Abstract
The crystal structures of rod-like molecules with nitro-biphenyl or nitro-phenyl end groups and attached n-alkyl units with terminal acid or ester groups are determined by single crystal analysis and their arrangements are compared. The molecules are linked by head-to-tail arrangements and form strings. They point in a single or two different directions and are placed side by side to create the crystal structure. Some of the space groups of the structures can only be determined by a statistical routine because strongly disordered structures prevent the use of extinction methods, since many extinction violations occur for monoclinic and orthorhombic unit cells. An agreement between experimental and calculated X-ray reflection intensities serves as proof of the correctness of the method as well as a test of the existence of an inversion center. The single crystals are grown in solution with ethanol, isopropanol, DMAc, and toluene as solvents. Cocrystals are formed in DMAc solutions by the dissolved acid compounds. The two-molecule asymmetric unit of the acid compound is reduced to a one-molecule asymmetric unit with one DMAc included which forms a hydrogen bond with the acid group of the biphenyl molecule. These changes alter the hydrogen bonding scheme along a string. Some structural similarities as the head-to-tail arrangement in the strings are maintained between the terminal acid and ester compounds despite disordered ester groups in the compounds and the ester molecules themselves at ambient temperature.
1. Introduction
The structures of materials of rod-like (calamitic) molecules in the crystalline and liquid crystalline states are of particular interest for application and can be investigated by available spectroscopic methods (NMR, IR, Raman, etc.), elemental analysis, X-ray, electron, and neutron scattering, etc. X-ray investigations are preferred if highly ordered specimens are available as single crystals which allow the determination of the shape and packing of the molecules. If the crystals are transformed by heating into the liquid crystal state, these data can be used to approximate the structure of these less ordered phases as demonstrated by many researchers, i.e., by Doucet et al. [1]. He determined the crystal structure in the first step and then applied these findings to modeling a smectic phase in a succeeding step. However, a complete description should also consider the influence of the conformation of the molecules and their arrangements, accessible by comparing similar constitutions of molecules in the crystalline state, which was achieved by Heiske [2], Thyen [3], Roman et al. [4], and Zugenmaier [5].
In the present investigation, we were able to synthesize and study the influence of small alternations in the available compounds concerning the conformation and arrangements of the molecules in the crystalline state which will also affect the liquid crystalline behavior. It is not possible at the present time to predict any of the changes which are observed, and the study must be regarded as a collection of basic data which can be considered as a first step for possible applications by many scientists. Basic research is vividly supported by the great Dutch theoretical physicist Casimir who later became the director of the Philips-Research-Laboratory expressing that many important inventions have started this way. Einstein’s introduction of stimulated emission of light in 1916 on a purely theoretical basis led to the invention of lasers in 1960 with numerous applications.
2. Materials and Methods
Single crystals of the following selected compounds are synthesized by procedures using the published routes [6,7,8] and the improvements of [2]:
- (i)
- Pure crystals with acid terminal groups: 4-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)butanoic acid (NO2-Bi-3S) and 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoic acid (NO2-Bi-5S).
- (ii)
- Cocrystals with acid terminal groups: 2-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)ethanic acid N,N-dimethylacetamide (NO2-Bi-1S-DMAc), 4-((4′-nitro-[1,1′-biphenyl]-4-yl)oxy)butanoic acid N,N-dimethylacetamide (NO2-Bi-3S-DMAc), and 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoic acid N,N-dimethylacetamide (NO2-Bi-5S-DMAc).
- (iii)
- Phenyl replacing biphenyl: 2-(4-nitrophenoxy)acetic acid (NO2-Ph-1S), 6-(4-nitrophenoxy)hexanoic acid (NO2-Ph-5S), 1-ethoxy-4-nitrobenzene (NO2-Ph-1).
- (iv)
- Ether groups replacing acids: methyl 2-((4′-cyano-[1,1′ biphenyl]-4-yl)oxy)ethanate (CN-Bi-1S-M), ethyl 5-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)pentanoate (NO2-Bi-4S-E) and ethyl 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoate (NO2-Bi-5S-E).
They have been obtained by growing crystals from solutions by the well-known techniques of crystal growth from solution by the method of slow evaporation [9]. The compounds are dissolved in capillaries using ethanol as a solvent close to saturation. The solvent vaporizes slowly, and single crystals are formed. Besides ethanol, isopropanol as solvent also gives good results for the ester compounds, toluene for NO2-Bi-3-S, and N,N-dimethylacetamide (DMAc) is used to create the DMAc cocrystals. However, at ambient temperature, the solvent DMAc evaporates slowly from the cocrystals during the X-ray measurements. Some of the crystal structures are highly disordered and produce broad and less intense X-ray reflections at higher angles.
The X-ray data were collected with a CAD4 Enraf-Nonius single crystal diffractometer at T = 293 (2) K. The unit cells and further basic crystallographic data are summarized for the selected compounds in Table 1, Table 2, Table 3 and Table 4. The intensities of the reflections are processed with the MolEN package [10,11] on a Micro VAX computer and the structures are solved by direct methods [12]. The space group was determined with a statistical routine provided by SIR14 [12] and used in further procedures despite the many space group extinction violations found for monoclinic and orthorhombic space groups which are due to the disorder. A slightly better agreement factor and structure with an additional molecule introduced are neglected. All compounds showed excellent starting models which allowed the hydrogen atoms to be placed at their respective positions. The structural models were refined [13,14] on a personal computer with or without hydrogens included in the refinement procedure depending on the quality of the data. The conformational and packing models were produced and drawn with the Schakal-99 [15] and Ortep3 programs [16]. The molecular structure and packing arrangements of the different compounds will be compared and discussed. The data describing the structures such as coordinates, bond lengths, angles, etc., are collected in the Crystallography Open Database (COD).

Table 1.
Summary of crystallographic data collected at ambient temperature for 4-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)butanoic acid (NO2-Bi-3-S) and 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoic acid (NO2-Bi-5-S).

Table 2.
Summary of crystallographic data collected at ambient temperature for 2-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)ethanic acid N,N-dimethylacetamide (NO2-Bi-1-S-DMAc), 4-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)butanoic acid N,N-dimethylacetamide (NO2-Bi-3-S-DMAc) and 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoic acid N,N-dimethylacetamide (NO2-Bi-5-S-DMAc).

Table 3.
Summary of crystallographic data collected at ambient temperature for (4-nitrophenoxy)acetic acid (NO2-Ph-1-S), 6-(4-nitrophenoxy)hexanoic acid (NO2-Ph-5-S) and 1-ethoxy-4-nitrobenzene (NO2-Ph-1, 17.5 °C).

Table 4.
Summary of crystallographic data collected at ambient temperature for methyl 2-((4′-cyano-[1,1′ biphenyl]-4-yl)oxy)ethanate (NC-Bi-1-S-M), ethyl 5-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)pentanoate (NO2-Bi-4-S-E) and ethyl 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoate (NO2-Bi-5-S-E).
3. Results and Discussion
Figure 1a,b depict the asymmetric unit (2 molecules) with atom labeling and thermal ellipsoids of 50% for NO2-Bi-n-S, n = 3, 5, and Figure 2 and Figure 3 show two projections of the arrangement of the structure for these two similar compounds. The packing of the molecules discloses an arrangement in strings with one molecule in the string connected to the next one by a hydrogen bond (c.f. Table 5) between the hydroxyl groups O104 and O205 and O204 … O105 of molecules 1 and 2 in Figure 2 and the hydroxyl group O104 and O105, and O204 … O205 in Figure 3. The hydrogen bonds formed by the molecules of the two structures are of the same type and strength and cause dimeric molecules to be formed. The two adjacent dimers in the string are connected by van der Waals interaction of the nitro moiety of molecules with group number one and two in the same string in Figure 2 but with the molecule group number one and one, or two and two in Figure 3. The projection on a perpendicular plane to the strings provides differences in the cross sections for the two structures which is caused by a shift in the connecting NO2 groups. Two adjacent chains are placed in antiparallel fashion in Figure 2a and Figure 3a to fulfill the requirement of the space group P-1 with four molecules in the unit cell.

Figure 1.
(a,b). Asymmetric unit (2 molecules) with atom labeling and thermal ellipsoids of 50% for a) 4-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)butanoic acid (NO2-Bi-3-S) and b) 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoic acid (NO2-Bi-5-S). Drawn with Ortep3 [16] using the stored COD data.
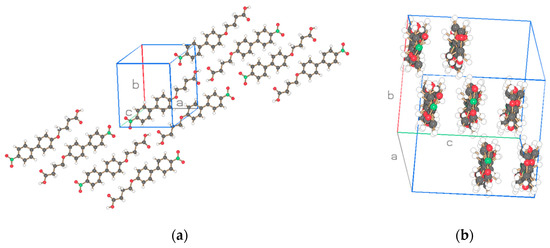
Figure 2.
Two projections of the two molecules of the asymmetric unit for NO2-Bi-3-S of Figure 1a. Drawn with Schakal [15].
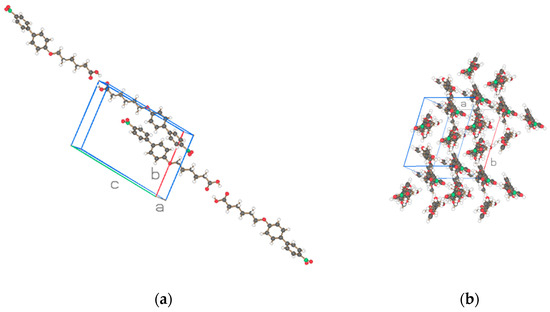
Figure 3.
Two projections of the two molecules of the asymmetric unit for NO2-Bi-5-S of Figure 1b. Drawn with Schakal [15].

Table 5.
Hydrogen bonds and twisting angles between phenyl groups and NO2, attained by the refinement program of Sheldrick [14].
The basic structure of the cocrystalline material is represented with the same compounds n = 3 including the terminal acid groups of Figure 1a,b but with DMAc incorporated into the unit cell in Figure 4 and the result for n = 1 added. The projection of the arrangements of the molecules is shown in Figure 5, Figure 6 and Figure 7. The cocrystals exhibit triclinic unit cells P-1 for alkyl lengths of n = 1, 3 but a biological monoclinic one P21 of the Sohnke type for n = 3. In contrast to the discussed structures with two molecules as asymmetric units, only one molecule and one DMAc unit are detected as an asymmetric unit, thus forming a structure with only two molecules in the unit cell instead of 4 as above. Molecular strings are observed, and a continuous sequence of biphenyl molecules and solvent DMAc occurs. The hydrogen bonds are missing between the acid groups of neighboring biphenyl molecules; instead, a hydrogen bond is formed between the terminal acid group of the biphenyl compound and DMAc (c.f. Table 5). The next biphenyl molecule is attached by van der Waals interaction, as depicted in Figure 5, Figure 6 and Figure 7 for the structures considered belonging to different space groups and packing arrangements. A straight string formation for the three structures is also verified by a projection along the string axis in contrast to the above-discussed structures. A difference occurs in the string arrangement for NO2-Bi-3-S-DMAc compared with all the discussed structures so far. The strings point in two directions as shown in projection on the c, b-plane of Figure 6a. A projection down one string direction shows in Figure 6c the cross sections of the strings and simultaneously a projection of overlapping elongated strings pointing in the second observed direction. A further remarkable feature is observed. The crystallization of the compounds in a centrosymmetric space group requires this symmetry element to be present in the packing arrangement and is fulfilled by an antiparallel arrangement of neighboring molecules or strings. Such a symmetry element is absent for space group P21 and neighboring molecules or strings are placed in a parallel direction as seen in Figure 6b.
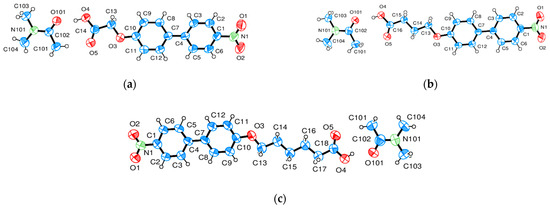
Figure 4.
(a–c). Representation and numbering of the asymmetric unit (1 molecule) of (a) NO2-Bi-1 S-DMAc, (b) NO2-3-S-DMAc and (c) NO2-Bi-5-S-DMAc (Ortep3 [16]).
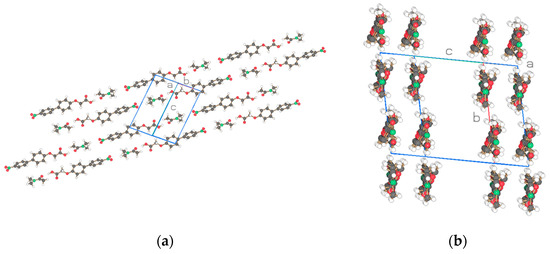
Figure 5.
Two projections of the structure for NO2-Bi-1-S-DMAc of Figure 4a with one biphenyl molecule and one DMAc as the asymmetric unit. Drawn with Schakal [15].
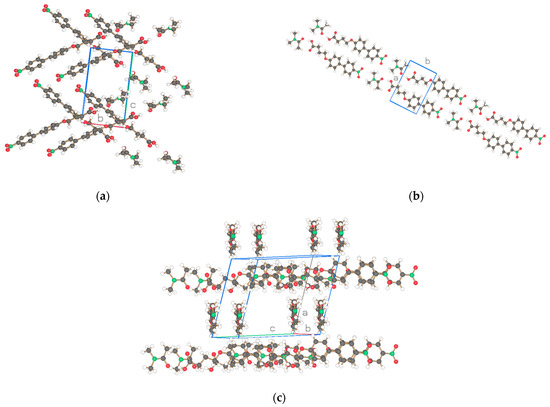
Figure 6.
Three projections of the structure for NO2-Bi-3-S-DMAc of Figure 4b with one biphenyl molecule and one DMAc as the asymmetric unit. Drawn with Schakal [15].
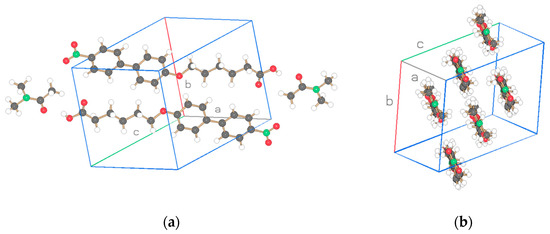
Figure 7.
Two projections of the structure for NO2-Bi-5 S-DMAc of Figure 4c. Drawn with Schakal [15].
The flexibility of the rod-like compounds can be changed by replacing the biphenyl moiety of the molecules with a phenyl group. This effect was investigated by the determination of three structures: NO2-Ph-n-S, n = 1, 5 and NO2-Ph-1 omitting also the acid group S in this compound. The conformation and numbering of atoms are shown in Figure 8. The space groups of the three crystalline structures are determined to be P-1 for NO2-Ph-1-S (asymmetric unit one molecule), Pbca for NO2-Ph-5-S (asymmetric unit two molecules), and P21/c for NO2-Ph-1 (asymmetric unit one molecule) with two, eight, and four molecules in the unit cell. Projections of the crystal structures are represented in Figure 9, Figure 10 and Figure 11. They are not comparable with similarly discussed structures. The monoclinic or orthorhombic structures are severely distorted with many systematic absence violations required by the space group. Nevertheless, the listed space groups are clearly supported by a statistical routine [12], the excellent X-ray R-value for the monoclinic NO2-Ph-1, and an acceptable R-value for the orthorhombic NO2-Ph-5-S structure.
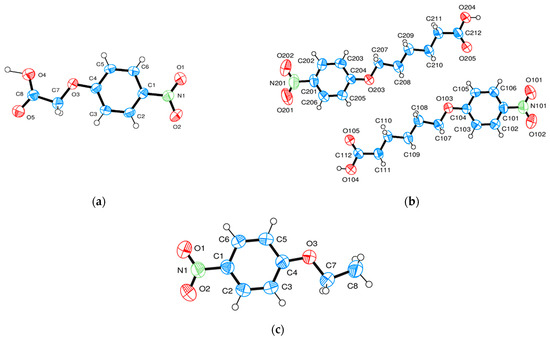
Figure 8.
(a–c) Representation and numbering of the asymmetric unit for (a) NO2-Ph-1 S, (b) NO2-Ph-5-S, and (c) NO2-Ph-1 (Ortep3 [16]).
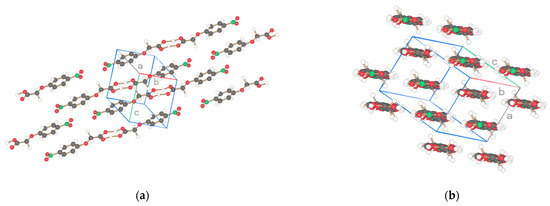
Figure 9.
Two projections of the molecules of the asymmetric unit (1 molecule) for NO2-Ph-1-S of Figure 8a. Drawn with Schakal [15].
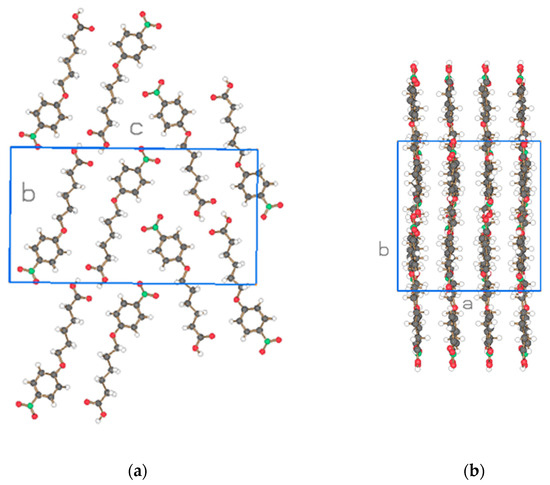
Figure 10.
Two projections of the molecules of the asymmetric unit (2 molecules) for NO2-Ph-5-S of Figure 8b. Drawn with Schakal [15].

Figure 11.
Two projections of the molecules of the asymmetric unit (1 molecule) for NO2-Ph-1 of Figure 8c. Drawn with Schakal [15].
Molecular strings are formed for the compound NO2-Bi-1-S by linking two molecules with a hydrogen bond to form dimers with an elongation of the rod-like character (Figure 9a) and established in the discussed structures. Straight strings are confirmed by a projection of the strings (Figure 9b). The hydrogen of the hydroxyl group seems to be delocalized since only a long O-H bond and partial hydrogen position lead to a reasonable result in the structure refinement. The centrosymmetric character of the space group is expressed by an antiparallel position of neighboring dimeric molecules.
The crystal structure of NO2-Ph-5-S resembles in part the one of NO2-Bi-3-S-DMAc as two different molecular directions are detected (Figure 10) but with antiparallel placement of neighboring dimeric molecules consistent with the orthorhombic, centrosymmetric space group Pbca. The molecules form planes as represented in Figure 10b with various placements of the molecules in the b-direction.
The structure of NO2-Ph-1 without the acid group and therefore, no hydrogen bonds available, provided straight molecular strings with single molecules connected by van der Waals interactions shown in the projections of Figure 11. Antiparallel neighboring molecules are present, located in strings as required by the space group. The strings are positioned in planes with various arrangements the in the b-direction as for NO2-Ph-5-S, however, with a single orientation.
It remains to study the structure of similar compounds without hydrogen bonding, i.e., replacing the acid terminal group with an ester moiety: ethyl 5-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)pentanoate (NO2-Bi-4-S-E), crystallizing in the triclinic space group P-1; ethyl 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoate (NO2-Bi-5-S-E), monoclinic space group P21/c; and methyl 2-((4′-cyano-[1,1′ biphenyl]-4-yl)oxy)ethanate (CN-Bi-1-S-M) also replacing the nitro group with a cyano one, crystallizing in the monoclinic space group P21/a. Crystallographic data are collected in Table 4. The conformation and numbering of atoms of the molecules are represented in Figure 12 forming two molecules, with one molecule as an asymmetric unit.
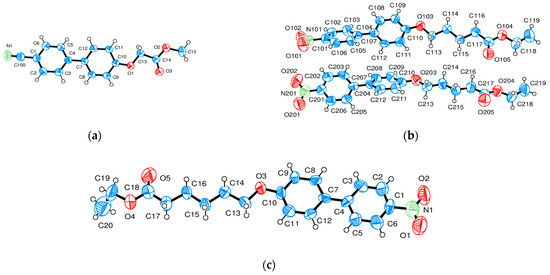
Figure 12.
(a–c) Representation and numbering of the asymmetric unit of (a) CN-Bi-1-S-M, (b) NO2-Bi-4-S-E (2 molecules), and (c) NO2-Bi-5S-E (Ortep3 [16]).
The structure of methyl CN-Bi-1-S-M shown in Figure 13 resembles the one for NO2-Bi- 3-S- DMAc in Figure 6. Straight strings pointing in two directions are formed by van der Waals interactions between the molecules. The structure of space group P21/a is proposed by Sir14 [12] despite many violations of the required extinction due to a disordered structure. The proposal is also supported by a low R-value from the shelxl-19 [13] refinement (Table 4). Contrary to the Sohnke space group P21 of the structure in Figure 6 with parallel neighboring molecules, the molecules considered here are placed in an antiparallel arrangement, also supporting the choice of symmetry for P21/a.
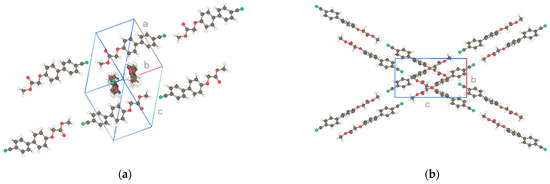
Figure 13.
Two projections of the molecules of the asymmetric unit (1 molecule) for CN-Bi-1-S-M of Figure 12a. Drawn with Schakal [15].
The projections of the two NO2-Bi-n-S-E, n = 4, 5 crystal structures, one with triclinic space group P-1, two molecules in the asymmetric unit (n = 4, Figure 14), and the other structure with monoclinic space group P21/c and one molecule in the basic unit (n = 5, Figure 15) are comparable with undulated strings for n=4 and straight strings for n = 5. The molecules in the strings are arranged in a parallel fashion with van der Waals interaction of succeeding molecules. Pairs of neighboring strings with molecules of n = 4 and single strings with neighboring molecules of n = 5 are placed in an antiparallel fashion due to an inversion symmetry element of the space groups.
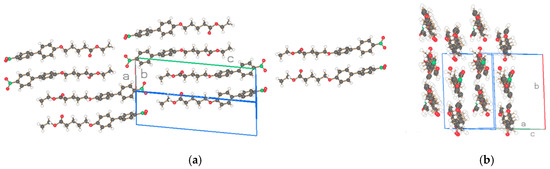
Figure 14.
Two projections of the molecules of the asymmetric unit (2 molecules) for (b) NO2-Bi-4-S-E of Figure 12b. Drawn with Schakal [15].

Figure 15.
Two projections of the molecules of the asymmetric unit (1 molecule) for (c) NO2-Bi-5-S-E of Figure 12c. Drawn with Schakal [15].
The crystallographic and structural data of the selected compounds are stored in the Crystallography Open Database (COD), i.e., the coordinates, bond lengths, angles, etc. The O..O distances of the hydrogen bonds (HB) considered as the strength of the HB bonds compare well and are listed in Table 5. However, the twisting angles between the nitro moiety and the adjacent phenyl ring as well as between the two phenyl rings of the molecules are clearly influenced by the packing of the molecules. The O–N distances in the nitro group and O–C in the acid group as well as O–C of DMAc are listed in Table 6 and show values expected for disordered structures.

Table 6.
Selected bonds.
4. Conclusions
A comparison of the determined crystalline structures shows that they have a continuous string-like appearance as a basic feature in common which is formed by head-to-tail or head-to-head arrangements of succeeding molecules. These strings point either in a single, or in two directions and form by van der Waals interactions, the three-dimensional crystal structures with distinct symmetries, unit cells, and space groups ranging from the triclinic space group P-1 to a monoclinic P21/a or P21/c and to an orthorhombic Pbca, all structures with either one or two molecules as asymmetric units. All these structures have an inversion symmetry element in common and adjacent molecules of two close strings or molecules of two pairs of close strings (NO2-Bi-4-S-E, Figure 14) are placed in antiparallel fashion, except NO2-Bi-3-S-DMAc crystallizing in the biological space group P21 and neighboring molecules of two close strings placed parallel.
The terminal acid group of several compounds gives rise to hydrogen bonding between the head-to-head of the molecules in one string. Dimers are formed with a polar group in the center and an investigation should be conducted to study this structural feature in the liquid crystalline state. This head-to-head hydrogen bond is lost in the cocrystal with DMAc as well as the dimer formation and a hydrogen bond observed between the terminal acid group and DMAc. The molecules in the strings are arranged in head-to-tail fashion as all the structures missing a terminal acid group. A hydrogen bond involving the nitro group was not observed as for the crystal structure of 4-hydroxy-4′-nitro-biphenyl (NO2-Bi-OH) [17] where the string exhibits a continuous head-to-tail hydrogen bond (NO2…HO).
The collected X-ray data with the CAD4 single crystal diffractometer point towards lattice distortions for many structures investigated: 1. A decrease in intensities for the reflections at higher angles beyond normal is observed. 2. The symmetrical element selection for the crystal structures for space group determination by the extinction method is difficult because too many possible extinct reflections are visible for a chosen space group. The description and discussion of lattice distortions was provided by polymer crystallography a long time ago by introducing distortions of the first and second kind being present in polymer structures [18]. A distortion of the first kind preserves the long-range order and results from a displacement of structural elements, i.e., atoms etc., from the equilibrium positions and is still described by ideal lattice points, comparable with a frozen-in thermal displacement. The temperature exponent for the structure factor calculation is increased. The long-range order is lost in a lattice with second kind distortions and each lattice point varies its position only in relation to its nearest neighbor to form a distorted lattice. It was shown that such a distortion influences the shape of a reflection and causes a decrease in intensity especially noticed at higher reflection angles. This observation can be approximated by an additional term in the exponent of the temperature factor—also described as the disorder factor- leading to a reduction of intensities of the X-ray reflections.
The structure solution package SIR2014 [12] provides not only starting models for the structures but also an excellent statistical routine for the determination of space groups, necessary for a refinement of the structures [13].
Despite the many observed reflections which should be extinct by the space group symmetry, the described procedure leads to a low X-ray R-value and excellent structural models. It remains to investigate the influence of the approximations introduced for a description of observed distortion on the fine structure of the molecules as bond lengths and angles. A method for describing disordered structures was proposed by Hermann [19] but was not accepted at his time.
A collection of new crystal structures with similar compounds may lead by further comparing investigations either to new perceptions of structure forming interactions, phase behavior at the transition to the liquid crystalline state as well as the formation of various liquid crystals phases with polar groups and their influence of optical properties.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and (Crystallography Open Database) COD 3000573-3000583.
Acknowledgments
The author thanks Andreas Pfeil for providing the samples.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Doucet, J.; Mornon, J.P.; Chevalier, R.; Lifchitz, A. Structure de la Phase Cristalline à Température Ambiante (20 °C) du Dérivé Bis-p-butylanilino de l’Acide Téréphtalique (TBBA). I. Acta Cryst. 1977, B33, 1701–1710. [Google Scholar] [CrossRef]
- Heiske, A. Untersuchungen zur Struktur-Eigenschaftskorrelation Kalamitischer Biphenylderivate. Ph.D. Dissertation, TU Clausthal, Clausthal, Germany, 1994. [Google Scholar]
- Thyen, W. Korrelation von Struktur und Packung in Kristallinen und Geordneten Smektischen Phasen. Ph.D. Dissertation, TU Clausthal, Clausthal, Germany, 1995. [Google Scholar]
- Roman, M.; Kaeding-Koppers, A.; Zugenmaier, P. Mixed dimer formation in binary systems of 4-substituted benzoic acids. Can. J. Chem. 2008, 86, 528–532. [Google Scholar] [CrossRef]
- Zugenmaier, P. Review of Crystalline Structures of Some Selected Homologous Series of Rod-Like Molecules Capable of Forming Liquid Crystalline Phases. Int. J. Mol. Sci. 2011, 12, 7360–7400. [Google Scholar] [CrossRef] [PubMed]
- Shibaev, V.P.; Kostromin, S.G.; Plate, N.A. Tthermotropic liquid-crystalline polymers. 6. Comb-like liquid-crystalline polymers of the smectic and nematic types with cyanobiphenyl groups in the side-chains. Eur. Polym. J. 1982, 18, 651–659. [Google Scholar] [CrossRef]
- Rötz, U.; Lindau, J.; Weissflog, W.; Reingold, G.; Unseld, W.; Kuschel, F. Synthesis and properties of liquid-crystalline polymers with laterally and terminally linked mesogenic units. Mol. Cryst. Liq. Cryst. 1988, 170, 185–193. [Google Scholar] [CrossRef]
- Oku, A.; Harada, T.; Kita, K. Selective cleavage of ethers by sodium iodide-acyl chloride. Tetrahydron Lett. 1982, 23, 681–684. [Google Scholar] [CrossRef]
- Rychkov, D.A.; Sergey, G.; Arkhipov, S.G.; Elena, V.; Boldyreva, E.V. Simple and efficient modifications of well known techniques for reliable growth of high-quality crystals of small bioorganic molecules. J. Appl. Cryst. 2014, 47, 1435–1442. [Google Scholar] [CrossRef]
- Fair, C.K. MolEN: Crystal Structure Analysis; Enraf-Nonius: Delft, The Netherlands, 1990. [Google Scholar]
- Harms, K. XCAD4NV: A Program for Extracting Intensity Data from CAD4 (Enraf-Nonius) Files; University of Marburg: Marburg, Germany, 1993. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-2019: A Program for Refinement of Single Crystal Diffraction Data; University of Göttingen: Göttingen, Germany, 2019. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Keller, E. SCHAKAL-99: A Computer Program for the Graphic Representation of Molecular and Crystallographic Models; Kristallographisches Institut der Universität Freiburg i. Br.: Freiburg, Germany, 1999. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Pfeil, A.; Zugenmaier, P. The crystal and Molecular Structure of 4-Hydroxy-4′-nitro-biphenyl and its Potassium Salt. Cryst. Res. Technol. 1996, 31, 863–873. [Google Scholar] [CrossRef]
- Alexander, L.E. X-Ray Diffraction Methods in Polymer Science; John Wiley & Sons, Inc.: New York, NY, USA, 1969; pp. 423–429. [Google Scholar]
- Hermann, C. The symmetry groups of amorphous and mesomorphous phases. Z. Kristallogr. 1931, 79, 186–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).