Hydrogen Storage Capacity and Optoelectronic Response of Mechanically and Thermally Stable Lithium-Based Tetrahydrates (LiXH4, X = B, Al, Mn), a DFT Approach
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Structural Properties
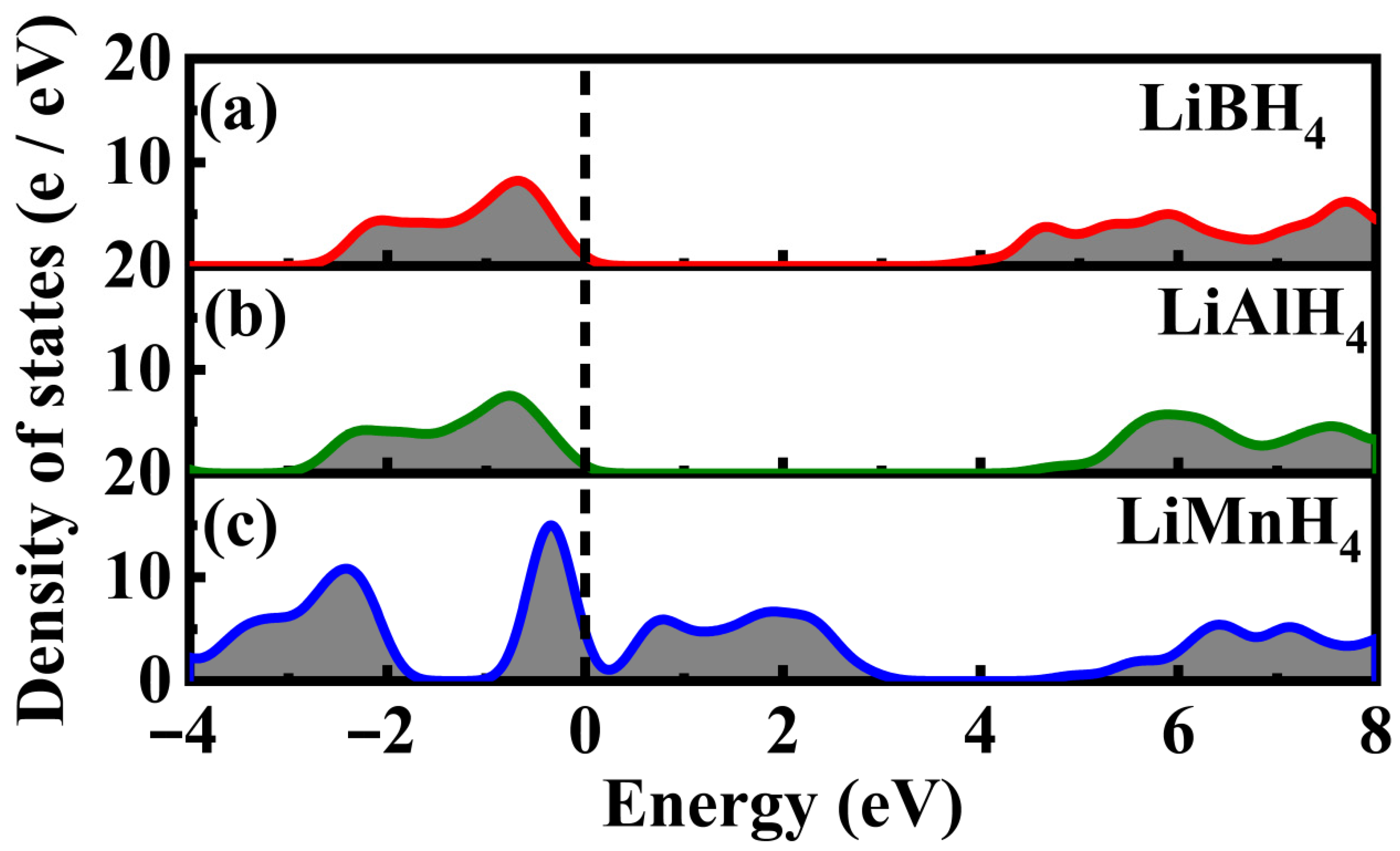
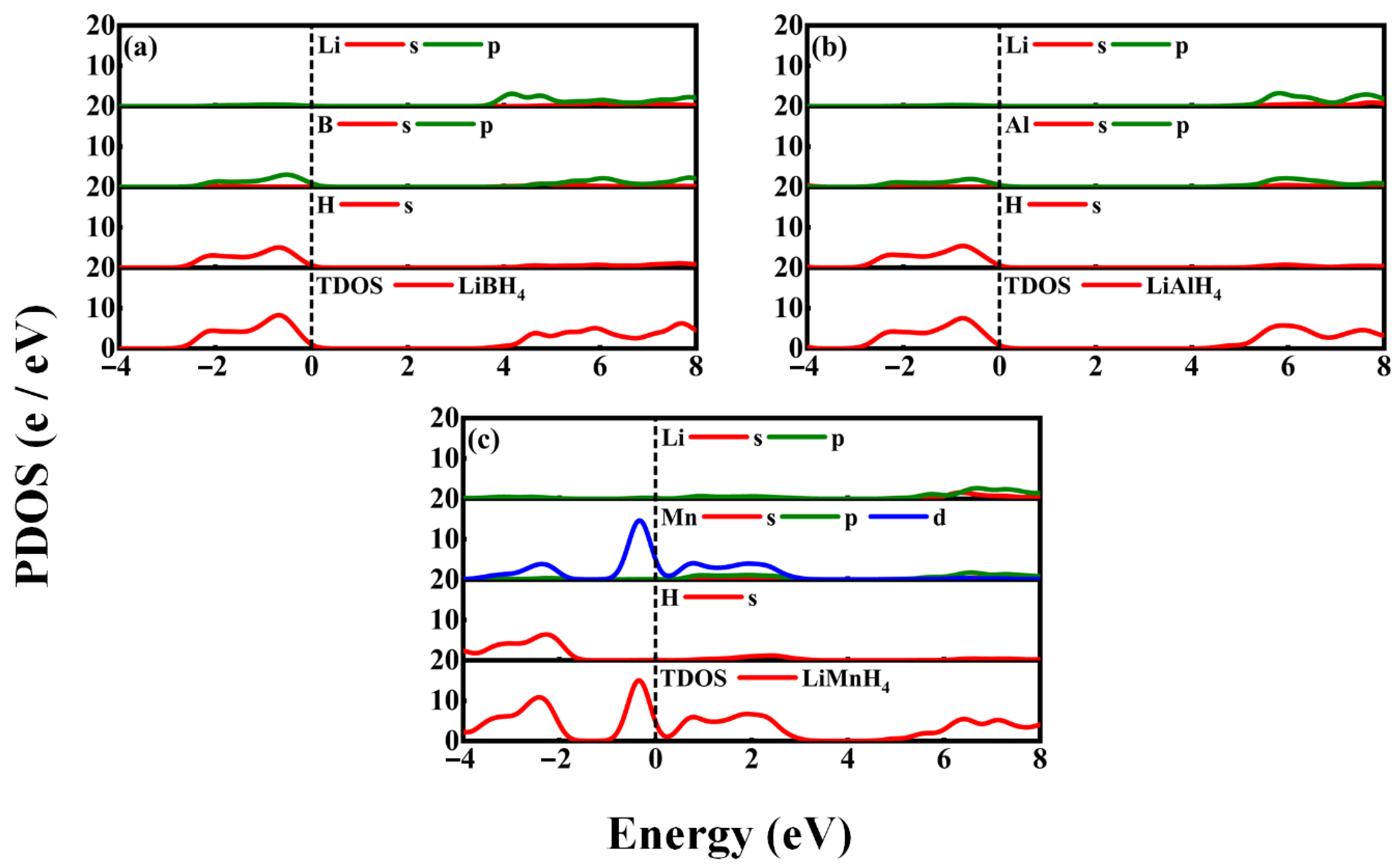
3.2. Electronic Properties
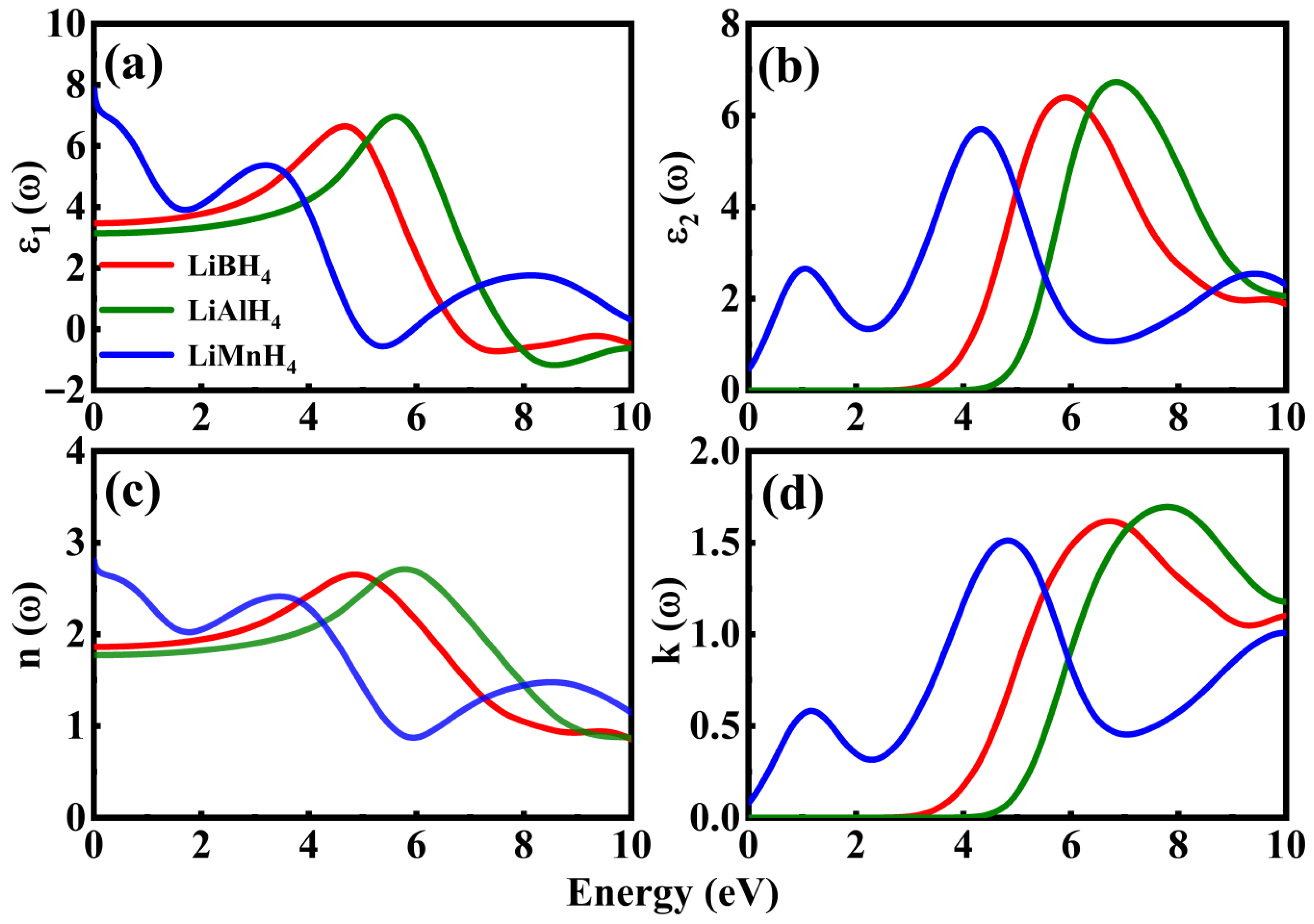
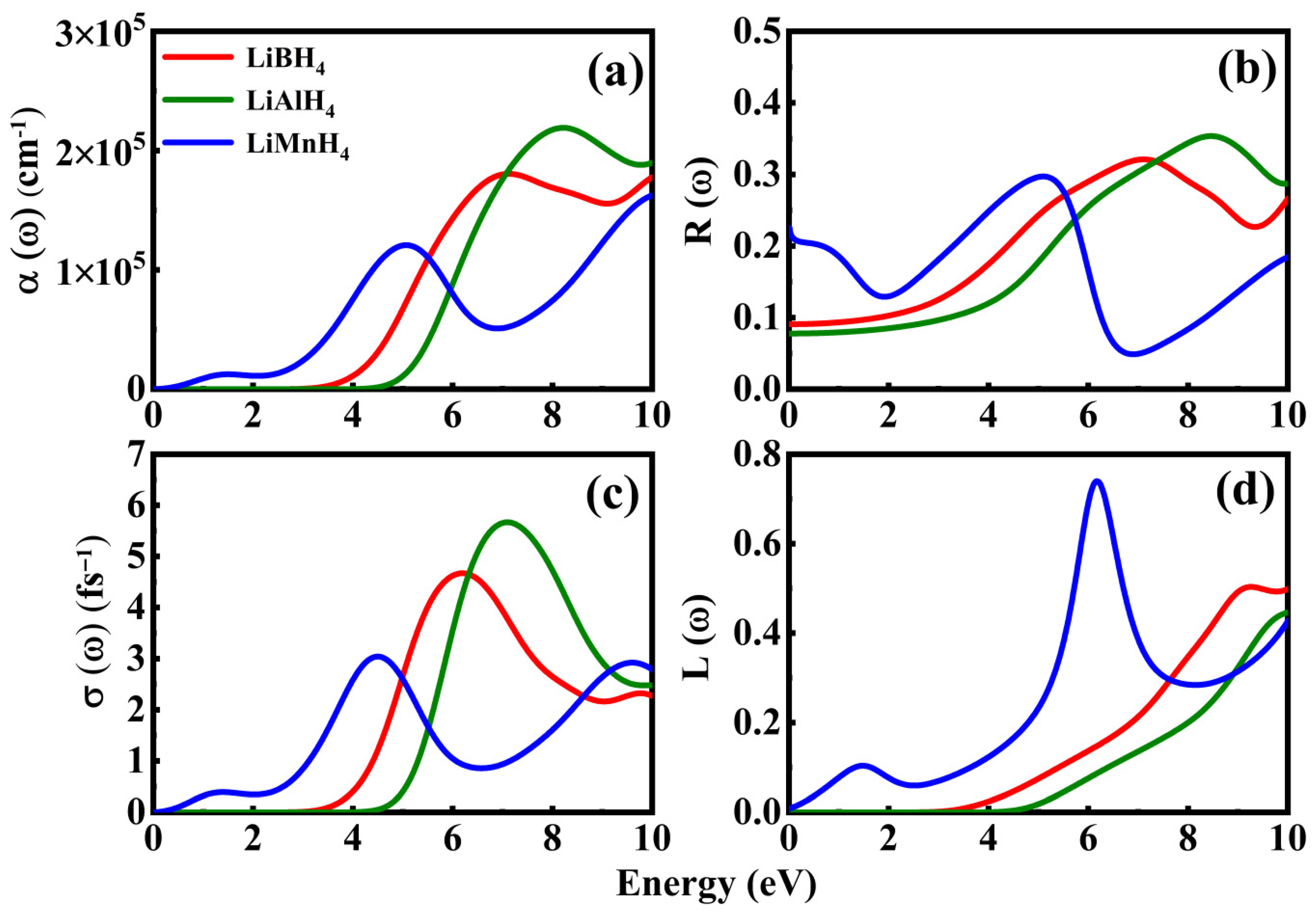
3.3. Optical Properties
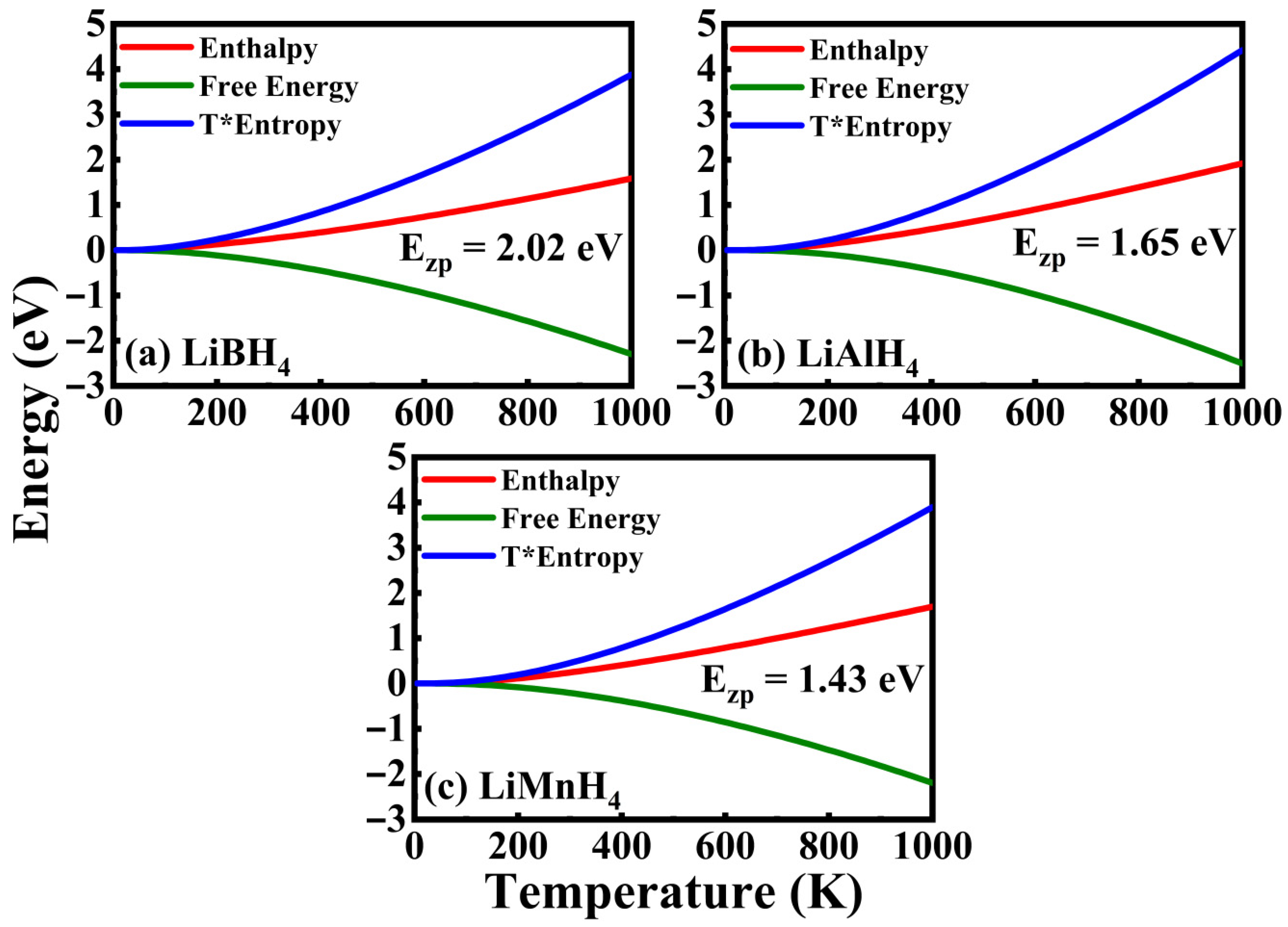
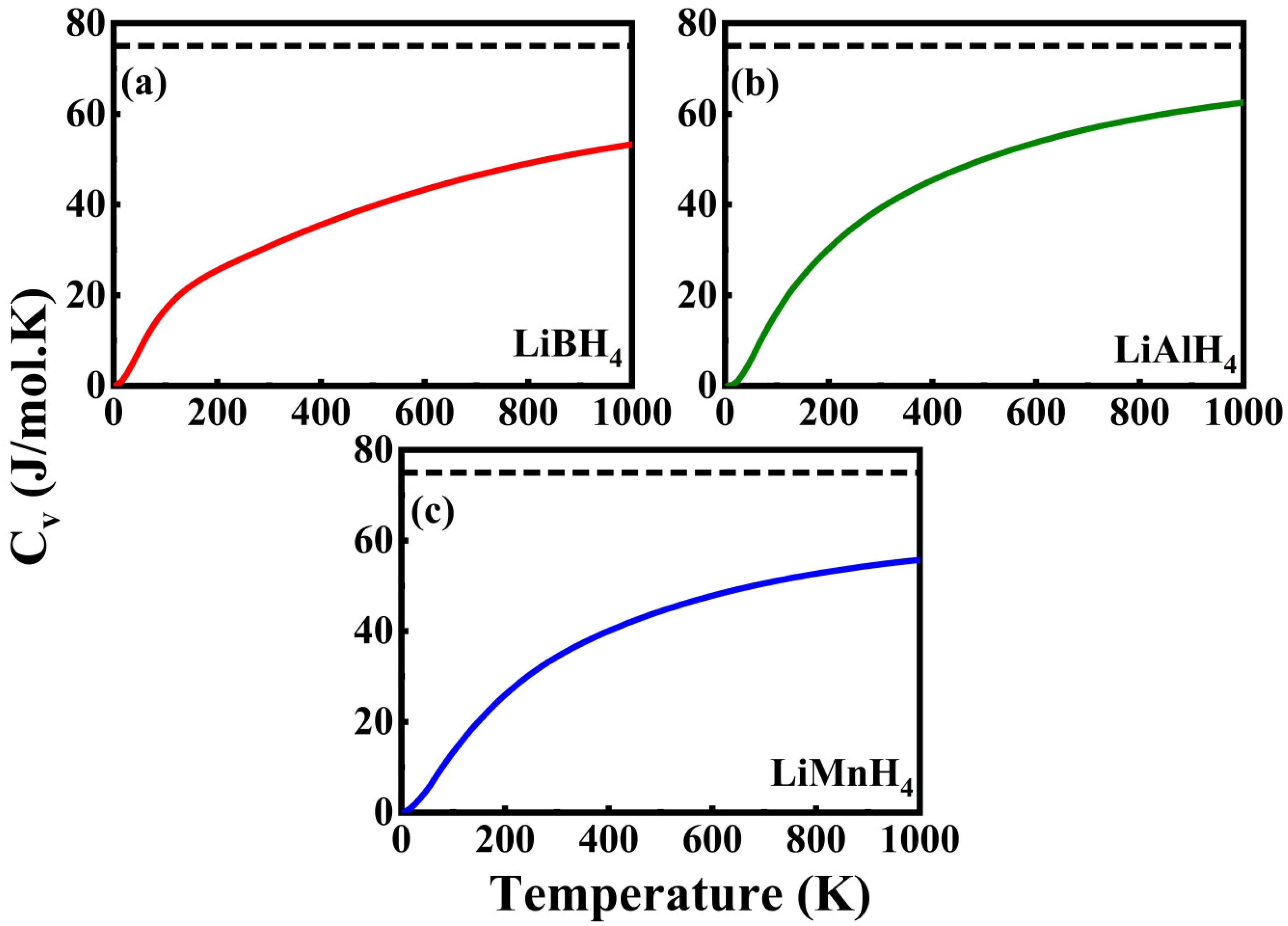
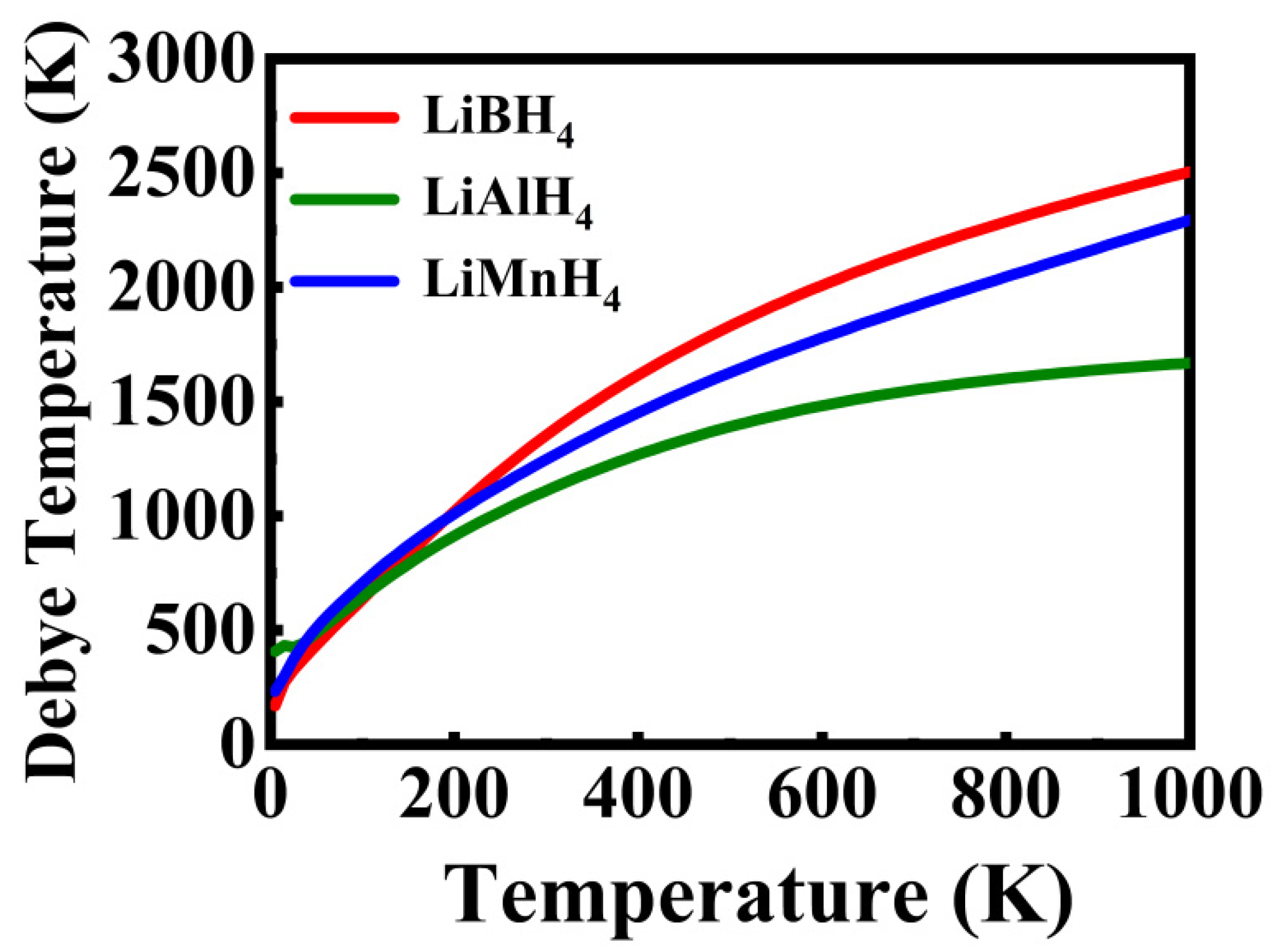
3.4. Thermodynamics Properties
3.5. Mechanical Properties
3.6. Hydrogen Storage Capacities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Shetwi, A.Q. Sustainable development of renewable energy integrated power sector: Trends, environmental impacts, and recent challenges. Sci. Total Environ. 2022, 822, 153645. [Google Scholar] [CrossRef]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Inumaru, J.; Hasegawa, T.; Shirai, H.; Nishida, H.; Noda, N.; Ohyama, S. Fossil fuels combustion and environmental issues. In Advances in Power Boilers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–56. [Google Scholar]
- Sarfraz, M. Global Warming Cause and Impact on Climate Change. Int. J. Emerg. Knowl. Stud 2024, 3, 198–204. [Google Scholar]
- Freedman, B.; Dorsey, W.; Frazier, A.; Kambhampati, M.; Galiotos, J.; Mukherjee, S. Renewable and Non-renewable Energy Sources. Environ. Sci. 2024. [Google Scholar]
- Rampai, M.M.; Mtshali, C.B.; Seroka, N.S.; Khotseng, L. Hydrogen production, storage, and transportation: Recent advances. RSC Adv. 2024, 14, 6699–6718. [Google Scholar] [CrossRef]
- Rolo, I.; Costa, V.A.; Brito, F.P. Hydrogen-based energy systems: Current technology development status, opportunities and challenges. Energies 2023, 17, 180. [Google Scholar] [CrossRef]
- Gupta, P.; Toksha, B.; Rahaman, M. A critical review on hydrogen based fuel cell technology and applications. Chem. Rec. 2024, 24, e202300295. [Google Scholar] [CrossRef]
- Qasem, N.A.; Abdulrahman, G.A. A recent comprehensive review of fuel cells: History, types, and applications. Int. J. Energy Res. 2024, 2024, 7271748. [Google Scholar] [CrossRef]
- Rehman, M.A.; Rehman, Z.U.; Usman, M.; Alomar, S.Y.; Khan, M.J.; Fatima, J. Exploring the hydrogen storage in novel perovskite hydrides: A DFT study. Int. J. Hydrogen Energy 2024, 84, 447–456. [Google Scholar] [CrossRef]
- Deen, S.M.; Usman, M.; Rehman, J.U.; Ali, S.M.; Ali, M. A DFT study for physical properties and hydrogen storage capability of indium-based hydride perovskites XInH3 (X = Li, K) for hydrogen storage application. Solid State Commun. 2024, 384, 115488. [Google Scholar] [CrossRef]
- Hu, J.; Shen, H.; Jiang, M.; Gong, H.; Xiao, H.; Liu, Z.; Sun, G.; Zu, X. A DFT study of hydrogen storage in high-entropy alloy TiZrHfScMo. Nanomaterials 2019, 9, 461. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.; Probert, M.A.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M.J.P.R.L. Perdew, burke, and ernzerhof reply. Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Nawi, N.M.; Ransing, M.R.; Ransing, R.S. An improved learning algorithm based on the Broyden-Fletcher-Goldfarb-Shanno (BFGS) method for back propagation neural networks. In Sixth International Conference on Intelligent Systems Design and Applications; IEEE: Jian, China, 2006; Volume 1, pp. 152–157. [Google Scholar]
- MacDonald, A.H.; Picket, W.E.; Koelling, D.D. A linearised relativistic augmented-plane-wave method utilising approximate pure spin basis functions. J. Phys. C Solid State Phys. 1980, 13, 2675. [Google Scholar] [CrossRef]
- Jabeen, N.; Zafar, S.; Hussain, A.; Abdullah, A.I.; Kumar, A.; Ahmad, H. A DFT Analysis on the Electronic, Mechanical, Optical, Elastic, Structural and Thermodynamic Properties of XBiNb2O7 (X= Cs, Rb, K and Na) for Photovoltaic Applications. Comput. Condens. Matter 2025, 44, e01063. [Google Scholar] [CrossRef]
- Pallikara, I.; Kayastha, P.; Skelton, J.M.; Whalley, L.D. The physical significance of imaginary phonon modes in crystals. Electron. Struct. 2022, 4, 033002. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Kainat, F.; Jabeen, N.; Yaqoob, A.; Hassan, N.U.; Hussain, A.; Khalifa, M.E. Effect of Ca, Ba, Be, Mg, and Sr substitution on electronic and optical properties of XNb2Bi2O9 for energy conversion application using generalized gradient approximation–Perdew–Burke–Ernzerhof. Crystals 2024, 14, 710. [Google Scholar] [CrossRef]
- Griffiths, D.J. Introduction to Electrodynamics; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar]
- Hussain, A.; Jabeen, N.; Yaqoob, A.; Zafar, S.; Khan, M.U.; Ayob, E.A.; Khalifa, M.E. First-principles investigation of diverse properties of X2CaTa2O7 (X= Li, Na, K, and Rb) Ruddlesden–Popper compounds for photovoltaic applications. Crystals 2025, 15, 228. [Google Scholar] [CrossRef]
- Moore, E.A.; Smart, L.E. Optical properties of solids. In Solid State Chemistry; CRC Press: Boca Raton, FL, USA, 2020; pp. 283–314. [Google Scholar]
- Jabeen, N.; Hussain, A.; Hamza, A.; Musa, O.; Beemkumar, N.; Nanda, J. Multifunctional analysis of CaX2Bi2O9 (X= Nb, V, Ta, and Pa) compound by first principles framework for photovoltaic applications. AIP Adv. 2025, 15, 055015. [Google Scholar] [CrossRef]
- Yaqoob, A.; Jabeen, N.; Hamza, A.; Haider, I.; Kainat, F.; Hussain, A. A Theoretical Study for Investigation of Structural, Optical, Electronic, and Mechanical Properties of Double Perovskites Halide for Solar Cell Application. Conclus. Eng. 2025, 1, 72–81. [Google Scholar] [CrossRef]
- Moreira, E.; Barboza, C.A.; Albuquerque, E.L.; Fulco, U.L.; Henriques, J.M.; Araújo, A.I. Vibrational and thermodynamic properties of orthorhombic CaSnO3 from DFT and DFPT calculations. J. Phys. Chem. Solids 2015, 77, 85–91. [Google Scholar] [CrossRef]
- Günther, E.; Hiebler, S.; Mehling, H.; Redlich, R. Enthalpy of phase change materials as a function of temperature: Required accuracy and suitable measurement methods. Int. J. Thermophys. 2009, 30, 1257–1269. [Google Scholar] [CrossRef]
- Hussain, A.; Kainat, F.; Jabeen, N.; Yaqoob, A.; Abbas, T.; Khan, M.U.; Qaiser, M.A.; Mahmoud, M.H.H. First-principles calculations of the structural, mechanical, optical, and electronic properties of X2Bi4Ti5O18 (X = Pb, Ba, Ca, and Sr) bismuth-layered materials for photovoltaic applications. Crystals 2024, 14, 870. [Google Scholar] [CrossRef]
- Born, M.; Huang, K. Dynamical Theory of Crystal Lattices; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Ding, Y. Theoretical study on stability, mechanical properties and thermodynamic parameters of the orthorhombic-A2N2O (A=C, Si and Ge). Phys. B Condens. Matter 2012, 407, 2190–2200. [Google Scholar] [CrossRef]
- Hussain, A.; Kainat, F.; Hamza, A.; Naz, A.; Jabeen, N.; Munawar, T.; Qaiser, M.A. A DFT study on the structural, electronic, optical, and elastic properties of BLSFs XTi4Bi4O15 (X = Sr, Ba, Be, Mg) for solar energy applications. Ceramics 2024, 7, 1727–1741. [Google Scholar] [CrossRef]
- Cao, J.; Li, F. Critical Poisson’s ratio between toughness and brittleness. Philos. Mag. Lett. 2016, 96, 425–431. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Yang, X.; Zhang, J. Review on hydrogen storage performance of MgH2: Development and trends. Chem. Sel. 2021, 6, 1589–1606. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Mao, J.F.; Dou, S.X. The hydrogen storage properties and reaction mechanism of the MgH2–NaAlH4 composite system. Int. J. Hydrogen Energy 2011, 36, 9045–9050. [Google Scholar] [CrossRef]
- Yaqoob, A.; Hussain, A.; Jabeen, N.; Fallatah, A.M. DFT Study of Physical Properties for A2H3X (A = Sr, Ba; X = Cl, Br) Compounds: Their Possible Applications for Hydrogen Storage and Optoelectronic Devices. Chem. Sel. 2025, 10, e03422. [Google Scholar] [CrossRef]
- Masood, M.K.; Khan, W.; Bibi, S.; Khan, N.; Pingak, R.K.; Tahir, K.; Rehman, J.; Bahajjaj, A.A.A. The structural, elastic, optoelectronic properties and hydrogen storage capability of lead-free hydrides XZrH3 (X: Mg/Ca/Sr/Ba) for hydrogen storage application: A DFT study. Comput. Theor. Chem. 2024, 1242, 114941. [Google Scholar] [CrossRef]
- Pela, R.R.; Hsiao, C.L.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Electronic and optical properties of core–shell InAlN nanorods: A comparative study via LDA, LDA-1/2, mBJ, HSE06, G 0 W 0 and BSE methods. Phys. Chem. Chem. Phys. 2024, 26, 7504–7514. [Google Scholar] [CrossRef] [PubMed]

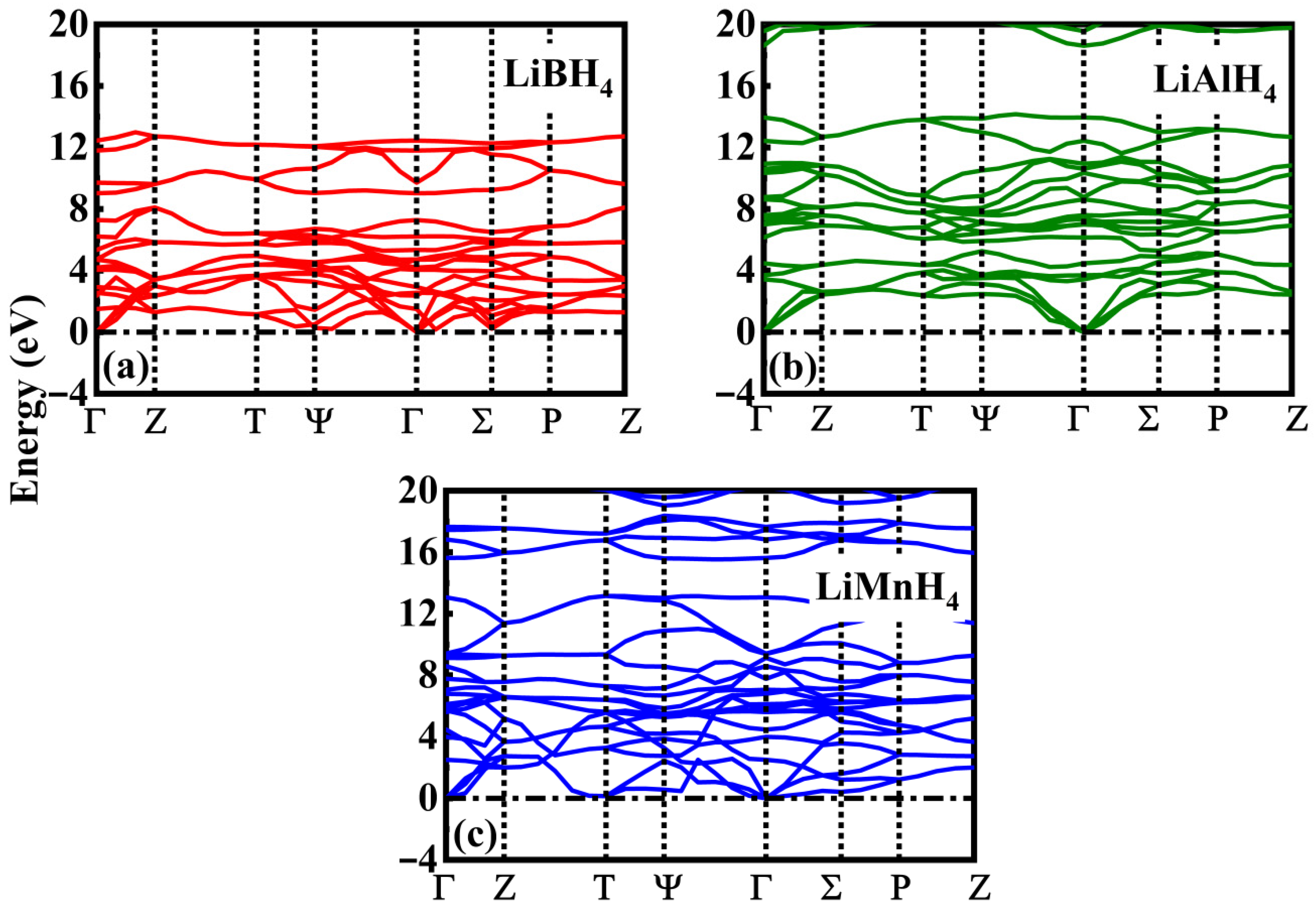
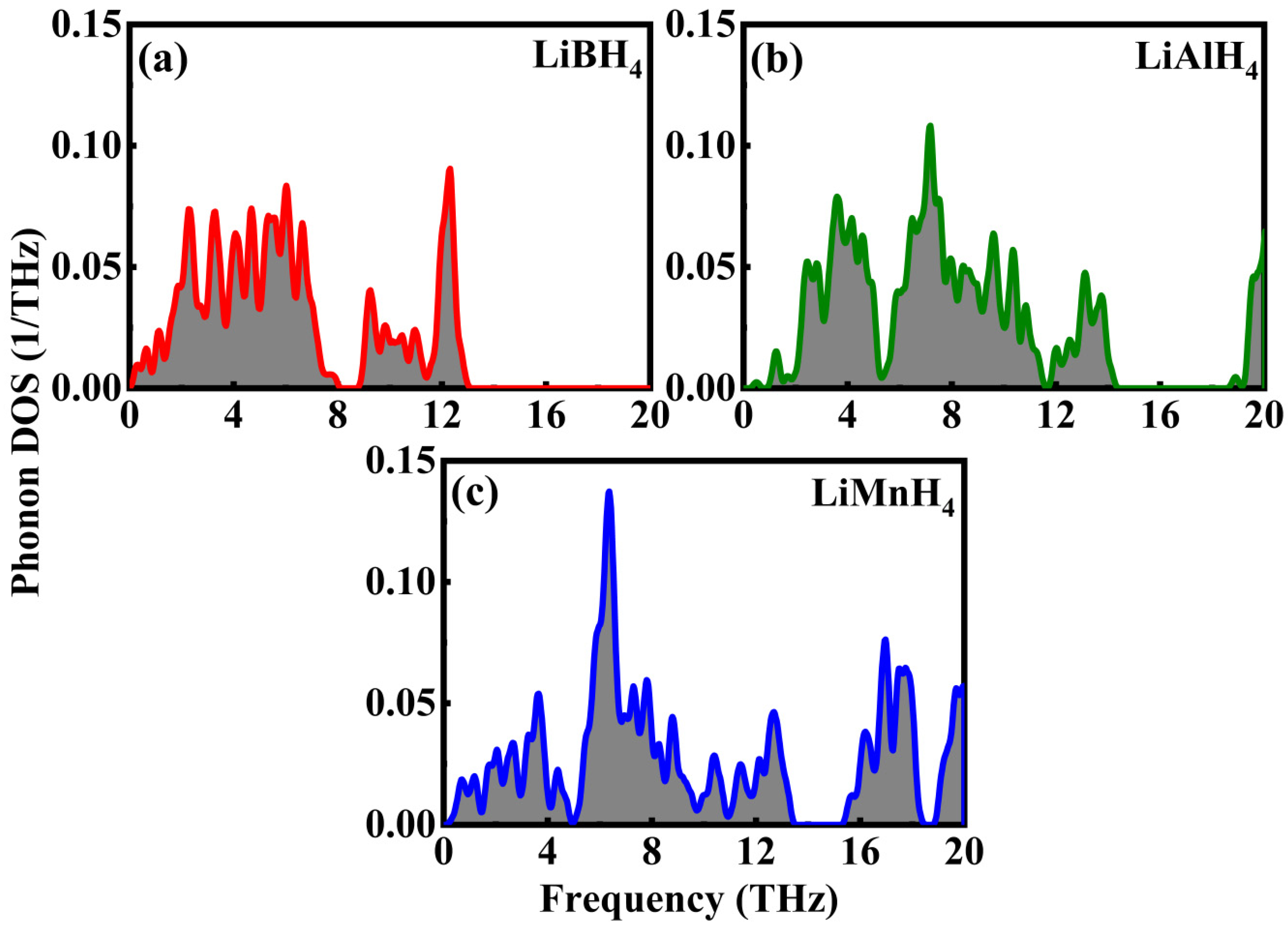
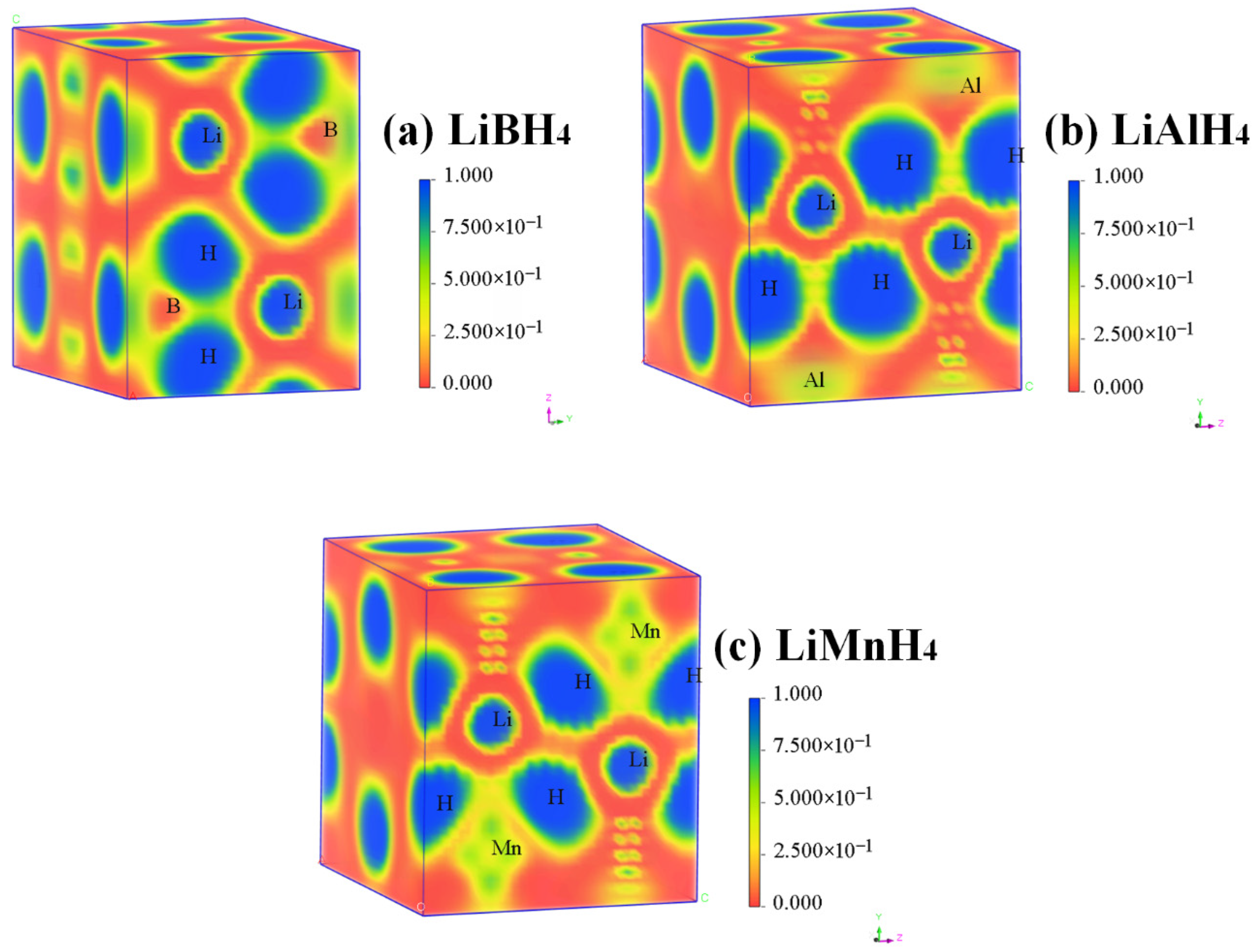
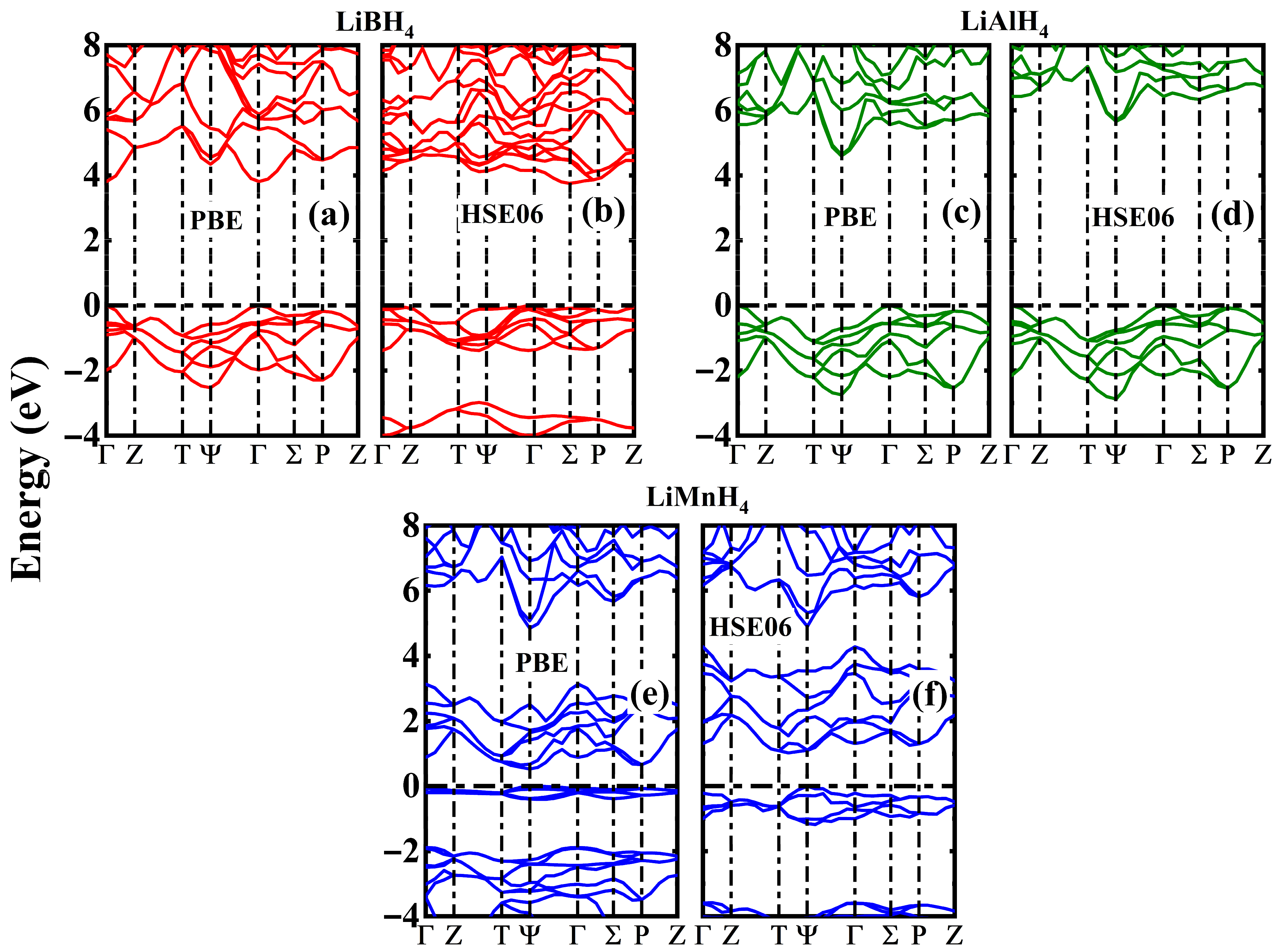
| Compound | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Volume (Å3) |
|---|---|---|---|---|---|---|---|
| LiBH4 | 6.3641 | 4.8092 | 6.6053 | 90 | 90 | 90 | 202.16 |
| LiAlH4 | 6.4707 | 7.2182 | 6.0795 | 90 | 90 | 90 | 283.95 |
| LiMnH4 | 6.5500 | 7.4000 | 6.2000 | 90 | 90 | 90 | 300.51 |
| Column1 | Species | Ion | s | p | d | f | Total | Mulliken Charge (e) | Hirshfeld Charge (e) |
|---|---|---|---|---|---|---|---|---|---|
| LiBH4 | H | 1–16 | 1.22 | 0 | 0 | 0 | 1.22 | −0.22 | −0.05 |
| Li | 1–4 | 1.71 | −0.24 | 0 | 0 | 1.47 | 1.53 | 0.25 | |
| B | 1–4 | 0.93 | 2.72 | 0 | 0 | 3.65 | −0.65 | −0.07 | |
| LiAlH4 | H | 1–4 | 1.49 | 0 | 0 | 0 | 1.49 | −0.49 | −0.13 |
| H | 5–8 | 1.46 | 0 | 0 | 0 | 1.46 | −0.46 | −0.13 | |
| H | 9–12 | 1.49 | 0 | 0 | 0 | 1.49 | −0.49 | −0.13 | |
| H | 13–16 | 1.46 | 0 | 0 | 0 | 1.46 | −0.46 | −0.13 | |
| Li | 1–4 | 1.8 | 0.01 | 0 | 0 | 1.81 | 1.19 | 0.17 | |
| Al | 1–4 | 0.89 | 1.39 | 0 | 0 | 2.27 | 0.73 | 0.34 | |
| LiMnH4 | H | 1–4 | 1.46 | 0 | 0 | 0 | 1.46 | −0.46 | −0.11 |
| H | 5–8 | 1.45 | 0 | 0 | 0 | 1.45 | −0.45 | −0.1 | |
| H | 9–12 | 1.46 | 0 | 0 | 0 | 1.46 | −0.46 | −0.11 | |
| H | 13–16 | 1.45 | 0 | 0 | 0 | 1.45 | −0.45 | −0.1 | |
| Li | 1–4 | 1.97 | 0.46 | 0 | 0 | 2.43 | 0.57 | 0.17 | |
| Mn | 1–4 | 2 | 6 | 5.76 | 0 | 13.75 | 1.25 | 0.21 |
| Property/Condition | LiBH4 | LiAlH4 | LiMnH4 |
|---|---|---|---|
| C11 | 292.5032 | 112.8912 | 10.3946 |
| C22 | 275.7758 | 78.2088 | 11.5606 |
| C33 | 305.938 | 82.1848 | 11.9533 |
| C12 | 185.387 | 77.8179 | 7.4317 |
| C13 | 189.5727 | 89.475 | 6.9144 |
| C23 | 212.5106 | 75.8357 | 7.1179 |
| C44 | 59.8856 | 15.811 | 0.5015 |
| C55 | 62.2655 | 14.0753 | 1.4352 |
| C66 | 5.5946 | 8.0513 | 0.4317 |
| C11 + C22 − 2C12 | 197.505 | 35.4642 | 7.0918 |
| C11 + C33 − 2C13 | 219.2958 | 16.126 | 8.5191 |
| C22 + C33 − 2C23 | 156.6926 | 8.7222 | 9.2781 |
| C11 + C22 + C33 + 2C12 + 2C13 + 2C23 | 2049.158 | 759.542 | 76.8365 |
| P1 = C12 − C66 | 179.7924 | 69.7666 | 7 |
| P2 = C13 − C55 | 127.3072 | 75.3997 | 8.3496 |
| P3 = C23 − C44 | 152.625 | 60.0247 | 6.6164 |
| Property | LiBH4 | LiAlH4 | LiMnH4 |
|---|---|---|---|
| Bulk modulus B (GPa) | 225.855 | 90.483 | 8.357 |
| Shear modulus G (GPa) | 17.136 | 0.682 | 1.180 |
| Young’s modulus E (GPa) | 50.140 | 2.040 | 3.381 |
| Poisson’s ratio (ν) | 0.4630 | 0.4962 | 0.4326 |
| Compound | H wt % (Gravimetric Ratio) |
|---|---|
| LiBH4 | 18.5% |
| LiAlH4 | 10.6% |
| LiMnH4 | 6.1% |
| Material | Storage Capacity (wt%) | Band Gap (eV) | Mechanism | Reference |
|---|---|---|---|---|
| LiBH4 | 18.5 | 3.91 | Complex hydride | Present study |
| LiAlH4 | 10.6 | 5.66 | Complex hydride | Present study |
| LiMnH4 | 6.1 | 1.02 | Complex hydride | Present study |
| MgH2 | 7.6 | ~5.6 | Hydride | [36] |
| NaAlH4 | 5.5 | ~4.6 | Complex hydride | [37] |
| Sr2H3Cl | 1.41 | 2.97 | Complex hydride | [38] |
| Sr2H3Br | 1.17 | 2.26 | Complex hydride | [38] |
| MgZrH3 | 2.55 | Metallic | Perovskite hydride | [39] |
| CaZrH3 | 2.22 | Metallic | Perovskite hydride | [39] |
| InAlN core–shell nanorods | – | ~2.3 | Semiconductor nanorod | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Jabeen, N.; Yaqoob, A.; Smerat, A.; Qaiser, M.A.; Aldawsari, N.A. Hydrogen Storage Capacity and Optoelectronic Response of Mechanically and Thermally Stable Lithium-Based Tetrahydrates (LiXH4, X = B, Al, Mn), a DFT Approach. Crystals 2025, 15, 990. https://doi.org/10.3390/cryst15110990
Hussain A, Jabeen N, Yaqoob A, Smerat A, Qaiser MA, Aldawsari NA. Hydrogen Storage Capacity and Optoelectronic Response of Mechanically and Thermally Stable Lithium-Based Tetrahydrates (LiXH4, X = B, Al, Mn), a DFT Approach. Crystals. 2025; 15(11):990. https://doi.org/10.3390/cryst15110990
Chicago/Turabian StyleHussain, Ahmad, Nawishta Jabeen, Ali Yaqoob, Aseel Smerat, Muhammad Adnan Qaiser, and Naflaa A. Aldawsari. 2025. "Hydrogen Storage Capacity and Optoelectronic Response of Mechanically and Thermally Stable Lithium-Based Tetrahydrates (LiXH4, X = B, Al, Mn), a DFT Approach" Crystals 15, no. 11: 990. https://doi.org/10.3390/cryst15110990
APA StyleHussain, A., Jabeen, N., Yaqoob, A., Smerat, A., Qaiser, M. A., & Aldawsari, N. A. (2025). Hydrogen Storage Capacity and Optoelectronic Response of Mechanically and Thermally Stable Lithium-Based Tetrahydrates (LiXH4, X = B, Al, Mn), a DFT Approach. Crystals, 15(11), 990. https://doi.org/10.3390/cryst15110990









