Abstract
Tantalum carbide MXenes, notably Ta4C3Tx and Ta2CTx, exhibit distinctive physicochemical properties that distinguish them from the well-studied Ti3C2Tx MXene. The combination of exceptional electrochemical properties, efficient photothermal conversion, and tunable surface terminations highlights the versatility of Ta-MXenes. These characteristics render them highly valuable for versatile applications. This minireview summarizes recent progress in tantalum carbide MXenes and their composites, focusing on applications in energy storage, conversion, sensing, and biomedicine. First, synthesis methods for tantalum carbide MXenes are summarized. Subsequently, their key properties are discussed, followed by a systematic review of diverse applications. Finally, this review offers a summary and outlook on the challenges and opportunities in the field of tantalum carbide MXenes research.
1. Introduction
MXenes, first discovered in 2011 [1], are a group of 2D transition metal carbides, nitrides, and carbonitrides exhibiting excellent electrical conductivity, remarkable mechanical strength, tunable hydrophilic/hydrophobic properties, and unique optical, electrical, and electromechanical characteristics, rendering them promising for a wide range of applications [2,3,4,5,6]. The general formula for MXene is Mn+1Xn or Mn+1XnTx (M stands for transition metals, X is C and/or N, n = 1–3, and Tx represents surface terminations [-OH, -F, -O, -Cl, etc.]) [7]. These terminations facilitate further surface modifications, enhancing the material’s versatility. Since their discovery, MXenes have garnered significant attention for their adjustable composition and layered structure, enabling property customization and broad application potential.
So far, most studies in the MXene field have focused on Ti3C2Tx due to its relative ease of synthesis and well-documented properties. Recently, tantalum-based MXenes have garnered significant attention due to their notable attributes compared to Ti3C2Tx. Tantalum-based MXenes follow the general formula Tan+1CnTx, where n represents the number of carbon layers. To date, two representative compositions, Ta4C3Tx and Ta2CTx, have been successfully synthesized. These materials exhibit distinctive physicochemical properties. For example, Ta4C3Tx MXene features a significantly larger interlayer distance than Ti3C2Tx [8], which facilitates enhanced ion intercalation and diffusion processes, crucial for the performance of energy storage devices. The biocompatibility of elemental tantalum, which has driven its extensive use in clinical applications, also extends to its MXene derivatives, enabling their application in biological systems with minimal cytotoxicity concerns. Due to its high atomic number (Z = 73), tantalum provides superior CT imaging effects compared to iodine-based contrast agents, enhancing its utility in diagnostic imaging. In contrast to the extensively researched Ti3C2Tx MXene, Ta4C3Tx exhibits distinct advantages, including an electrical conductivity six times higher than that of other MXene types [9]. These superior properties render it particularly suitable for applications in electrocatalysis, photocatalysis, and other energy conversion processes [10]. Moreover, the remarkable attributes of tantalum carbide MXenes have ensured their applicability across a range of areas, including energy storage devices [9,11], sensors [12,13], surface-enhanced Raman spectroscopy (SERS) [14,15], biomedicine [16,17], photonics and optoelectronics [18,19], and so on (Figure 1). This paper provides an overview of the advancements in tantalum carbide MXene preparation, properties, and applications. Significant attention is devoted to the innovative synthesis techniques, their wide-ranging applications, and novel strategies spanning various fields.
Figure 1.
Brief description of tantalum carbide MXenes in various applications (pink area: the content of this review, green area: the application domain of tantalum carbide MXenes).
2. Synthesis Procedures
2.1. Synthesis of Tantalum Aluminum Carbide MAX Phase
Tantalum carbide MXenes are typically synthesized by selectively etching aluminum (Al) from their tantalum aluminum carbide MAX phase precursors. Specifically, Ta4AlC3 and Ta2AlC serve as the precursors for the synthesis of Ta4C3Tx and Ta2CTx MXenes, respectively. A variety of approaches have been adopted for the synthesis of Ta4AlC3 and Ta2AlC MAX phases so far, most involving high-temperature sintering treatment of tantalum (Ta), aluminum (Al), and carbon (C) powders, and other materials. For example, Dai et al. employed a ball-milling technique to mix Ta, Al, and C powders in a 4:1.75:3 molar ratio at a speed of 800 rpm for 12 h. Following this, the mixed powders were pressed into a disc shape and then heated to 1500 °C in an argon-filled tube furnace at a rate of 10 °C/min for 2 h, resulting in the formation of Ta4AlC3 MAX phase ceramics [20]. Beyond traditional sintering approaches, hot-press sintering represents another frequently utilized technique in the fabrication of the tantalum aluminum carbide MAX phase. Lin et al. prepared Ta4AlC3 ceramic through a hot-pressing method, where elemental Ta, Al, and C powders were first homogenized via ball milling for 10 h, followed by heating to 1500 °C at 15 °C/min in a flowing argon atmosphere and applying 30 MPa pressure for 1 h [21]. Syamsai et al. synthesized a Ta4AlC3 MAX phase through a solid-state technique, initially involving ball milling of Ta, Al, and C powders at 800 rpm, followed by hot pressing the resultant powder at 900 °C under 0.5 ton and 10−3 base vacuum [22]. Despite the effectiveness of hot-pressing sintering techniques in the synthesis of tantalum aluminum carbide MAX, their high costs and requirement for specialized equipment have led researchers to explore more cost-effective alternatives. Yeh et al. present a novel approach for synthesizing Ta2AlC MAX through self-propagating high-temperature synthesis [23]. It shows that the ideal Al content (Ta:Al:C = 2:1.6:1) promotes single-phase Ta2AlC MAX formation through exothermic combustion reactions that take place at temperatures between 1050 and 1250 °C.
2.2. Synthesis of Tantalum Carbide MXene
After the successful preparation of tantalum aluminum carbide MAX phase precursors, a widely adopted technique for producing tantalum carbide MXenes is the targeted etching of the Al components in their MAX phase. Various etching methods have been developed for tantalum carbide MXenes, with different etchants significantly influencing their morphological structures. HF etching is one of the primary methods for etching tantalum carbide MXenes, utilizing concentrated hydrofluoric acid directly. Another approach is in situ HF etching, which employs a combination of acid and fluorine-containing salts. Additionally, alkali etching and the acid-base treatment method without fluorine have been explored as viable alternatives. These approaches to synthesizing tantalum carbide MXenes from the etching of tantalum aluminum carbide MAX are depicted in Table 1.
2.2.1. Direct Hydrofluoric Acid (HF) Etching
HF is employed as a most common selective etchant to facilitate the conversion of Ta4AlC3 and Ta2AlC MAX phases into Ta4C3Tx and Ta2CTx MXene. The selective etching is driven by the preferential removal of Al layers, which is attributed to the comparatively lower bond energy of Al-Ta or Al-C bonds relative to the robust Ta-C bonds. This selective etching process facilitates the transformation of layered MAX phases into 2D MXene materials, ensuring the structural integrity of the transition metal carbide layers. In most cases, the etching process after HF treatment leads to the formation of a distinctive accordion-like morphology tantalum carbide MXene, which significantly enhances its surface area and expands the interlayer spacing, thereby improving its functional properties [16,24,25]. Moreover, the direct HF etching process influences the surface chemistry of tantalum carbide MXene by introducing a variety of terminal functional groups, primarily -OH, -O, and -F, which significantly influence the MXene’s physicochemical properties [9,24]. This direct HF etching process consists of simple yet effective steps, including HF treatment and subsequent washing, making it accessible. However, the permeability and high toxicity of HF require that strict safety measures must be followed during use and handling.
2.2.2. In Situ HF Etching (Fluoride Salt)
Liu et al. developed a method to synthesize Ta4C3Tx MXene etching Al from the Ta4AlC3 MAX phase using in situ HF generated from LiF and HCl [26]. This approach replaces the use of highly toxic HF with milder HCl for the synthesis of Ta4C3Tx MXene. Considering the higher exfoliation energy of Ta4AlC3 relative to conventional MAXs (such as Ti3AlC2, Zr3AlC2, Ti2AlN and Ti2AlC2) [27], the milder HCl/LiF etching process was usually conducted under high temperature and pressure. Specifically, a hydrothermal treatment at 180 °C for 20 h was implemented to achieve the effective etching [13,26]. Rafieerad et al. developed a method to etching Ta4AlC3 using 12 M HCl and 4 M NaF as etchants at 60 °C for 48 h [28]. The resultant MXene nanosheets were further treated by hydrothermal process to obtain Ta4C3Tx MXene quantum dots.
2.2.3. Fluorine-Free Etching
For safer and more practical applications, fluorine-free etching synthesis has utilized alkali species to extract Al from tantalum aluminum carbide and synthesize tantalum carbide MXene. Vijayaprabhakaran et al. developed a straightforward alkali-assisted hydrothermal method for synthesizing Ta2C MXene [10]. Ta2AlC MAX phase powder was uniformly dispersed in a concentrated KOH solution, subjected to hydrothermal treatment at 150 °C for 24 h in an autoclave, centrifuged, and thoroughly washed with deionized water to achieve a neutral pH, ensuring complete removal of residual alkaline components, ultimately yielding etched Ta2C MXene. Rafieerad et al. employed a modified alkaline-based etching method to synthesized Ta4C3Tx MXene through a two-step acidic/alkaline (HCl/KOH) etching [11]. The Ta4AlC3 MAX phase powder underwent a sequential chemical treatment involving 6 M HCl followed by 6 M KOH to produce an exfoliated nanocomposite material. These fluorine-free etching methods provide environmentally friendly alternatives for the synthesis of tantalum carbide MXene.

Table 1.
The different preparation approaches for synthesizing tantalum carbide MXene.
Table 1.
The different preparation approaches for synthesizing tantalum carbide MXene.
| Precursor | Etchant | Reaction Condition | Ref. |
|---|---|---|---|
| 10 g Ta4AlC3 power | 40% HF | Stirred for 72 h at room temperature (RT) | [8] |
| 1 g Ta4AlC3 powder | 20 mL 20% HF | Stirred for 4 h | [29] |
| 2 g Ta4AlC3 powder | 50 mL HF | Stirred at 45 °C for 96 h | [9] |
| 1 g of Ta4AlC3 precursor | 20 mL 45% HF | Stirred for 120 h at 50 °C | [30] |
| 2 g of Ta4AlC3 | 50 mL 45% HF | Stirred at 45 °C for 120 h | [12] |
| 2 g Ta4AlC3 | 20 mL 9 M HCl + 2 g LiF | Stirred at 35 °C for 72 h | [31] |
| 1 g Ta4AlC3 | 50 wt% HF | Stirred at 40 °C for 96 h | [24] |
| 0.5 g Ta4AlC3 | 20 mL of 40% HF | Stirred for 96 h at RT | [22] |
| 1 g Ta4AlC3 powder | 40 mL 49 wt% HF | Stirred for 72 h at RT | [32] |
| Ta4AlC3 | 40% HF | Stirred for 96 h at RT | [20] |
| Ta4AlC3 | HF | Etched for 4 days at RT | [33] |
| 2 g Ta4AlC3 | 40 mL 40 v/v% HF | Stirred for 4 days | [34] |
| 1 g Ta4AlC3 | 12 mL 40 wt% HF | Stirred for 4 days | [14] |
| Ta4AlC3 | 40% HF | Etched for 4 days | [18] |
| 1 g Ta2AlC | 5 mL 49 wt% HF | Stirred for 1 day at RT | [16] |
| Ta2AlC | HF | Etching | [35] |
| 1 g Ta4AlC3 | 30 mL 40% HF | Stirred for 72 h at RT | [17] |
| 1 g Ta4AlC3 | 20 mL 50 wt% HF | Stirred at 40 °C for 96 h | [36] |
| 0.5 g Ta4AlC3 | 15 mL (36−38 wt%) + 0.6 g LiF | Hydrothermal reacted at 180 °C for 20 h | [13] |
| 0.5 g Ta4AlC3 | 15 mL~38 wt% HCl + 0.6 g LiF | Hydrothermal reacted at 180 °C for 20 h | [26] |
| 2 g Ta4AlC3 | 40 HCl + mL 3.2 g LiF | Reacted for 48 h | [37] |
| Ta4AlC3 | 12 M HCl and 4 M NaF | Reacted at 60 °C for 48 h | [28] |
| Ta4AlC3 | 6 M HCl, 6 M KOH | Shaking in HCl at 37 °C for 72 h, Freeze dried, treating in KOH at RT for 90 h | [11] |
| 1.5 g Ta2AlC | KOH | Hydrothermal reacted at 150 °C for 24 h | [10] |
2.3. Delamination of Tantalum Carbide MXene
Following the etching process, tantalum carbide MXene commonly presents an accordion-like morphology. The accordion-like multilayered MXene requires delamination for effective exploration of its properties. For multilayer MXene, increasing the interlayer spacing weakens van der Waals forces, enabling easy separation into single or few-layered flakes through simple methods like shaking or ultrasonication. Delamination of multiple layers further requires intercalating agents to expand the interlayer spacing (Figure 2). The most commonly used intercalating agents are tetramethylammonium hydroxide (TMAOH), tetrapropylammonium hydroxide (TPAOH), and tetrabutylammonium hydroxide (TBAOH). These intercalating agents can further increase the interlayer distance of MXene, promoting the exfoliation of MXene nanosheets, thereby obtaining thinner MXene nanosheets. The enlarged interlayer can be identified by XRD. For example, in Liu’s report, the diffraction peak of the tantalum carbide (002) crystal plane exhibited a shift of 2° toward the lower-angle region after etching and intercalation, indicating an expansion in the interlayer spacing [26]. The multiple preparation techniques for delamination of tantalum carbide MXene are depicted in Table 2.
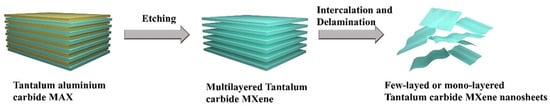
Figure 2.
Schematic illustration of the synthesis of tantalum carbide MXene. (brown: the Al layer, blue: the tantalum carbide MXene).

Table 2.
Multiple preparation techniques for delamination of tantalum carbide MXene.
3. Structure of Tantalum Carbide MXene
Ta4C3Tx MXene is generally prepared by selectively etching the Al layer in its MAX phase using HF [38]. Figure 3a,b show SEM images of the microstructure of a multilayer of Ta4C3Tx after HF etching, illustrating the successful realization of decrustation for individual grain. The HRTEM image clearly demonstrates that the crystal lattice of multilayered nanosheet with hexagonal structure and the basal plane hexagonal symmetry of the MAX phase is preserved during HF treatment (Figure 3c and inset). TEM images reveal that thin electron-transparent sheets of the delaminated Ta4C3Tx nanosheets (Figure 3d,e) inform a typical sheet-like morphology with an average size of approximately 100 nm. Viewing the basal planes in the cross section of the TEM image (Figure 3f), the single-layered Ta4C3Tx nanosheet is ~1 nm thick. The XRD pattern shows the comparison of the Ta4AlC3 MAX phase with Ta4C3Tx (Figure 3g). The peak intensity of Ta4AlC3 was greatly reduced after HF treatment. The enlarged c lattice parameter was found to be 30.15 Å. For most of the reported HF-etched MXenes, an emerging low-angle (002) peak is observed, which implies that the sample has been converted into MXene [25]. As illustrated in the atomic structure image, an atomic model of a free-standing Ta4C3 nanosheet was constructed from the Ta4AlC3 phase. Both top and side views of the stable atomic configuration of the 2D Ta4C3 nanosheet (a = 3.13 Å) are illustrated in Figure 3h and Figure 3i, respectively.
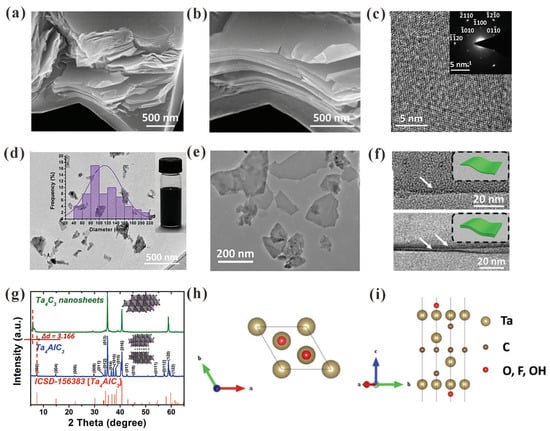
Figure 3.
Structural features of Ta4C3Tx. (a,b) SEM images of layered Ta4C3Tx after HF etching. (c) HRTEM image of multilayer Ta4C3Tx after HF etching. Inset shows the overall SAED pattern. (d,e) TEM images of ultrathin Ta4C3Tx nanosheets after probe sonication. Middle inset shows lateral size distribution of the Ta4C3Tx nanosheets and right inset is a digital photo of Ta4C3Tx nanosheets dispersed in water. (f) HRTEM images of ultrathin Ta4C3Tx nanosheets with one layer and two layers. (g) XRD patterns of Ta4AlC3 phase and Ta4C3Tx nanosheets. (h) Top and (i) side views of the surface terminated Ta4C3Tx (T = O, F, and OH) [25]. Copyright (2017), Wiley-VCH.
4. Properties of Tantalum Carbide MXene
The crystal structures of Ta2C and Ta4C3 MXene are illustrated in Figure 4. Ta2C demonstrates the fundamental building block with a symmetric tri-layer sequence (Ta-C-Ta), featuring two Ta layers coordinating a single C interlayer. Ta4C3 extends this stacking principle into a seven-layer architecture with four Ta layers establishing three distinct carbon intercalation planes [39]. XRD shows that Ta4C3Tx crystallizes in a hexagonal close-packed configuration [33]. The density functional theory calculations reveal that the total and partial density of states (DOS) of Tan+1Cn Mxene exhibit non-zero density of states at the Fermi level, revealing metallic conductivity characteristics. The Ta 5d orbital electrons constitute the predominant contribution to the electronic states near the Fermi level [39].
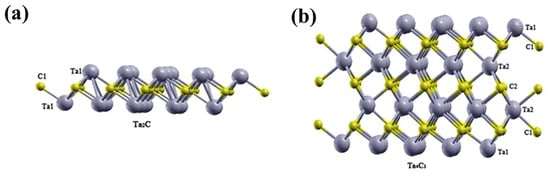
Figure 4.
The atomic structure of pristine (a) Ta2C and (b) Ta4C3 Mxene. Permission granted for reproduction [39]. Copyright (2018), Wiley-VCH.
As for optical properties, Ta2CTx MXene demonstrates strong and relatively flat absorption characteristics spanning from the UV to the NIR region (400–2000 nm) [19]. Ta4C3 MXene displays a broad and intense optical absorption, similar to other 2D nanomaterials, including graphene and MoS2 [25]. Moreover, Ta2CTx and Ta4C3Tx MXene demonstrate outstanding nonlinear optical characteristics, highlighting their promise as photonic application materials [35,37].
Surface terminations (Tx) significantly influence the properties of tantalum carbide MXene materials. According to the research of Dong et al., the electronic properties of tantalum carbide MXenes can be modified by surface terminations [18]. As for Ta4C3, upon adsorption of -O, the electron energy states in the upper conduction band were notably reduced, indicating a profound impact on the electronic properties. After O-termination, the overall energy bands were concentrated within the range of −8.5 eV to approximately 3 eV, with a significant downward shift in the energy level of the highest conduction band [18]. In the case of -OH terminated Ta4C3, a novel band δ emerged at approximately −10 eV, originating from the orbital hybridization of Ta 3d, C 2s, O 2p, and H 1s, which would endow it with broadband absorption. As for optical properties, -O termination significantly enhances the absorption of Ta4C3 in the near-infrared region (800–1600 nm). -OH termination specifically boosts absorption within the 1200–1600 nm wavelength range.
5. Multifunctional Applications of Tantalum Carbide MXene
5.1. Energy Storage
5.1.1. Lithium-Sulfur Batteries
Lithium-sulfur (Li-S) batteries are a promising next-generation energy storage technology offering ultrahigh theoretical capacity, superior energy density, and significant cost and environmental advantages over conventional lithium-ion systems. Despite their potential, Li-S batteries suffer from the polysulfide shuttle effect, slow redox kinetics, and lithium dendrite growth that raises safety risks. Ta4C3Tx MXene has emerged as a novel Li-S battery component due to its excellent electrical conductivity and good mechanical strength. Liang et al. presents a novel bifunctional barrier design for Li-S batteries using a Ta4C3Tx-Ta2O5 heterostructure [9]. The heterostructure was fabricated by H2O2-mediated oxidation of Ta4C3Tx MXene and subsequent calcination, yielding Ta2O5 quantum dots anchored on Ta4C3Tx. The high conductivity of Ta4C3Tx MXene enhances Li+ diffusion and storage. Moreover, the excellent mechanical strength suppresses Li dendrite formation, while Ta2O5 efficiently anchored polysulfides (Figure 5a). Owing to these merits, the Ta4C3Tx-Ta2O5@carbon polypropylene (C-PP) heterostructure exhibits remarkable electrochemical performance, with an initial capacity of 801.9 mAh·g−1 at 1 C and a remarkably low capacity degradation rate of just 0.086% across 500 cycles (Figure 5b).
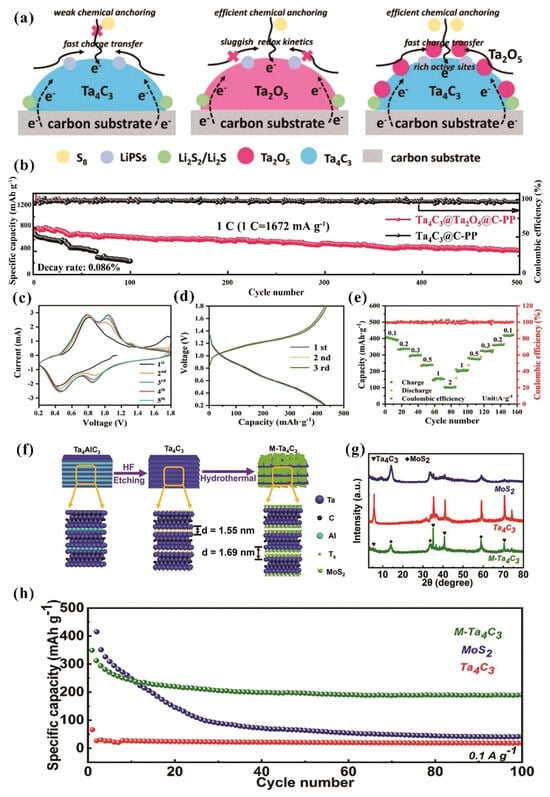
Figure 5.
(a) Schematic illustration of the synthesis for Ta4C3-Ta2O5 heterostructure architectures. (b) Electrochemical cycling performance of Ta4C3-Ta2O5@C-PP and Ta4C3@C-PP cells over 500 cycles at 1 C. Permission granted for reproduction [9]. Copyright (2023), Elsevier. (c) CV curves of VO2(B)@Ta4C3 for the first five cycles, obtained at a scan rate of 1 mV/s. (d) GCD profiles at a current density of 0.1 A/g for the initial three cycles of the VO2(B)@Ta4C3 composite. (e) Rate performance of VO2(B)@Ta4C3 cathode across current densities ranging from 0.1 to 2 A/g. Permission granted for reproduction [26]. Copyright (2023), American Chemical Society. (f) A schematic representation of the synthetic process for MoS2-Ta4C3 (M-Ta4C3). (g) The XRD patterns of MoS2, Ta4C3, and M-Ta4C3. (h) The cycling stability of MoS2, Ta4C3, and M-Ta4C3 at a current density of 0.1 A/g. Permission granted for reproduction [8]. Copyright (2021), Elsevier.
5.1.2. Zinc Ion Batteries
Zinc ion batteries (ZIBs) exhibit multiple advantages, including low-cost zinc anodes, high theoretical capacity (820 mAh·g−1), and low equilibrium potential (−0.76 V), coupled with inherent safety from aqueous electrolytes, making them significant promise for both grid storage and electric vehicles. However, ZIBs face critical challenges, including vanadium oxide cathode dissolution, structural instability during Zn2+ intercalation, and rapid capacity fading. Ta4C3Tx MXene exhibits superior electrical conductivity, mechanical stability, and a unique electronic structure compared to conventional MXenes, enabling it to serve as an ideal conductive substrate for cathode of ZIBs. The research work by Liu et al. shows that Ta4C3 MXene serves as a good substrate for growing vanadium metal-organic framework (V-MOF) [26]. The introduction of Ta4C3 modulated the phase evolution of V-MOF toward VO2(B) instead of V2O5, resulting in a 3D network cross-linked structure with superior electrochemical performance. This VO2(B)@Ta4C3 composite effectively reduces the Zn2+ diffusion barrier to 0.34 eV, outperforming both V2O5 (0.70 eV) and VO2(B) (0.51 eV). Figure 5c illustrates the cyclic voltammetry (CV) curves of the VO2(B)@Ta4C3 cathode, obtained at a scan rate of 1 mV/s over a voltage range spanning from 0.2 V to 1.8 V, revealing excellent reversibility in the Zn2+ insertion/extraction processes, as evidenced by the consistent peak positions observed over multiple cycles, which confirms the VO2(B)@Ta4C3 composite’s high structural stability and efficient electrochemical performance. As shown in Figure 5d, the GCD profiles measured at a current density of 0.1 A/g demonstrate an initial discharge capacity of 437 mAh/g for the VO2(B)@Ta4C3 cathode. Figure 5e illustrates the rate capability of the VO2(B)@Ta4C3 cathode material across a wide range of current densities, spanning from 0.1 to 2 A/g, exhibiting excellent capacity retention and recovery when returning to lower current densities. The consistently high Coulombic efficiency, approaching almost 100% throughout the cycling process, underscores the excellent electrochemical reversibility and robust structural stability of the VO2(B)@Ta4C3 cathode, demonstrating superior electrochemical reversibility for ZIBs applications.
5.1.3. Sodium Ion Capacitors
Sodium ion capacitors offer a cost-effective and resource-abundant alternative to lithium-ion systems. MoS2 nanosheets demonstrate a theoretical capacity of 670 mAh/g for Na+ storage [40]. Yet, the electrochemical performance of MoS2 nanosheets is significantly hindered by agglomerative stacking, cycling-induced mechanical degradation, low conductivity, and kinetically limited Na+ intercalation processes. Liu et al. first employed Ta4C3Tx MXene in Na+ storage applications and found that the Ta4C3Tx MXene can address critical challenges by spatially confined growth of 2D MoS2 nanosheets both on the surface and within the interlamellar of layered Ta4C3Tx MXene [8]. The accordion-like architecture of Ta4C3Tx MXene functions as both a conductive scaffold and physical constraint matrix, simultaneously mitigating MoS2 nanosheet aggregation and volume expansion while enhancing electron conduction (Figure 5f). The XRD pattern (Figure 5g) shows a (002) plane of MoS2-Ta4C3 shifting from 5.7° to 5.3° compared to pristine Ta4C3, indicating an enlarged interlayer spacing from 1.55 nm to 1.69 nm due to the pillar effect of intercalated MoS2 nanosheets. The Na+ ion capacitor obtained by using 2D MoS2/Ta4C3Tx as anode and active carbon as cathode demonstrates excellent energy storage capabilities, achieving 87.6 Wh kg−1 specific energy density alongside an impressive 3937.3 W kg−1 power density. Figure 5h shows the cycling stability of MoS2, Ta4C3, and MoS2-Ta4C3 at 0.1 A/g over 100 cycles, revealing superior electrochemical performance of MoS2-Ta4C3 with a high capacity of 218.8 mAh g−1 after 100 cycles. This significantly surpasses pure MoS2 (fading to 41.2 mAh g−1) and Ta4C3 (18.6 mAh g−1), highlighting the synergistic enhancement achieved by employing Ta4C3 in Na+ storage.
5.2. Energy Conversion and Environmental Remediation
5.2.1. Hydrogen Production
Hydrogen is a clean and sustainable energy source with zero emissions and high energy density, emerged as a promising substitute for traditional fossil fuels in sustainable energy systems. Electrochemical water splitting represents a highly viable approach for scalable hydrogen generation. The research work of Wu et al. demonstrates a one-step synthesis of 2D Ta2CS2 with ordered sulfur termination (Figure 6a), exhibiting superior conductivity and bifunctional catalytic activity (hydrogen evolution reaction, HER) for overall water splitting in 1 M KOH [41]. The layered Ta2CS2 was exfoliated into ultrathin two-dimensional nanosheets (Ta2CS2-E) using N,N-dimethylformamide (DMF) (Figure 6b). The polarization curves depicted in Figure 6c show that the HER performance of Ta2CS2-E is close to that of conventional Pt/C material. Ta2CS2-E demonstrates an overpotential of 73 mV at 10 mA/cm2, approaching commercial Pt/C (44 mV). Ta2CS2-E demonstrates a Tafel slope that is comparable to that of Pt/C among various catalysts (Figure 6d), indicating its effectiveness in promoting the HER. The long-term stability performance of Ta2CS2-E indicates that it exhibits nearly 100% retention of current density, maintaining a value of 10 mA/cm2 at an overpotential of 73 mV (Figure 6e) over a period of 48 h. This study demonstrates Ta2CS2-E as an excellent catalyst, offering good potential for future electrochemical water splitting.
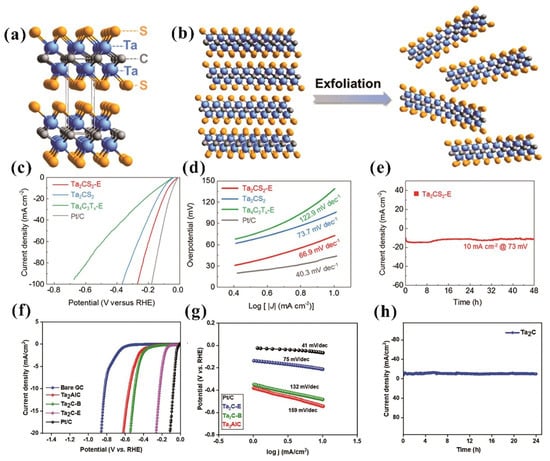
Figure 6.
(a) Crystal structure of Ta2CS2. (b) The schematic illustration of exfoliation route for generating Ta2CS2-E nanosheets. (c) HER polarization curves and (d) Tafel slopes of Ta2CS2-E compared with other samples. (e) Stability study of Ta2CS2-E at an overpotential of 73 mV. Permission granted for reproduction [41]. Copyright (2021), Wiley-VCH. (f) HER polarization curves and (g) Tafel slopes of Ta2C-E compare with other samples. (h) Time-dependent current density curves of Ta2C-E. Permission granted for reproduction [10]. Copyright (2021), Elsevier.
In another research work, Vijayaprabhakaran et al. synthesized 2D Ta2C via a fluorine-free etching method and found that Ta2C MXene served as an efficient electrocatalyst for both HER and nitro compound reduction [10]. The LSV curve reveals that (Ta2C-E) MXene exhibits excellent HER kinetics in 0.5 M H2SO4, demonstrating performance comparable to that of Pt-C catalysts (Figure 6f). The Tafel slopes of the electrocatalysts were determined to be 75 mV/dec for Ta2C-E MXene and 44 mV/dec for Pt-C (Figure 6g). Notably, the Tafel slope of Ta2C-E MXene is closely aligned with that of Pt-C, suggesting its favorable HER kinetics. The chronoamperometric study (Figure 6h) revealed that Ta2C-E MXene exhibited remarkable stability for 24 h under HER conditions without any loss of activity, alongside its enhanced electrocatalytic performance, evidenced by a lower onset potential and higher cathodic peak current in 0.5 M H2SO4. These research findings indicate that Ta2C is a promising candidate for efficient electrocatalytic hydrogen production.
5.2.2. Photo/Electrothermal Conversion
In recent years, significant attention has been focused on developing multifunctional photothermal/electrothermal conversion materials to address critical environmental and health challenges, such as freshwater scarcity and tumor treatment. Liu et al. fabricated novel Ta4C3Tx/graphene aerogel composites via a hydrothermal method followed by freeze-drying, which exhibit superior photo/electrothermal conversion performance and multifunctional applicability [42]. As depicted in the inset of Figure 7a, Ta4C3Tx/graphene aerogel (TGA-6) appears as a black cylinder with an extremely low density of 24.6 mg/cm3. Its microporous network structure, clearly revealed in the FESEM images, enhances sunlight scattering and optimizes solar energy utilization. The photothermal performance of Ta4C3Tx/graphene aerogel was tested under 1 sun simulated sunlight, showing the sample reached 85.3 °C in 3 s and up to 90.2 °C in 50 s, with temperatures stabilizing after 1 min (Figure 7b). Figure 7c illustrates the relationship between the surface temperature and irradiation time, demonstrating its rapid photothermal conversion capability. By utilizing its photo/electrothermal properties, this Ta4C3Tx/graphene aerogel can be successfully applied in seawater steam generation, thermal therapy, deicing, and the absorption of high-viscosity oil. The enhanced photothermal conversion efficiency of the Ta4C3Tx/graphene aerogel composites can be attributed to the synergistic effects between Ta4C3Tx and graphene aerogel, where the porous structure of graphene aerogel acts as light-trapping caves for maximizing solar energy utilization through multiple reflections and scattering, while Ta4C3Tx, with its superior conductivity and stability in air, further improves the electrical conductivity and sunlight absorption capability of the composite. Additionally, the hierarchical porous structure facilitates water transport and enhances overall solar energy utilization efficiency.
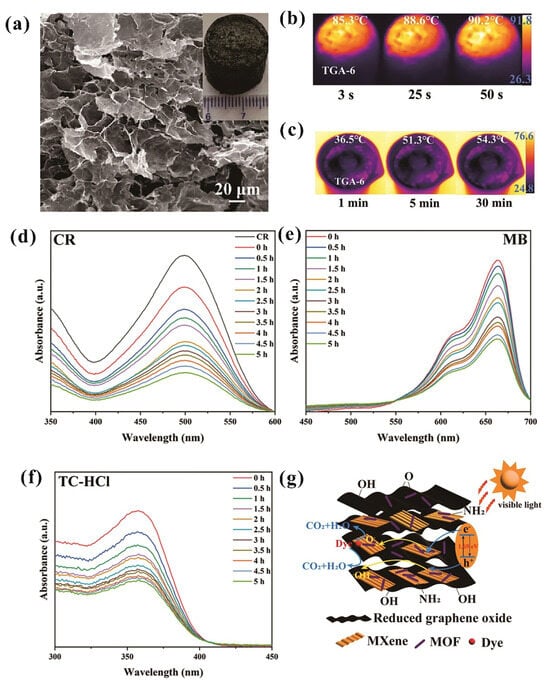
Figure 7.
(a) SEM images of Ta4C3Tx/graphene aerogel. (b,c) Thermal infrared imaging and surface temperature analysis of Ta4C3Tx/graphene aerogel composites in the dry state at varying time intervals. Permission granted for reproduction [42]. Copyright (2024), Elsevier. UV–visible absorption spectra of (d) CR, (e) MB, and (f) TC-HCl with respect to irradiation time, demonstrating the photocatalytic degradation activities of NH2-MIL-88B/Ta4C3Tx/graphene. (g) Photocatalytic degradation mechanism of dye/drug on NH2-MIL-88B/Ta4C3Tx/graphene under visible light illumination. Permission granted for reproduction [43]. Copyright (2025), Elsevier.
5.2.3. Pollutant Degradation
Environmental pollution is a significant challenge, especially concerning wastewater that contains with dyes and antibiotics, which negatively impacts ecosystems and health. Zhang et al. developed innovative NH2-MIL-88B/Ta4C3Tx/graphene aerogels with remarkable capabilities for photocatalytic applications and thermal energy storage using solar energy [43]. By utilizing a hydrothermal method and subsequent freeze-drying process, the MOF material NH2-MIL-88B was successfully integrated with Ta4C3Tx to prepare the NH2-MIL-88B/Ta4C3Tx/graphene composite. The combination of NH2-MIL-88B as a photocatalyst, Ta4C3Tx as a co-catalyst and adsorbent, and graphene aerogel provided a 3D porous structure that generates a significant synergistic effect, leading to superior physicochemical properties of the composite. The NH2-MIL-88B/Ta4C3Tx/graphene composite exhibited superior degradation efficiencies for Congo red (GR), methylene blue (MB), and tetracycline hydrochloride (TC-HCl) (Figure 7d–f). Under visible light irradiation, NH2-MIL-88B generates electron-hole pairs owing to its 1.69 eV bandgap, and the electrons transfer to reduced graphene oxide and Ta4C3Tx to form a conductive network that suppresses charge recombination. Simultaneously, holes on NH2-MIL-88B produce hydroxyl radicals (•OH) by reacting with OH−, and electrons on graphene/MXene generate superoxide radicals (•O2−) through oxygen adsorption, leading to the mineralization of pollutants into CO2 and H2O (Figure 7g). This study demonstrates that Ta4C3Tx MXene exhibits significant promise for photocatalytic pollutant degradation.
5.3. Detection and Sensing
5.3.1. SERS
Surface-enhanced Raman scattering (SERS) is a powerful spectroscopic tool distinguished by its sensitivity, specificity and rapid detection, making it ideal for biological and environmental applications. Wang et al. synthesized a Ta4C3 MXene/gold nanostar hybrid as a SERS substrate, as shown in Figure 8a [15]. Figure 8b demonstrates that Au nanostars (AuNSs) are attached to the Ta4C3 MXene surface, ensuring both the effective attachment of AuNSs to the two-dimensional substrate and the interaction between adjacent nanoparticles, thereby generating SERS hot spots. UV−vis spectroscopy reveals the blue shift in absorption peaks of AuNSs when combined with Ta4C3 MXene (Figure 8c), indicating tighter connections in the composite structure. The Ta4C3 MXene/AuNSs hybrids substrate exhibits superior SERS performance for detecting 4-aminothiophenol (PATP) molecules compared to individual components (Au nanostars or Ta4C3 MXene) (Figure 8d). The Ta4C3 MXene facilitates charge transfer between its 2D electron gas structure and adsorbed PATP molecules via molecular orbital interactions, amplifying Raman signals through chemical enhancement. Moveover, the high specific surface area and surface hydroxyl/carboxyl groups of Ta4C3 MXene enhance molecular adsorption capacity, accumulating more analyte molecules on the composite substrate and boosting detection sensitivity. Figure 8e presents the Raman spectra of Ta4C3 MXene/AuNSs used for detecting various concentrations of PATP, demonstrating that the detection limit can reach as low as 10−9 M. In another study by Lan et al., it was demonstrated that 2D Ta4C3 nanosheet by itself is a superior material for SERS sensing [14], removing the dependence on noble metals (Figure 8f). A 2D Ta4C3 nanosheet exhibited excellent detection performance for both Rhodamine 6G (R6G) and crystal violet (CV), with detection limits of 1 × 10−7 M for R6G (Figure 8g) and 1 × 10−6 M for CV (Figure 8h), demonstrating its high sensitivity in SERS measurements.
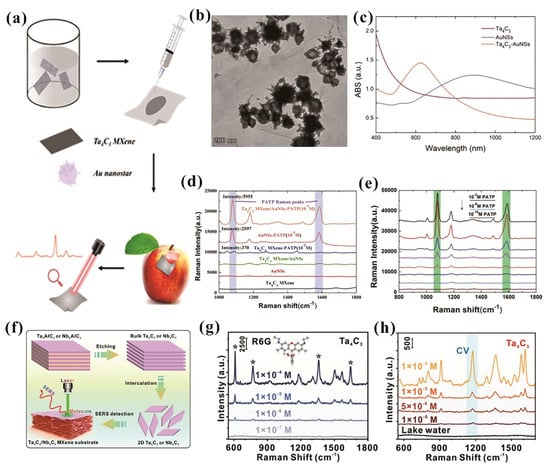
Figure 8.
(a) Preparation of 2D Ta4C3 MXene/ANSs composites as SERS substrates. (b) TEM images of Ta4C3 MXene/AuNSs composites. (c) UV–vis absorption spectra of Ta4C3 MXene, AuNSs, and the Ta4C3 MXene/AuNSs composite structure. (d) Raman spectra of Ta4C3 MXene, AuNSs, Ta4C3 MXene/AuNSs, Ta4C3 MXene/AuNSs with 10−5 M PATP, AuNSs with 10−5 M PATP, and Ta4C3 MXene with 10−2 M PATP. (e) Raman spectra of Ta4C3 MXene/AuNSs for detecting various concentrations of PATP. Permission granted for reproduction [15]. Copyright (2024), American Chemical Society. (f) Schematic representation of the synthesis and SERS application for 2D Ta4C3 and Nb4C3 nanosheet. (g) Raman spectra of R6G with concentrations ranging from 10−4 to 10−7 M adsorbed on Ta4C3. (h) Raman spectra of CV with concentrations ranging from 10−4 to 10−6 M adsorbed on Ta4C3. Permission granted for reproduction [14]. Copyright (2024), American Chemical Society.
5.3.2. Gas Sensors
Tantalum Carbide MXenes can be utilized as efficient gas sensors, utilizing their unique properties for sensitive and selective detection of various gas molecules. The gas-sensing performance of tantalum carbide MXenes is mainly driven by the chemisorption of gases and the interaction with surface functional groups. Kong et al. fabricated gas sensors by drop-casting monolayers of Ta4C3Tx onto interdigital electrodes, which exhibited high sensitivity and selectivity to ammonia at room temperature with a low detection limit of 0.72 ppm. TEM in Figure 9a reveals that few-layer or monolayer Ta4C3Tx forms a relatively uniform film without obvious defects. Figure 9b shows the resistance change of the Ta4C3Tx sensor during recovery at room temperature for NH3 concentrations ranging from 65 to 325 ppm. The cyclability of the sensor was measured at 100 ppm ammonia over three cycles, as shown in Figure 9c, where the consistent response amplitude indicates good repeatability of the sensor [12].
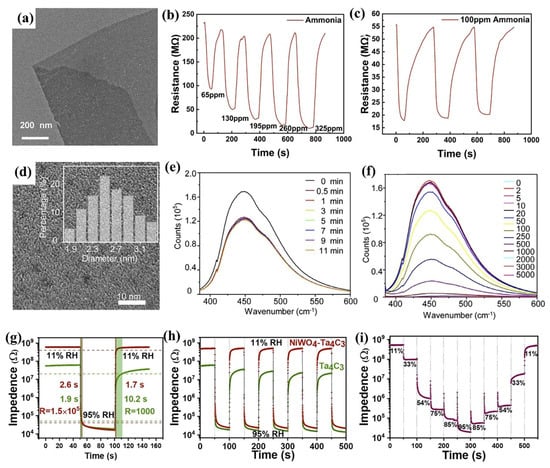
Figure 9.
(a) TEM of Ta4C3Tx nanosheet. (b) Dynamic response curves of Ta4C3Tx to NH3 concentrations ranging from 65 to 325 ppm. (c) Repeatability test of Ta4C3Tx at 100 ppm NH3 over multiple cycles. Permission granted for reproduction [12]. Copyright (2024), Elsevier. (d) TEM of N-MQDs. (e) Time-dependent fluorescence quenching of N-MQDs by 100 μmol/L Fe3+. (f) PL emission profiling of N-MQDs with different concentrations (μmol/L) o of Fe3+. Permission granted for reproduction [44]. Copyright (2022), Elsevier. (g) The response and recovery durations, along with the response levels, for the NiWO4-Ta4C3 material (red curve) and Ta4C3 MXene nanosheet (green curve) humidity sensors. (h) Continuous working stability of the NiWO4-Ta4C3 (red curve) and Ta4C3 MXene (green curve) humidity sensors. (i) Resistance values of NiWO4-Ta4C3 humidity sensor at different humidities. Permission granted for reproduction [13]. Copyright (2023), American Chemical Society.
5.3.3. Ion Detection
Li et al. developed an innovative synthesis strategy to prepare nitrogen-doped Ta4C3 quantum dots (N-MQDs) with a uniform particle size of 2.60 nm (Figure 9d), achieving remarkable fluorescence quantum yields [44]. The synthesized N-MQDs exhibit outstanding photoluminescent (PL) characteristics, emitting intense blue fluorescence with a remarkably high quantum yield of 23.4%. The abundant -NH2 and -OH groups, along with N and O atoms on N-MQDs surfaces, provide a strong electron absorbing capability that effectively passivates surface defects. The N-MQDs exhibit good functionality as an advanced fluorescent nanosensor, demonstrating highly selective Fe3+ detection capability. The adsorption of Fe3+ induces efficient electron transfer, accelerating electron/hole recombination on surface of N-MQDs, thereby altering electronic state and enabling selective fluorescence quenching for sensing applications. The rapid fluorescence quenching of N-MQDs within 0.5 min upon Fe3+ addition (Figure 9e) demonstrates their high detection efficiency, with a significant signal reduction at 100 μmol/L and a detection limit as low as 2 μmol/L (Figure 9f), confirming strong potential of N-MQDs for Fe3+ sensing.
5.3.4. Humidity Sensing
Liu et al. reported that a humidity sensor utilized accordion-like Ta4C3 MXene nanosheets as its sensing material, demonstrating excellent performance characterized by ultralow loading, high sensitivity, and fast response/recovery kinetics [13]. First-principles calculations demonstrate that Ta4C3 MXene nanosheets exhibit good water molecule adsorption capabilities, with an adsorption energy reaching 2.01 eV, substantially exceeding those observed for NiWO4 (0.37 eV) and Ti3C2 (1.08 eV). Moveover, Ta4C3 MXene offers a high specific surface area, which accelerates the initial adsorption process. NiWO4 nanoparticles enhance the desorption performance of water molecules and the linear response characteristics of the composite material. The hybrid structure combines the high electrical conductivity of Ta4C3 MXene with the stability of NiWO4, resulting in superior humidity sensing capabilities of the composite material. As illustrated in Figure 9g, the resistance variations of both the NiWO4-Ta4C3 and Ta4C3 MXene nanosheet-based humidity sensors are presented within the relative humidity (RH) range of 11% to 95%. Figure 9h illustrates the repeatability of NiWO4-Ta4C3 and Ta4C3 MXene nanosheet humidity sensors over 5 cycles between 11% and 95% RH, showing that the NiWO4-Ta4C3 sensor maintains stable performance with little change, whereas the Ta4C3 MXene sensor fails to retain its initial response due to incomplete desorption of water molecules within the given time. Figure 9i presents the resistance values of the NiWO4-Ta4C3 humidity sensor at relative humidities of 11%, 33%, 54%, 75%, 85%, and 95%, demonstrating the excellent response performance of the sensors across different humidity levels. This study establishes Ta4C3 MXene as a promising humidity sensor material.
5.3.5. Piezoresistive Sensing
The continuous monitoring of human physiological parameters via high-precision sensing technologies has garnered considerable research interest. Ta4C3 nanosheets exhibit superior properties for piezoresistive sensing applications, including excellent electrical conductivity, good mechanical stability, abundant functional groups facilitating sponge integration, and enhanced air stability compared to conventional MXene materials, collectively enabling high-performance, durable pressure sensors suitable for diverse environmental conditions. According to the research of Wu et al., Ta4C3 nanosheet/melamine sponge piezoresistive sensors were successfully fabricated via the integration of etching-intercalation and dipping-drying techniques [30]. This sensor exhibits superior performance metrics, such as a high sensitivity of 4.01 kPa−1, a broad linear range of 0.018–12.06 kPa, a low detection limit of 18.5 Pa, and rapid response/recovery times of 35.4/27.88 ms. With their excellent stability and durability, these sensors are well-suited to real-time physiological signal monitoring and have the potential for wireless detection of human and robotic motions.
5.4. Advantages over Ti-Based MXene
Ta-based MXenes are a newer member of the MXene family, exhibiting unique advantages in terms of structure and functionality compared to common titanium (Ti)-based MXenes, such as Ti3C2Tx. For example, according to Lin et al.’s report, the Ta4C3 nanosheets demonstrated an high photothermal conversion efficiency (η) of 44.7%, outperforming Ti3C2 nanosheets (30.6%) [25]. Stavrou et al. studied ultrafast ultrafast nonlinear optical (NLO) properties and carrier dynamics of Ta4C3Tx. They found that Ta4C3Tx’s NLO response surpasses Ti3C2Tx, attaining exceptionally high third-order susceptibility (χ(3)) values on the order of 10−13 esu [45]. Guo et al. investigated the Tan+1Cn as anode materials for rechargeable metal ion batteries by DFT. The findings reveal that Tan+1Cn MXenes exhibit outstanding adsorption capabilities and remarkable electronic conductivities when it comes to accommodating Li, Na, K, Mg, and Ca atoms. Moreover, the presence of ultra-low diffusion barriers for alkali metal ions suggests that Tan+1Cn MXenes hold significant potential for achieving high-performance characteristics, particularly in the context of ultra-fast charging and discharging processes [46]. Rafieerad et al. demonstrated a fluorine-free exfoliation method for synthesizing Ta4C3Tx MXene-tantalum oxide. This material exhibits better biocompatibility than other MXene materials, making it highly suitable for implantable bioelectronic devices and tissue engineering applications [11]. Liu et al. successfully fabricated a NiWO4-Ta4C3-based humidity sensor, which demonstrated superior performance compared to the humidity sensors prepared with Ti3C2 MXene, NiWO4, and NiWO4-Ti3C2 [13]. For a comparison highlighting the superiority of tantalum carbide MXenes, the properties of tantalum carbide MXenes are presented alongside those counterparts (Table 3).

Table 3.
Comparison of the properties of tantalum carbide MXenes in various applications.
5.5. Other Applications
Tantalum carbide MXene demonstrates extensive applicability across multiple advanced technological domains, including energy storage, energy conversion, environmental remediation, detection and sensing, and various other frontier applications. Owing to its tunable physicochemical characteristics and the ability to be hybridized with a variety of other materials, tantalum carbide MXene has emerged as a multifunctional platform material with burgeoning applications in biomedical applications, optics and photonics, as well as nuclear and radiation applications.
Due to its high atomic number (Z = 73) and impressive X-ray attenuation coefficient (4.3 cm2/kg at 100 eV, compared to 1.94 cm2/kg for iodine), tantalum has been identified as a promising candidate for use in CT contrast agents [47,48]. Ta4C3 MXene exhibits remarkable photothermal conversion efficiency, surpassing traditional photothermal agents such as Au nanorods, making Ta4C3 MXene a promising material for multiple imaging-guided photothermal tumor ablation. Dai et al. modified MnOx/Ta4C3 by soybean phospholipid (SP), yielding MnOx/Ta4C3-SP composites with tumor theranostic capabilities [20]. The Ta4C3 demonstrated a photothermal conversion efficiency of 34.9%. Within 10 min, the tumor surface temperature in MnOx/Ta4C3-SP treated mice increased to around 55 °C, as observed via infrared thermal imaging, achieving a level sufficient for effective tumor ablation. Blood and organ function analyses at 30 days post-injection showed no significant differences between the MnOx/Ta4C3-SP treated groups and the control group, indicating the safety of the composite nanosheet. This research work demonstrates that Ta4C3 holds great potential for both photoacoustic imaging and photothermal ablation of tumors. In addition to the application in tumor ablation, excellent photothermal conversion and biocompatibility make tantalum carbide MXene a promising material for therapeutic platforms against antibiotic-resistant infections. According to the report by Wang et al., Ta4C3 exhibits remarkable photothermal conversion efficiency, significant reactive oxygen species scavenging abilities, and notable immunomodulatory characteristics [29]. Comprehensive in vitro and in vivo analyses demonstrated the effectiveness of Ta4C3 in eradicating methicillin-resistant staphylococcus aureus infections and promoting wound healing.
Ta4C3Tx demonstrates excellent potential for optoelectronic applications across a broad spectral range. For example, Dong et al. conducted a theoretical study using DFT-based first-principles calculations to study the influence of surface functionalization on Ta4C3 MXene’s electronic structure and optical response. The results show that oxidized/hydroxylated Ta4C3 variants exhibit significantly enhanced near-infrared absorption compared to bare Ta4C3 [18]. Terminated Ta4C3 demonstrate broadband near-infrared nonlinear absorption and effective mode-locking at 1044/1557 nm, suggesting potential for optical modulators. Du et al.’s research also demonstrates that Ta4AlC3 and Ta4C3 possess outstanding nonlinear absorption capabilities in the ultraviolet range, particularly showing remarkable optical limiting performance, making them highly promising materials for optical limiting applications and advancing their potential in photonics and nonlinear optics for laser protection [37].
Owing to their elevated atomic number (Z = 73) of Ta element, tantalum carbide MXenes exhibit exceptional potential for nuclear and radiation applications. Ta displays significant γ-ray characteristics within the 67.4–88 keV energy range [24], which makes it an ideal candidate for the fabrication of multifunctional radiation protection materials. Liu et al. developed a flexible, self-healing, thermal-managing, and responsive radiation protection material using Ta4C3Tx MXene, hydrogen-terminated phenyl polysiloxane, and polyborosiloxane, which exhibits enhanced tensile strength, high sensitivity, and superior γ-ray attenuation compared to Ta2O5 composites, demonstrating the potential of Ta4C3Tx MXene in nuclear environments [24]. In another research work of this group, they fabricated multilayer composites with an interlocked structure through vacuum filtration and layer-by-layer assembly, utilizing Ta4C3Tx MXene as the filler, multi-crosslinked elastomers as the matrix, and abrasive cloths as templates to enhance mechanical and attenuation properties [36]. The composites exhibited superior thermal management and radiation protection properties, with a mass attenuation coefficient of 0.927 cm2/g for 59.6 keV γ-rays, demonstrating considerable promise in diverse applications in low-temperature nuclear environments, particularly as wearable radiation protection materials.
6. Summary and Outlook
This review provides summary of the advancing research on tantalum carbide MXenes, with particular focus on Ta2CTx and Ta4C3Tx. The synthesis, properties, and applications have been explored across various fields, highlighting their remarkable potential in energy storage, energy conversion, sensing, biomedicine, and so on.
The production of tantalum carbide MXenes is mainly achieved through a top-down etching process that has evolved from conventional HF-etching methods towards more eco-friendly alternatives. Fluorine-free synthesis methods, including KOH and HCl etching, have emerged as viable alternatives, addressing safety challenges and environmental concerns simultaneously. The employment of advanced exfoliation techniques involving intercalants like TMAOH, TPAOH, and TBAOH has facilitated the synthesis of few-layer and monolayer tantalum carbide MXenes with enlarged interlayer spacing, thereby considerably improving their functional properties.
The intrinsic properties of tantalum carbide MXenes highlight their remarkable potential. Theoretical and experimental studies confirm their metallic conductivity, adjustable electronic structures. Their optical behaviors extend across the ultraviolet to the infrared spectrum, exhibiting notable nonlinear optical responses and efficient photothermal conversion capabilities. Additionally, the surface chemistry of Ta-based MXenes, particularly their terminal functional groups, influences their overall physicochemical behavior and performance.
The range of applications for tantalum carbide MXenes continues to grow rapidly. Within energy storage, they showcase excellent electrochemical behavior across multiple battery systems and capacitors, offering high capacity, excellent rate performance, and outstanding cycling stability. Their catalytic activity toward HER demonstrates promising efficiency towards energy conversion. The sensing capabilities of tantalum carbide MXenes are highly versatile, covering gas detection, humidity sensing, and SERS, with detection limits reaching trace amount. Photothermal properties are utilized in biomedical applications like cancer theranostics and wound healing. Emerging applications further include nonlinear optics for laser protection radiation shielding and so on.
Currently, the exploration of tantalum carbide MXenes is still in the early stages. They still face insufficient structural stability, primarily manifested in the tendency of surface terminal groups (-O, -F, -OH) to dissociate in oxidizing environments. This leads to the degradation of their layered structure, along with a gradual decay in conductivity and optical performance over time, thereby limiting their long-term reliability in optoelectronic devices. Moreover, breakthroughs in efficient, environmentally friendly, and low-cost large-scale preparation techniques remain unrealized. Several issues related to synthesis, properties, and practical applications should be addressed in order to fully unlock their potential. Synthetic approaches require ongoing optimization to develop scalable and environmentally sustainable production methods. Exploring alternative etching techniques beyond conventional fluorine-based routes is a crucial research focus, as it may facilitate industrial-scale synthesis while reducing environmental impact. Precise regulation of surface termination groups remains of importance, as these groups profoundly affect the functional attributes of tantalum carbide MXenes and dictate their performance across various applications. The integration of computational modeling with advanced characterization methods will remain critical in understanding the atomic-scale mechanisms behind their excellent performance. The integration of tantalum carbide MXenes with other functional materials into composite materials represents a promising research direction. Combining tantalum carbide MXenes with other substances such as metal oxides, chalcogenides, and polymers can synergistically improve performance attributes while addressing the limitations of individual components.
In summary, tantalum carbide MXenes represent a high-potential group of 2D materials, distinguished by their extraordinary properties and versatile applications. Their unique electronic, mechanical, optical, and chemical properties, alongside customizable surface chemistry, establish them as pivotal components for advancing next-generation energy, environmental, biomedical, and optics technologies. Ongoing studies aimed at optimizing synthesis strategies, improving structural designs, and customizing properties for specific purposes will further unlock their full potential.
Author Contributions
Investigation, M.L., X.L. and Y.M.; writing—original draft, M.L. and L.X.; writing—review and editing, M.G. and Y.M.; supervision, Y.M.; project administration, Y.M.; conceptualization, Y.M. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Startup Fund (BK202112, BK202319, BK202363) from Hubei University of Automotive Technology, Research Project of Hubei Provincial Department of Education (No. D20231803) and open fund of Hubei Key Laboratory of Energy Storage and Power Battery (ZDK22023B03, ZDK22024A01, ZDK22024A03).
Acknowledgments
We would like to express our appreciation for the assistance rendered by Wenqiao Liu, who contributed to both literature retrieval and the preparation of certain figures and tables in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wen, Y.; Qi, Y.; Zhao, Q.; Qu, L.; Li, C. Pristine titanium carbide mxene films with environmentally stable conductivity and superior mechanical strength. Adv. Funct. Mater. 2020, 30, 1906996. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Liu, Y.; Xu, Y.; Chang, Z.; Wang, D.; Li, Q. Expanded Polytetrafluoroethylene/Mxene Nanosheet Composites with Hydrophilicity and Lipophilicity for Purification of Oil Spills and Wastewater. ACS Appl. Nano Mater. 2022, 5, 2483–2491. [Google Scholar] [CrossRef]
- Oh, T.; Lee, S.; Kim, H.; Ko, T.Y.; Kim, S.J.; Koo, C.M. Fast and high-yield anhydrous synthesis of Ti3C2T mxene with high electrical conductivity and exceptional mechanical strength. Small 2022, 18, 2203767. [Google Scholar] [CrossRef]
- Bagheri, S.; Abourahma, J.; Lu, H.; Vorobeva, N.S.; Luo, S.; Gruverman, A.; Sinitskii, A. High-yield fabrication of electromechanical devices based on suspended Ti3C2Tx MXene monolayers. Nanoscale 2023, 15, 1248–1259. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Fan, X.; Zhang, X.; Fan, S.; Bei, G. Two-dimensional carbonitride MXenes: From synthesis to properties and applications. Carbon. Energy 2024, 6, e609. [Google Scholar] [CrossRef]
- Liu, M.-C.; Zhang, Y.-S.; Zhang, B.-M.; Zhang, D.-T.; Tian, C.-Y.; Kong, L.-B.; Hu, Y.-X. Large interlayer spacing 2D Ta4C3 matrix supported 2D MoS2 nanosheets: A 3D heterostructure composite towards high-performance sodium ions storage. Renew. Energy 2021, 169, 573–581. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, S.; Jia, X.; Yang, J.; Li, Y.; Shao, D.; Feng, L.; Liao, J.; Song, H. MXene derivative Ta4C3-Ta2O5 heterostructure as bi-functional barrier for Li-S batteries. J. Mater. Sci. Technol. 2023, 151, 89–98. [Google Scholar] [CrossRef]
- Vijayaprabhakaran, A.; Kathiresan, M. Fluorine-free synthesized tantalum carbide (Ta2C Mxene) as an efficient electrocatalyst for water reduction and nitro compound reduction. Mater. Adv. 2023, 4, 3593–3602. [Google Scholar] [CrossRef]
- Rafieerad, A.; Amiri, A.; Sequiera, G.L.; Yan, W.; Chen, Y.; Polycarpou, A.A.; Dhingra, S. Development of fluorine-free tantalum carbide Mxene hybrid structure as a biocompatible material for supercapacitor electrodes. Adv. Funct. Mater. 2021, 31, 2100015. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Huang, P.; Qin, F.; Liu, J.; Lin, J.; Lin, Y.; Huang, H.; Wang, W.; Han, C.; Zhang, S. Exploring monolayer Ta4C3Tx MXene for quick ammonia detection at room temperature. Mater. Lett. 2024, 363, 136250. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Feng, X.; Yin, H.; Gong, S.; Yu, K.; Zhu, Z. Microgram-level Ta4C3 nanosheets decorated with NiWO4 nanoparticles as a high-performance humidity sensor. ACS Appl. Nano Mater. 2023, 6, 20970–20981. [Google Scholar] [CrossRef]
- Lan, L.; Ni, Z.; Zhao, C.; Gao, J.; Tang, X.; Qu, Z.; Zheng, L.; Fan, X.; Qiu, T. Photoinduced charge transfer empowers Ta4C3 and Nb4C3 MXenes with high SERS performance. Langmuir 2024, 40, 20945–20953. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, C.; Dong, P. Preparation and application of two-dimensional Ta4C3 MXene/gold nanostar composite SERS substrates for thiram detection. Langmuir 2024, 40, 22015–22026. [Google Scholar] [CrossRef]
- Song, X.; Huang, Q.; Yang, Y.; Ma, L.; Liu, W.; Ou, C.; Chen, Q.; Zhao, T.; Xiao, Z.; Wang, M.; et al. Efficient therapy of inflammatory bowel disease (IBD) with highly specific and durable targeted Ta2C modified with chondroitin sulfate (TACS). Adv. Mater. 2023, 35, 2301585. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, S.; Ding, K.; Jin, P.; Zheng, Y.; Wu, Z. Photothermal controlled antibacterial Ta4C3Tx-AgNPs/nanocellulose bioplastic food packaging. Food Chem. 2024, 448, 139126. [Google Scholar] [CrossRef]
- Dong, L.; Chu, H.; Li, Y.; Ma, X.; Pan, H.; Zhao, S.; Li, D. Surface functionalization of Ta4C3 MXene for broadband ultrafast photonics in the near-infrared region. Appl. Mater. Today 2022, 26, 101341. [Google Scholar] [CrossRef]
- Li, G.; Xu, Q.; Nie, H.; Wang, C.; Wang, R.; Yang, K.; He, J.; Zhang, B. Few-layer Ta2CTx nanosheets-based mode-locked fiber lasers. Opt. Mater. Express 2022, 12, 1731–1740. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhuo, M.J.; Zhou, Y.C.; Li, M.S.; Wang, J.Y. Structural characterization of a new layered-ternary Ta4AlC3 ceramic. J. Mater. Res. 2006, 21, 2587–2592. [Google Scholar] [CrossRef]
- Syamsai, R.; Vijay, V.; Easwaran, S.K.; Navaneethan, M. Evaluation of 2D Tantalum Carbide MXene for Room to Mid-temperature Thermoelectric Applications. ChemNanoMat 2024, 10, e202400391. [Google Scholar] [CrossRef]
- Yeh, C.L.; Shen, Y.G. Effects of Al content on formation of Ta2AlC by self-propagating high-temperature synthesis. J. Alloys Compd. 2009, 482, 219–223. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Mai, F.; Li, X.; Pu, G.; Deng, Z.; Ji, L.; Bai, X.; Zhang, Q.; Zhou, Y. Multifunctional Ta4C3Tx MXene/HTPP—PBS composites with multi cross-linking systems to cope with complex nuclear environments. J. Alloys Compd. 2024, 1004, 175734. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D tantalum carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Liu, W.; Zong, H.; Li, M.; Zeng, Z.; Gong, S.; Yu, K.; Zhu, Z. Ta4C3-modulated MOF-DErived 3D crosslinking network of VO2(B)@Ta4C3 for high-performance aqueous zinc ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 13554–13564. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Esfarjani, K.; Bogdanovski, D.; Dronskowski, R.; Yunoki, S. Insights into exfoliation possibility of MAX phases to MXenes. Phys. Chem. Chem. Phys. 2018, 20, 8579–8592. [Google Scholar] [CrossRef]
- Rafieerad, A.; Yan, W.; Alagarsamy, K.N.; Srivastava, A.; Sareen, N.; Arora, R.C.; Dhingra, S. Fabrication of Smart Tantalum Carbide MXene Quantum Dots with Intrinsic Immunomodulatory Properties for Treatment of Allograft Vasculopathy. Adv. Funct. Mater. 2021, 31, 2106786. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Ma, Y.; Liu, J.; Wang, P.; Luo, J.; Rui, Y.; Wu, Y. Ta4C3 Nanosheets as a novel therapeutic platform for photothermal-driven ros scavenging and immune activation against antibiotic-resistant infections in diabetic wounds. Small 2024, 20, 2400741. [Google Scholar] [CrossRef]
- Wu, F.; Meng, X.; Liu, Z.; Lv, T.; Yu, L.; Zhang, J.; Zhao, Y.; Zhao, C.; Xing, G. Ta4C3 Nanosheet/Melamine Sponges with High Sensitivity and Long-Term Stability for Wearable Piezoresistive Sensors. ACS Appl. Nano Mater. 2024, 7, 695–704. [Google Scholar] [CrossRef]
- He, S.; Lv, Y.; Qiu, J.; Cui, S.; Gao, Z.; Peng, L. Ta4C3 MXene Slows Progression of Fatty Liver Disease through Its Anti-Inflammatory and ROS-Scavenging Effects. ACS Appl. Mater. Interfaces. 2025, 17, 17217. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, H.; Liu, W.; Wu, Z.; Shang, C.; Wang, S.; Kang, Z.; Yue, H.; Wang, D.; Wei, C.; et al. Single-Frequency Pulsed Fiber Laser Enabled by Tantalum Carbide Featuring 2.8 μm Wavelength Tunability. J. Light. Technol. 2025, 43, 2277–2283. [Google Scholar] [CrossRef]
- Syamsai, R.; Grace, A.N. Ta4C3 MXene as supercapacitor electrodes. J. Alloys Compd. 2019, 792, 1230–1238. [Google Scholar] [CrossRef]
- Maldonado-Lopez, D.; Rodriguez, J.R.; Pol, V.G.; Syamsai, R.; Andrews, N.G.; Gutiérrez-Ojeda, S.J.; Ponce-Pérez, R.; Moreno-Armenta, M.G.; Guerrero-Sánchez, J. Atomic-scale understanding of li storage processes in the Ti4C3 and chemically ordered Ti2Ta2C3 MXenes: A theoretical and experimental assessment. ACS Appl. Energy Mater. 2022, 5, 1801–1809. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Wageh, S.; Al-Hartomy, O.A.; Al-Sehemi, A.G.; Ge, Y.; He, W.; Wei, S.; Bao, W.; Zhang, H. Ta2C MXene: Nonlinear optical properties and application in femtosecond fiber laser. Opt. Laser Technol. 2023, 161, 109178. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Lu, Y.; Huang, R.; Mai, F.; Li, X.; Deng, Z.; Ji, L.; Bai, X. Multi-crosslinked Ta4C3TX MXene composites with “interlocked structure” for efficient gamma-ray shielding, behavioural detection and thermal management in nuclear environments. Compos. Part A Appl. Sci. Manuf. 2025, 189, 108597. [Google Scholar] [CrossRef]
- Du, B.; Zhao, Z.; Ren, Z.; Liu, Q.; Zhang, F. 2D Ta4AlC3 and Ta4C3 nanosheets with excellent ultraviolet optical limiting behavior for laser protection. J. Mater. Chem. C 2024, 12, 7748–7758. [Google Scholar] [CrossRef]
- Sahu, S.; Purkayastha, D.D. 2D Titanium Carbide MXene-Interfaced Zinc Oxide/Tungstite Architectures Adorned Mixed Matrix Polymer Membranes for Oily Wastewater Treatment. ACS Appl. Mater. Interfaces 2025, 17, 5278–5289. [Google Scholar] [CrossRef]
- Wang, S.; Guan, C.; Zhao, Z.; Wang, R.; Tian, Y.; Du, Y. Density functional theory analysis of electronic and optical properties of two-dimensional tantalum carbides Tn+1Cn (n = 1, 2, 3). Phys. Status Solidi (b) 2019, 256, 1800457. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Pang, H.; Xue, H.; Yu, Y. MoS2-based nanocomposites for electrochemical energy storage. Adv. Sci. 2017, 4, 1600289. [Google Scholar] [CrossRef]
- Wu, T.; Pang, X.; Zhao, S.; Xu, S.; Liu, Z.; Li, Y.; Huang, F. One-Step Construction of Ordered Sulfur-Terminated Tantalum Carbide MXene for Efficient Overall Water Splitting. Small Struct. 2022, 3, 2100206. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Zhao, Y.; Wu, F.; Lv, T.; Yu, L.; Yu, C.; Zhao, C.; Xing, G. Ta4C3TX/graphene aerogels with combined photo/electro-thermal conversion performances for multifunctional applications. Chem. Eng. J. 2024, 489, 151196. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Zhao, Y.; Zhao, T.; Yang, Y.; Yu, C.; Zhao, C.; Xing, G. Synergistic NH2-MIL-88B/Ta4C3TX/graphene aerogels for sustainable wastewater treatment and thermal energy storage. Carbon 2025, 232, 119823. [Google Scholar] [CrossRef]
- Li, S.; Ma, J.; Zhao, X.; Zhu, P.; Xu, M.; Niu, Y.; Luo, D.; Xu, Q. Highly fluorescence Ta4C3 MXene quantum dots as fluorescent nanoprobe for heavy ion detection and stress monitoring of fluorescent hydrogels. Chin. Chem. Lett. 2022, 33, 1850–1854. [Google Scholar] [CrossRef]
- Stavrou, M.; Chacon, B.; Farsari, M.; Pappa, A.; Delogu, L.; Gogotsi, Y.; Gray, D. Emerging Ta4C3 and Mo2Ti2C3 MXene Nanosheets for Ultrafast Photonics. Adv. Optical Mater. 2025, 13, 2403277. [Google Scholar] [CrossRef]
- Guo, J.; Hu, D.; Bai, C.; Xu, L.; Xiao, H.; Shi, Q.; Li, X.; Chen, X.; Ma, Y.; Fang, G. Structural and electrochemical properties of Tan+1Cn MXene anode materials for metal-ion batteries. Inorg. Chem. Front. 2024, 11, 2945–2956. [Google Scholar] [CrossRef]
- Pan, X.; Siewerdsen, J.; La Riviere, P.J.; Kalender, W.A. Anniversary Paper: Development of X-ray computed tomography: The role of Medical Physics and AAPM from the 1970s to present. Med. Phys. 2008, 35, 3728–3739. [Google Scholar] [CrossRef]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).