Abstract
The reactions of UO2(NO3)2·6H2O or UO2(O2CMe)2·2H2O and 2,2′-{(1,2-ethanediyl)bis[nitrilo(phenyl)methylidene]}bisphenol (H2L) in MeOH and DMF have provided access to complexes [UO2(L)(MeOH)] (1) and [UO2(L)(DMF)]·DMF (2·DMF), respectively. The molecular structures of the complexes are similar. The central UVI atom is surrounded by five oxygen and two nitrogen atoms in a distorted pentagonal bipyramidal geometry; the two uranyl oxygen atoms are at the axial positions. Two phenolato oxygen and two imino nitrogen atoms from the tetradentate chelating (1.1111 using Harris notation) L2− ligand are located at the equatorial plane, which is completed by the oxygen atom of a terminally ligated solvent (MeOH, DMF) molecule. Interestingly, the L2− ligand adopts a chair (or stepped) conformation in 1 and a boat conformation in 2·DMF. The supramolecular features of 1 and 2·DMF are distinctly different due to the different H-bonding abilities of coordinated MeOH and DMF, and the presence of an extra-lattice solvent molecule in the latter. The solid complexes were studied by IR, Raman, electronic (UV/Vis), and emission spectroscopic techniques. Complex 1 decomposes in CHCl3 and DMSO, whereas the molecular structure of 2 is retained in these solvents. A new polymorph of the free ligand, H2L(B), has also been discovered and its crystal structure is described.
1. Introduction
The intense interest for actinoid chemistry started during the Manhattan Project in the 1940–1945 period, when scientists were trying to identify volatile compounds suitable for the separation of fissionable isotopes for nuclear applications. Uranium (U), named so because of the planet Uranus, has a rich chemical “history”, being the most important element in the 5f-series of the periodic table. The most important milestones in this “history” were the discovery of this radioactive decay by Becquerel in 1895 and the experiments which revealed that 235U is capable of undergoing fission with slow neutrons in 1938; the latter was responsible for the production of nuclear weapons [1,2,3]. Today, it is recognized that U can have a “Dr. Jekyll and Mr. Hyde” character. From the applications’ viewpoint, U can be used as a useful nuclear fuel but also for the production of destructive weapons [4]. From the chemistry viewpoint, U can behave as a transition element, but sometimes as a lanthanoid [4]. The element can be found in a variety of oxidation states ranging from +1 to +6 [5,6,7,8], mainly due to the radial extension of the 5f atomic orbitals and to relativistic effects. The oxidation states +4 and +6 are the most common [9]. The great scientific interest for molecular U chemistry is proven by the fact that nearly 60% of all entries for this element in the Cambridge Structural Database have been deposited in the last 20 years [10].
The linear, thermodynamically stable trans-dioxidouranium(VI) cation, trans-{UVIO2}2+ (uranyl ion), dominates the chemistry of U(VI) in aqueous solution and in the environment [9]; however, there are few exceptions, such as the molecular compounds UX6 (X = F, Cl) and the alkoxido species U(OMe)6. The uranyl ion has been used in paints since the ancient Romans and its green fluorescence (a consequence of a UVI-to-ligand charge transfer transition) led to the discovery of radioactivity and the Stokes shift. The uranium(VI)-oxido bond (always trans) is very short (~1.78 Å), as a result of a formally triple character; two π bonds arise from 6dxz − 2px and 5fxz2 − 2px overlaps, while one σ bond is formed from the overlap of O(2p) σ orbitals with a 6dz2 orbital of the metal ion and a hybrid orbital derived by mixing 6pz and 5fz3. In coordination complexes, the equatorial sites are occupied by 4 (octahedral geometry), 5 (pentagonal bipyramidal geometry), or 6 (hexagonal bipyramidal geometry) donor atoms from various ligands. The two oxido groups are most often inert with no (or little) chemical reactivity [9,10]. The coordination chemistry of the uranyl cation continues to attract the research interest (in fact, there has been a renaissance in the last 15 years or so) of many inorganic chemistry groups around the world for a variety of reasons including: (a) the uptake of {UVIO2}2+ by natural and artificial proteins [11]; (b) the synthesis of heterometallic uranyl-transition metal [12] and uranyl-lanthanoid(III) [13] complexes that behave as molecular hybrid materials; (c) the excellent photocatalytic activity (i.e., UV or Vis light-driven catalysis) of some uranyl complexes for the destruction of several dyes [14]; (d) the chemistry of peroxido [15] and polycarboxylato [16] uranyl complexes; (e) the preparation of porous anionic uranyl (uranylate) complexes that can efficiently remove the hazardous radiotoxic 127Cs+ ion [17]; (f) the selective complexation and separation of U(VI) from Th(IV), a challenge in the thorium-uranium fuel cycle [18]; and (g) the study of the synthetic and structural chemistry of uranyl-amidoxime complexes towards understanding the molecular basis and improving the recovery of uranium from seawater using amidoxime-functionalized synthetic polymers [10,19,20].
Crucial to the progress of uranyl chemistry is the selection of the organic ligands that are coordinated to {UVIO2}2+. A particularly interesting family of ligands appropriate for this goal is the family of polydentate O,N-based Schiff bases. These molecules possess one or more azomethine (-HC=N-) or imine (R2-C=N-) groups. Due to their highly modular synthesis that allows a strict control over the nature of donor sites, denticity, chelating and/or bridging behavior, structural features, and electronic characteristics Schiff bases have played a central role in the development of modern inorganic chemistry [21,22,23,24,25,26,27]; for this reason, they are often considered as “privileged” ligands [28]. Metal complexes containing Schiff bases as ligands are of great importance in a number of interdisciplinary research areas such as molecular magnetism, bioinorganic chemistry, molecular energetic materials, medicinal chemistry, multifunctional molecular materials, catalysis (both homogenous and heterogenous), sensors, photo- and electroluminescence, and multielectron redox chemistry [21,22,23,24,25,26,28,29,30,31,32]. The most well studied group of Schiff bases is the so-called “salen-type” [33,34] (Scheme 1); the mother (H2salen) is bis(salicylaldehyde)ethylenediamine (X = H, R = H, Z = CH2CH2), which is potentially tetradentate (2N, 2O). This work concerns a member of this group (vide infra) and its complexes with the uranyl ion.
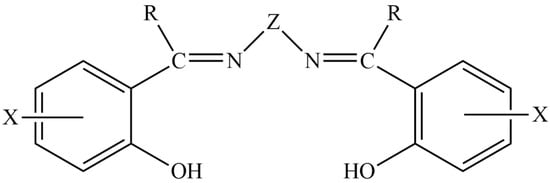
Scheme 1.
The general structural formula of the “salen-type”-Schiff-base ligands; X, Z, and R are non-donor sites (including H). In the ligand of the present work, abbreviated as H2L, X = H, Z = CH2CH2, and R = Ph (phenyl). In a more general view, X, R, and Z may contain donor groups, and thus the denticity of the molecules is increased and the properties of the resultant Schiff bases alter.
Uranyl complexes with Schiff bases as ligands is a “hot” research theme in inorganic chemistry [35]. Areas of interest are: (i) {UVIO2}2+ is a good template agent for 2:2 condensation of dialdehydes or diketones and diamines, leading to uranyl complexes with macrocyclic Schiff-base ligands [36,37]; (ii) planar pentadentate Schiff bases form a suitable pocket capable of accommodating the size and the preference for a pentagonal bipyramidal environment of the UVI center in uranyl complexes [21]; (iii) the ability of neutral uranyl complexes with Schiff bases (of the “salen-type”) to behave as selective receptors of anions, thus providing a platform for advanced supramolecular chemistry [38]; (iv) the utility of uranyl/Schiff-base complexes to act as excellent homogenous catalysts, for example, in 1,4-thiol addition reactions [39]; (v) the employment of such complexes in separation processes which are very useful in nuclear fuel technology and nuclear waste management [40,41]; (vi) the success of “salen-type” Schiff bases in promoting reduction and functionalization (i.e., reactivity) of the, otherwise, thermodynamically stable and chemically inert {UVIO2}2+ [42,43], and (vii) theoretical studies on uranyl complexes with Schiff-base ligands [44].
Despite the research devoted to uranyl complexes with ligands derived from the condensation of salicylaldehyde or its derivatives and 1,2-diaminoethane (ethylenediamine), i.e., Schiff bases with R = H, X = a non-donor site, and Z = CH2CH2 (Scheme 1) [45,46,47,48,49], the coordination chemistry of such ligands with R ≠ H towards the uranyl ions is completely unexplored. We decided to start our efforts with the bulky ligand H2L (see caption of Scheme 1; X = H, R = Ph, Z = CH2CH2); the IUPAC name of this compound is 2,2′-((1E, 1′E)-ethane-1,2-diylbis(azan-1-yl-1-ylidene))bis(phenylmethan-1-yl-1-ylidene)diphenol, while a simpler and often used name is 2,2′-{(1,2-ethanediyl)bis[nitrilο(phenyl)methylidyne]}bisphenol. The crystal structure of the free ligand was reported in 1997 [50], while its coordination chemistry has escaped the attention of inorganic chemistry, being restricted to only three Mn complexes [51,52,53]. Thus, this work describes the synthesis, crystal structures, and characterization of two uranyl complexes isolated from the reactions of uranyl sources and H2L in two different solvents. In the course of our efforts, we also obtained (although this was not our goal!) crystals of H2L. The unit cell was different from that reported in the literature [50] and we thus proceeded to a crystal structure determination which revealed a polymorph (called hereafter polymorph B) of the published structure (called hereafter polymorph A); a comparison of the structural features of the two polymorphs is also described. Finally, since synthetic and spectroscopic data for the free ligand have not been available (only its crystal structure had been investigated), we also report here its detailed synthesis and brief spectroscopic characterization. This study is a continuation of the interest of our groups in the uranyl/Schiff base chemistry [35,54,55].
2. Materials and Methods
2.1. Materials
All experimental procedures were performed under aerobic conditions using reagents and solvents (Alfa Aesar, Sigma-Aldrich, Karlsruhe, Germany) as received. The deuteration degree of d6-DMSO was ~99.5%. The actual H2O percentage might be higher than 0.5% because the solvent is hygroscopic. Deionized water was produced in-house. Warning: Depleted uranium was used in all experiments; also, this has a very long half-life. However, precautions for working with radioactive substances should be strictly followed. Manipulations and synthetic studies were carried out in a fume hood which contains α- and β-counting equipment. In all the experiments, masks and gloves were used, because uranyl compounds can affect breathing and may be absorbed through the skin; in addition, contact can irritate and burn the skin with possible eye damage.
2.2. Microanalyses
Microanalyses of carbon, hydrogen, and nitrogen were performed by the instrumental analysis service of the University of Patras using a Carlo-Erba, model EA1108 analyzer (Milan, Italy).
2.3. Conductivity Measurements
Conductivity experiments were carried out at 25 ± 0.5 °C with a Metrohm-Herisau E-527 bridge and a cell of standard value. The concentrations of the solutions were ~10−3 M.
2.4. Infrared Spectra
Fourier-transform infrared spectra (4000–400 cm−1) were recorded using a Perkin Elmer 16PC spectrometer (Waltham, MA, USA), with the samples being in the form of KBr pellets. The spectra of some samples were also recorded using the attenuated total reflectance (ATR) on a Bruker Optics Alpha-P Diamond spectrometer (Billerica, MA, USA).
2.5. Raman Spectra
Raman spectra were obtained using a T-64000 Jobin Yvon-Horiba micro-Raman setup (Kyoto, Japan), employed in a single-spectrograph configuration. The excitation wavelength for the complexes was 632.8 nm emitted from a He-Ne laser (Optronics Technologies SA, model HLA-20P, 20 mW) (Moschato, Greece). The laser power on the samples was 1 mW. In order to eliminate or minimize the overlapping fluorescence effect, the photobleaching of the samples for 30 min, prior to the Raman measurements, was necessary. The backscattered radiation was collected from the monochromator after passing through a suitable edge filter (LP02-633RU-25, Laser2000 Ltd., Huntigdon, Cambridgeshire, UK). The excitation wavelength for the free ligand H2L was 514 nm emitted from a DPSS laser (Cobolt Fandango TMISO Laser) (Kassel, Germany). The collected backscattered radiation was directed to the monochromator (single configuration) and a Spectraview-2DTM liquid N2-cooled detector. The laser was focused on the sample using a 50× microscope objective with a power of 2 mW on the sample. The calibration of the instrument was made via the Raman peak position of Si at 520.5 cm−1. The spectra resolution was 3 ± 1 cm−1.
2.6. Photoluminescence Experiments
Solid-state excitation and emission spectra were recorded at room temperature using a Cary Eclipse fluorescence spectrometer (Santa Clara, CA, USA) with a Xenon flash lamp and monochromators for the detection of light intensity. The operational wavelength range (emission and excitation) is 200–900 nm with an accuracy of ±1.5 nm. The wavelengths of the excitation and emission maxima are written as recorded by the instrument.
2.7. NMR Spectra
1H and 13C room-temperature NMR spectra were recorded on a Bruker Avance DPX spectrometer (Billerica, MA, USA) at resonance frequencies of 600.13 and 100.62 MHz, respectively. The solvents used were d6-DMSO and CDCl3, and the signals of their non-deuterated forms were used as references.
2.8. Electrospray Ionization Mass Spectra
The electrospray ionization mass spectrum (ESI-MS) of a sample of the free ligand H2L in MeOH was recorded on an Amazon SL spectrometer (Billerica, MA, USA).
2.9. Ultraviolet-Visible Spectra
Solution UV-Vis spectra (DMSO, CHCl3) were recorded on a Hitachi-U300 spectrometer (Tokyo, Japan) in the 220–800 nm spectroscopic window. The absorption solid-state UV-Vis spectrum of one uranyl complex was obtained using an Agilent Cary 60 spectrometer (Santa Clara, CA, USA), again in the same spectroscopic region. For the solution spectra, the absorbance maxima are written as recorded by the instrument. In the solid-state spectrum, the bands’ position was determined after deconvolution.
2.10. Synthetic Procedures
H2L: To a yellow slurry of 2-hydroxybenzophenone (5.95 g, 30.0 mmol) in EtOH (15 mL) was slowly added ethylenediamine (1.00 mL, 15.0 mmol) and the resultant mixture was refluxed for 2 h, during which time a clear yellow solution was obtained. The solution was cooled to room temperature, and a yellow solid started to precipitate. The precipitation was completed at −20 °C for a period of 30 min. The solid was obtained by filtration, washed with Et2O (2 × 10 mL), recrystallized from boiling EtOH, and dried at 60 °C overnight. The yield (after recrystallization) was 62%. Anal. Calcd. (%) for C28H24N2O2: C, 80.0; H, 5.8; N, 6.7. Found (%) C, 79.8; H, 5.7; N, 6.5. IR (KBr, cm−1): ~3390wb, 3056w, 2940w, 2906w, 1607s, 1573m, 1495m, 1447m, 1330m, 1301m, 1258m, 1221w, 1174w, 1150w, 1113w, 1070w, 1029w, 954w, 920m, 849w, 825m, 764s, 702m, 642w, 601w, 544w, 470w, 444w, 421w. Raman peaks (2000–200 cm−1): 1596s, 1546m, 1472s, 1443m, 1333s, 1263w, 1172w, 1143m, 1035w, 1002m, 943w, 846w, 775w, 727w, 591w, 479w, 386w, 257w, 233w. Room-temperature, solid-state excitation spectrum (λ/nm, maximum emission at ~520 nm): 355, 384, 418 and 457. Room-temperature, solid-state emission spectrum (λ/nm, maxima excitation at 355, 384, 418 and 457): ~520. 1H NMR (d6-DMSO, δ/ppm): 15.26 (s, 2H), 7.55 (t, 6H), 7.33 (mt, 2H), 7.23 (dd, 4H), 6.91 (d, 2H), 6.72 (mt, 4H), 3.70 (s, 4H). 1H NMR (CDCl3, δ/ppm): 15.12 (sb, 2H), 7.52 (mt, 6H), 7.30 (mt, 2H), 7.17 (dd, 4H), 7.01 (d, 2H), 6.78 (dt, 2H), 6.65 (mt, 2H), 3.65 (s, 4H). 13C NMR (CDCl3, δ/ppm): 175.45, 162.87, 133.99, 132.45, 131.63, 129.01, 128.92, 128.83, 128.36, 127.24, 119.86, 117.88, 117.46, 52.34. 13C NMR (d6-DMSO, δ/ppm): 175.34, 162.75, 133.10, 131.59, 129.73, 129.39, 127.52, 119.77, 118.06, 52.24. ESI(+)-MS (MeOH): Representative signals at {1344.23}+, {863.25}+, {421.19}+ and {274.28}+. The assignments of the peaks are given in the 3.1 paragraph of “Results and Discussion” (Section 3).
Crystals of H2L (polymorph B): Single crystals of this polymorph were obtained during our efforts to prepare a Sm(III) complex of H2L (vide infra)! Thus, to a yellow slurry of H2L (0.08g, 0.2 mmol) in MeOH (15 mL) was slowly added Et3N (0.06 mL, 0.4 mmol); no dissolution or color change were observed. The slurry was heated at 40–45 °C for 30 min and a yellow solution was formed. Solid Sm(O2CMe)3·6H2O (0.08g, 0.2 mmol) was then added and a new yellow slurry was obtained which was stirred for a further 30 min under reflux, filtered with a filter-paper and the resultant solution was stored in a closed vial at room temperature. After 2 d, small yellow crystals had been precipitated, which were suitable for X-ray crystallography. The crystals were taken directly from the mother liquor, cooled to −113 °C, and used for data collection (without recording their IR spectra!). Solution of the structure revealed a new polymorph(B) of the published [50] structure of H2L. For bulk characterization, the crystals were collected by filtration, washed with Et2O (3 × 2 mL), and dried in air. The yield (without taking into account the measured crystal) was low (10–15%). The crystalline powder was analyzed satisfactorily. Anal. Calcd. (%) for C28H24N2O2: C, 80.0; H, 5.8; N, 6.7. Found (%): C, 81.0; H, 5.9; N, 6.6. The IR, Raman, 1H NMR, and 13C NMR spectra of the powder were almost identical to those described above for the crude H2L.
[UO2(L)(MeOH)] (1) and [UO2(L)(DMF)]·DMF (2·DMF) in a mixture: To a yellow slurry of H2L (0.09 g, 0.2 mmol) in MeOH (16 mL) was added solid UO2(O2CMe)2·2H2O (0.08 g, 0.2 mmol). The orange slurry obtained was refluxed for 75 min with no noticeable change. At the end of this time, MeOH (8 mL) and DMF (8 mL) were added, and the system was retained under reflux and stirring for a further 15 min. The solids were dissolved and the orange solution was filtered and stored in a closed vial at room temperature. After 1–2 d, orange crystals had been precipitated. Careful inspection of the reaction mixture revealed the presence of two kinds of crystals with slightly different morphologies and sizes; longer and thinner crystals, and smaller and thicker ones in a visually estimated ratio of approximately 4:1. Single-crystal X-ray data were collected for both kinds of crystals which proved that the crystals in excess represent complex [UO2(L)(MeOH)] (1), whereas fewer crystals correspond to compound [UO2(L)(DMF)]·DMF. Following exactly the same procedure, but layering the final solution with Et2O (20 mL), a mixture of the same crystals was obtained and checked by unit cell determination. This time, the main batch of crystals represented complex 2·DMF and fewer crystals corresponded to 1 (visual ratio of approximately 5:1).
[UO2(L)(MeOH)] (1) (Method A): To a stirred yellow solution of H2L (0.09 g, 0.2 mmol) in warm MeOH (30 mL) was added solid UO2(NO3)2·6H2O (0.10 g, 0.2 mmol). The color of the reaction system was changed to orange and immediately a small quantity of an orange microcrystalline solid was precipitated. The reaction mixture was stirred for a further 15 min, and the solid was isolated by filtration and dried at 40 °C. The filtrate was stored in a closed vial at −20 °C for 2 d and was then slowly evaporated at room temperature. Orange crystals of the product were formed after 1 d, which were collected by filtration and dried in air. The IR spectrum of the crystals was identical with that of the orange microcrystalline solid. The total yield was 25–30%. The solid and the crystals were analyzed satisfactorily. For the microcrystalline sample: Anal. Calcd. (%) for C29H26N2UO5: C, 48.3; H, 3.6; N, 3.9. Found (%): C, 48.1; H, 3.6; N, 3.8. For the crystals: Anal. Calcd. (%) for C29H26N2UO5: C, 48.3; H, 3.6; N, 3.9. Found (%): C, 48.2; H, 3.5; N, 4.0. The following data were obtained from the microcrystalline solid. IR (KBr, cm−1): ~3445wb, 3052w, 2943w, 2813w, 1597s, 1520s, 1487sh, 1454w, 1412w, 1382w, 1344m, 1269s, 1214m, 1152m, 1118w, 1074w, 1023m, 917s, 838m, 804w, 759m, 697m, 628w, 600w, 520w, 455wb. Raman peaks (2500–200 cm−1): 1600w, 1577w, 1540w, 1462w, 1443w, 1323m, 1260w, 1174w, 1158w, 1142w, 1046w, 1038w, 1021w, 1000m, 986w, 978w, 844w, 817s, 772w, 717m, 590w, 347w, 255w, 233w, 217w. Solid-state UV/Vis (λ/nm): 253, 290sh, 351, 395sh, 510. UV-Vis (DMSO, λ/nm): 247, 280sh, 360, 385sh, 430. Room-temperature, solid-state excitation spectrum (λ/nm, maximum emission at 598 nm): 336, 350sh, 402. Room-temperature, solid-state emission spectrum (λ/nm, maximum excitation at 400 nm): 465, 560, 598. 1H NMR (CDCl3, δ/ppm, poor quality spectrum due to solubility reasons): 7.64–7.47 (mt), 7.14 (s), 4.13 (s), 3.49 (s), 1.25 (s). 1H NMR (d6-DMSO, δ/ppm): 15.19 (s, 2H), 7.55 (t, 6H), 7.31 (ddd, 2H), 7.22 (mt, 4H), 6.91 (d, 2H), 6.69 (mt, 4H), 4.00 (s, 1H), 3.57 (s, 4H), 3.17 (s, 3H), 3.06 (s, 2H). 13C NMR (CDCl3, δ/ppm, poor quality spectrum due to solubility reasons): Only signals at ca. 136, 130, 129, 118, 51 and 34 ppm are clearly seen, 13C NMR (d6-DMSO, δ/ppm): 175.62, 163.05, 134.14, 133.32, 131.94, 130.00, 129.68, 127.90, 120.15, 118.37, 118.31, 52.67, 49.01. ΛΜ (DMSO, 25 °C, 10−3 M) = 9 S cm2 mol−1.
[UO2(L)(MeOH)] (1) (Method B): To a stirred yellow solution of H2L (0.09g, 0.2 mmol) in warm MeOH (30 mL) were added Et3N (52 μL, 0.4 mmol) and solid UO2(NO3)2·6H2O (0.10 g, 0.2 mmol). UO2(NO3)2·6H2O was dissolved and the resultant orange solution was stirred. An orange microcrystalline solid was immediately precipitated, and the reaction mixture was stirred for a further 1 h. The solid was collected by filtration, washed with cold MeOH (2 × 1 mL) and Et2O (4 × 2 mL), and dried in air overnight. The yield was 70%. Anal. Calcd. (%) for C29H26N2UO5: C, 48.3; H, 3.6; N, 3.9. Found (%): C, 48.6; H, 3.7; N, 4.1. The IR, Raman, and 1H NMR (d6-DMSO) spectra of the solid were identical with those of the authentic material prepared by method A.
[UO2(L)(MeOH)] (1) (Method C): Solids H2L (0.17 g, 0.4 mmol) and UO2(O2CMe)2·2H2O (0.17 g, 0.4 mmol) were suspended in MeOH (50 mL). The resultant orange slurry was refluxed for 2 h, during which time most quantity of the solids were dissolved. The insoluble yellow–orange material was removed by filtration, and the filtrate was cooled to room temperature and stored in a closed vial at room temperature. An orange microcrystalline solid was obtained almost immediately. When precipitation was judged to complete (i.e., after 1 d), the solid was collected by filtration, washed with cold MeOH (2 × 2 mL) and Et2O (5 × 3 mL), and dried in air overnight. The yield was in the range 40–45%. Anal. Calcd. (%) for C29H26N2UO5: C, 48.3; H, 3.6; N, 3.9. Found (%): C, 47.6; H, 3.5; N, 3.8. The IR, Raman and 1H NMR (d6-DMSO) spectra of the orange solid were identical with those of the authentic material prepared by method A. The IR spectrum of the original yellow–orange material had the bands of the product and bands of the UO2(O2CMe)2·2H2O starting material, indicating that it is a mixture.
[UO2(L)(DMF)] (2·DMF) (Method A): To a stirred yellow solution of H2L (0.17 g, 0.4 mmol) in DMF (8 mL) was added solid UO2(NO3)2·6H2O (0.20 g, 0.4 mmol). The solid was immediately dissolved to give an orange solution. The solution was stirred for a further 15 min, filtered and stored in a closed vial at room temperature. Orange crystals of the product, suitable for X-ray crystallography, were formed after 1 week. The crystals were collected by filtration, washed with Et2O (5 × 2 mL) and dried at 120 °C for 2 d. The yield was 20%. The sample was analyzed satisfactorily without the lattice DMF solvent (i.e., as 2). Anal. Calcd. (%) for C30H27N3UO5: C, 48.2; H, 3.6; N, 5.6. Found (%): C, 48.6; H, 3.8; N, 5.4. IR (KBr, cm−1): 3020w, 2928wb, 1649s, 1587s, 1537m, 1493w, 1450sh, 1435s, 1377m, 1321s, 1243m, 1143w, 1116w, 1095w, 1043wb, 974w, 893s, 843m, 807w, 757m, 705m, 634w, 587m, 531w, 473m, 415w. Raman peaks (cm−1): 3055w, 3047w, 2960w, 2941w, 2906w, 1640m, 1595m, 1581sh, 1540m, 1466m, 1442m, 1329s, 1286w, 1241w, 1171w, 1149m, 1118w, 1095w, 1046w, 1028w, 1002m, 940w, 866w, 845w, 812s, 764w, 725w, 683w, 660w, 638w, 615w, 592m, 445w, 405w. UV/Vis (CHCl3, λ/nm): 287, 345, 398, ~500. Room-temperature, solid-state excitation spectrum (λ/nm, maximum emission at 598 nm): 335, 353, 400. Room-temperature, solid-state emission spectrum (λ/nm, maximum excitation at 400 nm): 599. 1H NMR (CDCl3, δ/ppm, poor quality spectrum for solubility reasons): 8.10 (s), 7.64–7.43 (mt), 6.98 (s), 2.98 (s), 2.93 (s), 1.59 (s). 1H NMR (d6-DMSO, δ/ppm): 7.95 (s, 1H), 7.63 (t, 4H), 7.56 (t, 2H), 7.45 (mt, 6H), 7.04 (d, 2H), 6.75 (dd, 2H), 6.43(mt, 2H), 4.02 (s, 4H), 2.89 (s, 3H), 2.73 (s, 3H). 13C NMR (CDCl3, δ/ppm, poor quality spectrum due to solubility reasons): Only signals at ca 163, 151, 138, 134, 129, 128, 42, 37 and 32 ppm are clearly seen. 13C NMR (d6-DMSO δ/ppm): 169.96, 163.23, 138.72, 134.13, 133.79, 129.80, 129.65, 127.94, 126.30, 121.47, 116.44, 57.28, 36.25, 31.55. ΛΜ (DMSO, 25 °C, 10−3 M) = 4 S cm2 mol−1.
[UO2(L)(DMF)] (2·DMF) (Method B): To a stirred yellow solution of H2L (0.17 g, 0.4 mmol) in DMF (7 mL) was added Et3N (104 μL, 0.8 mmol) and solid UO2(NO3)2·6H2O (0.20 g, 0.4 mmol). The solid was easily dissolved to give an orange solution, from which an orange solid was immediately precipitated. The reaction mixture was stirred for a further 45 min, and the precipitate was collected by filtration, washed with Et2O (5 × 3 mL), and dried at 100 °C for 1 week. The yield was 72%. The sample was analyzed satisfactorily as lattice DMF-free. Anal. Calcd. (%) for C30H27N3UO5: C, 48.2; H, 3.6; N, 5.6. Found (%): C, 48.6; H, 3.7; N, 5.4. The IR and Raman spectra of the solid were almost identical with those obtained for the authentic dried solid prepared by method A.
[UO2(L)(DMF)] (2·DMF) (Method C): Solids H2L (0.17 g, 0.4 mmol) and UO2(O2CMe)2·2H2O (0.17 g, 0.4 mmol) were suspended in DMF (8 mL). The resultant orange slurry was stirred at 90 °C overnight, during which time most of the quantity of the solids were dissolved. The insoluble yellowish–orange solution was removed by filtration. The filtrate was cooled to room temperature, and stirring provided an orange solid almost immediately. The solid was collected by filtration upon further stirring overnight, collected by filtration, washed with Et2O (4 × 4 mL), and dried at 110 °C for 1 d. The yield was ~50%. The IR spectrum of the solid was identical with that obtained for the authentic material prepared by method A.
2.11. Single-Crystal X-Ray Crystallography
A yellow crystal of H2L (polymorph B) (0.21 × 0.21 × 0.36 mm), and orange crystals of 1 (0.10 × 0.13 × 0.41 mm) and 2·DMF (0.14 × 0.22 × 0.38 mm), all with parallelepiped habit, were taken directly from the mother liquor and immediately cooled to −113 °C (H2L, (B), 2·DMF) or −93 °C (1). Diffraction measurements were made on a Rigaku R-Axis Spider Image Plate diffractometer using graphite-monochromated Mo Kα radiation. Data collection (ω-scans) and processing (cell refinement, data reduction, and empirical absorption correction) were performed using the CrystalClear program package (version 1.4.0) [56]. Important crystallographic data are gathered in Table 1. The structures were solved by direct methods using SHELXS, ver. 2013/1 [57] and refined by full-matrix least-squares methods on F2 with SHELX, ver. 2014/6 [58]. The data for 1 were refined using the non-merohedral twin law of a 2-fold axis parallel to the [1,0,5] crystallographic axis expressed by the matrix [(−1.0, 0.0, 0.0), (0.00, −1.00, 0.00), (0.315, 0.0, 1.0)] and a BASF parameter equals to 0.257(2). The H atoms were either located by difference maps and refined isotropically or introduced at calculated positions as riding on their bonded atoms All non-H atoms were refined using anisotropic thermal parameters. Plots of the structures were constructed using the Diamond 3 program package [59].

Table 1.
Crystallographic data and refinement parameters for compounds H2L (polymorph B), 1 and 2·DMF.
The CCDC reference numbers 2493661, 2493662, and 2493663 entries contain the supplementary crystallographic data for compounds H2L (polymorph B), 1 and 2·DMF included in this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures (7 October 2025).
2.12. Powder X-Ray Diffraction
The powder X-ray diffraction measurement was performed using a Rigaku SmartLab diffractometer (Neu-Isenburg, Germany) in θ/θ geometry with Brag-Brentano optics. The instrument was equipped with a secondary beam pyrolytic graphite monochromator and utilized Cu Kα radiation (Cu Κα1 = 1.54060 Å, Cu Κα2 = 1.54439 Å). The powder sample was analyzed under generator settings of 40 kV and 35 mA, using slits of 2/3°, 2/3°, and 0.6°. Data were collected over the 2θ range 2.0–90.0° with a step size of 0.03° and a counting time of 2 s per step.
3. Results and Discussion
3.1. Synthetic and Spectroscopic Comments for the Free Ligand H2L
As mentioned in the “Introduction”, the structure of the free H2L ligand (Scheme 1; X = H, Z = CH2CH2, R = Ph) had been solved, but no synthetic and characterization data were reported. Full details are available in Section 2.10, and we now briefly comment on them; data are shown in Figure 1, Figure 2, Figure 3 and Figures S1–S6. The compound was synthesized by the 2:1 condensation of 2-hydroxybenzophenone and ethylenediamine in refluxing EtOH in good yield (62% after recrystallization). Unfortunately, the recrystallization procedure gave crystals of poor quality and, thus, determination of the crystal structure was not possible. In the IR spectrum (Figure S1), the region of the carbonyl stretching vibration is free (this band appears at ~1635 cm−1 in the spectrum of the 2-hydroxybenzophenone starting material), confirming the formation of the imine groups. The ν(C=N) vibration is located at 1607 cm−1 [54,55]. This mode appears at 1596 cm−1 as a strong peak in the Raman spectrum (Figure 1) [60]. The purity of the ligand is confirmed by the ESI-MS(+) which shows the molecular ion at m/z 421.19 (Figure 2). The signals at m/z values of 1344.23, 863.25, 650.18, and 274.28 are tentatively assigned to the species [3M + K+ + 2Na+], [2M + Na+], [M + C13H10O2− + 2Na+], and [C16H14N22+ + K+]+, respectively; M is the molecular ion. The C13H10O2− and C16H14N22+ species are fragments of the original ligand. The compound emits visible light at ~520 nm after excitation at 355, 384, 418, or 457 nm (Figure 3). Using all four different wavelengths for excitation, we always recorded emission with the same “structure” and intensity. In the 1H NMR spectra of H2L in d6-DMSO (Figures S2 and S3) and CDCl3, the -OH signal appears at δ 15.26 and 15.12 ppm, respectively [61]; the low-field value of δ is indicative of the remarkable acidity of this group. In the aromatic region, there are five (d6-DMSO, δ 7.55–6.72 ppm) and six (CDCl3, δ 7.52–6.65 ppm) signals with the expected integration ratio. The singlet signal at δ 3.70 (d6-DMSO) and 3.68 (CDCl3) ppm is due to the equivalent aliphatic protons [61]. The 13C{1H} NMR spectrum of H2L in CDCl3 (Figure S6) exhibits fourteen resonances attributable to the 28 equivalent carbon atoms, the signal at δ 52.34 ppm being assigned to the aliphatic -CH2CH2- atoms [62]. Only nine resonances (out of the expected thirteen) are observed in the aromatic region of the spectrum recorded in d6-DMSO, presumably due to overlapping signals; the signal for the aliphatic carbons appears at δ 52.24 ppm.
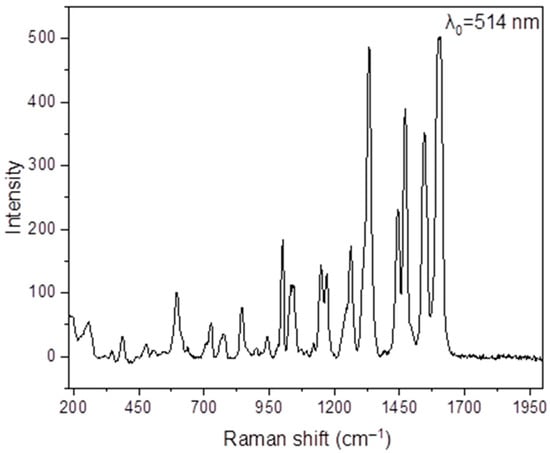
Figure 1.
The Raman spectrum of H2L in the 2000–200 cm−1 region.
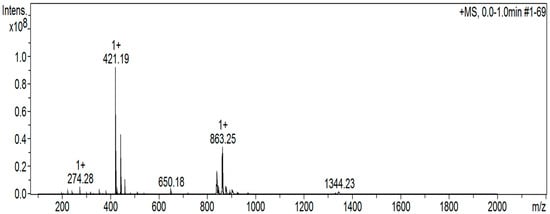
Figure 2.
The ESI-MS(+) of H2L in MeOH. The assignments of the peaks are given in the text.
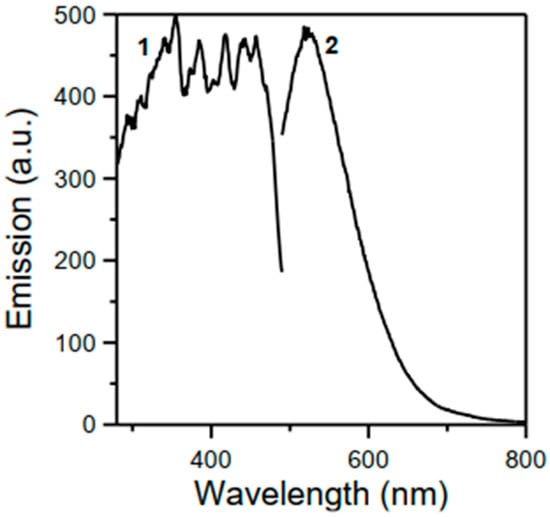
Figure 3.
Solid-state, room-temperature excitation (λ/nm, maximum emission at ~520 nm) (curve 1) and emission (maxima excitation at 355, 384, 418, and 457 nm) (curve 2) spectra of H2L. Under excitation by using the four different wavelengths, emission with the same “structure”, and intensity was always recorded.
3.2. Isolation of a New Polymorph (Polymorph B) of H2L
A new polymorph B of H2L (we call the previously reported structure [50] as polymorph A) was isolated during our efforts to prepare a Sm(III) complex of L2−. The Sm(O2CMe)3/H2L/Et3N (1:1:2) reaction system in MeOH provided access to single crystals of B. Polymorph A had been isolated [50] from slow evaporation of a saturated methanolic solution of the crude product. Thus, it is evident that the presence of extra chemical species in the reaction solution affects the crystal structure. Recrystallization of our crude product from MeOH (i.e., by slow evaporation of a saturated methanolic solution) gave single crystals of H2L(A), as expected (confirmation by unit cell determination). Polymorphs have the same chemical composition, but different crystal structures and therefore differ in their physiochemical properties such as solubility, density, and dissolution rates [63]. The density of A is 1.250 g cm−3 [50], while that of B is 1.222 g cm−3 (Table 1). Polymorphism is of great importance in pharmaceutical, chemical, and food industries.
3.3. Comments Concerning the Preparation of the Uranyl Complexes 1 and 2·DMF
Complexes [UO2(L)(MeOH)] (1) and [UO2(L)(DMF)]·DMF (2·DMF) were first isolated as a mixture of single crystals from the UO2(O2CMe)2·2H2O/H2L (1:1) reaction system in a solvent mixture comprising MeOH and DMF under reflux. Full data collection was carried out for the two (slightly different) kinds of crystals, which revealed their identity. Then, we attempted to devise clean preparations for the two complexes. Each complex was prepared by three procedures (methods A, B, and C), see general Equations (1)–(3), respectively, where S is MeOH or DMF. Complex 2 was crystallized as a DMF solvate; this extra-lattice DMF molecule does not appear in the equations.
The authentication of the single crystals isolated by method A (Equation (1)) was performed through unit cell determination. The identity of the microcrystalline solids obtained by methods B and C (Equations (2) and (3), respectively) was confirmed by spectroscopic methods. A further point deserves comment. Under the concentrations mentioned in paragraph 2.10, the precipitation of the products is slow (thus leading to single crystals) only using method A, i.e., in the absence of an external base. The precipitation is fast (thus leading to microcrystalline solids and higher yields) when an external base (Et3N, method B; MeCO2−, method C) is present. It is evident that Et3N and MeCO2− favor rapid kinetics of the precipitation.
3.4. Description of the Crystal Structure of H2L (Polymorph B) and Comparison with Polymorph A; Powder X-Ray Diffraction Pattern of the Crude Product
Views of the molecular structure of H2L(B) (space group Pbca) are presented in Figure 4 and Figure S7. The structures of the two independent molecules of H2L(A) (space group Pna21) [50] are shown in Figure 5. In our compound, the C7-N1 and C16-N2 bond lengths (1.289(2) and 1.288(2) Å, respectively) are indicative of double carbon–nitrogen bonds, whereas the C14-N1 and C15-N2 distances (1.463(2) and 1.472(2) Å) clearly suggest single bonds. The C1-O1 and C28-O2 bonds are single, as evidenced by the corresponding distances of 1.342(2) and 1.355(2) Å. There are two strong intramolecular H bonds with the phenol oxygen atoms (O1, O2) as donors and the imino nitrogen atoms (N1, N2) as acceptors; their dimensions are O1⋯N1 2.537(2) Å, H1⋯N1 1.57(2) Å, O1-H1⋯N1 147.2(2)°, and O(2)⋯N2 2.541(2) Å, H2⋯N2 1.56(2) Å, O(2)-H2⋯N2 151.1(2)°. The angle between the planar C1C2C3C4C5C6 and C23C24C25C26C27C28 phenol rings is 74.4°. As a result, the four potentially donor atoms (O1, O2, N1, N2) deviate significantly from their best mean plane, i.e., by 0.418 (O1), 0.235 (O2), 0.748 (N1), and 0.566 (N2) Å.
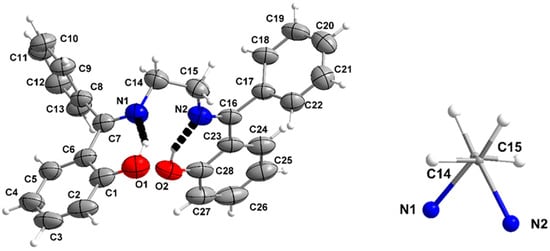
Figure 4.
(Left). ORTEP-type plot of the structure of the molecule in H2L(B). The dashed black lines indicate intramolecular H bonds (Right). The conformation of the central NCCN skeleton of the molecule. Displacement ellipsoids are drawn at the 50% probability level.

Figure 5.
(Left). ORTEP-type plots of the structures of the N11⋯N12 (a)- and N21⋯N22 (b)- containing molecules of H2L(A) [50]. The dashed black lines indicate intramolecular H bonds; their dimensions are listed in Table S2. (Right). The conformation of the central NCCN skeleton of the two molecules. Displacement ellipsoids are drawn at the 50% probability level.
The two independent molecules of H2L(A) adopt a gauche conformation, since the dihedral angles N21-C214-C215-N22 and N11-C114-C115-N12 take the values 62.5(6)° and −63.4(6)°, respectively (right parts in Figure 5a,b). In the molecule of H2L(B), the dihedral angle N1-C14-C15-N2 takes the value 64.9(2)° (right part in Figure 4), which means that this molecule has a similar conformation of the N21C214C215N22-containing molecule in H2L(A). Although this is evident by comparing the right parts in Figure 4 and Figure 5b, it is becoming clear by the overlay presentation of the three molecules in Figure 6.
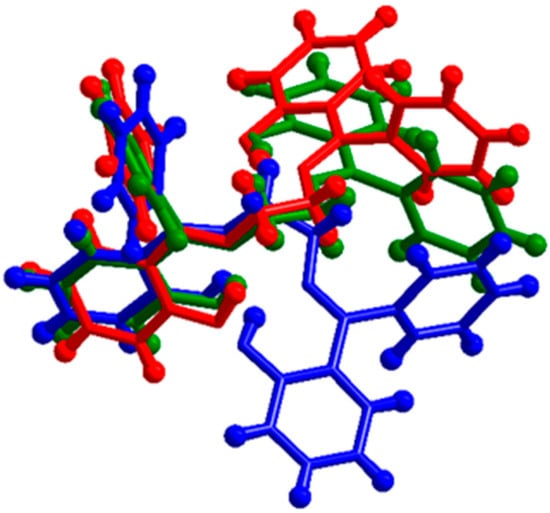
Figure 6.
Overlay presentation of the three N1⋯N2 (present study, H2L(B), red)-, N11⋯N12 (H2L(A), blue)-, and N21⋯N22 (H2L(A), green)- containing molecules in the two polymorphs H2L(A) and H2L(B).
The crystal packing features of H2L(B) are interesting; their geometrical characteristics appear in Table S1. The intermolecular interactions were also studied by the use of the Hirshfeld analysis tools. The percentage contributions of the different types of H⋯H, C⋯H/H⋯C, O⋯H/H⋯O, N⋯H/H⋯N, C⋯N/N⋯C, and C⋯C interactions are 57.1, 32.5, 9.2, 0.6, 0.5, and 0.1%, respectively. The crystal architecture of the compound is mainly based on C⋯H/H⋯C and O⋯H/H⋯O types of interactions. Through the C11-H11⋯O2, C26-H26⋯O1, and C9-H9⋯Cg1* intermolecular interactions, layers of molecules parallel to the (001) crystallographic direction are formed (Figure S8a,b); Cg1* is the centroid of the C1C2…C6 ring. These layers are stacked along the α axis (Figure 7) and interact through the rest of the C-H⋯π interactions listed in Table S1.
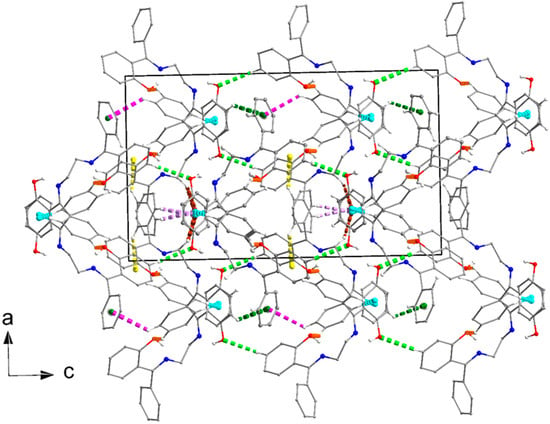
Figure 7.
Layers of molecules in the crystal structure of H2L(B) stacked along the α axis. Dashed violet, brown, pink, dark green, and yellow lines indicate the C22-H22⋯Cg1*, C15-H15B⋯Cg1*, C12-H12⋯Cg2**, C3-H3⋯Cg2**, and C18-H18⋯Cg3*** intermolecular interactions, respectively. Cg1*, Cg2**, and Cg3*** are the centroids of the C1C2…C6, C17C18…C22, and C23C24…C28 rings, respectively. Geometrical characteristics are listed in Table S1. The intramolecular H bonds (see Figure 4) and the H atoms not involved in any interaction are not shown for clarity reasons.
All the types of interactions discussed above are also clearly seen on the dnorm Hirsfeld surface (HS) representations (Figure 8a,b,d). On the Shape decorated HS, the characteristic complementary red (concave) and blue (convex) areas characteristic of the C-H⋯π interactions are present (Figure 8c,e). The drawing f in the same figure shows the fingerprint plot for the structure of H2L(B).
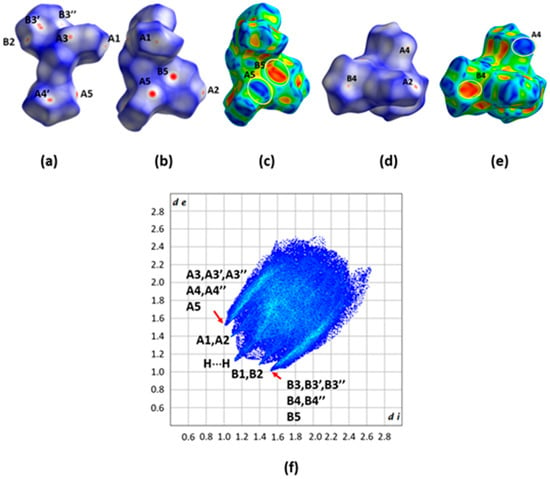
Figure 8.
(a,b,d) Different views of the dnorm decorated HS. (c,e) Two views of the Shape decorated HS. (f) The fingerprint plot of H2L(B). A1, A2, A3, A3′, A3″, A4, A4″, A5 and B1, B2, B3, B3′, B3″, B4, B4″, B5 are the donor and acceptor contact points of the C11-H11⋯O2, C26-H26⋯O1, C9-H9⋯Cg1*, C22-H22⋯Cg1*, C15-H15B⋯Cg1*, C12-H12⋯Cg2**, C3-H3⋯Cg2**, and C18-H18⋯Cg3*** interactions, respectively (Table S1). Cg1*, Cg2**, and Cg3*** have been defined in the caption of Figure 7. The white and orange ellipses in (c,e) drawings indicate the characteristic complementary blue and red areas for the C-H⋯π-type interactions.
In the case of H2L(A) [50], as already mentioned previously, two symmetry-independent molecules exist in the asymmetric unit of the cell. The analysis with the HS tools for the N11⋯N12-containing molecule (Figure 5) gives for the interactions H⋯H, C⋯H/H⋯C, O⋯H/H⋯O, C⋯C, N⋯H/H⋯N, and C⋯N/N⋯C contributions of 57.8, 27.6, 9.5, 3.0, 1.7, and 0.3%, respectively. For the N21⋯N22-containing molecule (Figure 5), the contributions for the corresponding interactions are 55.9, 29.2, 9.5, 3.2, 1.7 and 0.3%. Figure 9 presents the fingerprint plots for the two molecules. By comparing the plots of this figure with the drawing of Figure 8, it is clear that differences in the distribution of di, de points result from structures with different packing. In the published structure of H2L(A), there is a decrease in the C-H/H⋯C interactions and an increase in the C⋯C type of interactions, which corresponds to π-π overlap. All the C-H⋯O, and almost all the C-H⋯π (except C210-H210⋯Cg3***, Table S2) and π-π, interactions result in the formation of layers parallel to the (001) plane (Figure S9). The π-π overlaps are between the rings C223C224…C228 (with the Cg4**** centroid) and C123iC124i…C128i (with the Cg2** centroid), the Cg4****⋯Cg2**I distance being 3.931(2) Å; the angle between the planes is 1.4° (symmetry code: i x, −1 + y, z). C210-H210⋯Cg3*** interactions (Table S2) contribute to the formation of the 3D architecture of the structure (Figure 10).
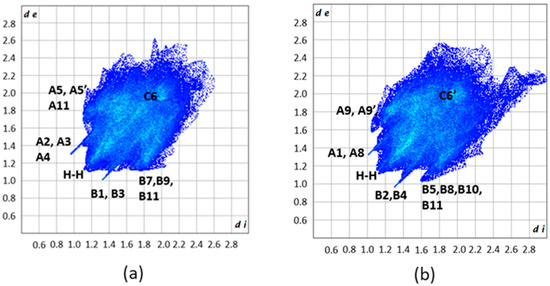
Figure 9.
Fingerprint plots of the N11⋯N12- (a) and N21⋯N22 (b)- containing molecules of H2L(A). A1, A2, A3, A4, A5, A5′, A8, A9, A9′, A11 and B1, B2, B3, B4, B5, B7, B8, B9, B10, B11 are the donor and acceptor contact points of the C113-H113⋯O21, C213-H213⋯O11, C226-H226⋯O11i, C215-H21D⋯Cg1*, C15-H15⋯Cg2**, C119-H119⋯O12ii, C121-H121⋯Cg1*ii, C219-H219⋯O22iii, C29-H29⋯Cg2**iv,and C210-H210⋯Cg3***, respectively. Cg1*, Cg2**, and Cg3*** are the centroids of the C11C12…C16, C217C218…C222 and C123C124…C128 rings, respectively. Symmetry codes: i x, −1 + y, z; ii −0.5 + x, 0.5 − y, z; iii 0.5 + x, −0.5 − y, z; iv − 0.5 + x, −0.5 − y, z. C6 and C6′ indicate areas of C⋯C contact points.
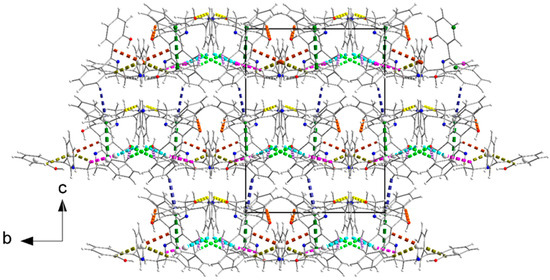
Figure 10.
The 3D architecture of the structure of H2L(A) [50]. Dashed orange, violet, light green, pink, brown, yellow, cyan, gray, and dark yellow lines represent the C113-H113⋯O21, C213-H213⋯O11, C226-H226⋯O11i, C215-H21D⋯Cg1*, C15-H15⋯Cg2**, C119-H119⋯O12ii, C121-H121⋯Cg1*ii, C219-H219⋯O22iii,and C29-H29⋯Cg2**iv intralayer interactions, respectively. The π-π interactions are indicated with dashed dark green lines. The C210-H210⋯Cg3*** interlayer interactions are indicated with dashed indigo lines. Cg1*, Cg2**, and Cg3*** are the centroids of the C11C12…C16, C217C218…C222, and C123C124…C128 rings, respectively. Symmetry codes: I x, −1 + y, z; ii −0.5 + x, 0.5 − y, z; iii 0.5 + x, −0.5 − y, z; iv −0.5 + x, −0.5 − y, z. Geometrical parameters are listed in Table S2.
All the types of interactions mentioned above are also clearly seen on the dnorm or Shape decorated HSs (Figure 11). On the Shape decorated HS, the characteristic complementary red (concave) and blue (convex) areas characteristic of C-H⋯π interactions are present (Figure 11c–e,g,i,l–n). In drawings c and g of this figure (areas indicated with C6 and C6′ labels), the diagnostic blue and red triangles of the π⋯π interactions are visible.
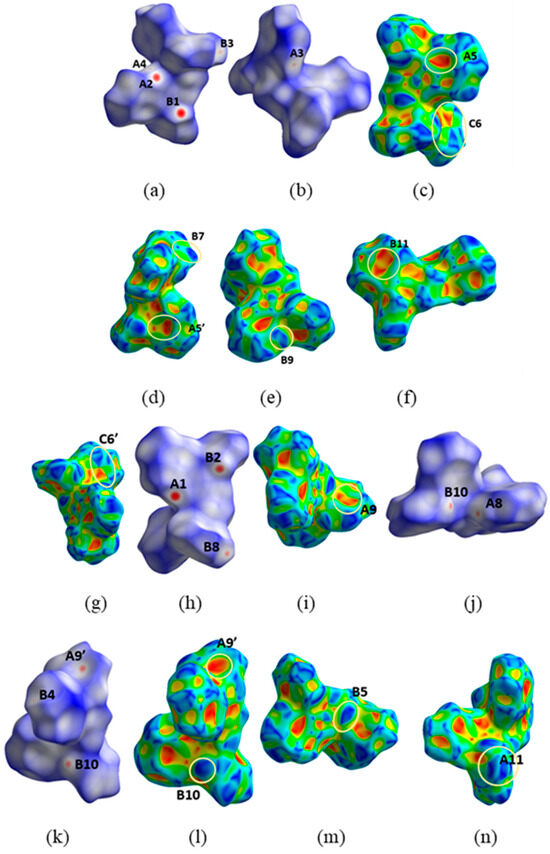
Figure 11.
Different views of the dnorm (a,b) and Shape (c–f) decorated HSs for the N11⋯N12-containing molecule of H2L(A) and the corresponding dnorm (h,j–l) and Shape (g,i,l–n) decorated HS for the N21⋯N22-molecule of the same polymorph. A1, A2, A3, A4, A5, A5′, A8, A9, A9′, A11 and B1, B2, B3, B4, B5, B7, B8, B9, B10, B11 are the donor and acceptor contact points of the C113-H113⋯O21, C213-H213⋯O11, C226-H226⋯O11i, C215-H21D⋯Cg1*, C15-H15⋯Cg2**, C119-H119⋯O12ii, C121-H121⋯Cg1*ii, C219-H219⋯O22iii, C29-H29⋯Cg2**iv, and C210-H210⋯Cg3*** interactions, respectively. For the assignment of the Cg points, see caption of Figure 9 and Figure 10. Areas in circles in the HS decorated with Shape indicate complementary red (concave) and blue (convex) areas of the characteristic blue and red triangles of π-π interactions. Symmetry codes: I x, −1 + y, z; ii −0.5 + x, 0.5 − y, z; iii 0.5+x, −0.5 − y, z; iv −0.5 + x, −0.5 − y, z.
Experimental X-ray diffraction data for the crude H2L sample prepared by us (Section 2.10, Section 2), and theoretical diffraction patterns (derived from the CIFs) for polymorphs H2L(A) and H2L(B) are presented in Figures S10–S13. It is evident that the crystalline powder of H2L prepared by us neither represents one of the polymorphs A or B, nor a mixture of them; on the contrary, it is probably a third polymorph whose crystal structure is still unknown. This experimental fact might be due to several reasons including (a) the absence of Sm(III) in the reaction mixture that led to the isolation of the crude ligand (this was present in the reaction system that gave the polymorph B), (b) the different solvent used for the synthesis of H2L (we used EtOH instead of MeOH which led to the polymorph A [50]), and (c) the flexibility of the Schiff-base H2L molecule which can give several conformers.
3.5. Description of the Molecular Structures of Complexes 1 and 2·DMF
Selected bond distances and angles are listed in Table 2 and Table 3, respectively. Structural plots are shown in Figure 12, Figure 13 and Figures S14–S22.

Table 2.
Selected bond lengths (Å) for complexes [UO2(L)(MeOH)] (1) and [UO2(L)(DMF)]·DMF (2·DMF).

Table 3.
Selected bond angles (°) for complexes [UO2(L)(MeOH)] (1) and [UO2(L)(DMF)]·DMF (2·DMF).
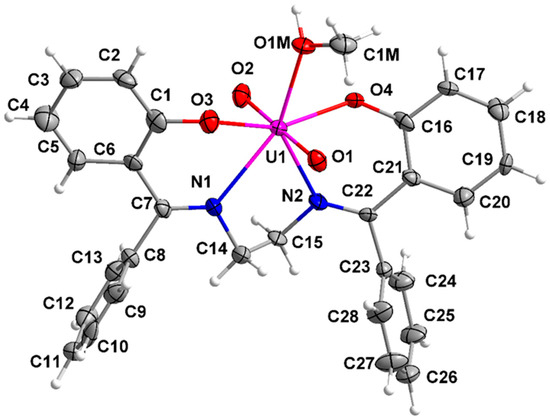
Figure 12.
Fully labeled, ORTEP-type plot of the molecular structure of [UO2(L)(MeOH)] (1). Displacement ellipsoids are drawn at the 50% probability level.
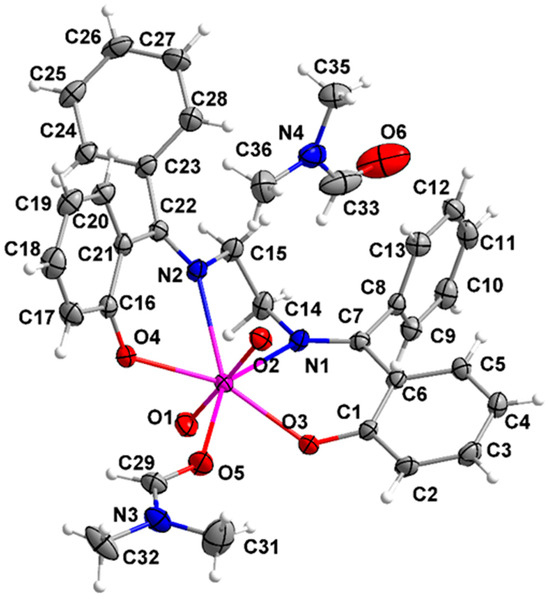
Figure 13.
Fully labeled, ORTEP-type plot of the structure of the molecule [UO2(L)(DMF)] that is present in complex 2·DMF; the lattice DMF solvent molecule is also shown.
The structures of the molecules [UO2(L)(MeOH)] and [UO2(L)(DMF) are similar and thus they are described together. Their asymmetric unit contains one complex molecule (1), and one complex molecule and one lattice solvent molecule (2·DMF). The same labeling scheme is used for the two molecules for convenience and easy comparison. The central UVI atom is surrounded by five oxygen and two nitrogen atoms in a distorted pentagonal bipyramidal geometry. This geometry has been confirmed by the application of the program SHAPE [64]. The two {UVIO2}2+ oxygen atoms (O1, O2) are at the axial positions, as expected. Two oxygen (O3, O4) and two nitrogen (N1, N2) from the tetradentate chelating L2− ligand (1.1111 using Harris notation [65]) are located at the equatorial plane, which is completed by an oxygen atom (O1M in 1, O5 in 2·DMF) of a terminally ligated solvent (MeOH, DMF) molecule.
The U=O bond distances (1.770(7)–1.794(7) Å) and O=U=O angles (178.5(3) and 177.9(1)°) are typical for most uranyl complexes [45,46,48,54,55,66]. The U1-O (L2−) and U1-N bond lengths fall within the typical ranges of mononuclear uranyl complexes with tetradentate N2O2 salen-type Schiff bases (U-O: 2.20–2.33 Å; U-N: 2.51–2.65 Å) [45,48,54,55,66]. The U1-O1M (2.443(7) Å) and U1-O5 (2.415(2) Å) bond lengths are similar with those found in the 7-coordinate complexes [UO2(salen)(MeOH)] [48] and [UO2(salen)(DMF)] [46]. The C14-N1 and C15-N2 distances (1.469(12)–1.486(3) Å) are indicative of single carbon–nitrogen bonds. On the other hand, the C7-N1 and C22-N2 bond lengths (1.289(12)–1.297(12) Å) suggest double bonds, in agreement with the formation of Schiff-base linkages; these short values suggest strong C=N bonds and this might explain their inhibition towards hydrolysis [45].
The bond angles between the neighboring donor atoms in the equatorial plane are in the range 65.2(1)–81.1(3)°. These angles deviate from the ideal value of 72° for a perfect pentagonal bipyramidal geometry, suggesting a distorted coordination polyhedron. The distortion is a consequence of the somewhat small bite angles (65.2(1)–70.3(1)°) which arise from the formation of the N1C14C15N2U1, N1C7C6C1O3U1, and N2C22C21C16O4U1 chelating rings. As a result, the O3-U1-O1M/O5 and O4-U1-O1M/O5 angles are larger than 72° (76.0(2)–81.1(3)°). Furthermore, the five equatorial donor atoms deviate from their best mean plane by 0.060(U1), 0.119(O1M), 0.024(O3), 0.240(O4), 0.155(N1), and 0.252(N2) Å for 1 and 0.028(U1), 0.125(O5), 0.010(O3), 0.223(O4), 0.141(N1), and 0.229 (N2) Å for 2·DMF. The angles between the axial uranyl oxygen atoms and the five equatorial donor atoms for 1 and 2·DMF are in the ranges 81.8(3)–97.1(3)° and 81.8(1)–96.1(1)°, respectively. The angles between the phenol C1C2C3C4C5C6 and C16C17C18C19C20C21 rings is 24.7° for 1 and 78.6° for 2·DMF. The torsion angles N1-C14-C15-N2 are +64.1°(1) and −51.4° (2·DMF). The salen-type ligands with an aliphatic central backbone (like H2L) have advantages over ligands with an aromatic central backbone in that free rotation between the phenol planes allows the ligand freedom to orientate the two donor oxygens for favorable U-O interactions, thus alleviating excess steric repulsions around the UVI center [45].
Tetradentate N2O2 chelating Schiff bases, derived from the 2:1 condensation of salicylaldehyde (or its derivatives) and a diamine, may exhibit a chair (stepped) or a boat conformation in uranyl complexes [46,48,66]. This depends on the relative orientation of the two aromatic phenol rings about the N2O2 plane. When the salicylidene units are at opposite sides of the equatorial plane, the chair conformation is present; on the contrary, if these units are lying on the same side of the N2O2 plane, the ligand adopts a boat conformation. As it is clearly seen in Figure S18, the L2− ligand has a chair conformation in 1 and a boat one in 2·DMF. This is probably due to the different (MeOH, DMF) coordinated solvent molecules. In 1, the atoms U1, O1, O2, O1M, and C1M define a plane, favoring the chair conformation.
Complexes 1 and 2·DMF are only the fourth and fifth members of the family of metal complexes containing H2L or its anions as ligands. The previously structurally characterized compounds are [MnII(L)(py)2] [51], {[MnIII(N3)(L)]·H2O}n [52] and [MnIII(ClO4)(L)(MeOH)] [53], where py = pyridine; the latter can activate dioxygen in the presence of aliphatic aldehydes.
3.6. Supramolecular Features in the Crystal Structures of 1 and 2·DMF
Structural plots are shown in Figure 14, Figure 15, Figure 16, Figure 17 and Figures S19–S22, while numerical data are listed in Tables S3 and S4. Based on the analysis of the fingerprint plots, derived from HS tools, for 1, the interactions H⋯H, C⋯H/H⋯C, O⋯H/H⋯O, N⋯H/H⋯N and C⋯C contribute 53.3, 26.8, 18.2, 1.0, and 0.4%, respectively. All the C-H⋯O and two C-H⋯π interactions (except C4-H4⋯Cg2**, Table S3) result in the formation of layers parallel to the (100) crystallographic plane (Figure S19); Cg2** is the centroid of the C8C9⋯C13 ring. The O1M-H(O1M)⋯O4 H bond with the small donor⋯acceptor distance and high directionality (Table S3) is strong. This interaction appears in pairs, and these are indicated with orange dashed lines in Figures S19 and Figure 14; through this H bond, neighboring centrosymmetrically related molecules form dimers. The layers are stacked along the α axis interacting through the C4-H4⋯Cg2** interactions and thus the 3D architecture of the structure of 1 is built (Figure 14).
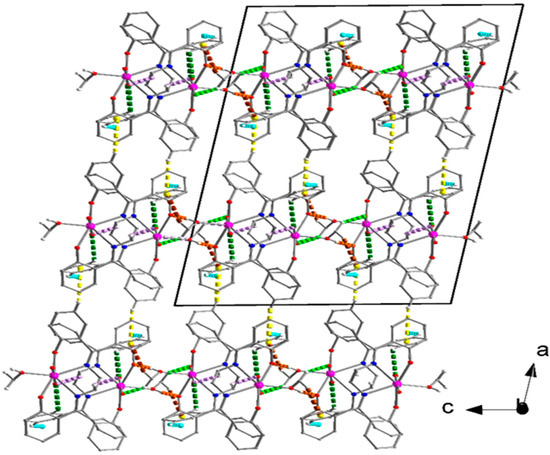
Figure 14.
The 3D architecture of the structure of compound 1. Dashed orange, light green, cyan, violet, green, and brown lines indicate the intralayer O1M-H(O1M)⋯O4, C1M-H3(C1M)⋯O2, C10-H10⋯Cg1*, C15-H15B⋯O1, C13-H13⋯O1, and C1M-H2(C1M)⋯Cg2** interactions, respectively. The interlayer C4-H4⋯Cg2** interactions are represented with dashed indigo lines. Cg1* and Cg2** are the centroids of the C16C17…C21 and C8C9…C13 rings, respectively. Geometrical characteristics are listed in Table S3.
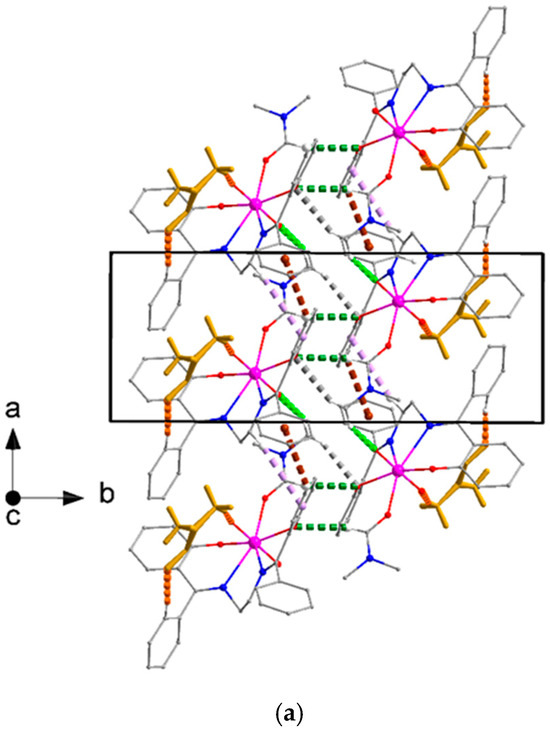
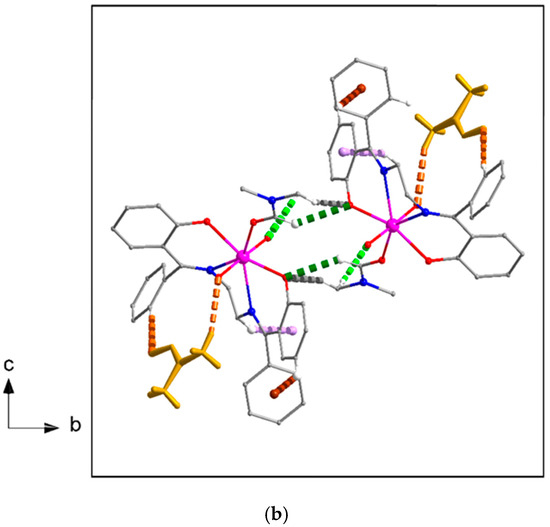
Figure 15.
(a) Top and (b) top and side views of the arrangement of [UO2(L)(DMF)] molecules in stripes parallel to the α axis in the structure of compound 2·DMF. Dashed light green, violet, brown, green, and gray lines indicate the C32-H32A⋯O1, C15-H15B⋯Cg1*, C19-H19⋯Cg2**, C29-H29⋯O4, and C32-H32B⋯O4 interactions, respectively. Lattice DMF solvent molecules are represented in capped stick style with dark yellow color, dashed orange lines indicate the C13-H13⋯O6 and C36-H36A⋯O2 H-bonding interactions. Cg1* and Cg2** are the centroids of the C16C17⋯C21 and C23C24⋯C28 rings, respectively. Geometrical characteristics are listed in Table S4.
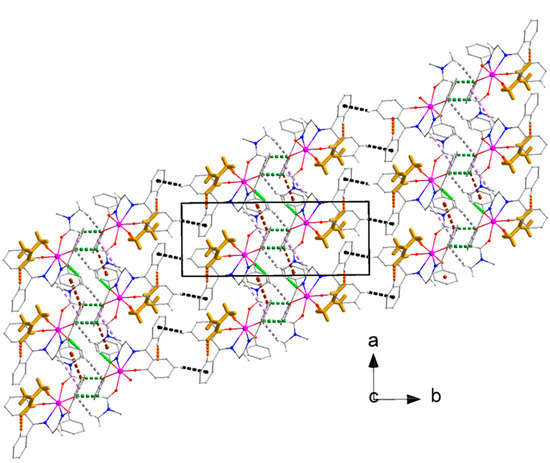
Figure 16.
Layers of molecules [UO2(L)(DMF)] parallel to the (001) plane in the crystal structure of compound 2·DMF. The color assignments of the lattice DMF solvent molecules, the intrastripe interactions, and the lattice DMF solvent⋯[UO2(L)(DMF)] interactions are as in Figure 15. The C4-H4⋯Cg3*** interactions are indicated with dashed black lines. The Cg1* and Cg2** centroids have been defined in the caption of Figure 15. Cg3*** is the centroid of the C8C9…C13 ring. Geometrical characteristics are listed in Table S4.
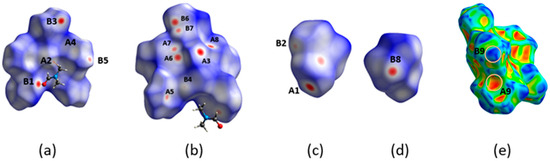
Figure 17.
(a,b) Different views of the dnorm decorated, (c,d) respective views for the DMF solvent molecules and (e) shape decorated HSs for compound 2·DMF. A1, A2, A3, A4, A5, A6, A7, A8, A9 and B1, B2, B3, B4, B5, B6, B7, B8, B9 are the donor and acceptor contact points of the C13-H13⋯O6, C36-H36A⋯O2, C32-H32A⋯O1, C15-H15B⋯Cg1*, C19-H19⋯Cg2**, C29-H29⋯O4, C32-H32B⋯O4, C35-H35B⋯O3, and C4-H4⋯Cg3*** interactions, respectively (Table S4). The orange ellipses in drawing (e) indicate the characteristic complementary blue and red areas for the C4-H4⋯Cg3*** interaction. The Cg1*, Cg2**, and Cg3*** have been defined in the captions of Figure 15 and Figure 16.
All the types of interactions discussed above are also clearly seen on the dnorm and Shape decorated HSs (Figure S20). On the Shape decorated HS, the characteristic complementary red (concave) and blue (convex) areas, typical of the C-H⋯π interactions, are visible. The presence of the O1M-H(O1M)⋯O1 H bonds gives the characteristic sharp spikes (Figure S20a).
Based on the analysis of the fingerprint plots, derived from HS tools for 2·DMF, the interactions H⋯H, C⋯H/H⋯C, O⋯H/H⋯O, C⋯C, N⋯H/H⋯N, C⋯O/O⋯C, and N⋯C/C⋯N contribute 55.8, 21.4, 18.18, 1.8, 1.7, 0.4, and 0.1%, respectively. In the crystal structure, the molecules are arranged in double chains forming stripes along the α axis through the C32-H32A⋯O1, C15-H15B⋯Cg1*, C19-H19⋯Cg2**, C29-H29⋯O4, and C32-H32B⋯O4 interactions (Figure 15a, Table S4). The stripes are extended to layers parallel to the (001) plane through the C4-H4⋯Cg3*** interactions (Figure 16). For the assignments of Cg1*, Cg2**, and Cg3***, see the footnote in Table S4. The layers are stacked along the c axis, building the 3D architecture of the structure. For the formation of the 3D arrangement, molecules belonging to neighboring layers are connected via lattice DMF solvent molecules; the connection is achieved through the C13-H13⋯O6, C36-H36A⋯O2, and C35-H35B⋯O3 interactions (Figure S21). Thus, it is clear that the lattice DMF solvent molecules are involved in interactions with surrounding complex molecules with contributions of 52.2, 29.8, 14.6, 2.6, 0.7, and 0.1% for H⋯H, O⋯H/H⋯O, C⋯H/H⋯C, N⋯H/H⋯N, N⋯C/C⋯N, and C⋯C interactions, respectively, in the fingerprint plot diagram (Figure S22b). All the types of interactions discussed above, and originating from the complex and solvent molecules, are also clearly seen on the dnorm and Shape decorated HSs (Figure 17a–d). On the Shape decorated HS, the characteristic red (concave) and blue (convex) areas, typical of C-H⋯π interactions, are visible (Figure 17e). The red point that appears on the dnorm decorated HS in Figure 17b, which lies below the lattice DMF solvent molecule, indicates a probable interaction with the underneath phenyl ring of the complex.
3.7. Spectroscopic Characterization of 1 and 2·DMF in the Solid State
The two solid uranyl complexes were characterized by IR, Raman, UV-Vis (only for 1), and emission spectroscopic techniques in the solid state. Representative spectra are presented in Figures S23–S31.
The IR spectrum of 1 at ~3445 cm−1 is attributable to the ν(OH) of coordinated MeOH; its broadness indicates H-bonding interactions established by crystallography (vide supra). The presence of coordinated DMF in the analytically pure sample of 2·DMF (i.e., [UO2(L)(DMF)]) is reflected in the strong ν(C=O) band at 1649 cm−1; this mode appears at 1640 cm−1 in its Raman spectrum. The ν(C=N) IR vibration appears at 1597 (1) and 1587 (2) cm−1; this band has been shifted to a lower frequency compared to the free ligand (located at 1607 cm−1) due to deprotonation and coordination [66]. A similar shift is not observed in the Raman spectra. A strong IR band at 917 (1) and 893 (2) cm−1 is assigned to the IR-active antisymmetric stretching vibration (ν3) of the uranyl group, νas(O=UVI=O) [54,55,66,67]. Thus, it is evident that a change in the monodentate equatorial ligand has a remarkable effect on the force constant of the U=O bond, despite the similar U=O bond lengths in the two complexes (Table 2). A reason for this might be the non-pure character of this vibration in the spectra of the complexes, since the free ligand also exhibits a medium-intensity band at 920 cm−1. The ν3 band in the spectra of the complexes has been shifted to lower wavenumbers compared to this band in aquo uranyl compounds (>950 cm−1), suggesting weaker U=O bonds in 1 and 2. This has been explained in terms of the negative charges in the equatorial plane of our complexes (arising from the phenolato oxygen atoms) and the involvement of the Oyl atoms (the oxido atoms) in H-bonding interactions; both phenomena weaken the axial uranium(VI)-oxygen bonds. The strong Raman peaks at 817 (1) and 812 (2) cm−1 are safely assigned to the Raman-active symmetric stretching mode (ν1) of the uranyl group, ν5 (O=UVI=O) [54,55,66,67]. The νs(UO2) mode is also observed in the IR spectra of the two complexes as a weak band at ~805 cm−1; this suggests a change in the dipole moment for this stretching mode. This IR activation of the ν1 mode has been observed in several uranyl salts and was explained in terms of non-linearity of the O=U=O group [66]. In 1 and 2·DMF, however, the O=U=O angle does not deviate remarkably from 180° (Table 3), and each pair of U=O bond lengths are similar for the two complexes. This spectroscopic feature is most probably due to the lowering of the overall symmetry of each complex in the solid state [66], partially due to the presence of the fifth monodentate equatorial group.
The solid-state electronic spectrum of 1 (Figure S27) exhibits bands at 253, 290(sh), 351, 395(sh) and 510 nm; the bands’ position was determined after deconvolution. The first two bands are assigned to π → π* transitions involving molecular orbitals localized on the C=N group and the phenyl rings [68]. The other three bands have their origin in the uranyl group; exact assignments are risky. The spectrum of the linear uranyl cation can be assigned as ligand-to-metal charge-transfer transitions. These are excitations from the “yl-bonding” orbitals (σωσg, πωσg) to the U(VI)-centered non-bonding 5fδ and 5fφorbitals [69]. In most cases, the spectrum in the 500–310 nm region comes from two parity-conserving orbital excitations, σu → 5fδ and σu → 5fφ, superimposed with vibrational fine structures. These excited configurations (abbreviated as σuδ and σuφ) depend little on the nature of the equatorial donor atoms, but they can give rise to several excited states due to three perturbations of similar magnitudes: spin–orbit coupling, equatorial ligand field, and electron correlation [69]. The 510 nm absorption band gives rise to the orange color of the complex. The presence of such a band is evidence [70] of non-linearity within the uranyl group (not observed in the molecular structure of 1) and for weaking of the UVI-Oyl bonds (observed in the crystal structure of 1).
Luminescence spectroscopy of uranyl complexes helps the study of the chemical interactions between the metal ion and the equatorial ligands [69]. The characteristic well-resolved, five-peak green emission pattern exhibited by some uranyl compounds (due to ligand-to-metal charge-transfer transitions) [71,72,73,74] was not observed for solid samples of 1 and 2 at room temperature. Upon excitation at 400 nm, the two complexes exhibit a strong emission at 600 nm (Figures S29 and S31). With the data at hand, it is difficult to assign this common band. This well-resolved emission peak is tentatively attributed to the uranyl cation [73,74]. An alternative assignment is that this is (L)2-based; the red shift by ~80 nm in comparison with the emission of H2L (at ~520 nm, Figure 3) might be due to the deprotonation and simultaneous coordination of the ligand [55]. It should be mentioned at this point that the solid free ligand H2L emits light also at ~520 nm after excitation at 400 nm, i.e., its emission spectrum is practically the same as that shown in Figure 3 (recorded after excitations at 355, 384, 418, or 457 nm).
3.8. Solution Behavior of the Two Uranyl Complexes
Although this was not the goal of our work, an attempt was made to study the behavior of the complex in the solution. This was carried out by conductivity measurements, 1H and 13C NMR spectroscopy and UV-Vis technique. Data are presented in Figures S32–S35.
The molar conductivities, ΛΜ, in DMSO are 9 (for 1) and 4 (for sample 2) S cm2 mol−1, respectively, suggesting the presence of neutral species in this solvent [75]. The 1H and 13C NMR spectra of 1 and 2 in CDCl3 (see Section 2.10) are of poor quality due to solubility reasons. Somewhat to our surprise, the 1H and 13C NMR spectra of 1 and free H2L in d6-DMSO are practically identical, the only difference being the signals due to MeOH. Note that the color of the solutions was yellow rather than orange (the color of the solid complex). Thus, the 1H NMR spectrum of this complex exhibits extra singlet signals at δ 4.00 and 3.17 ppm (with an integration ratio of 1:3) due to the -OH and -CH3 protons of free MeOH [76]. Similarly, the 13C NMR spectrum displays an extra signal at δ 49.01 ppm assigned to the MeOH carbon [76]. The -OH protons of the neutral organic ligand (H2L) are clearly visible in the 1H NMR spectrum of 1 at δ 15.19 ppm, the integration with the other protons being the expected one. The UV-Vis spectrum (also in DMSO) of the complex shows the π → π* bands of the organic ligand at 247 and 280 nm. The spectrum of the yellow solution is different from that in the solid state (see Section 2.10). The combined interpretation of the above-mentioned solution data suggests that 1 decomposes in DMSO giving free H2L molecules in solution. The source of the protons (the complex contains L2−) cannot be MeOH because this is in the neutral form (1H NMR evidence). Thus, our conclusions that the protons come from H2O contained in DMSO. Therefore, our proposal for the decomposition is illustrated in Equations (4)–(6). The OH− protons in the final soluble uranyl species are responsible for the singlet signal at δ 3.06 ppm. Analogous decomposition processes have been observed for other uranyl complexes from our group [20].
The solution behavior of an analytically pure sample of 2·DMF (i.e., 2) is different and most probably the solid-state structure is retained in both chloroform and dimethylsulfoxide. In agreement with this, solutions of 2 in DMSO are non-conductive (vide supra). The -OH signals are lacking in the 1H NMR spectra of the complex in d6-DMSO and CDCl3 (despite the poor quality of the latter), indicating that the ligand is deprotonated. The spectra clearly show the DMF signals. The -CH- proton appears as a singlet resonance at δ 8.10 (CDCl3) and 7.95 (d6-DMSO) ppm, while the expected two singlet signals for the -CH3 protons are visible at δ 2.98, 2.93 (CDCl3) and 2.89, 2.73 (d6-DMSO) ppm. The corresponding 13C NMR signals in d6-DMSO are at δ 163.23 (-CH-) and 36.25, 31.55 ppm (-CH3). The fact that the 1H and 13C NMR -CH- and -CH3 resonances of DMF in the spectra of the complex appear at almost identical δ values with those of free DMF in d6-DMSO [76] might indicate the replacement of the coordinated solvent molecule by a d6-DMSO molecule; this is reasonable given the large excess of the deuterated solvent in the NMR tube. Moreover, the 1H and 13C NMR signals of 2 have been shifted compared to those in H2L (and 1) due to the deprotonation and coordination of the Schiff base [68]. Additional evidence for the retainment of the structure of 2 in CHCl3 (data in DMSO are not available) is the orange color of the solution and the ligand-to-metal charge-transfer transition at ~500 nm in its UV-Vis spectrum; this band also appears in the solid-state UV-Vis spectrum of 1 (vide supra).
4. Concluding Remarks
In this work, we believe that we have contributed to the area of the synthetic and structural chemistry of uranyl/Schiff-base complexes. According to our opinion, the important messages of this work are: (a) We have fully characterized the bulky Schiff base H2L by a variety of spectroscopic methods; for this compound only its crystal structure had been reported [50]. (b) A new polymorph, H2L(B), of the ligand has been discovered during our efforts to prepare a Sm(III) complex; the crystal structures reveal that the two polymorphs have, as expected, different packing patterns. (c) The uranyl complexes [UO2(L)(MeOH)] (1) and [UO2(L)(DMF)]·DMF (2·DMF), rare examples of metal complexes with H2L as ligand, possess rather similar molecular structures; the main difference is the chair and boat conformations for 1 and 2·DMF, which is most probably a consequence of the different nature of the coordinated solvent molecules. (d) The two complexes exhibit different supramolecular features due to the different H-bonding abilities of MeOH and DMF, the presence of lattice DMF molecule in 2·DMF and the different conformations of L2−. (e) An attempt has been made to probe the solution behavior of the two complexes which shows that only the molecular structure of 2 is retained in CHCl3 and DMSO. A clear explanation of this difference would be risky. We tentatively propose that the decomposition of 1 is due to the easy replacement of MeOH by H2O (contained in the solvent), Equations (4)–(6), compared with the replacement of DMF.
With the knowledge and experience obtained in this work, our future activities are directed to several avenues including: (1) The employment of H2L in first transition-metal (except manganese for which complexes are known [51,52,53]), lanthanoid(III) and thorium (IV) chemistries; (2) studies on the reactivity of coordinated H2L, i.e., metal ion-assisted/promoted transformations of the ligand; (3) preparation of homo- and heterometallic dinuclear and polynuclear complexes in noncoordinating solvents, exploiting the bridging ability of the phenolato oxygen atoms [77]; and, (4) the study of the coordination chemistry of other salen-type ligands towards the uranyl ion, e.g., the ligand with X = H, R = Me and Z = CH2CH2 in Scheme 1. Preliminary results on the goals 1, 2, and 4 above have been obtained and will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15110974/s1, Figures S1–S6: Spectroscopic data for the free ligand H2L, Figures S7–S9: Molecular and supramolecular plots for polymorphs H2L(B) and H2L(A), Figures S10–S13: Experimental and theoretical (derived from the CIFs) powder X-ray diffraction data for the bulk sample of H2L, and the polymorphs H2L(A) and H2L(B), Figures S14–S22: Molecular, supramolecular and Hirshfeld Surface structural plots for complexes [UO2(L)(MeOH)] (1) and [UO2(L)(DMF)]·DMF (2·DMF), Figures S23–S31: Solid-state spectroscopic data for complexes 1 and 2·DMF, Figures S32–S35: Solution spectroscopic data for complexes 1 and 2·DMF. Tables S1–S4: Numerical data for the supramolecular interactions in the polymorphs A and B, and complexes 1 and 2.DMF. CIF files of the structures of H2L(B), 1 and 2·DMF were deposited by the Cambridge Crystallographic Data Centre: 2493661 (polymorph H2L(B)); 2493662 (Complex 1); 2493663 (Complex 2·DMF). Copies can be obtained free of charge on written application to CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44-1223-336033); or by access to http://www.ccdc.cam.ac.uk (7 October 2025).
Author Contributions
Conceptualization, V.P., S.T.T. and S.P.P.; validation, S.G.S. and I.T.P.; methodology, C.P.R., Z.G.L. and V.B.; investigation, Z.G.L. and C.P.R.; writing—original draft preparation, V.P., S.T.T. and S.P.P.; writing—review and editing, S.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
Sotiris G. Skiadas was financially supported by the “Andreas Mentzelopoulos Foundation-2024”.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaltsoyannis, N.; Liddle, S.T. Catalyst: Nuclear Power in the 21st Century. Chem 2016, 1, 659–665. [Google Scholar] [CrossRef]
- Burns, C.J.; Neu, M.P.; Boukhalfa, H.; Gutowski, K.E.; Bridges, N.J.; Rogers, R.D. The Actinides. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 3, pp. 189–330. [Google Scholar]
- Gordon, J.C.; Czerwinski, K.; Francesconi, L. Preface: Forum on Aspects of Inorganic Chemistry Related to Nuclear Energy. Inorg. Chem. 2013, 52, 3405–3406. [Google Scholar] [CrossRef]
- Liddle, S.T. The Renaissance of Non-Aqueous Uranium Chemistry. Angew. Chem. Int. Ed. 2015, 54, 8604–8641. [Google Scholar] [CrossRef]
- Liddle, S.T. Progress in Nonaqueous Molecular Uranium Chemistry: Where to Next? Inorg. Chem. 2024, 63, 9366–9384. [Google Scholar] [CrossRef]
- Eralie, D.M.T.; Ducilon, J.; Gorden, A.E.V. Uranium Chemistry: Identifying the Next Frontiers. Inorg. Chem. 2025, 64, 767–784. [Google Scholar] [CrossRef]
- Tsoureas, N.; Vagiakos, I. Recent Advances in Low Valent Thorium and Uranium Chemistry. Inorganics 2024, 12, 275. [Google Scholar] [CrossRef]
- Barluzzi, L.; Giblin, S.R.; Mansikkamäki, A.; Layfield, R.A. Identification of Oxidation State +1 in a Molecular Uranium Complex. J. Am. Chem. Soc. 2022, 144, 18229–18233. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 5th ed.; Pearson: Harlow, UK, 2018; pp. 1053–1058. [Google Scholar]
- Tsantis, S.T.; Iliopoulou, M.; Tzimopoulos, D.I.; Perlepes, S.P. Synthetic and Structural Chemistry of Uranyl-Amidoxime Complexes: Technological Implications. Chemistry 2023, 5, 1419–1453. [Google Scholar] [CrossRef]
- Odoh, S.O.; Bondarevsky, G.D.; Karpus, J.; Cui, Q.; He, C.; Spezia, R.; Gagliardi, L. UO22+ uptake by proteins: Understanding the binding features of the super uranyl protein and design of a protein with higher affinity. J. Am. Chem. Soc. 2014, 136, 17484–17494. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, Z.-H.; Olds, T.; Stemiczuk, M.; Burns, P.C. Copper(I) and Copper(II) Uranyl Heterometallic Hybrid Materials. Inorg. Chem. 2014, 53, 7993–7998. [Google Scholar]
- Xie, J.; Wang, Y.; Silver, M.A.; Liu, W.; Duan, T.; Yin, X.; Chen, L.; Diwu, J.; Chai, Z.; Wang, S. Tunable 4f/5f Bimodal Emission in Europium-Incorporated Uranyl Coordination Polymers. Inorg. Chem. 2018, 57, 575–582. [Google Scholar] [CrossRef]
- Behera, N.; Sethi, S. Unprecedented Catalytic Behavior of Uranyl(VI) Compounds in Chemical Reactions. Eur. J. Inorg. Chem. 2021, 95–111. [Google Scholar] [CrossRef]
- Lobeck, H.L.; Isner, J.K.; Burns, P.C. Transformation of Uranyl Peroxide Studtite [(UO2(O2)(H2O)2](H2O)2 to a Soluble Nanoscale Cage Cluster. Inorg. Chem. 2019, 58, 6781–6789. [Google Scholar] [CrossRef]
- Harrowfield, J.; Thuery, P. Uranyl Ion Complexes of Polycarboxylates: Steps towards Isolated Photoactive Cavities. Chemistry 2020, 2, 63–79. [Google Scholar] [CrossRef]
- Ai, J.; Chen, F.-Y.; Gao, C.-Y.; Tian, H.R.; Pan, Q.-J.; Sun, Z.-M. Porous anionic uranyl-organic networks for highly efficient Cs+ adsorption and investigation of the mechanism. Inorg. Chem. 2018, 57, 4419–4426. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wu, Y.; Li, J.; Hu, B.; Cai, Y.; Yuan, L.; Feng, W. Selective Complexation and Separation of Uranium(IV) from Thorium(IV) with New Tetradentate N,O-Hybrid Diamide Ligands: Synthesis, Extraction, Spectroscopy, and Crystallographic Studies. Inorg. Chem. 2023, 62, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Abney, C.W.; Mayes, R.T.; Saito, T.; Dai, S. Materials for the Recovery of Uranium from Seawater. Chem. Rev. 2017, 117, 13935–14013. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Lada, Z.G.; Skiadas, S.G.; Tzimopoulos, D.I.; Raptopoulou, C.P.; Psycharis, V.; Perlepes, S.P. Understanding the Selective Extraction of the Uranyl Ion from Seawater with Amidoxime-Functionalized Materials: Uranyl Complexes of Pyrimidine-2-amidoxime. Inorganics 2024, 12, 82. [Google Scholar] [CrossRef]
- Liu, X.; Hamon, J.-R. Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Hernandez-Molina, R.; Mederos, A. Acyclic and Macrocyclic Schiff Base Ligands. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 441–446. [Google Scholar]
- Yamada, S. Advancement in stereochemical aspects of Schiff base metal complexes. Coord. Chem. Rev. 1999, 190, 537–555. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Long, J. Luminescent Schiff-Base Lanthanide Single-Molecule Magnets. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef]
- Constable, E.C. Metals and Ligands Reactivity; VCH: Weinheim, Germany, 1996; Volume 135–182, pp. 72–78. [Google Scholar]
- Gisbert, R.J. Coordination Chemistry; Wiley-VCH: Weinheim, Germany, 2008; Volume 133, p. 132. [Google Scholar]
- Cozzi, P.G. Metal-salen Schiff base complexes in catalysis. Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Righetto, S.; Pasini, A. Push-pull unsymmetrical substitution in nickel(II) complexes with tetradentate N2O2 Schiff base ligands. Synthesis, structures and linear-nonlinear optical studies. Dalton Trans. 2019, 48, 11217–11234. [Google Scholar] [CrossRef]
- Alexopoulou, K.I.; Terzis, A.; Raptopoulou, C.P.; Psycharis, V.; Escuer, A.; Perlepes, S.P. Ni20 “bowls” from the use of tridentate Schiff bases. Inorg. Chem. 2015, 54, 5615–5617. [Google Scholar] [CrossRef] [PubMed]
- Camp, C.; Guidal, V.; Biswas, B.; Pécaut, J.; Dubois, L.; Mazzanti, M. Multielectron redox chemistry of lanthanide Schiff-base complexes. Chem. Sci. 2012, 3, 2433–2448. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Li, Y.; Wei, P.; Sun, T.; Xiao, C.; Cheng, J.; Xu, C. Selective Separation and Complexation of Trivalent Actinide and Lanthanide by a Tetradentate Soft-Hard Donor Ligand: Solvent Extraction, Spectroscopy, and DFT Calculations. Inorg. Chem. 2019, 58, 4420–4430. [Google Scholar] [CrossRef]
- Costamagna, J.; Vargas, J.; Latorre, R.; Alvarado, A.; Mena, G. Coordination compounds of copper, nickel and iron with Schiff bases derived from hydroxynaphthaldehydes and salicylaldehydes. Coord. Chem. Rev. 1992, 119, 67–88. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 1999; Volume 375, p. 375. [Google Scholar]
- Tsantis, S.T.; Tzimopoulos, D.I.; Holynska, M.; Perlepes, S.P. Oligonuclear Actinoid Complexes with Schiff Bases as Ligands-Older Achievements and Recent Progress. Int. J. Mol. Sci. 2020, 21, 555. [Google Scholar] [CrossRef] [PubMed]
- Costisor, O.; Linert, W. 4f and 5f Metal Ion Directed Schiff Condensation. Rev. Inorg. Chem. 2004, 24, 61–95. [Google Scholar] [CrossRef]
- Maurya, M.R.; Maurya, R.C. Coordination Chemistry of Schiff Base Complexes of Uranium. Rev. Inorg. Chem. 1995, 15, 1–107. [Google Scholar] [CrossRef]
- Rudkevich, D.M.; Verboom, W.; Brzozka, Z.; Palys, M.J.; Stauthamer, W.P.R.V.; van Hummel, G.J.; Franken, S.M.; Harkema, S.; Engbersen, J.F.J.; Reinhoudt, D.N. Functionalized UO2 salenes: Neutral receptors for anions. J. Am. Chem. Soc. 1994, 116, 4341–4351. [Google Scholar] [CrossRef]
- Van Axel Castelli, V.; Cort Dalla, A.; Mandolini, L. Supramolecular catalysis of 1,4-thiol addition by salophen-uranyl complexes. J. Am. Chem. Soc. 1998, 120, 12688–12689. [Google Scholar] [CrossRef]
- Sahu, S.K.; Chakravorty, V. Extraction of uranium(VI) with binary mixtures of a quadridentate Schiff base and various neutral donors. J. Radioanal. Nucl. Chem. 1998, 227, 163–165. [Google Scholar] [CrossRef]
- Hawkins, C.A.; Bustillos, C.G.; Copping, R.; Scott, B.L.; May, I.; Nilsson, M. Challenging conventional f-element separation chemistry-reversing uranyl(VI)/lanthanide(III) solvent extraction selectivity. Chem. Commun. 2014, 50, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Cowie, B.E.; Purkins, J.M.; Austin, J.; Love, J.B.; Arnold, P.L. Thermal and Photochemical Reduction and Functionalization Chemistry of the Uranyl Dication, [UVIO2]2+. Chem. Rev. 2019, 119, 10595–10637. [Google Scholar] [CrossRef]
- Takeyama, T.; Tsushima, S.; Gericke, R.; Duckworth, T.M.; Kaden, P.; März, J.; Takao, K. A Series of AnVIO22+ Complexes (An = U, Np, Pu) with N3O2-Donating Schiff-Base Ligands: Systematic Trends in the Molecular Structures and Redox Behavior. Inorg. Chem. 2025, 64, 1313–1322. [Google Scholar] [CrossRef]
- Klamm, B.E.; Windorff, C.J.; Celis-Barros, C.; Marsh, M.L.; Albrecht-Schmitt, T.E. Synthesis, Spectroscopy and Theoretical Details of Uranyl Schiff-Base Coordination Complexes. Inorg. Chem. 2020, 59, 23–31. [Google Scholar] [CrossRef]
- Bharara, M.S.; Tonks, S.A.; Gorden, A.E.V. Uranyl stabilized Schiff base complex. Chem. Commun. 2007, 4006–4008. [Google Scholar] [CrossRef] [PubMed]
- Hazra, D.K.; Dinda, S.; Helliwell, M.; Bhattacharyya, R.; Mukherjee, M. Synthesis, spectroscopic characterization and X-ray structure analyses of two uranyl complexes. Z. Kristallogr. 2009, 224, 544–550. [Google Scholar] [CrossRef]
- Evans, D.J.; Junk, P.C.; Smith, M.K. The effect of coordinated solvent ligands on the solid-state structures of compounds involving uranyl nitrate and Schiff bases. Polyhedron 2002, 21, 2421–2431. [Google Scholar] [CrossRef]
- Bandoli, G.; Clemente, D.A.; Croatto, U.; Vidali, M.; Vigato, P.A. Crystal and Molecular Structure of [N,N′-Ethylenebis(salicylideneiminato)](methanol)dioxouranium. J. Chem. Soc. Dalton 1973, 2331–2335. [Google Scholar] [CrossRef]
- Bustillos, C.G.; Hawkins, C.A.; Copping, R.; Reilly, S.D.; Scott, B.L.; May, I.; Nilsson, M. Complexation of High-Valency Mid-Actinides by a Lipophilic Schiff Base Ligand. Synthesis, Structural Characterization and Progress toward Selective Extraction. Inorg. Chem. 2019, 58, 3559–3563. [Google Scholar] [CrossRef]
- Corden, J.P.; Errington, W.; Moore, P.; Wallbridge, M.G.H. Two Schiff Base Ligands Derived from 1,2-Diaminoethane. Acta Crystallogr. Sect. C 1997, 53, 486–488. [Google Scholar] [CrossRef]
- Srinivasan, K.; Michaud, P.; Kochi, J.K. Epoxidation of Olefins with Cationic (salen)MnIII Complexes. The Modulation of Catalytic Activity by Substituents. J. Am. Chem. Soc. 1986, 108, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Lim, K.S.; Ryu, D.W.; Yoon, S.M.; Suh, B.J.; Hong, C.S. Synthesis, Structures and Magnetic Properties of End-to-End Azide-Bridged Manganese(III) Chains: Elucidation of Direct Magnetostructural Correlation. Inorg. Chem. 2014, 53, 7936–7940. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ishikawa, T.; Harada, A.; Ohba, S.; Sakamoto, M.; Nishida, Y. Chemical mechanism of dioxygen activation by manganese(III) Schiff base compound in the presence of aliphatic aldehydes. Polyhedron 1997, 16, 2553–2561. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Bekiari, V.; Raptopoulou, C.P.; Tzimopoulos, D.I.; Psycharis, V.; Perlepes, S.P. Dioxidouranium(VI) complexes with Schiff bases possessing an ONO donor set: Synthetic, structural and spectroscopic studies. Polyhedron 2018, 152, 172–178. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Lada, Z.G.; Tzimopoulos, D.I.; Bekiari, V.; Psycharis, V.; Raptopoulou, C.P.; Perlepes, S.P. Two different coordination modes of the Schiff base derived from ortho-vanillin and 2-(2-aminomethyl)pyridine in a mononuclear uranyl complex. Heliyon 2022, 8, e09705. [Google Scholar] [CrossRef] [PubMed]
- Crystalclear; Rigaku: The Woodlands, TX, USA, 2005.
- Shedrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Diamond-Crystal and Molecular Structure Visualization, Version 3.1; Crystal Impact: Bonn, Germany, 2018.
- Dollish, F.R.; Fateley, W.G.; Bentley, F.F. Characteristic Raman Frequencies of Organic Compounds; Wiley: New York, NY, USA, 1974; pp. 134–137. [Google Scholar]
- Szarlan, B.; Robaszkiewicz, J.; Pawluc, P.; Kubicki, M.; Zaranek, M. Salen-type nickel(II) complexes for distinct selective hydrosilylation of alkenes under mild conditions. Dalton Trans. 2025, 54, 7088–7094. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 4th ed.; Wiley: New York, NY, USA, 1981; pp. 249–303. [Google Scholar]
- Bernstein, J. Polymorphism in Molecular Crystals; Clarendon Press: Oxford, UK, 2002. [Google Scholar]
- Llunell, M.; Casanova, D.; Girera, J.; Alemany, P.; Alvarez, S. SHAPE, Continuous Shape Measure Calculation, Version 2.0; Universitat de Barcelona: Barcelona, Spain, 2010. [Google Scholar]
- Coxall, R.A.; Harris, S.G.; Henderson, D.K.; Parson, S.; Tasker, P.A.; Winpenny, R.E.P. Inter-ligand reactions: In situ formation of new polydentate ligands. J. Chem. Soc. Dalton Trans. 2000, 14, 2349–2356. [Google Scholar] [CrossRef]
- Hardwick, H.C.; Royal, D.S.; Helliwell, M.; Pope, S.J.A.; Ashton, L.; Goodacre, R.; Sharrad, C.A. Structural, spectroscopic and redox properties of uranyl complexes with a maleonitrile containing ligand. Dalton Trans. 2011, 40, 5939–5952. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; pp. 109–113. [Google Scholar]
- Signorini, O.; Dockal, E.R.; Castellano, G.; Oliva, G. Synthesis and Characterization of Aquo[N,N′-ethylenebis(3-ethoxysalicylideneaminato)]dioxouranium(VI). Polyhedron 1996, 15, 245–255. [Google Scholar] [CrossRef]
- Oher, H.; Pereira Gomez, A.S.; Wilson, R.E.; Schnaars, D.D.; Vallet, V. How Does Bending the Uranyl Unit Influence its Spectroscopy and Luminescence? Inorg. Chem. 2023, 62, 9273–9284. [Google Scholar] [CrossRef]
- Silver, M.A.; Dorfer, W.L.; Cary, S.K.; Cross, J.N.; Lin, J.; Schelter, E.J.; Albrecht-Schmitt, T.E. Why is Uranyl Formohydroxamate Red? Inorg. Chem. 2015, 54, 5280–5284. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Kalaj, M.; Kerridge, A.; Ridenour, J.A.; Cahill, C.L. How to Bend the Uranyl Cation via Crystal Engineering. Inorg. Chem 2018, 57, 2714–2723. [Google Scholar] [CrossRef]
- Jørgensen, C.K.; Reisfeld, R. The uranyl ion, fluorescent and fluorine-like: A review. J. Electrochem. Soc. 1983, 130, 681–684. [Google Scholar] [CrossRef]
- Adelani, P.O.; Burns, P.C. One-dimensional Uranyl-2,2′-bipyridine Coordination Polymer with Cation-Cation Interactions: (UO2)2(2,2′-bpy)(CH3CO2)(O)(OH). Inorg. Chem. 2012, 51, 11177–11183. [Google Scholar] [CrossRef]
- Leung, A.F.; Hayashibara, L.; Spadoro, J. Fluorescence properties of uranyl nitrates. J. Phys. Chem. Solid. 1999, 60, 299–304. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Takao, K.; Ikeda, Y. Structural Characterization and Reactivity of UO2(salophen)L and [UO2(salophen)]2: Dimerization of UO2(salophen) Fragments in Noncoordinating Solvents (salophen = N,N′-disalicylidene-o-phenylenediamine, L = N,N-Dimethylformamide, Dimethyl Sulfoxide). Inorg. Chem. 2007, 46, 1550–1562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).