Comparison of Selected Crystal Structures of Rod-like Molecules with Acid and Ester Terminal Groups
Abstract
1. Introduction
2. Materials and Methods
- (i)
- Pure crystals with acid terminal groups: 4-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)butanoic acid (NO2-Bi-3S) and 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoic acid (NO2-Bi-5S).
- (ii)
- Cocrystals with acid terminal groups: 2-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)ethanic acid N,N-dimethylacetamide (NO2-Bi-1S-DMAc), 4-((4′-nitro-[1,1′-biphenyl]-4-yl)oxy)butanoic acid N,N-dimethylacetamide (NO2-Bi-3S-DMAc), and 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoic acid N,N-dimethylacetamide (NO2-Bi-5S-DMAc).
- (iii)
- Phenyl replacing biphenyl: 2-(4-nitrophenoxy)acetic acid (NO2-Ph-1S), 6-(4-nitrophenoxy)hexanoic acid (NO2-Ph-5S), 1-ethoxy-4-nitrobenzene (NO2-Ph-1).
- (iv)
- Ether groups replacing acids: methyl 2-((4′-cyano-[1,1′ biphenyl]-4-yl)oxy)ethanate (CN-Bi-1S-M), ethyl 5-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)pentanoate (NO2-Bi-4S-E) and ethyl 6-((4′-nitro-[1,1′ biphenyl]-4-yl)oxy)hexanoate (NO2-Bi-5S-E).
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doucet, J.; Mornon, J.P.; Chevalier, R.; Lifchitz, A. Structure de la Phase Cristalline à Température Ambiante (20 °C) du Dérivé Bis-p-butylanilino de l’Acide Téréphtalique (TBBA). I. Acta Cryst. 1977, B33, 1701–1710. [Google Scholar] [CrossRef]
- Heiske, A. Untersuchungen zur Struktur-Eigenschaftskorrelation Kalamitischer Biphenylderivate. Ph.D. Dissertation, TU Clausthal, Clausthal, Germany, 1994. [Google Scholar]
- Thyen, W. Korrelation von Struktur und Packung in Kristallinen und Geordneten Smektischen Phasen. Ph.D. Dissertation, TU Clausthal, Clausthal, Germany, 1995. [Google Scholar]
- Roman, M.; Kaeding-Koppers, A.; Zugenmaier, P. Mixed dimer formation in binary systems of 4-substituted benzoic acids. Can. J. Chem. 2008, 86, 528–532. [Google Scholar] [CrossRef]
- Zugenmaier, P. Review of Crystalline Structures of Some Selected Homologous Series of Rod-Like Molecules Capable of Forming Liquid Crystalline Phases. Int. J. Mol. Sci. 2011, 12, 7360–7400. [Google Scholar] [CrossRef] [PubMed]
- Shibaev, V.P.; Kostromin, S.G.; Plate, N.A. Tthermotropic liquid-crystalline polymers. 6. Comb-like liquid-crystalline polymers of the smectic and nematic types with cyanobiphenyl groups in the side-chains. Eur. Polym. J. 1982, 18, 651–659. [Google Scholar] [CrossRef]
- Rötz, U.; Lindau, J.; Weissflog, W.; Reingold, G.; Unseld, W.; Kuschel, F. Synthesis and properties of liquid-crystalline polymers with laterally and terminally linked mesogenic units. Mol. Cryst. Liq. Cryst. 1988, 170, 185–193. [Google Scholar] [CrossRef]
- Oku, A.; Harada, T.; Kita, K. Selective cleavage of ethers by sodium iodide-acyl chloride. Tetrahydron Lett. 1982, 23, 681–684. [Google Scholar] [CrossRef]
- Rychkov, D.A.; Sergey, G.; Arkhipov, S.G.; Elena, V.; Boldyreva, E.V. Simple and efficient modifications of well known techniques for reliable growth of high-quality crystals of small bioorganic molecules. J. Appl. Cryst. 2014, 47, 1435–1442. [Google Scholar] [CrossRef]
- Fair, C.K. MolEN: Crystal Structure Analysis; Enraf-Nonius: Delft, The Netherlands, 1990. [Google Scholar]
- Harms, K. XCAD4NV: A Program for Extracting Intensity Data from CAD4 (Enraf-Nonius) Files; University of Marburg: Marburg, Germany, 1993. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-2019: A Program for Refinement of Single Crystal Diffraction Data; University of Göttingen: Göttingen, Germany, 2019. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Keller, E. SCHAKAL-99: A Computer Program for the Graphic Representation of Molecular and Crystallographic Models; Kristallographisches Institut der Universität Freiburg i. Br.: Freiburg, Germany, 1999. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Pfeil, A.; Zugenmaier, P. The crystal and Molecular Structure of 4-Hydroxy-4′-nitro-biphenyl and its Potassium Salt. Cryst. Res. Technol. 1996, 31, 863–873. [Google Scholar] [CrossRef]
- Alexander, L.E. X-Ray Diffraction Methods in Polymer Science; John Wiley & Sons, Inc.: New York, NY, USA, 1969; pp. 423–429. [Google Scholar]
- Hermann, C. The symmetry groups of amorphous and mesomorphous phases. Z. Kristallogr. 1931, 79, 186–221. [Google Scholar] [CrossRef]

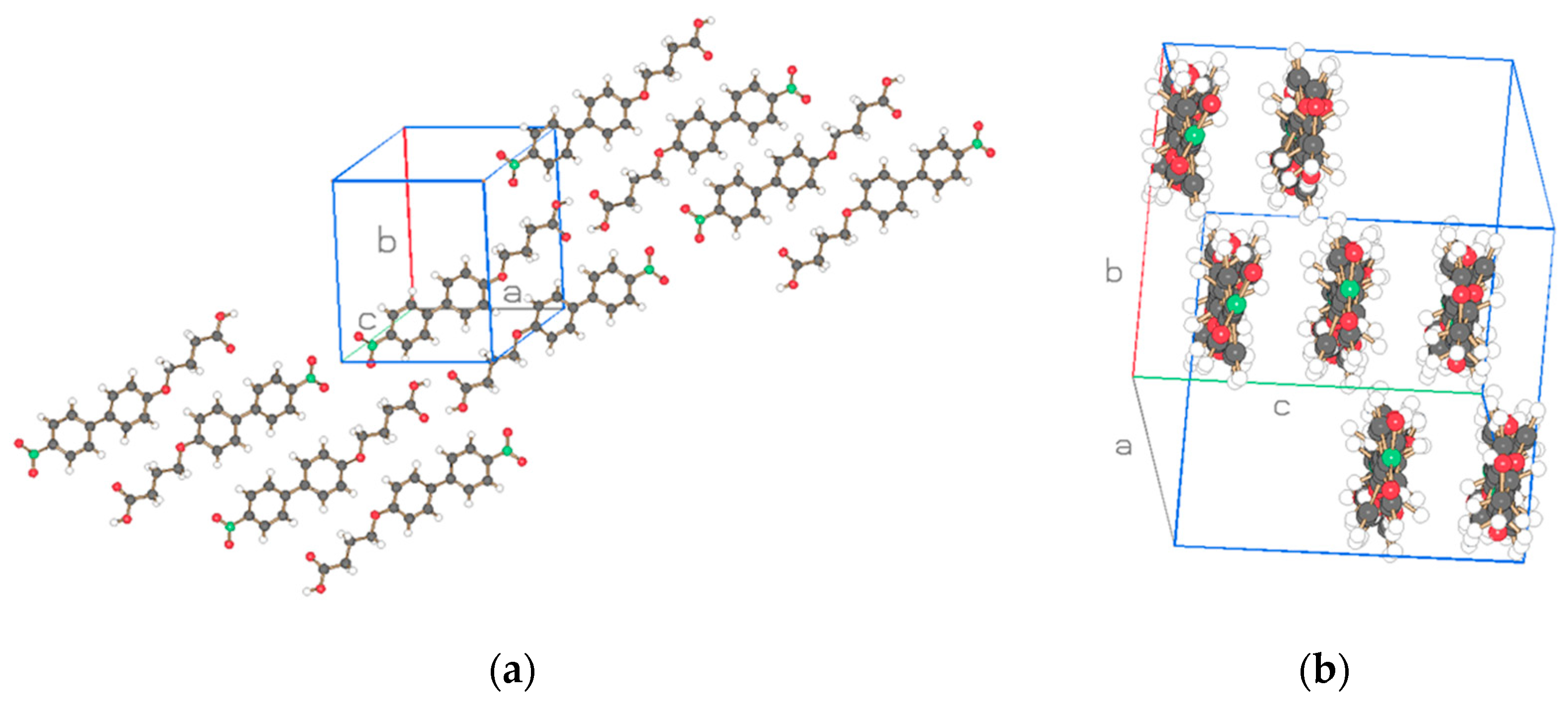
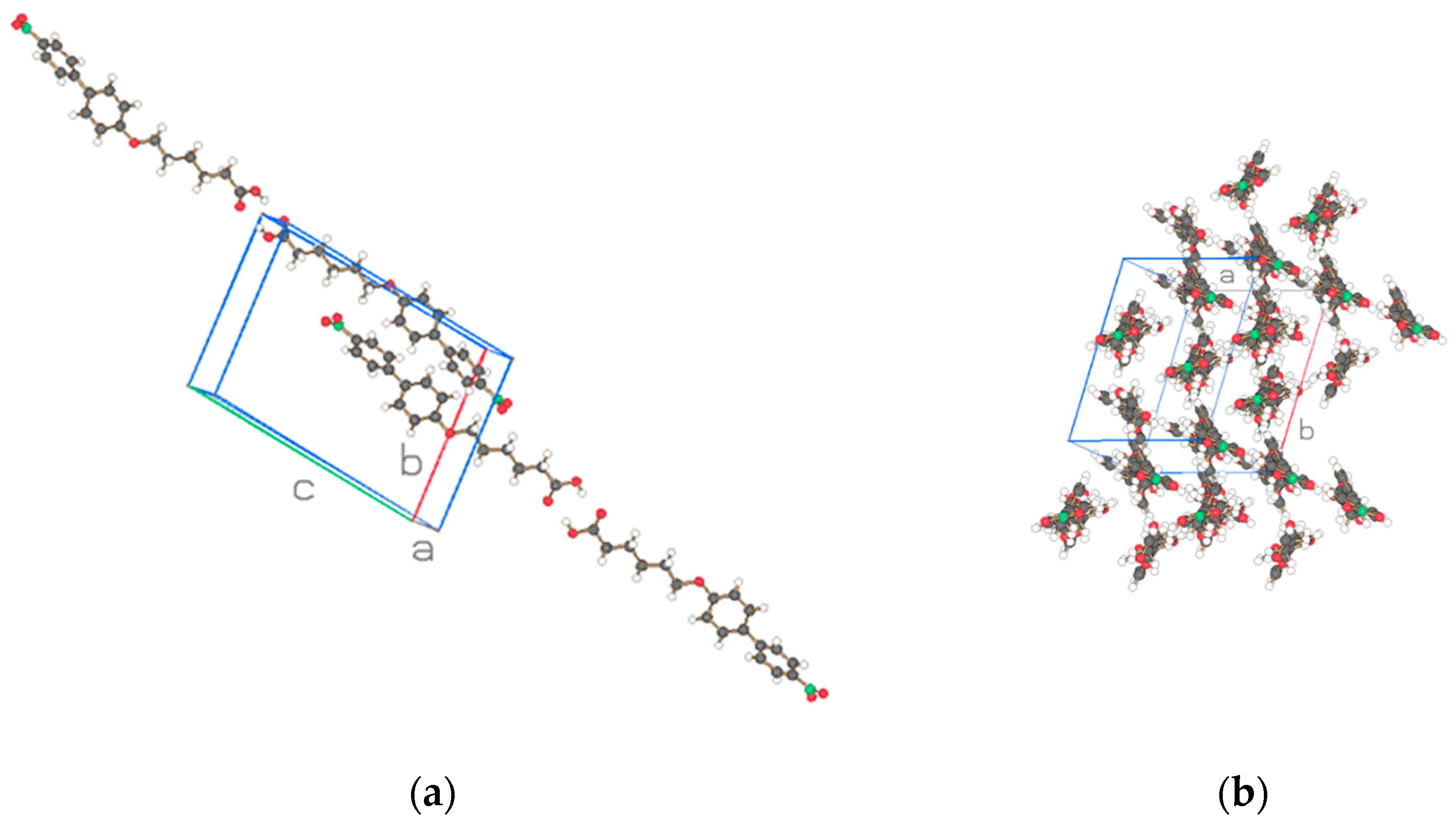
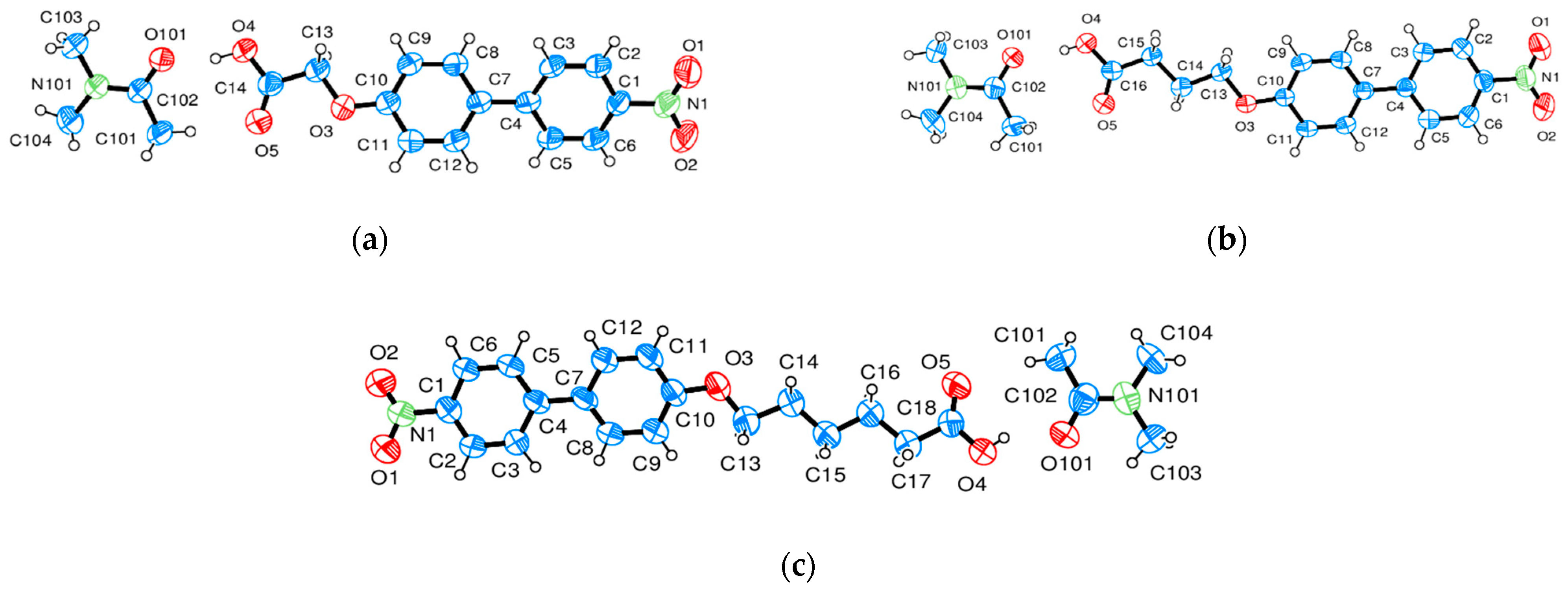
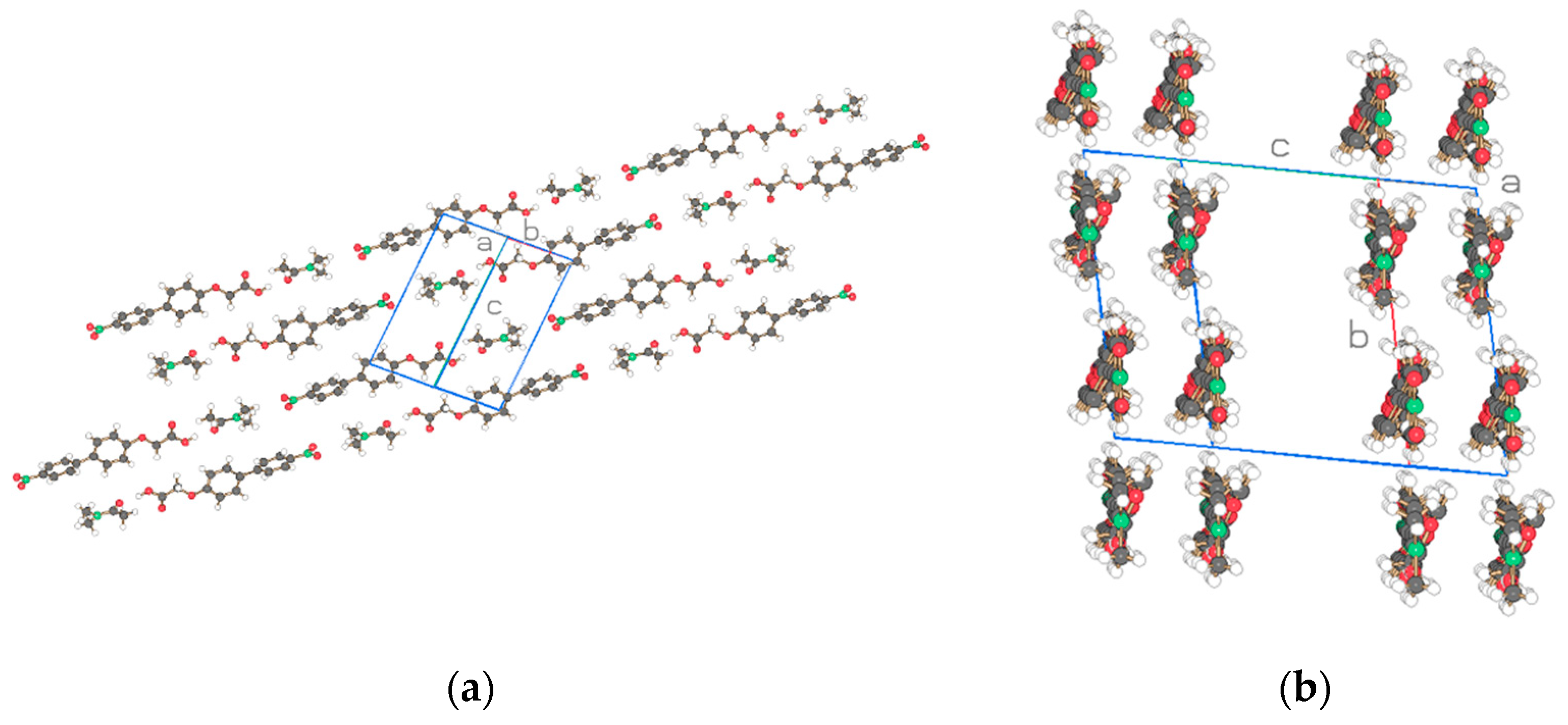
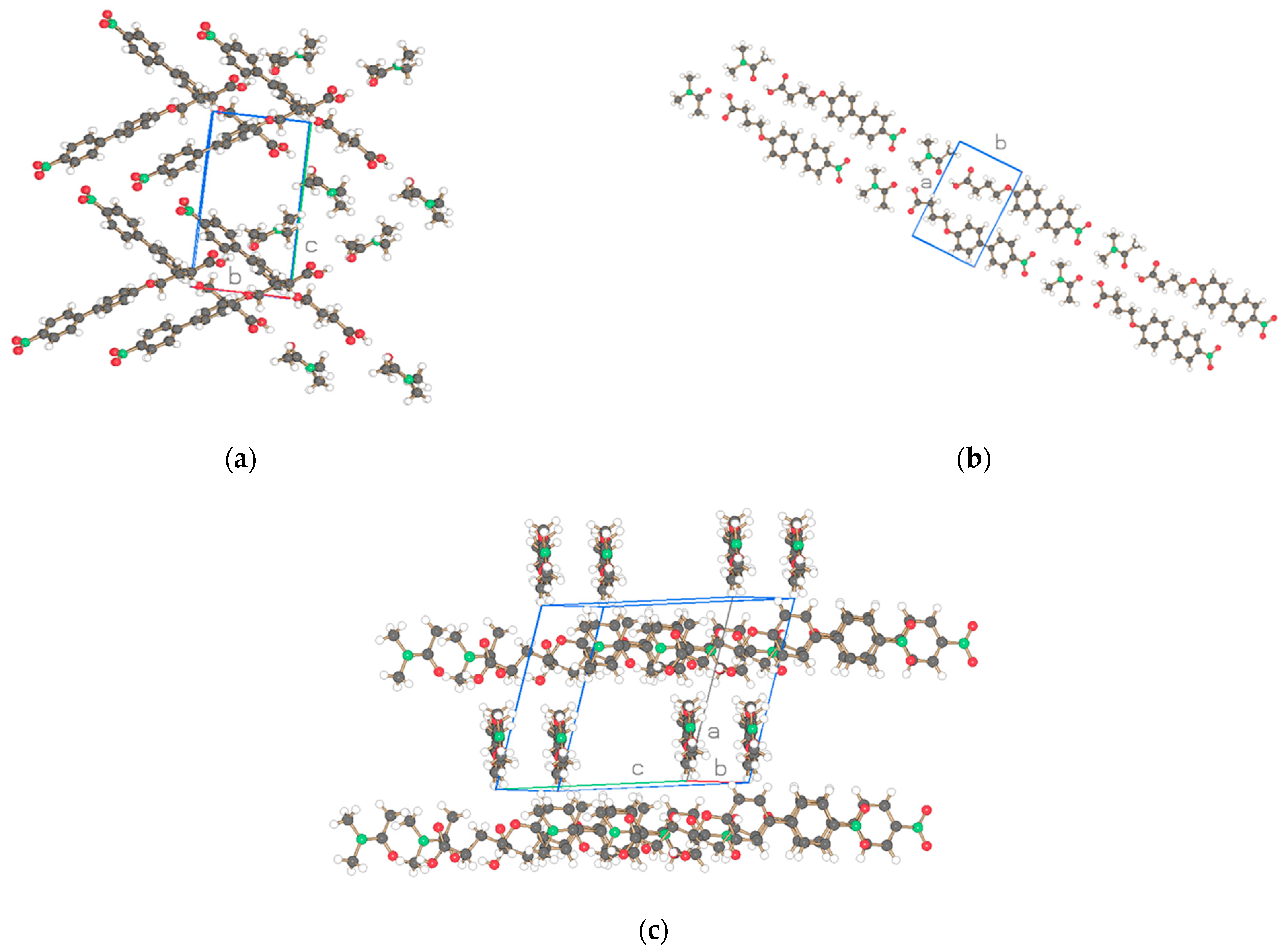

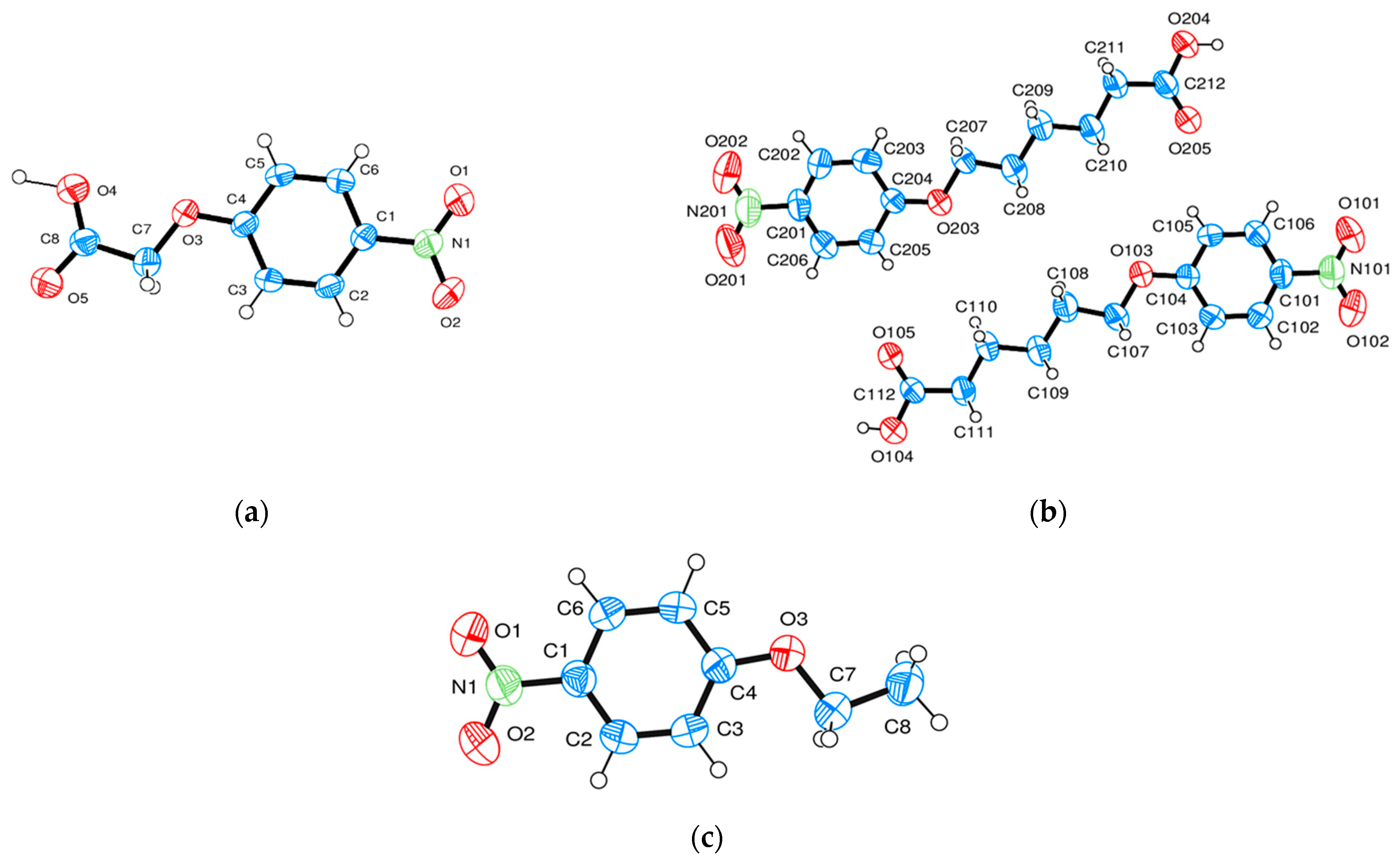
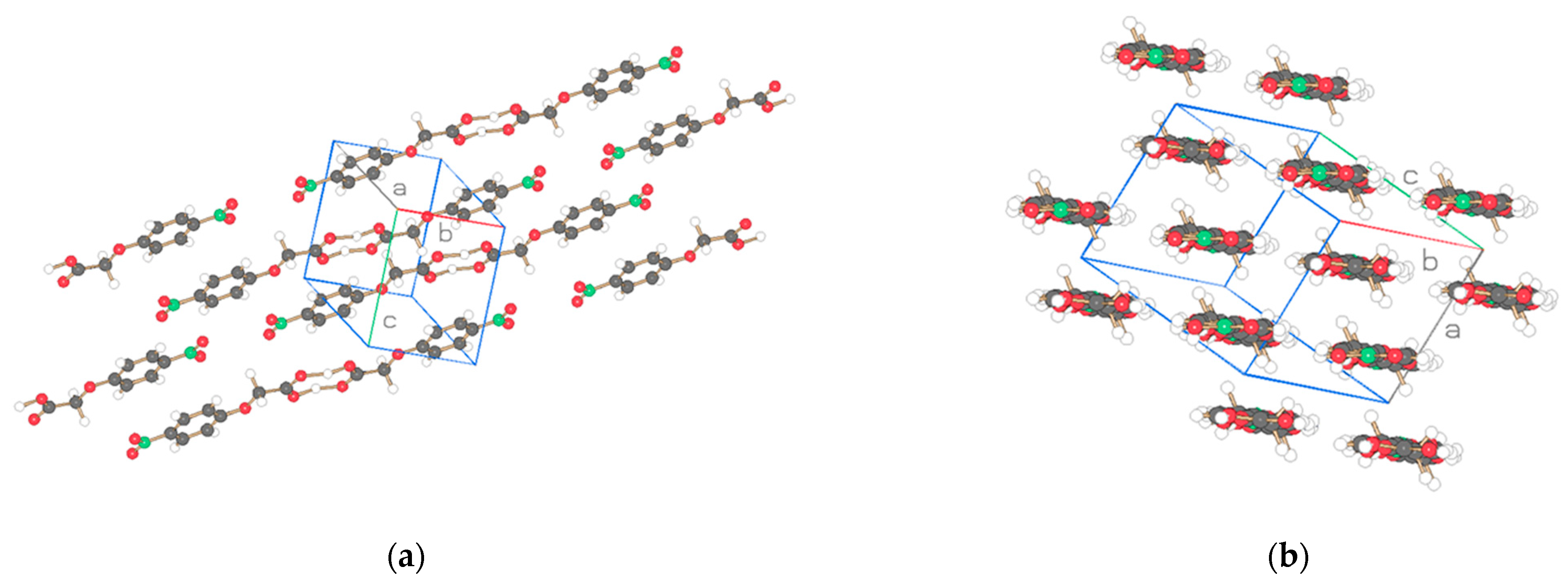
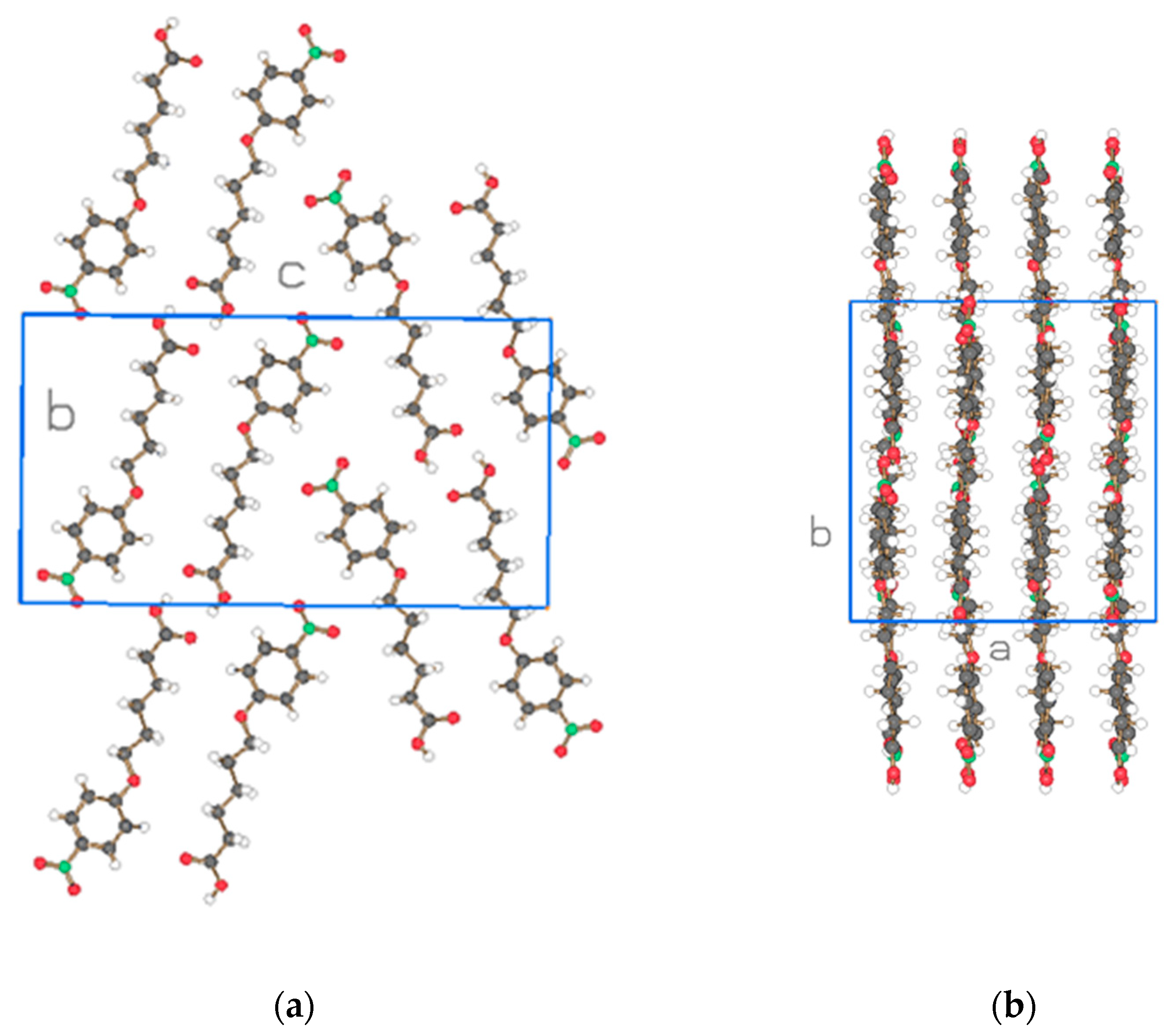
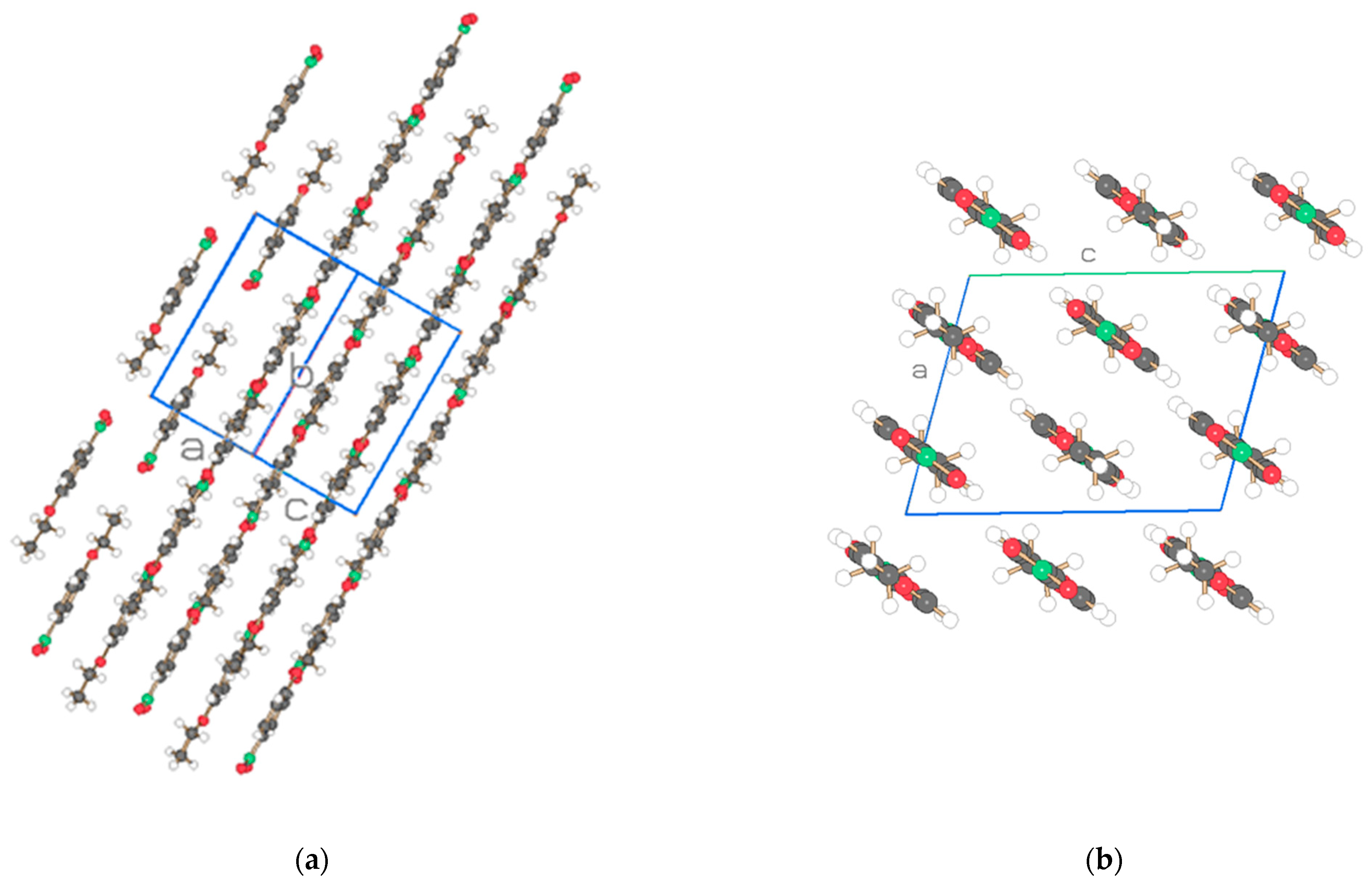
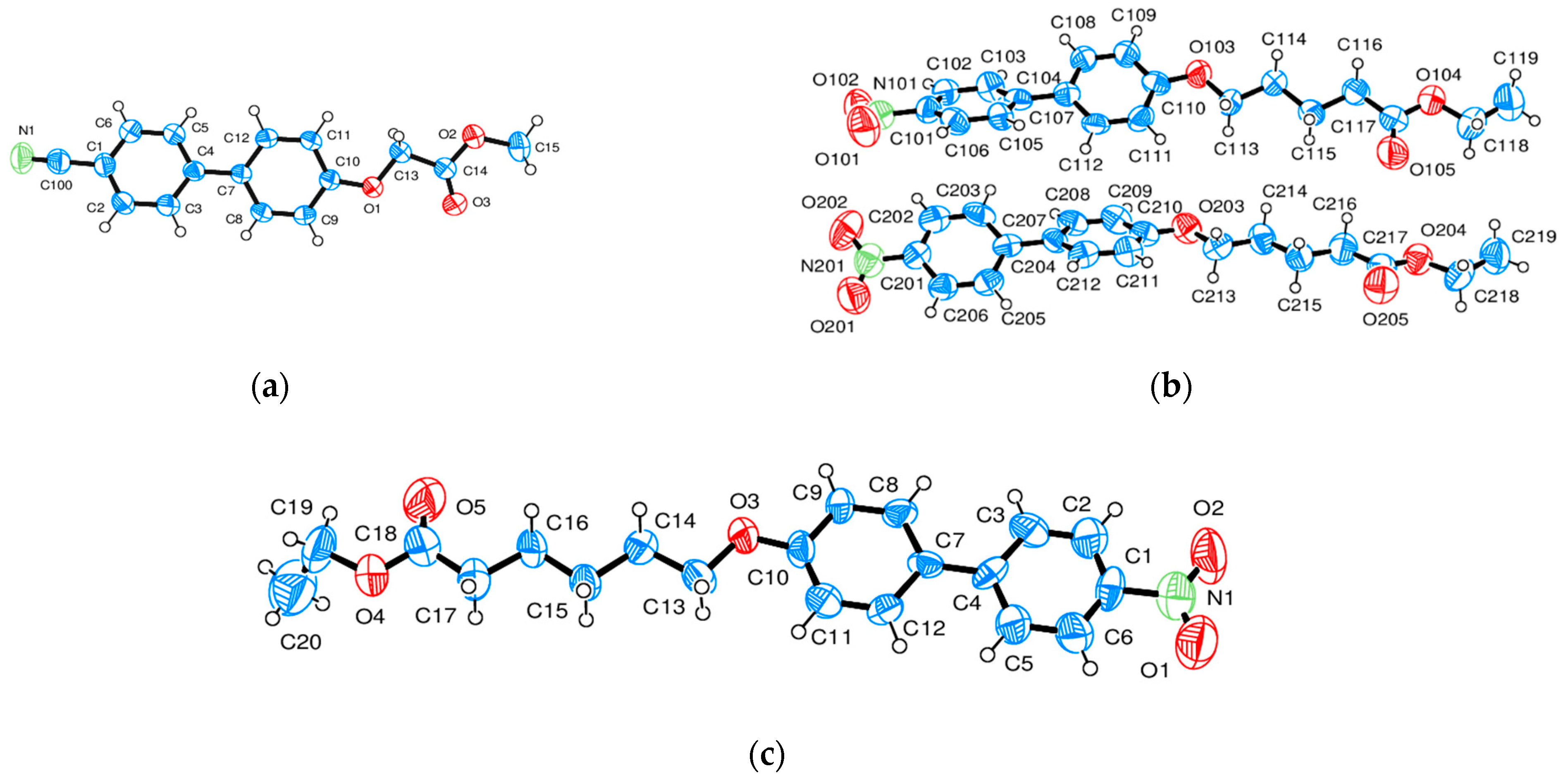
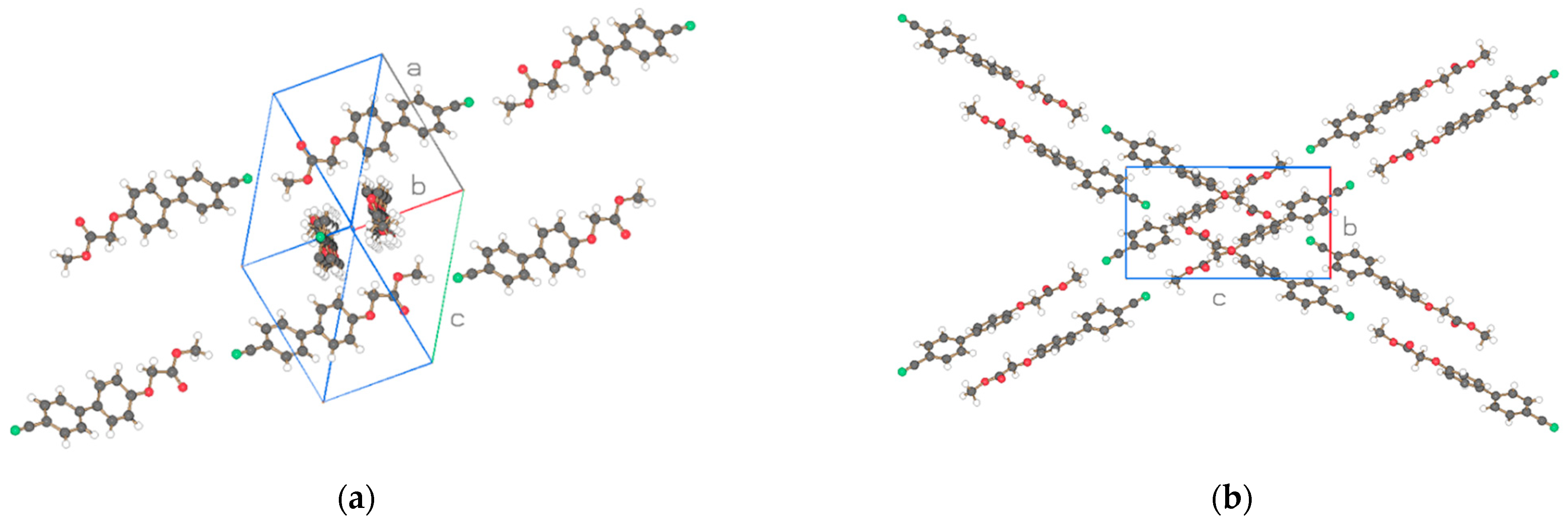

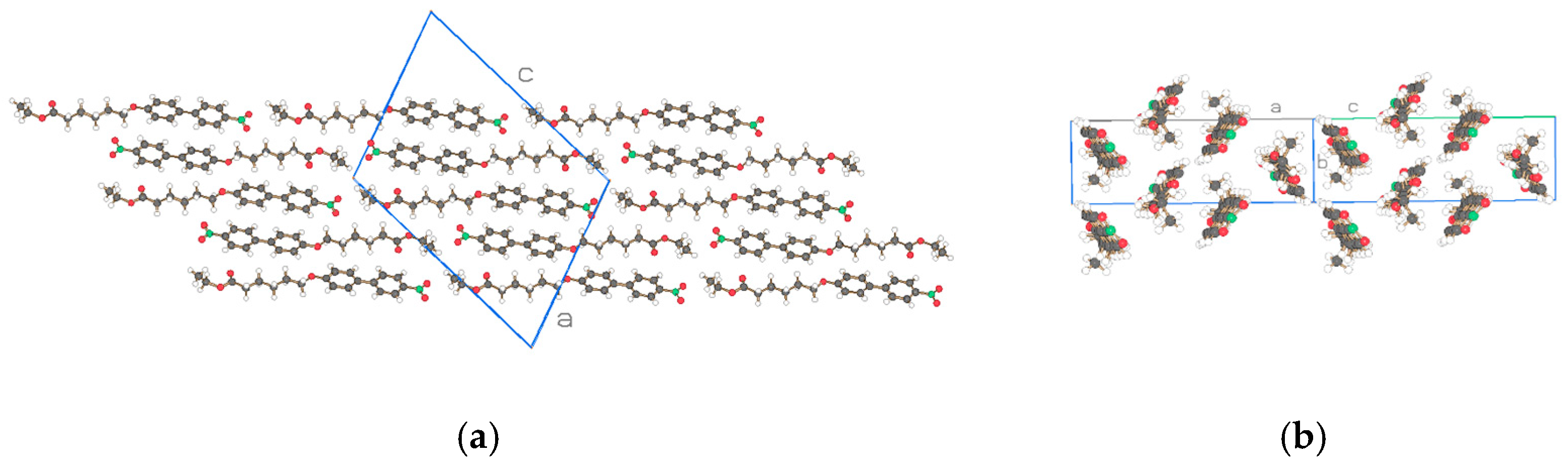
| Compound | NO2-Bi-3-S | NO2-Bi-5-S |
|---|---|---|
| Molecular formula | 2xC16H15NO5 | 2xC18H19NO5 |
| Formula mass/g·mol−1 | 602.58 | 658.68 |
| Crystal system | triclinic | triclinic |
| Space group/No. Int. Tab. | P-1/2 | P-1/2 |
| a/Å | 9.931 (3) | 8.407 (5) |
| b/Å | 11.924 (3) | 12.100 (6) |
| c/Å | 13.344 (6) | 17.651 (9) |
| α/° | 104.24 (2) | 77.36 (4) |
| ß/° | 110.67 (2) | 79.82 (4) |
| γ/° | 92.76 (2) | 74.59 (4) |
| V/Å3 | 1417.1 (9) | 1675.6 (16) |
| Dcal/g·cm−3 | 1.412 | 1.306 |
| Z (asymmetric unit) | 2 | 2 |
| CuKα/MoKα/Å | 0.71073 | 1.54184 |
| µ (CuKα/MoKα)/cm−1 | 0.106 | 0.793 |
| Number of reflections used for lattice parameter refinement | 21 | 19 |
| Scan range | 8° < θ < 20° | 13° < θ < 26° |
| F (000) | 632 | 696 |
| Reflections collected | 3960 | 2231 |
| Unique data | 3701 (Rint = 0.0134) | 2017 (Rint = 0.153) |
| Significant I’s (>2σ) | 3261 | 1319 |
| Data collection | 1.7° ≤ θ ≤ 22.5° | 2.6° ≤ θ ≤ 40.0° |
| Parameters refined/restraints | 518/0 | 438/0 |
| R1 | 0.035 | 0.065 |
| wR2 | 0.100 | 0.19 |
| Goodness-of-fit on F2 | 1.040 | 1.039 |
| Highest peak/e·Å−3 | 0.19 | 0.23 |
| Crystal color | opaque | opaque |
| Crystal shape | rod-like | rod-like |
| Compound | NO2-Bi-1-S-DMAc | NO2-Bi-3-S-DMAc | NO2-Bi-5-S-DMAc |
|---|---|---|---|
| Molecular formula | C14H11NO5 | C16H15NO5 | C18H19NO5 |
| x C4H9NO | x C4H9NO | x C4H9NO | |
| Formula mass/g·mol−1 | 360.36 | 388.41 | 416.46 |
| Crystal system | triclinic | monoclinic | triclinic |
| Space group/No. Int. Tab. | P-1/2 | P21/4 | P-1/2 |
| a/Å | 5.936 (2) | 11.265 (3) | 8.289 (4) |
| b/Å | 11.423 (3) | 6.999 (2) | 9.221 (4) |
| c/Å | 14.004 (3) | 13.202 (4) | 14.824 (7) |
| α/° | 109.41 (2) | 90 | 90.90 (3) |
| ß/° | 91.64 (2) | 108.08 (2) | 105.11 (3) |
| γ/° | 96.26 (2) | 90 | 97.65 (3) |
| V/Å3 | 888.1 (4) | 989.5 (5) | 1082.6 (9) |
| Dcal/g·cm−3 | 1.348 | 1.304 | 1.278 |
| Z (asymmetric unit) | 2 | 2 | 2 |
| MoKα/Å | 0.71073 | 0.71073 | 0.71073 |
| µ (MoKα)/mm−1 | 0.102 | 0.097 | 0.093 |
| Number of reflections used for lattice parameter refinement | 25 | 18 | 25 |
| Scan range | 10° < θ < 15° | 10° < θ < 15° | 10° < θ < 20° |
| F (000) | 380 | 412 | 444 |
| Reflections collected | 1865 | 1597 | 2863 |
| Unique data | 1650 (Rint = 0.008) | 1512 (Rint = 0.026) | 2635 (Rint = 0.066) |
| Significant I’s (>2σ) | 1436 | 1342 | 1235 |
| Data collection | 1.5° ≤ θ ≤ 20.0° | 1.6° ≤ θ ≤ 23.0° | 2.2° ≤ θ ≤ 22.0° |
| Parameters refined/restraints | 316/0 | 316/1 | 352/0 |
| R1 | 0.036 | 0.036 | 0.061 |
| wR2 | 0.103 | 0.097 | 0.19 |
| Goodness-of-fit on F2 | 1.039 | 1.064 | 1.019 |
| Highest peak/e·Å−3 | 0.17 | 0.16 | 0.15 |
| Crystal color | opaque | opaque | opaque |
| Crystal shape | platelet | rod-like | rod-like |
| NO2-Ph-1-S | NO2-Ph-5-S | NO2-Ph-1 | |
|---|---|---|---|
| Molecular formula | C8H6NO5 | C24H30N2O10 | C8H9NO3 |
| Formula mass/g·mol−1 | 196.64 | 506.50 | 167.16 |
| Crystal system | triclinic | orthorhombic | monoclinic |
| Space group/No. Int. Tab. | P-1/2 | Pbca/61 | P21/c/14 |
| a/Å | 6.9682 (9) | 13.745 (1) | 7.498 (5) |
| b/Å | 7.6771 (9) | 14.380 (2) | 11.813 (4) |
| c/Å | 8.3131 (9) | 26.310 (3) | 9.564 (7) |
| α/° | 71.470 (10) | 90.000 | 90.00 |
| ß/° | 88.693 (9) | 90.000 | 105.79 (3) |
| γ/° | 84.087 (9) | 90.000 | 90.00 |
| V/Å3 | 419.39 (9) | 5200.3 (10) | 815.2 (3) |
| Dcal/g·cm−3 | 1.557 | 1.294 | 1.362 |
| Z (asymmetric unit) | 2 | 8 | 4 |
| CuKα/MoKα/Å | 1.54184 | 1.54184 | 0.71073 |
| µ (CuKα/MoKα)/mm−1 | 1.15 | 0.86 | 0.105 |
| Number of reflections used for lattice parameter refinement | 18 | 20 | 25 |
| Scan range | 20o < θ < 25° | 18° < θ < 27° | 8° < θ < 21° |
| F (000) | 203 | 2144 | 352 |
| Reflections collected | 949 | 2094 | 1539 |
| Unique data | 855 (Rint = 0.041) | 2094 | 1428 (Rint = 0.012) |
| Significant I’s (>2σ) | 787 | 1499 | 1102 |
| Data collection | 5.6° ≤ θ ≤ 50.0° | 3.4° ≤ θ ≤ 45.0° | 2.8° ≤ θ ≤ 25.0° |
| Parameters refined/restraints | 156/0 | 446/0 | 146/0 |
| R1 | 0.040 | 0.039 | 0.037 |
| wR2 | 0.122 | 0.120 | 0.112 |
| Goodness-of- fit on F2 | 1.055 | 1.032 | 1.012 |
| Highest peak/e·Å−3 | 0.16 | 0.15 | 0.14 |
| Crystal color | opaque | opaque | opaque |
| Crystal shape | rod-like | platelet | rod-like |
| NC-Bi-1-S-M | NO2-Bi-4-S-E | NO2-Bi-5-S-E | |
|---|---|---|---|
| Molecular formula | C16H13NO3 | C38H42N2O10 | C20H23NO5 |
| Formula mass/g·mol−1 | 267.27 | 686.73 | 357.39 |
| Crystal system | monoclinic | triclinic | monoclinic |
| Space group/No. Int. Tab. | P21/a/14 | P-1/2 | P21/c/14 |
| a/Å | 10.775 (2) | 7.458 (5) | 16.695 (17) |
| b/Å | 8.291 (2) | 10.646 (8) | 5.238 (2) |
| c/Å | 15.753 (2) | 22.370 (10) | 22.312 (23) |
| α/° | 90.000 | 83.21 (5) | 90.000 |
| ß/° | 104.850 (5) | 86.70 (5) | 108.54 (5) |
| γ/° | 90.000 | 88.59 (4) | 90.000 |
| V/Å3 | 1360.3 (3) | 1760.5 (19) | 1850 (3) |
| Dcal/g·cm−3 | 1.305 | 1.296 | 1.283 |
| Z (asymmetric unit) | 4 | 2 | 4 |
| CuKα/MoKα/Å | 1.54184 | 0.71073 | 1.54184 |
| µ (CuKα/MoKα)/mm−1 | 0.745 | 0.094 | 0.758 |
| Number of reflections used for lattice parameter refinement | 25 | 22 | 25 |
| Scan range | 17° < θ < 48° | 6° < θ < 17° | 9° < θ < 25° |
| F (000) | 560 | 728 | 760 |
| Reflections collected | 1438 | 3620 | 1189 |
| Unique data | 1375 (Rint = 0.073) | 3280 (Rint = 0.071) | 1130 (Rint = 0.120) |
| Significant I’s (>2σ) | 1284 | 1370 | 1102 |
| Data collection | 2.9° ≤ θ ≤ 50.0° | 1.8° ≤ θ ≤ 20.0° | 2.7° ≤ θ ≤ 40.0° |
| Parameters refined/restraints | 234/0 | 455/0 | 241/0 |
| R1 | 0.041 | 0.069 | 0.063 |
| wR2 | 0.116 | 0.203 | 0.217 |
| Goodness-of- fit on F2 | 1.078 | 0.950 | 0.990 |
| Highest peak/e·Å−3 | 0.15 | 0.20 | 0.17 |
| Crystal color | opaque | opaque | opaque |
| Crystal shape | rod-like | platelet | rod-like |
| Hydrogen Bonds with H..A < r(A) + 2.000 Angstroms and <DHA > 110 deg | Twist (deg.) | ||||||
|---|---|---|---|---|---|---|---|
| D-H | d(D-H) d(H..A) <DHA d(D..A) A | Ph1-Ph2 | Ph1-NO2 | ||||
| NO2-Bi-3-S | |||||||
| O104-H014 | 0.914 | 1.757 | 171.73 | 2.665 | O205 [x + 2, y + 1, z] | 29.76(7) | 12.78(0.29) |
| O204-H024 | 0.861 | 1.774 | 173.68 | 2.632 | O105 [x − 2, y − 1, z] | 30.82(7) | 2.58(0.33) |
| NO2-Bi-5-S | |||||||
| O104-H104 | 0.820 | 1.797 | 164.29 | 2.596 | O105 [ x, −y + 2, −z + 2] | 34.05(0.31) | 11.23(1.02) |
| O204-H204 | 0.820 | 1.819 | 165.37 | 2.621 | O205 [−x + 1, −y + 1, −z − 1] | 38.41(0.34) | 10.42(1.04) |
| Cocrystalline NO2-Bi-1-S--DMAc | |||||||
| O5-H04 | 0.933 | 1.656 | 165.32 | 2.569 | O101 | 32.97(0.10) | 0.87(0.56) |
| Cocrystalline NO2-Bi-3-S--DMAc | |||||||
| O4-H04 | 0.820 | 1.831 | 161.08 | 2.621 | O101 [−x + 1, y + 3/2, −z + 1] | 14.91(0.17) | 7.36(0.64) |
| Cocrystalline NO2-Bi-5-S--DMAc | |||||||
| O4-H04 | 0.842 | 1.841 | 148.64 | 2.597 | O101 | 13.93(0.32) | 4.30(1.18) |
| NO2-Ph-1-S | |||||||
| O4-H04 | 1.341 | 1.303 | 169.38 | 2.632 | O5 [−x + 1, −y, −z + 1] | 4.83(0.19) | |
| O4-H04 | 1.341 | 2.583 | 113.98 | 3.359 | O4 [−x + 1, −y, −z + 1] | ||
| NO2-Ph-5-S | |||||||
| O104-H014 | 0.874 | 1.751 | 162.60 | 2.620 | O205 [x, y − 1, z] | 3.33(0.08) | |
| O204-H024 | 0.947 | 1.692 | 178.91 | 2.639 | O105 [x, y + 1, z] | 8.28(0.14) | |
| NO2-Ph-1 | 0.44(0.10) | ||||||
| CN-Bi-1-S-M | 27.54(0.10) | ||||||
| NO2-Bi-4-S-E | 35.94(0.18) | 7.98(0.75) | |||||
| 37.07(0.18) | 7.27(0.76) | ||||||
| NO2-Bi-5-S-E | 5.05(0.58) | 4.46(1.08) | |||||
| Compound | N-O1 | N-O2 | O4-C | O5-C | O101-C102 | N101-C102 |
|---|---|---|---|---|---|---|
| NO2-Bi-3-S | 1.222(2) | 1.219(2) | 1.298(2) | 1.225(2) | ||
| 1.232(2) | 1.228(2) | 1.289(2) | 1.227(2) | |||
| NO2-Bi-5-S | 1.217(1) | 1.222(1) | 1.321(1) | 1.245(1) | ||
| 1.218(1) | 1.233(1) | 1.302(1) | 1.215(1) | |||
| NO2-Bi-1-S—DMAc | 1.206(3) | 1.205(3) | 1.317(3) | 1.200(3) | 1.245(3) | 1.330(3) |
| NO2-Bi-3-S—DMAc | 1.213(4) | 1.219(4) | 1.310(4) | 1.195(4) | 1.245(4) | 1.321(5) |
| NO2-Bi-5-S—DMAc | 1.203(5) | 1.184(5) | 1.323(6) | 1.192(6) | 1.270(7) | 1.308(6) |
| NO2-Ph-1-S | 1.215(3) | 1.221(3) | 1.261(3) | 1.245(3) | - | |
| NO2-Ph-5-S | 1.225(4) | 1.215(4) | 1.305(4) | 1.207(4) | - | |
| 1.212(4) | 1.203(4) | 1.298(4) | 1.216(4) | - | ||
| NO2-Ph-1 | 1.215(2) | 1.224(2) | - | |||
| CN-Bi-1-S-M | - | - | 1.329(3) | 1.183(3) | - | |
| NO2-Bi-4-S-E | 1.218(8) | 1.219(8) | 1.330(9) | 1.206(9) | - | |
| 1.224(8) | 1.218(8) | 1.312(9) | 1.189(9) | - | ||
| NO2-Bi-5-S-E | 1.222(11) | 1.211(11) | 1.329(13) | 1.172(12) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zugenmaier, P. Comparison of Selected Crystal Structures of Rod-like Molecules with Acid and Ester Terminal Groups. Crystals 2025, 15, 102. https://doi.org/10.3390/cryst15020102
Zugenmaier P. Comparison of Selected Crystal Structures of Rod-like Molecules with Acid and Ester Terminal Groups. Crystals. 2025; 15(2):102. https://doi.org/10.3390/cryst15020102
Chicago/Turabian StyleZugenmaier, Peter. 2025. "Comparison of Selected Crystal Structures of Rod-like Molecules with Acid and Ester Terminal Groups" Crystals 15, no. 2: 102. https://doi.org/10.3390/cryst15020102
APA StyleZugenmaier, P. (2025). Comparison of Selected Crystal Structures of Rod-like Molecules with Acid and Ester Terminal Groups. Crystals, 15(2), 102. https://doi.org/10.3390/cryst15020102





