Isolation and Preliminary X-Ray Crystallographic Characterisation of the Periplasmic Ligand-Binding Domain of the Chemoreceptor Tlp3 from Campylobacter hepaticus
Abstract
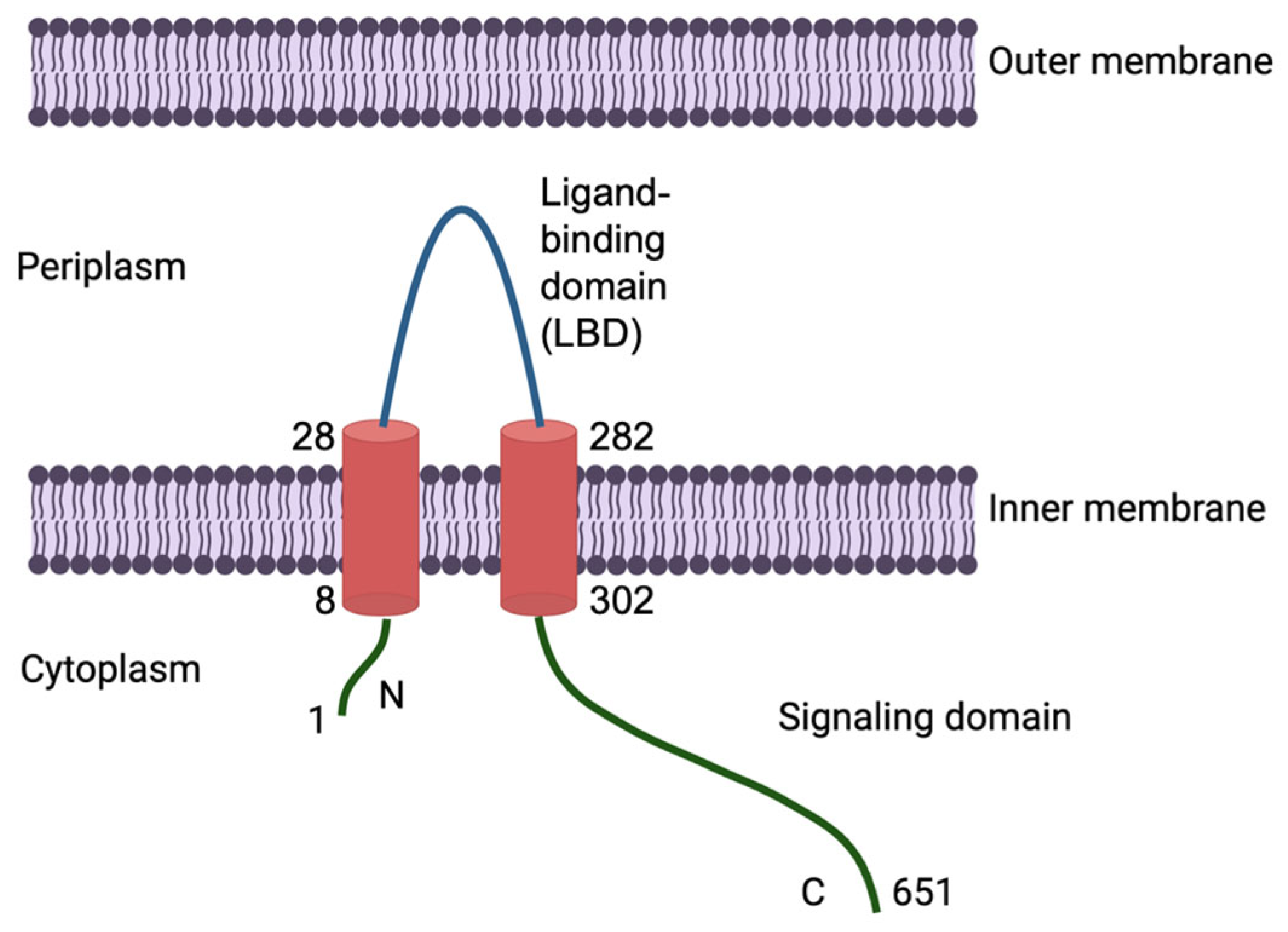
1. Introduction
2. Materials and Methods
2.1. Gene Cloning and Overexpression
2.2. Solubilisation of Inclusion Bodies
2.3. Refolding and Purification
2.4. Thermal Shift Assay
2.5. Crystallisation
2.6. Data Acquisition and Initial Crystallographic Analysis
3. Results
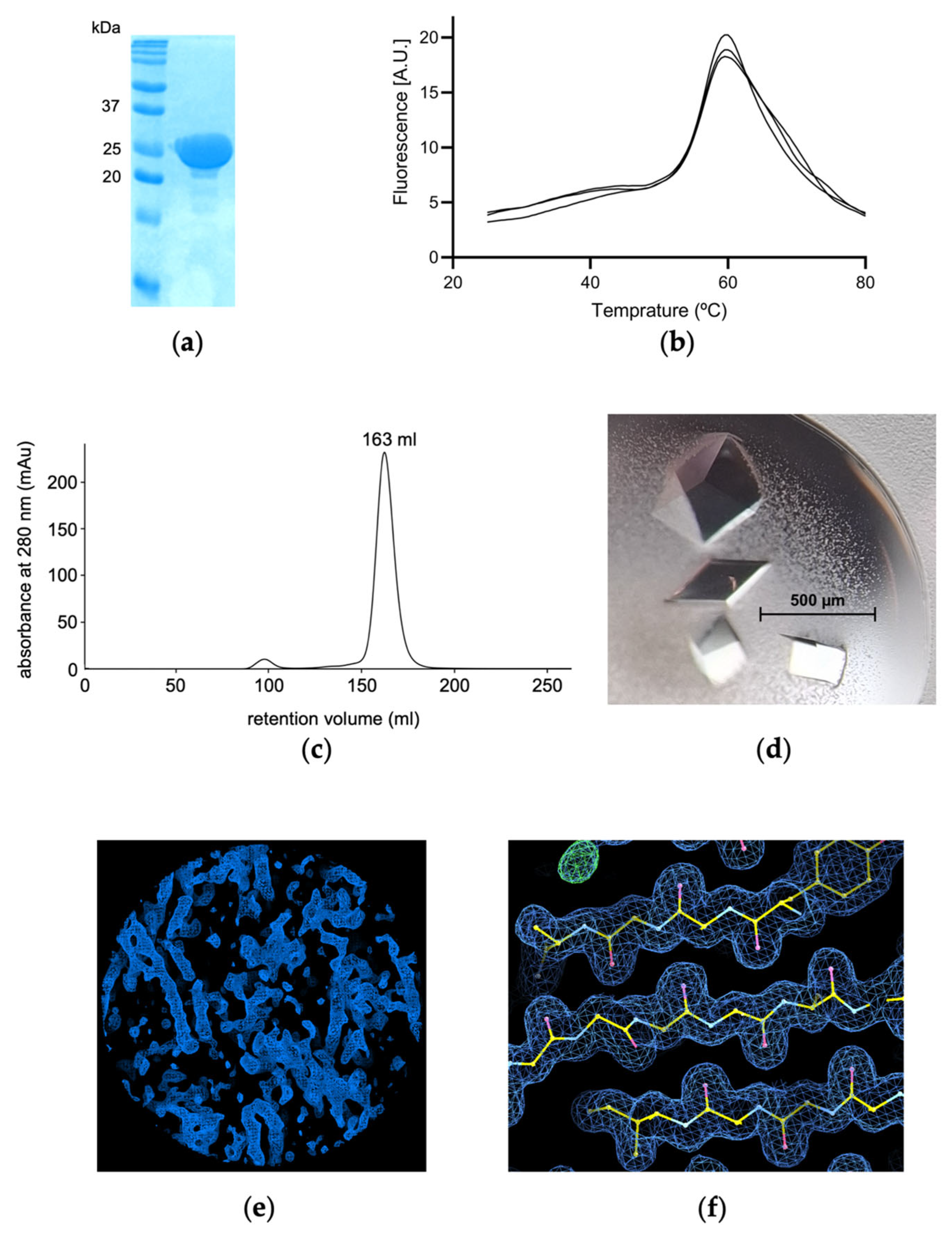
3.1. Cloning, Overproduction in Inclusion Bodies, Refolding, Purification and Thermal Shift Assay
3.2. Crystallisation and Intitial Crystallographic Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Tlp3 | Transducer-like protein 3 |
| SLD | Spotty liver disease |
| LBD | Ligand-binding domain |
| CcmL | Campylobacter chemoreceptor for multiple ligands |
| dCache | Double calcium channels and chemotaxis receptors |
| TEV | Tobacco etch virus |
| PMSF | Phenylmethanesulfonyl fluoride |
| DTT | Dithiothreitol |
| IBs | Inclusion bodies |
Appendix A
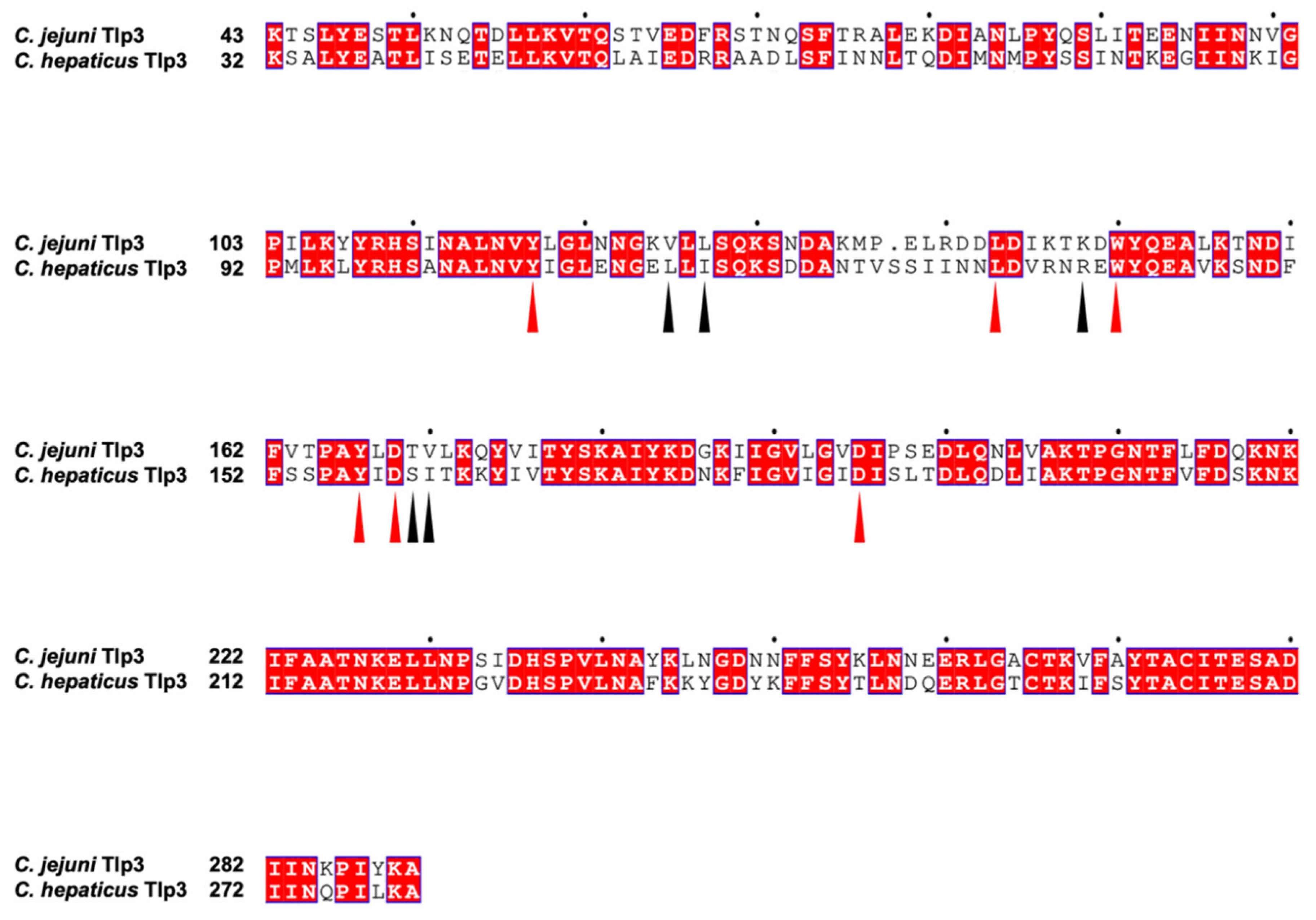

References
- Tikhomirova, A.; McNabb, E.R.; Petterlin, L.; Bellamy, G.L.; Lin, K.H.; Santoso, C.A.; Daye, E.S.; Alhaddad, F.M.; Lee, K.P.; Roujeinikova, A. Campylobacter jejuni virulence factors: Update on emerging issues and trends. J. Biomed. Sci. 2024, 31, 45. [Google Scholar] [CrossRef] [PubMed]
- Veronese, P.; Dodi, I. Campylobacter jejuni/coli Infection: Is It Still a Concern? Microorganisms 2024, 12, 2669. [Google Scholar] [CrossRef] [PubMed]
- Khairullah, A.R.; Yanestria, S.M.; Effendi, M.H.; Moses, I.B.; Jati Kusala, M.K.; Fauzia, K.A.; Ayuti, S.R.; Fauziah, I.; Martua Silaen, O.S.; Priscilia Riwu, K.H.; et al. Campylobacteriosis: A rising threat in foodborne illnesses. Open Vet. J. 2024, 14, 1733–1750. [Google Scholar] [CrossRef] [PubMed]
- Phung, C.; Vezina, B.; Anwar, A.; Wilson, T.; Scott, P.C.; Moore, R.J.; Van, T.T.H. Campylobacter hepaticus, the Cause of Spotty Liver Disease in Chickens: Transmission and Routes of Infection. Front. Vet. Sci. 2019, 6, 505. [Google Scholar] [CrossRef]
- Van, T.T.; Elshagmani, E.; Gor, M.C.; Anwar, A.; Scott, P.C.; Moore, R.J. Induction of spotty liver disease in layer hens by infection with Campylobacter hepaticus. Vet. Microbiol. 2017, 199, 85–90. [Google Scholar] [CrossRef]
- Van, T.T.H.; Elshagmani, E.; Gor, M.C.; Scott, P.C.; Moore, R.J. Campylobacter hepaticus sp. nov., isolated from chickens with spotty liver disease. Int. J. Syst. Evol. Microbiol. 2016, 66, 4518–4524. [Google Scholar] [CrossRef]
- Crawshaw, T.R.; Chanter, J.I.; Young, S.C.; Cawthraw, S.; Whatmore, A.M.; Koylass, M.S.; Vidal, A.B.; Salguero, F.J.; Irvine, R.M. Isolation of a novel thermophilic Campylobacter from cases of spotty liver disease in laying hens and experimental reproduction of infection and microscopic pathology. Vet. Microbiol. 2015, 179, 315–321. [Google Scholar] [CrossRef]
- Moore, R.J.; Scott, P.C.; Van, T.T.H. Spotlight on avian pathology: Campylobacter hepaticus, the cause of Spotty Liver Disease in layers. Avian. Pathol. 2019, 48, 285–287. [Google Scholar] [CrossRef]
- Van, T.T.H.; Lacey, J.A.; Vezina, B.; Phung, C.; Anwar, A.; Scott, P.C.; Moore, R.J. Survival Mechanisms of Campylobacter hepaticus Identified by Genomic Analysis and Comparative Transcriptomic Analysis of in vivo and in vitro Derived Bacteria. Front. Microbiol. 2019, 10, 107. [Google Scholar] [CrossRef]
- Matilla, M.A.; Krell, T. The effect of bacterial chemotaxis on host infection and pathogenicity. FEMS Microbiol. Rev. 2018, 42, 40–67. [Google Scholar] [CrossRef]
- Zhou, B.; Szymanski, C.M.; Baylink, A. Bacterial chemotaxis in human diseases. Trends Microbiol. 2023, 31, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef] [PubMed]
- Zhulin, I.B. The superfamily of chemotaxis transducers: From physiology to genomics and back. Adv. Microb. Physiol. 2001, 45, 157–198. [Google Scholar] [CrossRef] [PubMed]
- Elgamoudi, B.A.; Andrianova, E.P.; Shewell, L.K.; Day, C.J.; King, R.M.; Taha; Rahman, H.; Hartley-Tassell, L.E.; Zhulin, I.B.; Korolik, V. The Campylobacter jejuni chemoreceptor Tlp10 has a bimodal ligand-binding domain and specificity for multiple classes of chemoeffectors. Sci. Signal. 2021, 14, eabc8521. [Google Scholar] [CrossRef]
- Lopes, G.V.; Ramires, T.; Kleinubing, N.R.; Scheik, L.K.; Fiorentini, A.M.; Padilha da Silva, W. Virulence factors of foodborne pathogen Campylobacter jejuni. Microb. Pathog. 2021, 161, 105265. [Google Scholar] [CrossRef]
- Cha, G.; Liu, Y.; Yang, Q.; Bai, L.; Cheng, L.; Fan, W. Comparative Genomic Insights into Chemoreceptor Diversity and Habitat Adaptation of Archaea. Appl. Environ. Microbiol. 2022, 88, e0157422. [Google Scholar] [CrossRef]
- Rahman, H.; King, R.M.; Shewell, L.K.; Semchenko, E.A.; Hartley-Tassell, L.E.; Wilson, J.C.; Day, C.J.; Korolik, V. Characterisation of a multi-ligand binding chemoreceptor CcmL (Tlp3) of Campylobacter jejuni. PLoS Pathog. 2014, 10, e1003822. [Google Scholar] [CrossRef]
- Taha; Elgamoudi, B.A.; Andrianova, E.P.; Haselhorst, T.; Day, C.J.; Hartley-Tassell, L.E.; King, R.M.; Najnin, T.; Zhulin, I.B.; Korolik, V. Diverse Sensory Repertoire of Paralogous Chemoreceptors Tlp2, Tlp3, and Tlp4 in Campylobacter jejuni. Microbiol. Spectr. 2022, 10, e0364622. [Google Scholar] [CrossRef]
- Liu, Y.C.; Machuca, M.A.; Beckham, S.A.; Gunzburg, M.J.; Roujeinikova, A. Structural basis for amino-acid recognition and transmembrane signalling by tandem Per-Arnt-Sim (tandem PAS) chemoreceptor sensory domains. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 2127–2136. [Google Scholar] [CrossRef]
- Khan, M.F.; Machuca, M.A.; Rahman, M.M.; Koc, C.; Norton, R.S.; Smith, B.J.; Roujeinikova, A. Structure-Activity Relationship Study Reveals the Molecular Basis for Specific Sensing of Hydrophobic Amino Acids by the Campylobacter jejuni Chemoreceptor Tlp3. Biomolecules 2020, 10, 744. [Google Scholar] [CrossRef]
- Ortega, A.; Zhulin, I.B.; Krell, T. Sensory Repertoire of Bacterial Chemoreceptors. Microbiol. Mol. Biol. Rev. 2017, 81, e00033-17. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Liu, Y.C.; Roujeinikova, A. Expression, refolding, purification and crystallization of the sensory domain of the TlpC chemoreceptor from Helicobacter pylori for structural studies. Protein Expr. Purif. 2015, 107, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aydin, I.; Dimitropoulos, A.; Chen, S.H.; Thomas, C.; Roujeinikova, A. Purification, crystallization and preliminary X-ray crystallographic analysis of the putative Vibrio parahaemolyticus resuscitation-promoting factor YeaZ. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 604–607. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1204–1214. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef]
- Kantardjieff, K.A.; Rupp, B. Matthews coefficient probabilities: Improved estimates for unit cell contents of proteins, DNA, and protein-nucleic acid complex crystals. Protein Sci. 2003, 12, 1865–1871. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zwart, P.H.; Grosse-Kunstleve, R.W.; Lebedev, A.A.; Murshudov, G.N.; Adams, P.D. Surprises and pitfalls arising from (pseudo)symmetry. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.W. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
- Gavira, J.A.; Ortega, A.; Martin-Mora, D.; Conejero-Muriel, M.T.; Corral-Lugo, A.; Morel, B.; Matilla, M.A.; Krell, T. Structural Basis for Polyamine Binding at the dCACHE Domain of the McpU Chemoreceptor from Pseudomonas putida. J. Mol. Biol. 2018, 430, 1950–1963. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
| Diffraction source | MX1 beamline, Australian Synchrotron |
| Wavelength (Å) | 0.95 |
| Detector | EIGER2 9M |
| Total oscillation span (°) | 120 |
| Temperature (K) | 100 |
| Mosaicity (°) | 0.09 |
| Resolution range (Å) | 46.29–1.60 (1.63–1.60) |
| Space group | P21212 |
| Unit cell parameters | |
| a, b, c (Å) | 56.1, 137.7, 82.0 |
| α, β, γ (°) | 90, 90, 90 |
| Mean I/σ(I) | 18.8 (1.6) |
| Multiplicity | 4.3 (2.3) |
| Completeness (%) | 96 (86) |
| Observed reflections | 344,796 (7878) |
| Unique reflections | 81,116 (3462) |
| Rmerge | 0.023 (0.359) |
| Rmeas | 0.026 (0.445) |
| CC(1/2) (%) | 100 (83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovaleva, D.; Xin, Y.; Khan, M.F.; Chin, Y.H.; Roujeinikova, A. Isolation and Preliminary X-Ray Crystallographic Characterisation of the Periplasmic Ligand-Binding Domain of the Chemoreceptor Tlp3 from Campylobacter hepaticus. Crystals 2025, 15, 542. https://doi.org/10.3390/cryst15060542
Kovaleva D, Xin Y, Khan MF, Chin YH, Roujeinikova A. Isolation and Preliminary X-Ray Crystallographic Characterisation of the Periplasmic Ligand-Binding Domain of the Chemoreceptor Tlp3 from Campylobacter hepaticus. Crystals. 2025; 15(6):542. https://doi.org/10.3390/cryst15060542
Chicago/Turabian StyleKovaleva, Diana, Yue Xin, Mohammad F. Khan, Yu H. Chin, and Anna Roujeinikova. 2025. "Isolation and Preliminary X-Ray Crystallographic Characterisation of the Periplasmic Ligand-Binding Domain of the Chemoreceptor Tlp3 from Campylobacter hepaticus" Crystals 15, no. 6: 542. https://doi.org/10.3390/cryst15060542
APA StyleKovaleva, D., Xin, Y., Khan, M. F., Chin, Y. H., & Roujeinikova, A. (2025). Isolation and Preliminary X-Ray Crystallographic Characterisation of the Periplasmic Ligand-Binding Domain of the Chemoreceptor Tlp3 from Campylobacter hepaticus. Crystals, 15(6), 542. https://doi.org/10.3390/cryst15060542






