3]4 (Ln = Eu–Lu). Z. Naturforsch. 2024, 79b, 357–367. [Google Scholar] [CrossRef]
- Bevan, D.J.M.; Mann, A.W. The crystal structure of Y7O6F9. Acta Crystallogr. 1975, B31, 1406–1411. [Google Scholar] [CrossRef]
- Bevan, D.J.M.; Mohyla, J.; Hoskins, B.F.; Steen, R.J. The crystal structures of some Vernier phases in the yttrium oxide-fluoride system. Eur. J. Solid State Inorg. Chem. 1990, 27, 451–465. [Google Scholar]
- Pertlik, F. Verfeinerung der Kristallstruktur des Minerals Claudetit, As2O3 (Claudetit I). Monatsh. Chem.–Chem. Monthly 1978, 109, 277–282. [Google Scholar] [CrossRef]
- Pertlik, F. Die Kristallstruktur der monoklinen Form von As2O3 (Claudetit II). Monatsh. Chem.–Chem. Monthly 1975, 106, 755–762. [Google Scholar] [CrossRef]
- Pertlik, F. Structure refinement of cubic As2O3 (arsenolite) with single crystal data. Czech. J. Phys. 1978, B28, 170–176. [Google Scholar] [CrossRef]
- Goerigk, F.C.; Schander, S.; Ben Hamida, M.; Kang, D.-H.; Ledderboge, F.; Wickleder, M.S.; Schleid, T. Die monoklinen Seltenerdmetall(III)-Chlorid-Oxidoarsenate(III) mit der Zusammensetzung SE5Cl3[AsO3]4 (SE = La − Nd, Sm). Z. Naturforsch. 2019, 74b, 497–506. [Google Scholar] [CrossRef]
- Höppe, H.A. Rare-Earth Elements, 1st ed.; de Gruyter: Berlin, Germany, 2024. [Google Scholar]
- Netzsch, P.; Bariss, H.; Bayarjargal, L.; Höppe, H.A. Tb(HSO4)(SO4)—A green emitting hydrogensulfate sulfate with second harmonic generation response. Dalton Trans. 2019, 48, 16377–16383. [Google Scholar] [CrossRef] [PubMed]
- Netsch, P.; Hämmer, M.; Gross, P.; Bariss, H.; Block, T.; Heletta, L.; Pöttgen, R.; Bruns, J.; Huppertz, H.; Höppe, H.A. RE2[B2(SO4)6] (RE = Y, La–Nd, Sm, Eu, Tb–Lu): A silicate-analogous host structure with weak coordination behaviour. Dalton Trans. 2019, 48, 4387–4397. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Locke, R.J.C.; Ledderboge, F.; Goerigk, F.C.; Zimmer, F.C.; Schleid, T. About the Rare-Earth Metal(III) Bromide Oxoarsenates(III) RE5Br3[AsO3]4 with A- (RE = La and Ce) or B-Type Structure (RE = Pr, Nd, Sm–Tb) and RE3Br2[AsO3][As2O5] (RE = Y, Dy–Yb). Solids 2025, 6, 4. [Google Scholar] [CrossRef]
- Locke, R.J.C. Synthese und Charakterisierung von Seltenerdmetall-Halogenid-Oxoarsenaten(III) und -Oxoantimonaten(III) mit Ausläufern zu den entsprechenden -Oxobismutaten(III). Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2025. [Google Scholar]
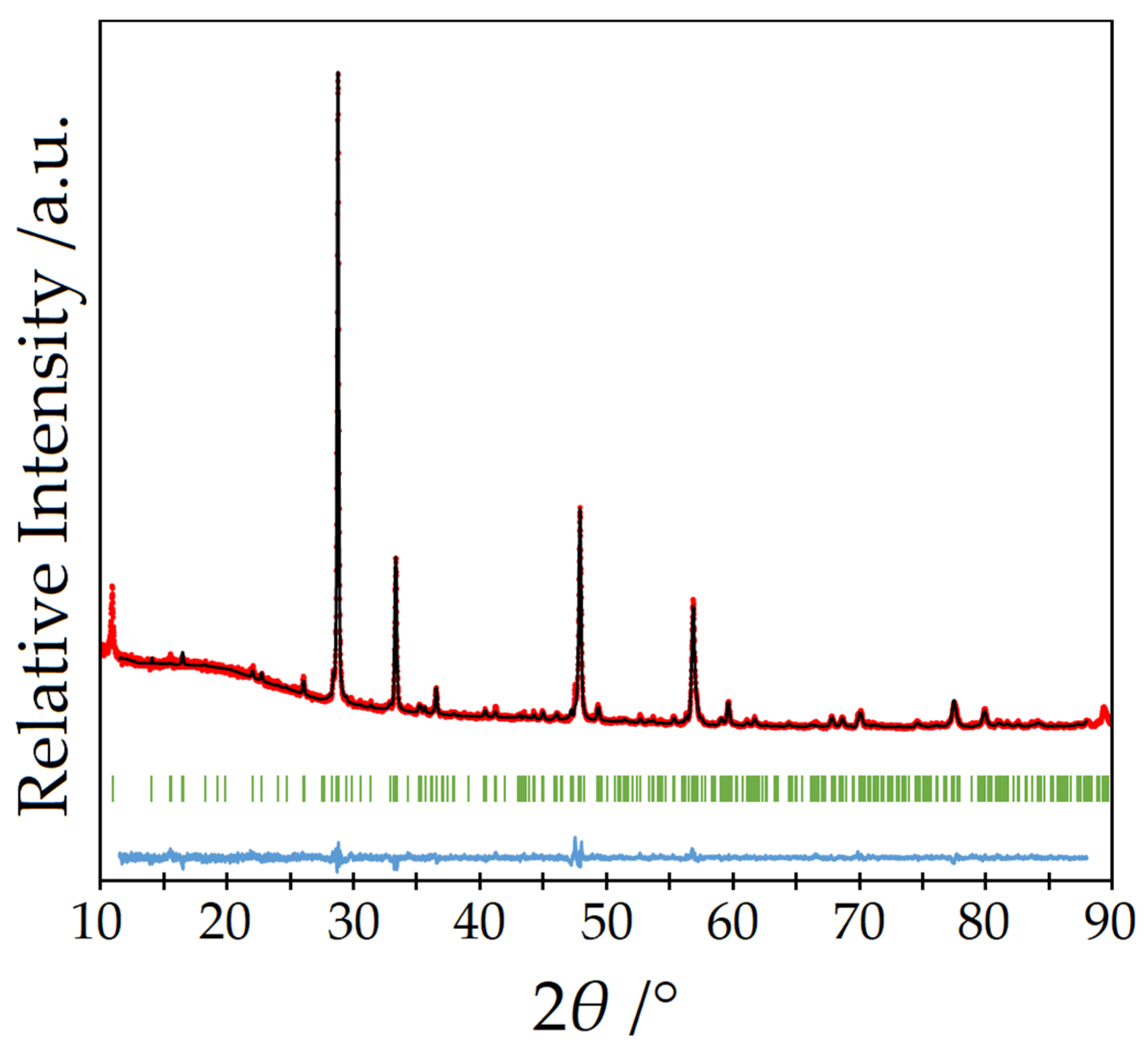










| Structured Formula | Y5F3[AsO3]4 | |
|---|---|---|
| Crystal system | tetragonal | |
| Space group | P4/ncc (no. 130) | |
| Lattice parameters, | a/pm c/pm c/a | 1143.80(8) 1078.41(7) 0.943 |
| Number of formula units, Z | 4 | |
| Calculated density, Dx/g∙cm−3 | 4.676 | |
| Molar volume, Vm/cm3∙mol−1 | 212.41 | |
| Index range, ±hmax, ±kmax, ±lmax | 14, 14, 13 | |
| Diffractometer limit, 2θ/° | 54.9 | |
| Electron sum, F(000)/e− | 1800 | |
| Absorption coefficient, μ/mm−1 | 29.75 | |
| Extinction coefficient, ε/pm−3 | 0.00106(7) | |
| Measured reflections | 20547 | |
| Independent ones | 810 | |
| Rint/Rσ | 0.096/0.041 | |
| Reflections with |Fo| ≥ 4σ(Fo) | 620 | |
| R1/R1 with |Fo| ≥ 4σ(Fo) | 0.056/0.034 | |
| wR2/GooF | 0.067/1.082 | |
| Residual electron density, ρmax/min/e− 10−6 pm−3 | 0.80/–0.85 | |
| CSD number | 2321105 | |
| Atom | Site | x/a | y/b | z/c | Ueq/pm2 |
|---|---|---|---|---|---|
| Y1 | 4c | 1/4 | 1/4 | 0.35328(11) | 134(3) |
| Y2 | 16g | 0.10339(5) | 0.07674(5) | 0.10733(6) | 130(2) |
| F1 | 4c | 1/4 | 1/4 | 0.1312(7) | 172(16) |
| F2 | 8f | 0.0517(4) | 0.9483(4) | 1/4 | 214(13) |
| As | 16g | 0.23168(6) | 0.54424(6) | 0.37332(7) | 147(2) |
| O1 | 16g | 0.0894(4) | 0.5477(4) | 0.4353(4) | 190(11) |
| O2 | 16g | 0.2702(4) | 0.4265(4) | 0.4764(4) | 131(10) |
| O3 | 16g | 0.2223(4) | 0.4409(4) | 0.2465(4) | 167(11) |
| [(Y1)F(1+1)O8]15− polyhedron | ||
|---|---|---|
| Y1–F1 | 1× | 239.5(7) |
| Y1–O2 | 4× | 242.8(4) |
| Y1–O3 | 4× | 248.9(5) |
| Y1⋯F1 | 1× | 299.7(7) |
| [(Y2)F2O6]11− polyhedron | ||
| Y2–F2 | 1× | 220.8(2) |
| Y2–O2 | 1× | 227.4(4) |
| Y2–O1 | 1× | 227.7(5) |
| Y2–O3 | 1× | 229.8(4) |
| Y2–O1′ | 1× | 234.3(4) |
| Y2–O2′ | 1× | 237.4(4) |
| Y2–O3′ | 1× | 250.4(4) |
| Y2–F1 | 1× | 260.9(1) |
| [AsO3]3− ψ1-tetrahedron | ||
| As–O1 | 1× | 176.0(5) |
| As–O2 | 1× | 180.1(4) |
| As–O3 | 1× | 181.1(4) |
| [(F1)Y5+1]17+ capped pyramid | ||
| F1–Y1 | 1× | 239.5(7) |
| F1–Y2 | 4× | 260.9(1) |
| F1–Y1′ | 1× | 299.7(7) |
| [(F2)Y2]5+ chevron | ||
| F2–Y2 | 2× | 220.8(2) |
| O1 | O2 | O3 | F1 | F2 | C.N. | |
|---|---|---|---|---|---|---|
| Y1 | 0/0 | 4/1 | 4/1 | 1 + 1/1 + 1 | 0/0 | 9 + 1 |
| Y2 | 2/2 | 2/2 | 2/2 | 1/4 | 1/2 | 8 |
| As | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 3 |
| C.N. | 3 | 4 | 4 | 5 + 1 | 2 |
| Structured Formula | Y5Cl3[AsO3]4 | |
|---|---|---|
| Crystal system | monoclinic | |
| Space group | C2/c (no. 15) | |
| Lattice parameters, | a/pm b/pm c/pm β/° | 1860.56(9) 536.27(3) 1639.04(8) 105.739(3) |
| Number of formula units, Z | 4 | |
| Calculated density, Dx/g∙cm−3 | 4.399 | |
| Molar volume, Vm/cm3∙mol−1 | 237.00 | |
| Index range, ±hmax, ±kmax, ±lmax | 24, 6, 20 | |
| Diffractometer limit, 2θ/° | 55.0 | |
| Electron sum, F(000)/e− | 1896 | |
| Absorption coefficient, μ/mm−1 | 27.14 | |
| Extinction coefficient, ε/pm−3 | 0.00026(6) | |
| Measured reflections | 19174 | |
| Independent ones | 1775 | |
| Rint/Rσ | 0.115/0.077 | |
| Reflections with |Fo| ≥ 4σ(Fo) | 1150 | |
| R1/R1 with |Fo| ≥ 4σ(Fo) | 0.095/0.054 | |
| wR2/GooF | 0.131/1.048 | |
| Residual electron density, ρmax/min/e− 10−6 pm−3 | 1.83/–1.74 | |
| CSD number | 2401390 | |
| Atom | Site | x/a | y/b | z/c | Ueq/pm2 |
|---|---|---|---|---|---|
| Y1 | 4e | 0 | 0.1972(3) | 1/4 | 158(4) |
| Y2 | 8f | 0.49908(6) | 0.2299(2) | 0.41353(6) | 168(3) |
| Y3 | 8f | 0.14507(6) | 0.2441(2) | 0.45963(6) | 198(3) |
| Cl1 | 4c | 1/4 | 1/4 | 0 | 708(19) |
| Cl2 | 8f | 0.25588(19) | 0.2504(7) | 0.3825(2) | 402(8) |
| As1 | 8f | 0.11822(6) | 0.2590(2) | 0.10200(7) | 164(3) |
| O1 | 8f | 0.0418(5) | 0.3322(14) | 0.1396(5) | 268(20) |
| O2 | 8f | 0.0772(4) | 0.0079(13) | 0.0325(4) | 185(18) |
| O3 | 8f | 0.0839(4) | 0.4931(13) | 0.0185(4) | 173(18) |
| As2 | 8f | 0.36980(6) | 0.2556(2) | 0.19349(7) | 161(3) |
| O4 | 8f | 0.4326(5) | 0.3543(14) | 0.2877(5) | 226(19) |
| O5 | 8f | 0.0844(5) | 0.4989(13) | 0.3399(4) | 186(18) |
| O6 | 8f | 0.4137(4) | 0.4718(12) | 0.1340(4) | 165(18) |
| [(Y1)O8]13− polyhedron | ||
|---|---|---|
| Y1–O1 | 2× | 227.4(7) |
| Y1–O4 | 2× | 239.9(8) |
| Y1–O5 | 2× | 244.9(7) |
| Y1–O6 | 2× | 244.9(7) |
| [(Y2)O8]13− polyhedron | ||
| Y2–O4 | 1× | 220.1(7) |
| Y2–O3 | 1× | 232.6(7) |
| Y2–O1 | 1× | 235.1(7) |
| Y2–O6 | 1× | 236.8(7) |
| Y2–O2 | 1× | 238.8(7) |
| Y2–O3′ | 1× | 248.4(8) |
| Y2–O2′ | 1× | 252.1(7) |
| Y2–O5 | 1× | 255.9(8) |
| [(Y3)O4Cl2Cl’2]9− polyhedron | ||
| Y3–O6 | 1× | 218.4(7) |
| Y3–O3 | 1× | 219.3(7) |
| Y3–O2 | 1× | 238.0(8) |
| Y3–O5 | 1× | 240.7(7) |
| Y3–Cl2 | 1× | 269.5(4) |
| Y3–Cl2‘ | 1× | 273.9(3) |
| Y3⋯Cl1 | 1× | 325.1(1) |
| Y3⋯Cl1‘ | 1× | 330.3(1) |
| [(As1)O3]3− ψ1-tetrahedron | ||
| As1–O1 | 1× | 174.0(8) |
| As1–O2 | 1× | 179.5(8) |
| As1–O3 | 1× | 183.9(7) |
| [(As2)O3]3− ψ1-tetrahedron | ||
| As2–O4 | 1× | 174.7(7) |
| As2–O5 | 1× | 178.2(7) |
| As2–O6 | 1× | 184.3(7) |
| [(Cl1)Y4As4]23+ polyhedron | ||
| Cl1⋯Y3 | 2× | 325.1(1) |
| Cl1⋯Y3′ | 2× | 330.3(1) |
| Cl1⋯As1 | 2× | 332.2(1) |
| Cl1⋯As2 | 2× | 334.5(1) |
| [(Cl2)Y2As4]17+ polyhedron | ||
| Cl2–Y3 | 1× | 269.5(4) |
| Cl2–Y3‘ | 1× | 273.9(3) |
| Cl2⋯As1 | 1× | 348.9(4) |
| Cl2⋯As1‘ | 1× | 355.9(4) |
| Cl2⋯As2 | 1× | 353.3(4) |
| Cl2⋯As2‘ | 1× | 357.5(4) |
| O1 | O2 | O3 | O4 | O5 | O6 | Cl1 | Cl2 | C.N. | |
|---|---|---|---|---|---|---|---|---|---|
| Y1 | 2/1 | 0/0 | 0/0 | 2/1 | 2/1 | 2/1 | 0/0 | 0/0 | 8 |
| Y2 | 1/1 | 2/2 | 2/2 | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 8 |
| Y3 | 0/0 | 1/1 | 1/1 | 0/0 | 1/1 | 1/1 | 0 + 2/0 + 4 | 2/2 | 6 + 2 |
| As1 | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3 |
| As2 | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 | 1/1 | 0/0 | 0/0 | 3 |
| C.N. | 3 | 4 | 4 | 3 | 4 | 4 | 0 + 4 | 2 |
| Y5Cl3[AsO3]4:Tb3+ | Y5F3[AsO3]4:Tb3+ | Tb2[B2(SO4)6] | |
|---|---|---|---|
| Transition exc. | Wavelength/nm | Wavelength/nm | Wavelength/nm |
| f–d | 233, 258 | 237, 262 | 212, 253 |
| 7F6 → 5H6 | – | 304 | 301 |
| 7F6 → 5H7 | – | 317 | 316 |
| 7F6 → 5L7, 5L8 | – | 341 | 339 |
| 7F6 → 5G5 | – | 351 | 356 |
| 7F6 → 5L10 | – | 371 | 366 |
| 7F6 → 5G6 | – | 377 | 373 |
| Y5Cl3[AsO3]4:Tb3+ | Y5F3[AsO3]4:Tb3+ | Y2[B2(SO4)6]:Tb3+ | |
|---|---|---|---|
| Transition em. | Wavelength/nm | Wavelength/nm | Wavelength/nm |
| 5D3 → 7F6 | 378, 382, 385 | 382 | – |
| 5D3 → 7F5 | 415, 420 | 417 | 411 |
| 5D3 → 7F4 | 436, 439 | 437, 443 | 434 |
| 5D3 → 7F3 | 456, 458, 460 | – | – |
| 5D4 → 7F6 | 482, 488 | 491 | 490 |
| 5D4 → 7F5 | 543, 547, 557 | 542, 547 | 541 |
| 5D4 → 7F4 | 586, 595 | 585, 594, 598 | 583, 588 |
| 5D4 → 7F3 | 621, 627 | 623 | 617, 621 |
| Y5Cl3[AsO3]4:Eu3+ | Y5F3[AsO3]4:Eu3+ | Eu2[B2(SO4)6] | |
|---|---|---|---|
| Transition exc. | Wavelength/nm | Wavelength/nm | Wavelength/nm |
| CT | 243, 278 | 258 | 265 |
| 7F0 → 5H4–7 | – | 319 | 316 |
| 7F0 → 5D4 | 361 | 362 | 360 |
| 7F0 → 5G3–6 | – | 376 | 374 |
| 7F0 → 5G2 | – | 384 | 381 |
| 7F0 → 5L6 | 393 | 394 | 392 |
| 7F1 → 5D3 | – | 415 | 412 |
| 7F0 → 5D2 | 464 | 465 | – |
| 7F0 → 5D1 | 526 | 526 | – |
| 7F1 → 5D1 | 535 | 536 | – |
| Y5Cl3[AsO3]4:Eu3+ | Y5F3[AsO3]4:Eu3+ | Eu2[B2(SO4)6] | |
|---|---|---|---|
| Transition em. | Wavelength/nm | Wavelength/nm | Wavelength/nm |
| 5D1 → 7F0 | – | 510 | – |
| 5D1 → 7F1 | – | 538 | – |
| 5D1 → 7F2 | – | 554, 558 | – |
| 5D0 → 7F1 | 587 | 590, 593 | 585, 591, 595 |
| 5D0 → 7F2 | 613, 618 | 614, 619, 623 | 610, 615, 621 |
| 5D0 → 7F3 | – | 651, 654 | 647, 650, 653 |
| 5D0 → 7F4 | 703 | 691, 701, 706 | 690, 697 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locke, R.J.C.; Mikuta, M.; Ledderboge, F.; Zimmer, F.C.; Höppe, H.A.; Schleid, T. Y5F3[AsO3]4 and Y5Cl3[AsO3]4: Two Non-Isostructural Yttrium Halide Oxoarsenates(III) and Their Potential as Hosts for Luminescent Eu3+- and Tb3+-Doping. Crystals 2025, 15, 611. https://doi.org/10.3390/cryst15070611
Locke RJC, Mikuta M, Ledderboge F, Zimmer FC, Höppe HA, Schleid T. Y5F3[AsO3]4 and Y5Cl3[AsO3]4: Two Non-Isostructural Yttrium Halide Oxoarsenates(III) and Their Potential as Hosts for Luminescent Eu3+- and Tb3+-Doping. Crystals. 2025; 15(7):611. https://doi.org/10.3390/cryst15070611
Chicago/Turabian StyleLocke, Ralf J. C., Martina Mikuta, Florian Ledderboge, Frank C. Zimmer, Henning A. Höppe, and Thomas Schleid. 2025. "Y5F3[AsO3]4 and Y5Cl3[AsO3]4: Two Non-Isostructural Yttrium Halide Oxoarsenates(III) and Their Potential as Hosts for Luminescent Eu3+- and Tb3+-Doping" Crystals 15, no. 7: 611. https://doi.org/10.3390/cryst15070611
APA StyleLocke, R. J. C., Mikuta, M., Ledderboge, F., Zimmer, F. C., Höppe, H. A., & Schleid, T. (2025). Y5F3[AsO3]4 and Y5Cl3[AsO3]4: Two Non-Isostructural Yttrium Halide Oxoarsenates(III) and Their Potential as Hosts for Luminescent Eu3+- and Tb3+-Doping. Crystals, 15(7), 611. https://doi.org/10.3390/cryst15070611







