Abstract
In the present study, we have developed a series of compounds [M(tcm)2(bix)4]n [where M = Co (1), Ni (2), and Cu (3)] using tricyanomethanide (tcm) and 1,4-bis(imidazol-1-ylmethyl)benzene (bix) ligands. The compounds were characterized by elemental analysis, PXRD, FT-IR and single-crystal X-ray crystallography. Single-crystal X-ray investigation of compounds 1, 2, and 3 shows the formation of the porous 2D structure. These 2D structures are further stacked to create a 3D network in the crystallographic space. All the compounds are thermally stable up to 300 °C, as revealed by the TGA. Hirshfeld surface analysis was carried out, and it reveals the existence of short intermolecular interactions between the layers.
1. Introduction
Recent advancements in metal–organic frameworks (MOFs) have brought porous layered coordination polymers (CPs) to the forefront, captivating researchers due to their modular nature and highly tunable structures [,,]. These remarkable features unlocked an impressive array of properties and applications, such as efficient gas and guest sorption, effective gas storage and separation, cutting-edge catalysis, luminescence, sensing capabilities, magnetism, and innovative energy storage and conversion solutions. Among the diverse spectrum of MOFs, two-dimensional (2D) compounds—known as 2D CPs or 2D MOFs—stand out for their extraordinary potential []. Since the ground-breaking discovery of graphene in 2004, the exploration of 2D materials has surged dramatically [,]. Many 2D MOFs can be exfoliated into ultrathin nanosheets that closely resemble graphene and other 2D materials, making these layered structures not just useful but essential for a wide range of technological applications []. Moreover, their intricate topological networks and unique entanglements add a captivating dimension to their functionality. A comprehensive overview of the multifaceted characteristics of 2D MOF layered architectures, focusing on critical aspects such as topology, interpenetration, structural transformations, properties, and their promising applications, was conducted [,].
This rapid growth is evident in the increasing number of structures, publications, and citations, as well as the continuous broadening of the research scope and engagement among researchers. The remarkable rise in MOF research can be attributed to five key developments: advancements in cluster chemistry; advancements in organic synthesis related to ligand preparation and post-synthetic modification; enhancements in structure determination, especially through X-ray crystallography, alongside improvements in the hardware and software used for assessing sorption properties; interdisciplinary expansion of MOF research with its associated fields; and the continually growing potential for various applications [,,,]. Overall, 2D metal–organic frameworks (MOFs) can be shaped into various shapes either in situ (direct approach) or through a post-synthesis method (indirect approach). In the first scenario, as the crystalline compound, the crystallites are promptly oriented into the required configurations, such as thin films or hollow superstructures. Then, for the second scenario, molding is performed as a second step on the already produced crystalline product. The selection of a shaping technique is influenced by the specific application, which dictates the preferred shape of the material (e.g., thin films for membranes and micronic spherical-shaped particles for fluidized beds), as well as by the stability and functionality of the MOF structure [,,,]. On the other hand, it is worth noting that for the development of coordination compounds, tricyanomethanide (tcm) played a pivotal role. Tcm shows interesting bridging modes, producing divergent multifunctional coordination polymers (Scheme 1). It is worth mentioning that till date, very limited structures with tcm and bridging co-ligands have been reported in the literature [,]. Few rutile-type structures have been reported with the formula M(tcm)2 (M = Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd, and Hg). The larger size of the bridging tcm ligand produced spacious network structures, but it can also form very tightly packed structures []. Kohout et al. collated a series of coordination compounds of Co(II), Ni(II), and Cu(II) containing tcm and co-ligands. In a feature article [], Prof. Batten and co-workers explored the chemistry and complexes of small cyano complexes []. In another study Julve and co-workers documented Cu(II)-tcm complexes along with their magnetic properties []. In a recent study, Rogers et al. explored the lanthanide–tcm coordination polymers using ionic liquids []. In the present study, we have developed a series of compounds [M(tcm)2(bix)4]n (where M = Co, Ni, and Cu) with an M-(tcm)2 moiety bridged by the 1,4-bis(imidazol-1-ylmethyl)benzene (bix) ligand. The bix ligand is one of the most interesting pillaring ligands that can adopt two kinds of bridging modes, the cis- and trans modes (Scheme 2). In the present compounds, it adopted the trans- configuration. Yao and co-workers reported the formation of six different coordination polymers with a v-shaped catboxylate ligand and different imidazolyl moieties []. In another study, Qin et al. reported three polymers with a bnn topology when they used a triazole-based carboxylate ligand with bis-imidazolyl co-linkers []. The structure presented in this paper clearly reveals that it adopts a 2D porous structure that is propagated in the crystallographic bc plane. Hirshfeld surface analysis was performed to evaluate the inter-layer interaction present in the structure.
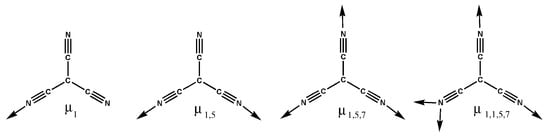
Scheme 1.
Possible coordination modes of the tricyanomethanide ligand (negative charges on central carbon atoms are not appended for clarity).
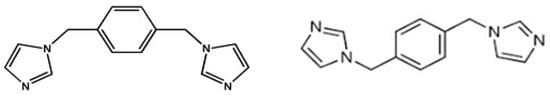
Scheme 2.
Possible coordination modes (cis form trans-) of the bix ligand.
2. Materials and Methods
2.1. Synthesis of Compounds 1, 2, and 3
Cobalt nitrate hexahydrate, nickel nitrate hexahydrate, and copper nitrate tetrahydrate were purchased from Avra Synthesis Pvt. Ltd. (Hyderabad, India), while 1,4-bis(imidazol-1-ylmethyl)benzene (bix) and sodium tricayanomethanide were procured from TCI Pvt. Ltd. (Tokyo, Japan). Solvents were purchased from commercial sources and used for synthesis as received. All chemicals were used without further purification.
All the compounds were synthesized by following the same procedures. For the synthesis process, we followed a layer diffusion technique in a crystal tube. For the synthesis of compound 1, in a 50 mL beaker, 0.5 mM of Co(NO3)2 was dissolved in 10 mL of distilled water, and in another beaker, 1,4-Bis-[(1H-imidazol-1-yl)methyl] benzene (0.5 mM) and sodium tricyanomethanide (0.5 mM) were taken and dissolved in 10 mL methanol and water solution in 1:1 ratio. A buffer solution was prepared in another beaker by mixing water and methanol in a 1:1 ratio (20 mL).
Initially, the metal solution was gently poured into the bottom of the crystal tube; then a layer of the buffer was carefully poured (approx. 5 cm in height) above the metal solution. Finally, the solution containing the ligands was carefully layered above the buffer solution.
The crystal tube was then sealed and kept undisturbed for 30 to 40 days. After one month, pink-colored single crystals grew in the middle of tube. The crystals were collected and washed with ethanol. The yield of compound 1 was 23% based on the bridging ligand. For compounds 2 and 3, Co(NO3)2 was replaced with Ni(NO3)2 and Cu(NO3)2, respectively. Blue-colored needle-shaped crystals grew with a yield of 25% (compound 2), and blue-colored needle-shaped crystals grew with a yield of 28% (compound 3). Elemental analysis was performed, and the results are collated in Table 1.

Table 1.
Elemental analyses of compounds 1, 2, and 3.
2.2. Characterization of Compounds 1, 2, and 3
To evaluate the structure of the compounds, various techniques were used. Elemental analysis, infrared spectroscopy (FT-IR) (Perkin–Elmer RX I FT-IR spectrometer, Shelton, CT, USA), powder X-ray diffraction (PANalytical Empyrean diffractometer equipped with an X’Celerator detector, Almelo, Netherland), and single-crystal X-ray diffraction (Bruker SMART APEX CCD X-ray diffractometer, Madison, WI, USA) were employed for these purposes.
2.2.1. Elemental Analyses of Compounds 1, 2, and 3
Elemental analyses of these compounds were performed to establish the formula of the compounds. CHN analyses were conducted with a Thermo Scientific Flash 2000 instrument (Thermo Scientific, Waltham, MA, USA). The results given in Table 1 clearly indicate the phase purity of the compounds.
2.2.2. FT-IR Analysis of Compounds 1, 2, and 3
The Fourier-transformed infrared (FT-IR) spectra of KBr pellets, containing compounds 1, 2, and 3, were obtained using a Perkin–Elmer RX I FT-IR spectrometer with a continuous flow of nitrogen gas with a flow rate of 20 mL/min. In the infrared spectra of compounds 1, 2, and 3 (Figure 1), the strong peaks obtained around 2180 and 2220 cm−1 correspond to the symmetric (υs(C≡N) and asymmetric (υas(C≡N) stretching vibrations of the tcm group []. The band at 2800 cm−1 indicates the presence of –CH2 groups present in the bix ligand, and broad bands around 3200–3500 cm−1 suggest the existence of water molecules in the bulk samples.
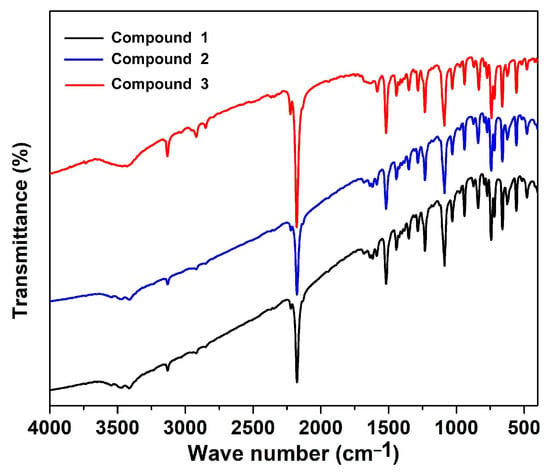
Figure 1.
Fourier-transformed infrared spectra of compounds 1, 2, and 3.
2.2.3. Thermal Stability Studies
Thermogravimetric analysis (TGA) was carried out to evaluate the stability of the frameworks. TG analysis of compounds 1, 2, and 3 was performed on a HITACHI STA300 thermal analysis system (Hitachi, Tokyo, Japan) with a heating rate of 5 °C/min, using powdered samples from room temperature to 750 °C under a nitrogen atmosphere (Figure 2). All the compounds start losing mass between 330 and 360 °C, and this continues up to ca. 530 °C. Initially the complex loses two tricyanomethanide molecules in all three compounds from the coordination spheres of the metal ions, which corresponds to a mass loss of around 21%. Complete decomposition occurs between 500 and 530 °C, leaving metal oxides.
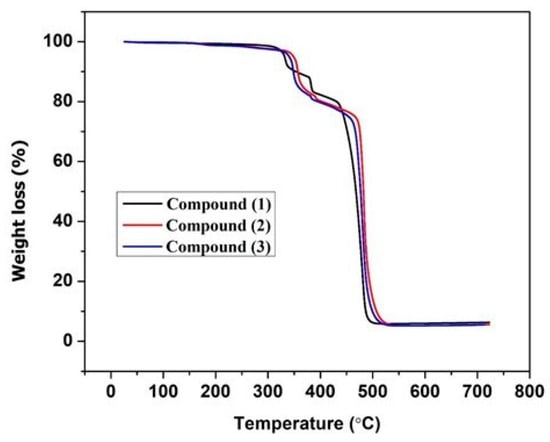
Figure 2.
Thermogravimetric curves of compounds 1, 2, and 3.
2.2.4. Single-Crystal X-Ray Crystallography
A CCD Bruker APEX II X-ray diffractometer (Bruker, Madison, WI, USA) was used to collect single-crystal X-ray diffraction data at 150 K using graphite-monochromated MoKα radiation (λ = 0.71073 Å). The SAINT32 program was used to determine integrated intensities and refine cells. The software’s program employs a narrow-frame integration technique []. It used an empirical absorption correction method (SADABS) []. Using the algorithms SHELXS97 and SHELXL97 [], the structure was solved directly, and then improved using the full-matrix least-squares methodology against F2 with anisotropic displacement parameters for non-hydrogen atoms. Based on Fourier differential maps, the hydrogen atoms in the ligands were added, and they were refined isotopically. In the final difference Fourier maps, there were no remarkable peaks except for the ghost peaks surrounding the metal centers in all the compounds. A summary of crystal data and relevant refinement parameters for compounds 1, 2, and 3 is given in Table 2.

Table 2.
Refinement parameters and crystal data of compounds 1, 2, and 3.
3. Results
3.1. Structure Description of [Co(tcm)2(bix)4] (1)
All the compounds crystallize in space group P-1. As all the compounds are isomorphous, only the structure of compound 1 has been discussed in detail. In all the compounds, the metal centers are in octahedral geometry. The coordination sphere of compound 1 is fulfilled by the four nitrogen atoms of the imidazolyl moieties of the bridging bis-imidazolyl ligand and two nitrogen atoms from two different tcm ligands. Interestingly the tcm ligand is attached to metal centers through the µ1 coordination mode (see Scheme 1). The basal plane is formed by the nitrogen atoms (N1, N1#, N3, and N3#) of the imidazolyl groups, and the apical positions are occupied by N5 and N5# of the tcm ligand (# = 1 − x, 2 − y, and 1 − z). An ORTEP diagram with an atom numbering scheme is shown in Figure 3. The Co-N bond distances are in the range of 2.1255(9) Å to 2.1462(10) Å, and bond angles are between 88.10(4)° and 180° (Table 3, and for compounds 2 and 3, see Table 4 and Table 5), which are consistent with the reported range of data for Co(II) centers. Four metal ions are further connected via the flexible bis-imidazolyl ligands, forming a hexagonal ring-type structure. These bridging units extend in two crystallographic directions and ultimately produce a porous 2D layer. The polyhedral 2D layer structure is shown in Figure 4. These 2D layers are stacked one over another along the crystallographic b-axis, and the distance between the layers formed by the basal plane of the octahedrons is around 8.820 Å. The layers form a 3D layered structure through weak interactions in the crystallographic bc plane (Figure 5). Compounds 2 and 3 possess the iso-structure of compound 1, but with slightly different cell volumes. The Ni compound has a smaller cell volume, while the Cu compound has a larger one.
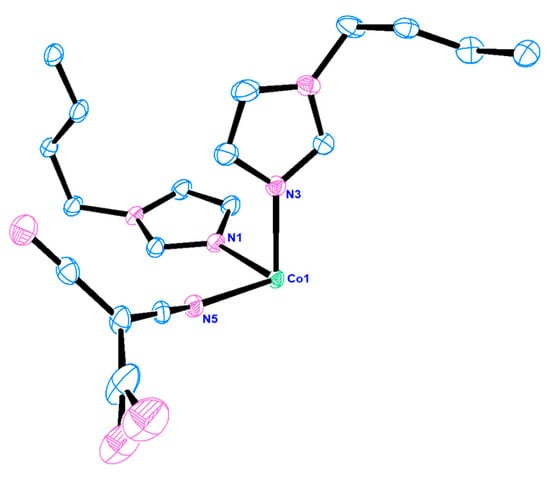
Figure 3.
ORTEP diagram of compound 1 with a 50% thermal ellipsoid probability.

Table 3.
Selected bond lengths [Å] and angles [°] for compound 1.

Table 4.
Selected bond lengths [Å] and angles [°] for compound 2.

Table 5.
Selected bond lengths [Å] and angles [°] for compound 3.
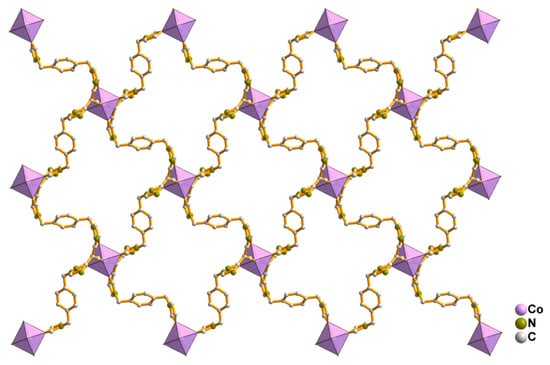
Figure 4.
Two-dimensional porous layer structure of compound 1.
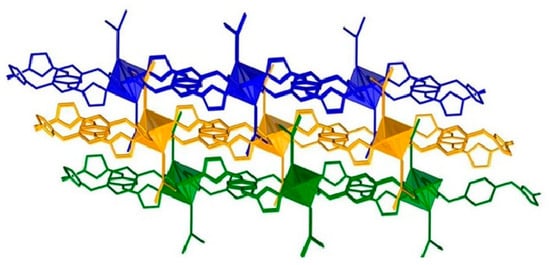
Figure 5.
Three-dimensional structure of compound 1 stacked along the crystallographic b-axis. The blue-, orange-, and green-colored moieties represent the 2D layers, which are stacked one over another with distances of 8.820Å.
The geometry of the central metal ions has been analyzed by continuous shape measurements (SHAPE 2.1) for all the compounds []. Generally, different values are obtained for the different geometries, and the lowest values are considered. In the present study, the lowest values of CSHM (continuous shape measure), 0.023, 0.032, and 0.821 for compounds 1, 2, and 3 were obtained, respectively, for the adoption of octahedral geometry, that are in agreement with the literature [,] (Table 6).

Table 6.
The continuous shape measure of the M(II) ions in compounds 1, 2, and 3.
3.2. Hirshfeld Surface (HS) Analysis
In order to better understand the intermolecular short contact in the reported molecular crystals of this work, the Hirshfeld surface was calculated using the program Crystal Explorer [] and visualized with a color gradient. This method was often used by researchers to illustrate the intermolecular short contact in molecular crystals [,,], which was introduced by Hirshfeld in 1977 [,]. The quantification of the interactions between the atoms is presented via different colors. According to the distance among the atoms between layers or molecules, those short, equal to, or greater than the sum of the Van der Waals radii of the atoms are represented by red, white, and blue. The compounds presented in this work have a 2D structure. Their layers stack along the b direction; therefore, their intermolecular short contacts can be visualized via front and back views of the same layer. Figure 6 presents the typical Hirshfeld surface, using compound 1 as an example, which was plotted over dnorm using a standard surface resolution with a color scale of −1.2399 (red) to 1.6717 (blue) a.u. (dnorm is the normalized sum of de and di). Almost no red spots can be seen on the surface. They are mainly white to blue, clearly indicating very weak interlayer interactions. Therefore, these compounds may be exfoliated into ultrathin nanosheets under some conditions. The side view of these layers pointed out that the surface of these layers is saw-toothed. From the structural point of view, these tooth parts are tcm ligands with a µ1 coordinate state. Figure 5 and Figure 6 indicate that the tooth parts entered into voids of neighboring bc layers.
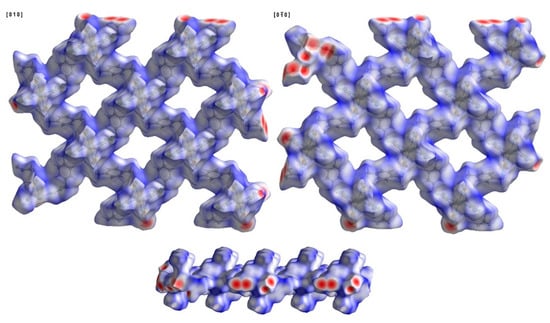
Figure 6.
Hirshfeld surface of compound 1 plotted over dnorm in the range of −1.2399 (red) to 1.6717 (blue) a.u.; view along the [010] and [0−10] directions and a side view of the layer.
Another way to visualize the interlayer interaction is by displaying the distance from HS to the nearest atoms, which can provide details of interlayer interactions. The distances from HS to the nearest atom inside the surface and the one outside the surface are presented as di and de. Figure 7 and Figure 8 display the analysis of HSs of compound 1 plotted over di and de. The reddest part represents the closest contacts, while blue represents the most distant contacts []. Both HSs in Figure 7 and Figure 8 are mainly green. Few light red spots and blue spots represent shorter and longer contact distances. This indicates that although few short contacts existed, the main distance is not very long. Figure 9 is the fingerprint plot of compound 1, showing the contributions of specific interacting pairs. The presence of spikes indicates the formation of H bonding. The Hirshfeld surface (HS) analysis of the other two materials is very similar to that of compound 1, where only the b- and c-axes of compound 3 are exchanged. Figure 10 gives the example of their similarity, showing the comparison of HSs plotted over dnorm and fingerprint plots of compounds 1 and 2. The similarity is visualized.
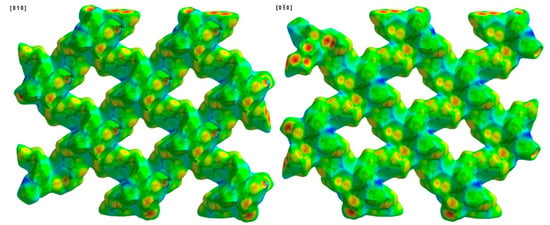
Figure 7.
Hirshfeld surface of compound 1 plotted over di in the range of 0.6894 to 2.8563 Å.
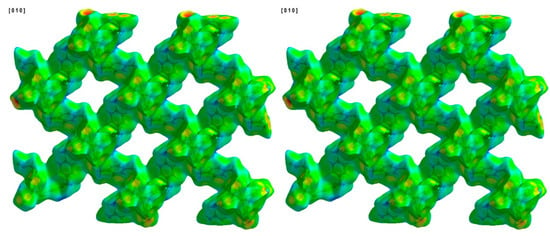
Figure 8.
Hirshfeld surface of compound 1 plotted over de in the range of 0.3733 to 2.9602 Å.
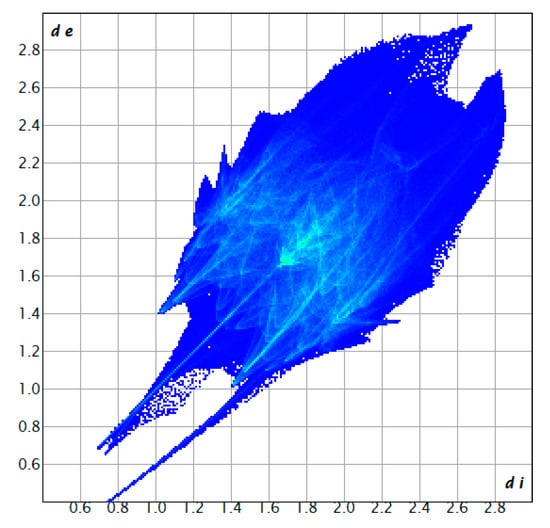
Figure 9.
Fingerprint plot of compound 1, showing the contributions of specific interacting pairs.
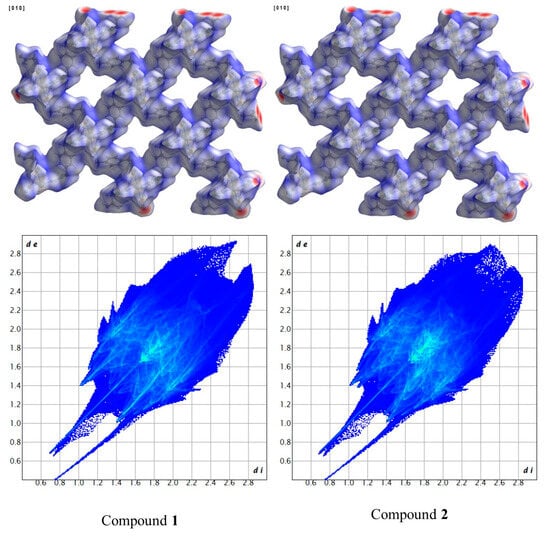
Figure 10.
Comparison of HSs plotted over dnorm and fingerprint plots of compounds 1 and 2.
3.3. Powder X-Ray Diffraction Study
Powder X-ray diffraction (PXRD) patterns of compounds 1, 2, and 3 were recorded at room temperature on a PANalytical Empyrean diffractometer in a Bragg–Brentano geometry using CuKα radiation, in a range of 5 to 50°. The pattern clearly shows that the compounds crystallized in their pure form. The simulated X-ray powder pattern of single crystal and the experimental powder X-ray pattern completely matched. The layered graph is shown in Figure 11.
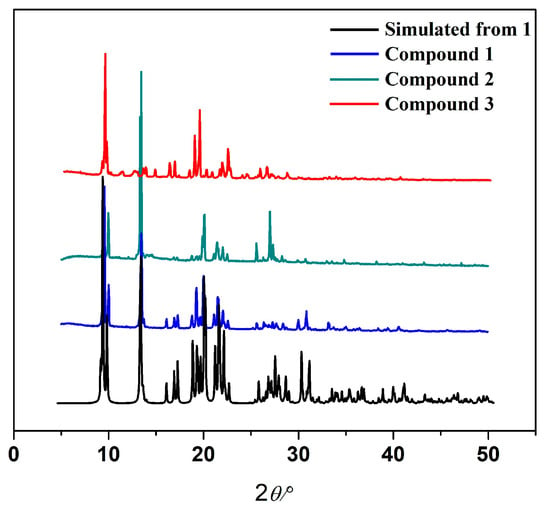
Figure 11.
The experimental PXRD patterns of compounds 1, 2, and 3 and the simulated PXRD pattern of compound 1 from single-crystal X-ray data.
4. Conclusions
Porous two-dimensional metal–organic frameworks (2D MOFs) have become a unique category of crystalline materials known for their remarkable structural flexibility, large surface area, and readily accessible active sites. The development of 2D MOFs, especially those incorporating transition metals like cobalt, nickel, and copper, has shown considerable promise for combining functional characteristics such as magnetism, redox behavior, and electrical conductivity within an organized porous structure. Two-dimensional MOFs provide a multifaceted foundation for material innovation at the crossroad of coordination chemistry and nanotechnology. Here we have developed a series of 2D porous frameworks containing Co(II), Ni(II), and Cu(II) ions as the central elements. These 2D sheets are further stacked along the b-axis (c-axis for compound 3), forming the 3D network. Stacking provides stability to the structure, despite only having weak interlayer interactions. TGA clearly reveals that all the structures are robust and exist above 300 °C. These 2D polymers are a wonderful addition to the family of tcm and co-ligand bridged coordination compounds. Considering the work performed by McKenzie et al. [], the materials presented in this work may also be exfoliated into ultrathin nanosheets under some conditions, forming a new material by coordinating free tcm to additional metals.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst15110989/s1. CCDC No. 2488565-2488567 contains the supplementary crystallographic data for these three compounds. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
Author Contributions
Conceptualization, R.S. and Z.L.; methodology, R.S. and Z.L.; software, R.S. and Z.L.; validation, R.S., S.S., A.D., P.B. and Z.L.; formal analysis, R.S., M.R., S.S., A.D., P.B. and Z.L.; investigation, R.S., M.R., S.S., A.D., P.B. and Z.L.; resources, R.S. and Z.L.; data curation, R.S., M.R., S.S., A.D., P.B. and Z.L.; writing—original draft, R.S. and Z.L.; writing—review and editing, R.S. and Z.L.; visualization, R.S. and Z.L.; supervision, R.S. and Z.L.; project administration, R.S. and Z.L.; funding acquisition, R.S. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
R.S. thanks DST-SERB for the ECR grant (ECR/2016/001572) and the Central Instrumentation Center (CIC) of Adamas University. The authors from Aveiro gratefully acknowledge the fundings from the Strategic Project of the CICECO-Aveiro Institute of Materials UID/50011/2025 (DOI 10.54499/UID/50011/2025) & LA/P/0006/2020 (DOI 10.54499/LA/P/0006/2020), which was financed by national funds through the FCT/MCTES (PIDDAC).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lin Foo, M.; Matsuda, R.; Kitagawa, S. Functional Hybrid Porous Coordination Polymers. Chem. Mater. 2014, 26, 310–322. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Chakraborty, G.; In-Hyeok, P.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Application. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Physics. 2010. Available online: https://www.nobelprize.org/prizes/physics/2010/summary/ (accessed on 26 October 2025).
- Majumdar, D.; Das, D.; Roy, S.; Dalai, S.; Hazra, S. A comprehensive review of synthesis, characterization, single-crystal X-ray diffraction, and applications of transition metal complexes with tricyanomethane anions. J. Mol. Struct. 2025, 1344, 142992. [Google Scholar] [CrossRef]
- Liu, J.; Xing, G.; Chen, L. 2D Conjugated Metal–Organic Frameworks: Defined Synthesis and Tailor-Made Functions. Acc. Chem. Res. 2024, 57, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Samorì, P.; Feng, X. Rational Construction of Two-Dimensional Conjugated Metal–Organic Frameworks (2D c-MOFs) for Electronics and Beyond. Acc. Chem. Res. 2024, 57, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Alezi, D.; Eddaoudi, M. A reticular chemistry guide for the design of periodic solids. Na.t Rev. Mater. 2021, 6, 466–487. [Google Scholar] [CrossRef]
- Lisensky, G.C.; Yaghi, O.M. Visualizing Pore Packing and Topology in MOFs. J. Chem. Educ. 2022, 99, 1998–2004. [Google Scholar] [CrossRef]
- Lee, S.J.; Doussot, C.; Shane, G.T. Architectural Diversity in Multicomponent Metal–Organic Frameworks Constructed from Similar Building Blocks. Cryst. Growth Des. 2017, 17, 3185–3191. [Google Scholar] [CrossRef]
- Qin, J.-S.; Du, D.-Y.; Li, M.; Lian, X.-Z.; Dong, L.-Z.; Bosch, M.; Su, Z.-M.; Zhang, Q.; Li, S.-L.; Lan, Y.-Q.; et al. Derivation and Decoration of Nets with Trigonal-Prismatic Nodes: A Unique Route to Reticular Synthesis of Metal–Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 5299–5307. [Google Scholar] [CrossRef]
- Goh, S.H.; Lau, H.S.; Yong, W.F. Metal-Organic Frameworks (MOFs)-Based Mixed Matrix Membranes (MMMs) for Gas Separation: A Review on Advance Materials in Harsh Environmental Applications. Small 2022, 18, 2107536. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, Z.; You, M.; Zhang, Y.; Feng, X.; Ma, X.; Meng, J. Formation of a thin and continuous MOF membrane with 2-D MOF nanosheets as seeds via layer-by-layer growth. Chem. Commun. 2019, 55, 10146–10149. [Google Scholar] [CrossRef]
- Li, W.; Samarasinghe, S.A.S.C.; Bae, T.-H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem. 2018, 67, 156–163. [Google Scholar] [CrossRef]
- Foziya, V.; Naved, Y.; Malek, I.; Kalisa, S.K. A Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Gandra, U.R.; Pandey, R.P.; Palanikumar, L.; Irfan, A.; Magzoub, M.; Belmabkhout, Y.; Hasan, S.W.; Mohideen, M.I.H. Cu-TCPP Metal–Organic Nanosheets Embedded Thin-Film Composite Membranes for Enhanced Cyanide Detection and Removal: A Multifunctional Approach to Water Treatment and Environmental Safety. ACS Appl. Mater. Interfaces 2025, 17, 9563–9574. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.R.; Chesman, A.S.R.; Murray, K.S.; Deacon, G.B.; Batten, S.R. The chemistry and complexes of small cyano anions. Chem. Commun. 2011, 47, 10189–10210. [Google Scholar] [CrossRef]
- Batten, S.R.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Kohout, J.; Jäger, L.; Hvastijová, M.; Kožíšek, J. Tricyanomethanide and Dicyanamide Complexes of Cu(II), Ni(II), Co(II), Their Structures and Properties. J. Coord. Chem. 2000, 51, 169–218. [Google Scholar] [CrossRef]
- Yuste, C.; Bentama, A.; Stiriba, S.-E.; Armentano, D.; Munno, G.D.; Lloret, F.; Julve, M. Ligand effects on the structures and magnetic properties of tricyanomethanid containing copper(II) complexes. Dalton. Trans. 2007, 5190–5200. [Google Scholar] [CrossRef]
- Wineinger, H.B.; Smetana, V.; Nayak, A.; Qu, F.; Mudring, A.-V.; Rogers, R.D. Accessing Lanthanide Tricyanomethanide Coordination Polymers Using Ionic Liquids. Cryst. Growth Des. 2022, 22, 2372–2381. [Google Scholar] [CrossRef]
- Yang, J.-X.; Zhang, X.; Qin, Y.Y.; Yao, Y.G. N-Donor Auxiliary Ligand Influence on the Coordination Mode Variations of V-Shaped Triazole Dicarboxylic Acid Ligand Affording Seven New Luminescent Zn(II) Compounds with Variable Structural Motifs. Cryst. Growth Des. 2020, 20, 6366–6381. [Google Scholar] [CrossRef]
- Yang, J.; Lin, X.-Q.; Miao, Z.-Y.; Chen, J.-S.; Sun, M.-L.; Zhang, X.; Qin, Y.-Y.; Yao, Y.-G. Metal Ion Modulation of Structural Interpenetration and Magnetic Properties in Mn(II), Co(II), and Ni(II) Coordination Polymers with bnn Topology. Cryst. Growth Des. 2025, 25, 6468–6477. [Google Scholar] [CrossRef]
- Mal, D.; Sen, R.; Brandão, P.; Lin, Z. Crystallization of five new supramolecular networks with both bipyridyl and dicyanamide ligands. Polyhedron 2013, 53, 249–257. [Google Scholar] [CrossRef]
- Bruker 2007 APEX 2 SAINT XPREP; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Bruker 2001 SADABS. Bruker AXS Inc.: Madison, WI, USA, 2001.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Sen, R.; Mondal, K.; dos Santos, A.M.; Escobar, L.B.L.; Brandão, P.; Reis, M.S.; Lin, Z. A chiral alkali metal capped Ni4 cubane complex: Synthesis, structure, magnetic and catalytic bromination studies. J. Mol. Struct. 2023, 1274, 134412. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Joubert, J.; Blacque, O.; Al-Shaalan, N.H.; El-Emam, A.A. Crystal structure, Hirshfeld surface analysis and DFT studies of 5-(adamantan-1-yl)-3-[(4-chlorobenzyl)sulfanyl]-4-methyl-4H-1,2,4-triazole, a potential 11β-HSD1 inhibitor. Sci. Rep. 2019, 9, 19745. [Google Scholar] [CrossRef] [PubMed]
- Kargar, H.; Ashfaq, M.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K.S.; Tahir, M.N. Unsymmetrical Ni(II) Schiffbase complex: Synthesis, spectral characterization, crystal structure analysis, Hirshfeld surface investigation, theoretical studies, and antibacterial activity. J. Mol. Struct. 2022, 1265, 133381. [Google Scholar] [CrossRef]
- Kargar, H.; Ashfaq, M.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K.S.; Tahir, M.N. Synthesis, crystal structure, spectral characterization, theoretical and computational studies of Ni(II), Cu(II) and Zn(II) complexes incorporating Schiff base ligand derived from 4-(diethylamino)salicylaldehyde. Inorg. Chim. Acta 2022, 536, 120878. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theoret. Claim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- McKenzie, J.; Pennington, D.L.; Ericson, T.; Cope, E.; Kaufman, A.J.; Cozzolino, A.F.; Johnson, D.C.; Kadota, K.; Hendon, C.H.; Brozek, C.K. Tunable Interlayer Interactions in Exfoliated 2D van der Waals Framework Fe(SCN)2(Pyrazine)2. Adv. Mater. 2024, 36, 2409959. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).