Abstract
Conversion-type transition metal oxides (TMOs) have emerged as promising anode materials for lithium-ion batteries (LIBs) owing to their high theoretical capacities and low cost. Intriguingly, many TMOs exhibit extra capacity that surpasses the limits predicted by conversion reaction mechanisms, challenging traditional electrochemical models and offering opportunities for next-generation high-energy storage. This review highlights the phenomenon of extra capacity in TMO anodes, emphasizing its mechanistic origins and practical implications. While these materials face well-known challenges such as low initial coulombic efficiency, solid electrolyte interphase (SEI) instability, and severe structural degradation due to large volume changes, they offer promising opportunities for achieving high energy density. Special emphasis is placed on understanding the underlying mechanisms that contribute to this anomalous capacity, including the role of reversible SEI formation, lithium-rich phases, reversible formation of LiOH, and interfacial storage phenomena. By clarifying these mechanisms and performance-enhancement approaches, this paper aims to guide future research toward the practical application of high-capacity conversion-type TMO anodes in next-generation LIBs.
1. Introduction
As science and technology continue to advance rapidly, the demand for energy is increasing. Fossil fuels, as the primary contributors to environmental pollution, are not sustainable in the long term. The need for clean and renewable energy has become increasingly critical. Renewable energy sources such as solar and wind are inherently intermittent and do not always align with real-time energy demand. To ensure a stable and reliable power supply, it is essential to develop efficient energy storage technologies that can support the growing demands of advanced technologies. There has been ongoing discussion about the need for storage devices and how to integrate them into existing power systems [1]. Key advancements in this area should focus on several factors, including a high energy storage capacity, fast charging capabilities, a long lifespan, and affordability. Meeting these demands will require continued innovation in materials, design, and manufacturing processes to realize the next generation of sustainable energy storage technologies.
Since their initial commercialization by Sony in 1991, rechargeable lithium-ion batteries (LIBs) have undergone continuous development and are now the dominant energy storage technology for portable electronics, power tools, and are becoming increasingly frequent for electric vehicles and grid storage [2]. Their widespread adoption is attributed to their stable power output, long cycle life, and relatively high energy density, making them superior to previous rechargeable battery systems [3]. As the world transitions toward a low-carbon economy, the next generation of LIBs is expected to play a vital role in smart grids and electric vehicles. However, the growing demand for improved energy storage technologies continues to outpace current capabilities [4]. To meet this demand, ongoing research focuses on developing new electrode materials with superior performance, aiming to produce LIBs that are lighter, more cost-effective, and capable of storing more energy [4]. Over the past three decades, advances in cathode chemistries, ranging from layered LiCoO2 to Ni-rich layered oxides, along with continuous improvements in electrolytes and manufacturing processes, have established LIBs as the leading commercial energy storage technology [2,5,6,7]. Graphite, the conventional anode material for LIBs, remains widely used due to its excellent cycling stability, high coulombic efficiency, and flat voltage profile [2,8,9]. Nonetheless, its relatively low theoretical capacity of 372 mAh g−1 limits the overall energy density of LIBs. Furthermore, the slow diffusion of lithium ions (Li+) within the graphite structure, estimated at 10−8 cm2 s−1, results in a low power density for LIBs, which continues to be a barrier for the widespread adoption of electric vehicles [10].
To overcome these limitations, extensive efforts have been made to explore alternative anode materials, such as silicon [11,12,13,14] and transition metal oxides (TMOs) [15,16,17,18,19,20,21]. TMOs used as anode materials in LIBs can generally be classified into two categories: intercalation-type and conversion-type LIBs. While most TMOs studied as anodes in LIBs are conversion-type materials, there is a small class that behaves primarily through intercalation. Intercalation-type TMOs, such as TiO2 [22,23,24], store lithium through reversible insertion into the host lattice with minimal structural change, offering excellent cycling stability but relatively low capacity. In contrast, conversion-type TMOs, such as CoO [25] and Fe2O3 [17,18,26,27], undergo reversible redox reactions with lithium to form metal nanoparticles dispersed in a Li2O matrix, enabling much higher theoretical capacities, though these are often accompanied by large volume changes and poor reversibility. Some TMOs, such as MoO2 [28,29], function as high-capacity anodes through a combination of intercalation and conversion reactions, where lithium ions are first inserted into the host structure via intercalation, followed by conversion that breaks down the oxide into metallic nanoparticles and Li2O.
Interestingly, many of these TMOs have demonstrated capacities that exceed their theoretical values in experiments, which is a phenomenon that remains the subject of active research and debate [30]. The observed excess capacity in TMO anodes is often referred to as over-capacity, extra capacity, or super-stoichiometric capacity. This unexpected behavior has prompted extensive investigation into the underlying lithium storage mechanisms beyond the classical conversion reaction [31].
Multiple hypotheses have been proposed to explain this extra capacity in TMO anodes, including the following:
- Formation of electrode–electrolyte interphases, such as the solid electrolyte interphase (SEI) or polymeric/gel-like films, results from electrolyte decomposition and associated side reactions [32].
- Li+ storage occurs at interfaces accompanied by charge separation across phase boundaries [33,34].
- Reversible reaction of LiOH with lithium takes place to form Li2O and LiH, as observed in RuO2-based electrodes [35].
- Lithium-rich phase formation: Under deep lithiation, some TMOs form amorphous or metastable lithium-rich phases that incorporate more Li than the stoichiometry of the conversion reaction products [36].
- Distinct structural architectures enable enhanced Li+ storage through features such as high specific surface areas, nanocavities, and interconnected meso- and/or nanoporous networks [37].
- The inherent lithium storage capability of conductive additives, which are often used in relatively large quantities (typically 15–50%) during cell fabrication, particularly for TMOs with low electronic conductivity such as Fe2O3 [38].
The objective of this review paper is to provide a comprehensive overview of the mechanisms underlying the extra capacity observed in TMO anodes. It aims to summarize the recent experimental and theoretical advances, explore the proposed lithium storage mechanisms beyond conventional conversion reactions, and highlight the challenges and opportunities for developing high-capacity, TMO-based anodes for next-generation LIBs. First, it provides a concise overview of the fundamental principles and electrochemical characteristics of TMO conversion reactions. It then examines the various mechanisms proposed to explain the observed extra capacity in these materials, including contributions from nanoscale effects, interfacial reactions, reversible SEI-related processes, and lithium-rich phases. The review also highlights recent experimental findings and theoretical insights that advance our understanding of these phenomena. Finally, it discusses the current challenges, future research directions, and the practical prospects of leveraging extra capacity in TMO anodes for next-generation high-energy LIBs.
2. Conversion Reaction of Transition Metal Oxides
2.1. Overview of Conversion Reaction Mechanism
Since the pioneering work by Tarascon in 2000, conversion reaction TMOs have attracted significant attention as potential anode materials for LIBs. These anodes operate through the reaction mechanism MaOb + (2b) Li+ + (2b) e− = aM0 + bLi2O, where M denotes a transition metal [15,16,17,18,19,20,21]. As shown in Figure 1 [39], this approach involves the electrochemical conversion of TMOs into a composite of metal nanoparticles and lithium oxide (Li2O), offering much higher theoretical capacities than conventional graphite anode. Conversion-reaction TMO anodes offer several advantages [15]. Their primary benefit is a significantly higher theoretical capacity, often two to three times greater than graphite, enabling much higher energy density in LIBs. In addition, many TMOs are abundant, low-cost, and environmentally benign, making them attractive for large-scale applications. TMOs also feature high corrosion resistance and facile operation in addition to their high specific capacities [15]. These features collectively make conversion-type TMOs a promising class of high-capacity anode materials.
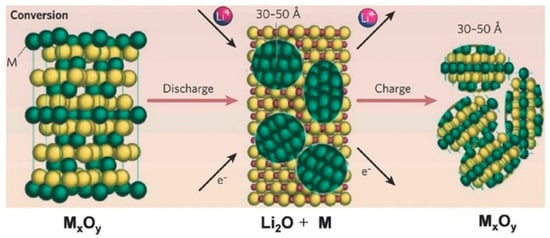
Figure 1.
Schematic illustration of the conversion reaction mechanism in TMO anodes. During discharge, the TMO is reduced to form metal and Li2O nanoparticles, while the subsequent charge process reverses this reaction, leading to the formation of a disordered TMO [39]. Copyright 2008 Nature Publishing Group.
Conversion-type TMOs can be classified by their metal species [15]. Manganese oxides (MnO, Mn3O4, Mn2O3, MnO2) offer high capacities, low cost, and environmental benignity [40,41,42]. Iron oxides (FeO, Fe2O3, Fe3O4) are attractive for their abundance, non-toxicity, and high theoretical capacities of 745–1007 mA g−1 [18,27,43]. Cobalt oxides (CoO, Co3O4) also deliver high capacities (715–890 mAh g−1) [44,45,46,47,48]. Nickel oxide (NiO) provides 718 mAh g−1 but suffers from poor conductivity and severe volume changes [49,50]. RuO2 is a promising anode material for LIBs due to its high theoretical capacity (1130 mAh g−1), good chemical and thermal stability, and high conductivity [51,52]. Molybdenum dioxide (MoO2) is known for its high capacity, metallic-like conductivity [29,53]. Tungsten trioxide (WO3) delivers a theoretical capacity of 693 mAh g−1 but suffers from significant volume changes during cycling [54,55,56,57].
2.2. Challenges of Conversion-Type TMO Anodes
Despite their potential, early studies also revealed critical challenges associated with conversion-reaction TMOs [15,21,58]. One of the primary obstacles is the low initial coulombic efficiency, which results from the irreversible consumption of lithium during the formation of the SEI and decomposition of electrolyte components in the first cycle. This irreversible lithium loss reduces the usable capacity of the cell and poses difficulties in full-cell configurations. Another major challenge is the large volume expansion associated with the conversion reaction, often reaching 100–200%, which can lead to mechanical degradation, particle pulverization, and loss of electrical contact. Poor electronic and ionic conductivity is a third challenge, as many TMOs are intrinsically insulating, and the formation of Li2O further impedes electron transport. Slow reaction kinetics and limited rate capability also hinder the performance of TMO anodes. Conversion reactions typically involve multistep processes and sluggish lithium diffusion, especially through dense oxide matrices. Finally, many TMO anodes suffer from poor cycling stability and fading capacity due to continuous mechanical and chemical degradation.
2.3. Solutions and Strategies
To address these issues, researchers have developed various nanostructures, such as nanoparticles, nanowires, and nanotubes, that help buffer volume expansion and improve cycling stability [21,59]. For instance, Xu et al. (2014) designed multi-shelled hollow microspheres of α-Fe2O3 as anode materials for LIBs, demonstrating a high reversible capacity and excellent cycling performance [60]. The electrochemical behavior of α-Fe2O3 follows a typical conversion reaction, where Fe2O3 is reduced to metallic Fe and Li2O during lithiation and is partially reversed during delithiation. The multi-shelled hollow structure plays a crucial role in mitigating the challenges commonly associated with conversion-type materials, such as large volume expansion, particle pulverization, and unstable SEI formation. These hollow architectures provide internal void spaces that buffer the volume change, maintain the structural integrity of the electrode, and facilitate faster lithium-ion diffusion and electron transport. In addition, the high surface area of the shells enhances electrode–electrolyte contact and reaction kinetics. As a result, the electrode exhibits high specific capacity (~1024 mAh g−1 after 100 cycles at 200 mA g−1), excellent rate performance, and remarkable charge retention. This study highlights how rational nano-structuring can overcome the intrinsic limitations of conversion-type anodes, making them more viable for long-term applications in LIBs. Huang et al. (2014) reported a novel design of micro/nano-structured Co3O4 cubes synthesized via a one-pot hydrothermal method followed by thermal treatment, using triethanolamine as a surfactant. The resulting morphology features micro-scale cubes (~2.37 µm) composed of densely packed Co3O4 nanocrystals (20–200 nm in diameter, 30–40 nm thick), forming a mesoporous architecture with an average pore size of ~3.08 nm and a surface area of ~5.1 m2 g−1. This tailored structure delivers high reversible capacities (~1298 mAh g−1 at 0.1 C and ~1041 mAh g−1 at 1 C in initial cycles) and excellent cycling stability, retaining over 89.9% of the first-charge capacity after 60 cycles at 1 C (and ≥97% at lower rates). The mesoporous nanocube assembly effectively buffers volume expansion, mitigates mechanical degradation, and promotes rapid lithium-ion transport [61]. Additionally, composite materials combining TMOs with carbon-based matrices have been designed to enhance electronic conductivity and reaction kinetics [16,62,63]. Beyond materials design, lots of efforts have been made to understand the complex mechanisms underlying conversion reactions. These include investigations into phase evolution, interfacial behavior, and the influence of particle size and morphology on electrochemical performance. Furthermore, the concept of conversion reactions has been extended to other battery systems, such as sodium-ion batteries, where layered TMOs are being explored for similar advantages [20,64,65,66].
3. Lithium Storage Mechanisms of Extra Capacity
3.1. Reversible SEI Formation
In addition to conventional lithium storage mechanisms, recent studies have identified the SEI as a contributor to extra capacity in certain anode materials. The formation, stabilization, and partial reversibility of the SEI play a crucial role in determining the electrochemical performance, coulombic efficiency, and long-term cycling stability of LIBs. During the first few charge–discharge cycles, electrolyte and lithium salt reduction lead to the formation of an inhomogeneous SEI, particularly on reactive anodes such as graphite, silicon, TMO, and lithium metal [67,68,69]. The initial formation of the SEI irreversibly consumes a significant amount of lithium ions and electrolyte solvents, resulting in capacity loss. However, studies have shown that the SEI evolves dynamically during cycling through structural rearrangement, thickening, and, in some cases, partial decomposition and reformation [70,71,72]. These processes can gradually release lithium ions previously trapped in the unstable SEI, contributing to a noticeable increase in reversible capacity over subsequent cycles, a phenomenon commonly observed as a capacity “activation period” in electrochemical testing.
The reversibility of SEI formation is largely influenced by the electrolyte composition, including the choice of solvents, lithium salts, and film-forming additives such as fluoroethylene carbonate (FEC) and vinylene carbonate (VC), which help promote a stable yet partially reversible SEI [73,74]. Electrode surface properties, such as roughness, crystallographic orientation, and surface functional groups, also significantly affect SEI uniformity and stability. Smoother surfaces with fewer defects tend to support more uniform SEI formation, reducing the risk of cracking and localized decomposition during cycling.
Advanced in situ and ex situ characterization techniques have significantly improved our understanding of SEI dynamics. High-resolution transmission electron microscopy (TEM) and scanning electron microscopy (SEM) enable direct visualization of morphological changes in the SEI, while surface-sensitive techniques like X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) reveal changes in chemical composition during cycling [75,76]. These studies provide strong evidence for the coexistence of stable and metastable SEI phases, some of which can reversibly accommodate lithium via redox-active species. Moreover, computational studies using density functional theory (DFT) and molecular dynamics simulations have shown that certain SEI components, such as lithium carbonate (Li2CO3) and organic lithium alkyl carbonates, can undergo reversible electrochemical reactions at low potentials. This redox activity enables the release of lithium ions previously trapped in the SEI, contributing to capacity increase or recovery during early cycles, as illustrated in Figure 2 [77]. These insights suggest that the SEI is not only an inert, passivating layer but a dynamic and electrochemically active interphase.
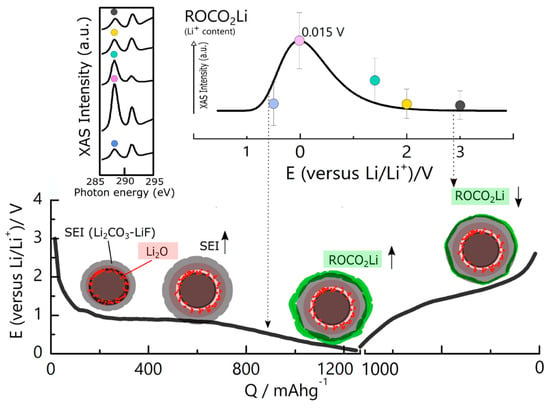
Figure 2.
Trend of Li storage in the superficial SEI layer (below 0.7 V) by alkyl lithium carbonates as monitored by the corresponding intensity of the ROCO2Li component at the XAS C K-edge (see marked points of different colors). The ROCO2Li thickness reaches its maximum at 0.015 V, corresponding to the maximum of Li+ content (right-hand panel). Sketch of the SEI formation on the ZFO-C particles during the charging-discharging cycle (E potential vs. Q capacity), distribution, and evolution at each stage of the lithiation-delithiation cycle obtained by the relevant experimental results. The arrows indicate an increase or decrease in the specified component thickness [77]. Copyright 2017 American Chemical Society.
Notably, Sun et al. (2014) demonstrated that high lithiation rates can intentionally induce structural reactivation in Co3O4 conversion anodes, transforming dense hollow spheres into hierarchically mesoporous nanocrystals [78]. This restructuring stabilizes a thin (~15 nm), robust, and reversible SEI that supports efficient Li+ transport, mitigates electrolyte decomposition, and endures thousands of cycles without cracking. The optimized architecture delivers > 900 mAh g−1 at 1.12 C for over 7000 cycles, exceeding Co3O4’s theoretical capacity. The results suggest that the engineered SEI not only protects the electrode but also contributes to extra capacity through interfacial or pseudocapacitive storage, offering a strategy for long-life, high-power LIBs. Li et al. (2015) developed a nanostructured α-Fe2O3@graphene composite via hydrothermal synthesis, freeze-drying, and annealing, yielding α-Fe2O3 nanoaggregates uniformly wrapped in reduced graphene oxide [79]. This core–shell architecture achieved 1787 mAh g−1 capacity after 90 cycles with excellent stability, far surpassing bare α-Fe2O3. The graphene shell enhanced conductivity, buffered volume changes, and stabilized a thin, uniform, and reversible SEI that remained intact under deep cycling, thereby preventing continuous cracking and reformation typical in conversion anodes. The porous, flexible graphene network also facilitated fast ion/electron transport while preserving interfacial integrity. These results highlight how graphene encapsulation enables SEI reversibility, linking interface engineering directly to high capacity, long cycle life, and superior rate performance in conversion-based LIB anodes.
In conclusion, while the traditional view of SEI has been that of a purely passive layer, emerging experimental and theoretical studies increasingly suggest that a dynamic, partially reversible SEI can be harnessed to improve battery performance. For high-capacity anodes prone to large volume changes (e.g., silicon, lithium metal, TMOs), reversible SEI formation is crucial to mitigating repeated cracking and electrolyte decomposition [70,80]. Tailoring the electrolyte formulation and cycling protocols to enhance SEI stability while enabling controlled reversibility is a promising strategy for advancing the cycle life and safety of next-generation LIBs [71,81].
3.2. Polymeric or Gel-like Film Formation
The formation of gel-like polymeric films on electrode surfaces has been identified as a key mechanism contributing to the extra reversible lithium storage capacity in TMO anodes. During the initial cycles, decomposition of electrolyte components leads to the development of a polymeric or gel-like layer, which is often considered part of the SEI. Unlike conventional SEI layers on graphite, this film in TMOs not only passivates the electrode but also actively participates in reversible lithium storage, contributing to capacities beyond the theoretical limits of conversion reactions.
The pioneering work by Laruelle et al. on CoO electrodes revealed that a gel-like polymeric film significantly contributed to the anomalous extra capacity observed at low potentials that is beyond what could be accounted for by the conventional conversion reaction of CoO to Co and Li2O [25]. As shown in Figure 3 [25], four CoO-based electrodes (a–d) were examined by TEM after different cycling conditions. A veil-type film formed after the first discharge to 0.02 V (Figure 3a), which condensed into a dense ~500 Å polymeric coating upon repeated cycling between 0.02–1.8 V (Figure 3b). Figure 3c demonstrates that this coating remains stable and does not further evolve when the cell continues cycling over the same voltage range. However, after full reoxidation to 3 V (Figure 3d), the thick coating disappeared, leaving only a thin (~30 Å) SEI on the reformed CoO nanoparticles. The TEM results demonstrate the appearance/disappearance of the polymer/gel-like coating, as CoO/Li cells were cycled over the 0.02 to 3 V range. This finding highlighted the essential role of surface phenomena in determining the total charge storage capacity of TMO anodes [25]. Further evidence was provided by Hu et al. (2013) using high-resolution solid-state NMR spectroscopy in combination with synchrotron X-ray diffraction and absorption techniques on RuO2 electrodes. Their comprehensive analysis revealed a reversible redox reaction involving lithium-containing gel-like species, which operated alongside the conventional conversion reaction and contributed to the enhanced lithium storage capacity of the material [35]. Similar behavior has been observed in α-Fe2O3 nanostructures. Yao et al. (2011) attributed the high capacity and rate capability of porous α-Fe2O3 nanorods partly to a soft, ionically conductive gel-like interphase formed from electrolyte decomposition, which accommodates volume change and provides pseudocapacitive storage [82]. In α-Fe2O3@graphene core–shell composites [83], the conductive shell likely stabilizes a thin, resilient gel-like film, suppressing unstable SEI growth while enabling fast transport and enhanced cycling stability.
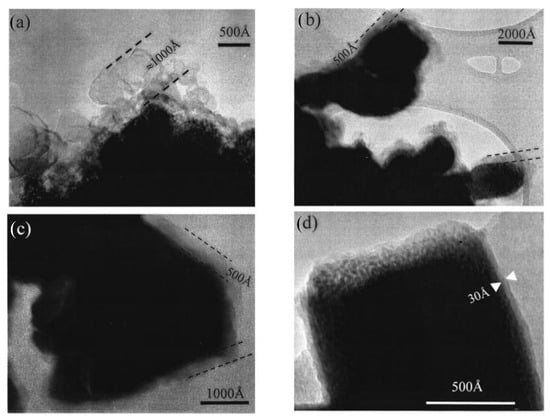
Figure 3.
TEM bright field images realized in (a) on an CoO-based electrode after its first discharge down to 0.02 V; in (b) on an electrode that was at 0.02 V after 10 cycles between 0.02 and 1.8 V; in (c) on an electrode that was at 1.8 V after 10 cycles between 0.02 and 1.8 V; and in (d) on an electrode that was cycled and fully re-oxidized up to 3 V. Note that only the polymeric layer disappears. The 30 Å SEI inorganic layer can be seen on the fully charged sample (d) [25]. Copyright 2024 IOP Publishing.
Recent studies have extended this mechanism to various materials. For instance, Deshmukh et al. (2020) investigated Fe3O4 nanoparticles embedded in nitrogen-doped carbon matrices and observed the formation of an electrolyte-derived polymeric film that enabled high-capacity retention even after 400 cycles. This finding directly linked the electrochemical activity of the film to long-term cycling stability [84]. Similarly, Tripathy et al. (2020) studied Cu3PS4-based electrodes using infrared spectroscopy and other analytical techniques, identified a gel-like film responsible for pseudocapacitive behavior and enhanced charge storage [85]. Xiao et al. (2021) developed WO3/C hybrids as promising anodes for lithium-ion batteries [57]. The incorporation of carbon not only enhances the intrinsic conductivity and structural stability of WO3 but also shortens the ion transport pathways owing to the reduced particle size of the active phase. These synergistic effects enable the hybrid anode to deliver outstanding lithium storage properties, including high efficiency, excellent cycling stability, and superior rate capability. The hybrid anode delivers a reversible capacity as high as 833.4 mAh g−1 with no noticeable capacity fading after 100 cycles, which surpasses the theoretical capacity of WO3 (693 mAh g−1). The authors attribute the extra capacity to the reversible formation and dissolution of gel-like polymeric films, together with interfacial lithium-ion storage. Additionally, Hu et al. (2013) reported novel CuO/Cu2O hollow polyhedrons with porous shells that delivered a reversible lithium storage capacity of 740 mAh g−1 at 100 mA g−1 after 250 cycles, significantly higher than the theoretical capacity of ~520 mAh g−1 expected from the conversion mechanism [86]. This excess capacity was attributed to the reversible formation and dissolution of a polymeric gel-like film generated from electrolyte degradation.
Recent studies have demonstrated that transition metals released from the dissolution of structures such as Fe3O4 in the electrolyte can act as catalysts for electrolyte decomposition [87]. This catalytic process leads to the formation of polymeric layers within the SEI that are redox-active in nature. These SEI layers are capable of reversibly accommodating lithium ions, thereby contributing to extra charge storage beyond the theoretical capacity of the active material. In situ spectroscopic and electrochemical analyses have confirmed that dynamic changes in SEI composition correlate closely with capacity activation during early cycles [88]. Further work on Co3O4 electrodes demonstrated that tailoring the organic-inorganic composition of gel-like SEI films can modulate pseudocapacitive behavior, improving both specific capacity and cycling stability [89]. Similar studies on RuO2 revealed that metastable SEI phases actively participate in reversible redox reactions at low potentials, with repeated formation and decomposition of polymeric components enabling reversible lithium exchange [90]. Zhou et al. (2025) developed a surfactant-free synthesis strategy to precisely control the morphology of hierarchical NiCo2O4 architectures. The 1D/3D flower-like structures demonstrated outstanding lithium storage properties, achieving 116% capacity retention after 200 cycles. The observed extra capacity was attributed to dynamic contributions from Li-containing gel-like films and reversible Co2+/Co3+ redox activity [91].
A 2025 review on amorphous TMOs revealed that their intrinsic pseudocapacitive behavior is closely associated with the formation of flexible, gel-like interfacial films. These films facilitate fast and reversible Li+ storage beyond traditional intercalation or conversion mechanisms, directly contributing to enhanced capacity and superior rate performance [92]. These studies highlight that gel-like polymeric films are not simply inert by-products of electrolyte decomposition but play an active role in reversible lithium storage in nanostructured TMO systems. Their dynamic behavior, lithium-ion storage capability, and contribution to reversible capacity suggest that optimizing the composition and stability of these films represents a promising strategy to improve both the capacity and cycle life of LIBs.
3.3. Interfacial Lithium Storage at Phase Boundaries
Interfacial charge storage is a widely recognized mechanism responsible for the extra reversible capacity observed in TMO conversion anodes [30,93]. This process occurs at the nanoscale interfaces formed between metallic nanoparticles and the lithium-containing matrix (e.g., Li2O) generated during conversion reactions. These interfacial regions act like nanoscale capacitors, enabling the efficient storage of lithium ions and electrons beyond the capacity limits of conventional bulk conversion or intercalation mechanisms.
This concept was initially proposed by Jamnik and Maier, who emphasized that the nature of interfacial lithium storage is influenced by material-specific factors such as particle size, transition metal electronegativity, and alloying ability with lithium [33]. As shown in Figure 4 [33], in systems where TMs are finely dispersed within a Li2O matrix, lithium ions are accommodated on the Li2O side of the interface, while electrons remain localized on the TM side. The interfacial mechanism involves the formation of a Li2O monolayer at the TM/Li2O interface, which acts as a host for lithium ions, while TM nanoparticles serve as electron sinks. It is proposed that one to three monolayers of lithium can be stored at this interface, with lithium ions saturating the Li2O layer and corresponding electrons transferred to the TM surface. Jamnik and Maier (2003) further introduced the concept of space-charge layers at metal/oxide boundaries, highlighting their ability to store charge independently of bulk reactions [33]. Initial experimental studies by Li et al. (2004) on metal fluoride/oxide systems revealed stable extra capacity associated with these interfacial zones [94]. Maier (2007) reinforced the concept of nano-ionics, emphasizing how nanoscale effects influence mass storage via interface-controlled charge localization [95]. DFT simulations by Zhukovskii et al. (2006) revealed the energetic stability of excess lithium at these interfaces [96]. Cherian et al. (2012) reported that electrospun α-Fe2O3 nanorods exhibit high capacity and excellent rate capability due to heterogeneous interfacial lithium storage at phase boundaries between Fe, Li2O, and residual Fe2O3 formed during cycling [97]. Similarly, Chaudhari et al. (2012) demonstrated that hollow α-Fe2O3 electrospun nanofibers achieve high reversible capacity and rate performance through the same interfacial mechanism [98]. In both studies, the synergy between bulk conversion and pseudo-capacitive interfacial storage that is enabled by high interface density and 1D morphology proved critical for achieving stable, high-performance LIB anodes.
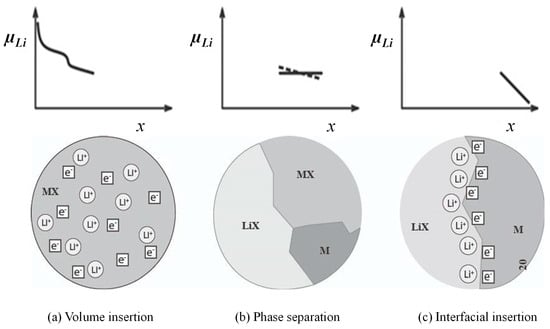
Figure 4.
A sequence of three different stages of lithium storage. Insertion in the bulk of MX (a). If the solubility limit is exceeded, a heterogeneous solid-state reaction characterized by phase separation occurs (b). Finally, the interfacial charging takes place (c) [33]. Copyright 2003 The Royal Society of Chemistry.
Recent advances have expanded and deepened this understanding. Zhao et al. (2024) demonstrated ultrafast, space-charge-enabled storage in Fe/Li2O electrodes, achieving a reversible capacity of 126 mAh g−1 within just six seconds and maintaining performance over 30,000 cycles [99]. Ahn et al. (2024) showed that size-controlled trilayer TMO nanoribbon yarns significantly enhance interfacial pseudo-capacitance and redox kinetics, resulting in high energy/power densities and excellent cycling stability [100]. Similarly, Li et al. (2022) revealed that reducing particle size in TiO2-based anodes increases interfacial area, thereby amplifying pseudocapacitive contributions and improving both reversible capacity and rate performance [101]. In addition, Zeng et al. (2024) demonstrated that tailoring SEI composition to favor Li2O-rich layers provides a stable host matrix for interfacial lithium storage, significantly improving coulombic efficiency and cycling stability [102]. Sun et al. (2025) further confirmed that dual electronic-ionic conductive interfaces promote space-charge formation, enhancing both lithium-ion transport and electron mobility, thus enabling faster charging and sustained high capacity in TiO2-based systems [103].
Spin-polarized electron storage at the phase boundary of metal nanoparticles and Li2O has emerged as a compelling mechanism to explain the anomalous extra capacity observed in TM conversion anodes. Using in situ magnetometry, Li et al. (2021) demonstrated that Fe nanoparticles in Fe/Li2O nanocomposites can store spin-polarized electrons during discharge in Fe3O4/Li model systems [104]. This phenomenon led to extra reversible capacity and measurable changes in interfacial magnetization. The mechanism involves spatial charge separation at the metal/Li2O interface: lithium ions migrate into the Li2O matrix, while spin-polarized electrons remain localized on the metallic Fe surface. Similar behavior has been observed in other TM-based electrodes such as CoO, NiO, FeF2 and Fe2N, suggesting that spin-polarized electron storage is a general and intrinsic property of many conversion-type systems. These findings sparked broad interest in the role of spin-polarized surface capacitance at TM/Li2O interfaces [104]. Li et al. (2021) confirmed that such interfaces can act as nanoscale, redox-active capacitors, enabling decoupled and reversible storage of lithium and electrons beyond the limits of classical conversion or intercalation reactions [104]. As illustrated in Figure 5 [105], this mechanism relies on the spatial separation of charge carriers: Li+ ions accumulate within the Li2O phase, while spin-polarized electrons are confined to the TM nanoparticle surface. A Li2O-rich SEI formed during early cycling helps stabilize the interface and mitigate electrolyte decomposition, allowing the interface to function as a reversible, redox-active region [105]. Particle size plays a key role in enhancing this effect. Bock et al. systematically showed that reducing Fe nanoparticle size increases interfacial area, which boosts the density of active charge storage sites and accelerates charge transport due to shorter diffusion paths. As shown in Figure 6, there is a clear inverse correlation between particle size and total reversible capacity, confirming the tunability of spin-polarized electron storage through nanoscale engineering [106].
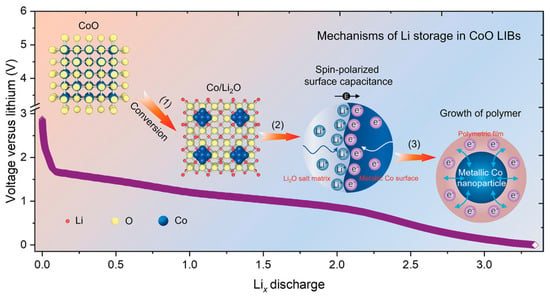
Figure 5.
Schematics of the lithium reaction mechanisms in CoO LIBs. The lithium storage in CoO across different voltage ranges involves conventional conversion reactions, interfacial charge storage via a spin-capacitor effect, and the formation of polymeric films [105]. Copyright 2021 Wiley-VCH GmbH.
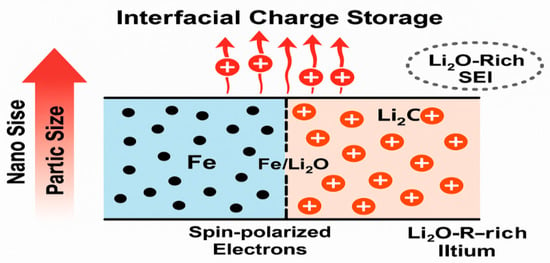
Figure 6.
Correlation between decreasing Fe nanoparticle size and increased interfacial charge storage capacity via spin-polarized electron accumulation.
From a theoretical perspective, Xia et al. (2024) provided a comprehensive framework for electron spin polarization in electrochemical systems. They proposed that localized spin states at TM-oxide and TM-sulfide interfaces serve as dynamic charge reservoirs, enabling pseudocapacitive behavior without requiring long-range structural reorganization and contributing to both high capacity and fast kinetics [107]. Operando studies further confirmed this mechanism. Zuo et al. (2023) and Kang et al. (2023) used advanced magnetometry and electronic structure analysis to investigate Co- and Mn-based nanostructured anodes. Their results showed that spin-polarized charge transport across engineered interfaces significantly improves rate performance and cycling stability, particularly when combined with conductive frameworks such as reduced graphene oxide (rGO) [108,109]. Building on this, Wang et al. (2024) fabricated Fe3O4/rGO 3D aerogels that offer enhanced surface area and structural flexibility, enabling more robust interfacial charge storage and mechanical stress buffering [110]. Teng et al. (2023) applied real-time magnetometry across a range of 3d transition metals, confirming a direct relationship between extra capacity and interfacial spin-polarized electron density [111].
Interface engineering has become a key strategy to optimize this mechanism. Chen et al. (2025) demonstrated that Li2O-rich SEI layers enhance spin-polarized charge storage and improve coulombic efficiency [112]. Thermodynamic modeling from a recent study predicted favorable spin-resolved charge localization at sharp metal-oxide junctions. Moreover, Ren et al. (2024) showed that embedding bimetallic sulfides in rGO shells improves both electronic and ionic conductivity, leading to higher active site density, reduced polarization, and enhanced rate performance, all tied to interfacial spin-polarized charge dynamics [113].
This concept also extends beyond lithium systems. Li et al. (2024) demonstrated that Fe3O4 anodes maintain strong sodium-ion storage performance under wide temperature ranges, attributed to the retention of spin-polarized surface capacitance at the Fe/Na2O interface [114]. Likewise, Zheng et al. (2024) found that differences in storage capacity across Li-, Na-, and K-ion batteries were directly linked to variations in spin-polarized electron storage and interfacial design in TM sulfide anodes [115].
3.4. Lithium-Rich Phase Formation
Lithium-rich phases refer to amorphous or metastable regions within electrode materials that accommodate more lithium than predicted by stoichiometric limits. In TMO systems, these phases are often observed during deep lithiation or extended cycling, particularly at the nanoscale or near electrode surfaces. They enable extra lithium storage beyond conversion mechanisms. The formation of a metallic lithium-rich phase was first proposed by Shon et al. [36], who reported mesoporous MoO2 exhibiting a capacity nearly twice the theoretical limit of the conversion reaction. Through in situ X-ray absorption spectroscopy, scanning transmission electron microscopy (STEM), Electron Energy Loss Spectroscopy (EELS), and computational modeling, they attributed the extra capacity to the formation of a metallic lithium-rich phase situated between the intercalated lithium-MoO2 domains. Alshareef et al. [116] also observed anomalous lithium storage in atomically thin, nonlayered 2D MoO2 sheets. Using ex situ XPS and XRD, they identified a dual mechanism consisting of lithium intercalation and the formation of a metallic lithium phase, further supporting the role of lithium-rich domains in enhancing capacity.
More recently, Wang et al. (2023) expanded this understanding by applying in situ and ex situ techniques, including XRD, XAS, SEM and TEM, to investigate MoO2 nanoparticle anodes [53]. Their findings revealed that no conversion reaction occurs during the initial discharge. Instead, lithiation results in the formation of a Li0.98MoO2 phase. Subsequent EELS and EDX analyses indicated the development of a metallic lithium-rich interfacial layer beyond Li0.98MoO2, which contributes to the extra lithium storage as shown in Figure 7 [53]. Electrochemical impedance spectroscopy, combined with SEM and TEM, further showed that changes in particle size, morphology, and SEI resistance significantly influence the evolution of capacity during cycling. In addition, DFT calculations support these observations, predicting that lithium-rich phases are energetically favorable, particularly on the surface of LiMoO2. A similar phenomenon has also been reported in another conversion-type anode material, MoS2 [117]. Li et al. (2025) discovered a unique lithium intercalation mechanism in MoS2, leading to the formation of reversible LixMoS2 phases that contribute to its extra capacity [117]. DFT simulations predict the formation of overlithiated phases such as Li5MoS2 as energetically favorable during cycling. Additionally, the formation of a lithium-rich surface phase on Li4MoS2 is also considered thermodynamically advantageous, highlighting the broader relevance of lithium-rich phases in enabling high-capacity behavior in TMO-based anodes.
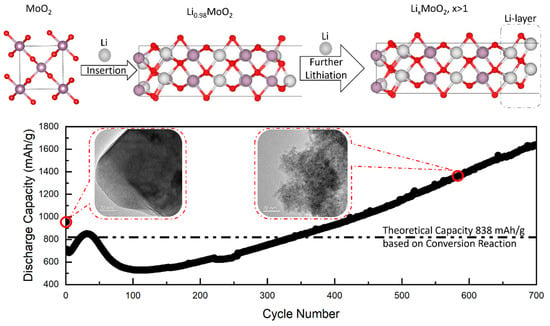
Figure 7.
MoO2 nanoparticles undergo lithium-ion intercalation during discharge without requiring a subsequent conversion reaction to produce Mo and Li2O. DFT calculations indicate that the formation of a lithium-rich surface phase on LiMoO2 is energetically favorable. The excess Li atoms accumulated at the surface can be readily stripped during charging, significantly contributing to the extra capacity of LiMoO2. Furthermore, variations in particle size and morphology, along with changes in SEI resistance, play an important role in the capacity evolution observed during cycling [53]. Copyright Royal 2025 Society of Chemistry.
3.5. Generation of LiOH and Its Subsequent Reversible Reaction
To identify the major sources of the extra capacity observed in RuO2/Li cells, Hu et al. (2013) applied a suite of short- and long-range structural characterization techniques to elucidate the charge-storage mechanism in this prototype conversion material [35]. RuO2 was chosen because it exhibits unusually high Coulombic efficiency and favorable mass transport properties relative to other TMOs/fluorides. Moreover, unlike Fe- and Co-based systems, RuO2 does not generate superparamagnetic nanoparticles, which enabled the acquisition of high-resolution NMR spectra essential for mechanistic insights. Their results revealed that during lithiation, LiOH forms at the electrode surface, likely through the reduction in surface hydroxyl groups or residual moisture; upon delithiation, LiOH is reversibly converted to Li2O and LiH according to the reaction: LiOH + 2Li+ + 2e− ⟷ Li2O + LiH. This redox-active pathway, distinct from the conventional RuO2-to-Ru conversion reaction, accounts for a substantial portion of the observed extra capacity. Supporting evidence from high-resolution 1H, 6Li, and 17O solid-state NMR, combined with in situ XRD and X-ray absorption spectroscopy, confirmed the dynamic and reversible nature of this LiOH-mediated process [35].
Su et al. (2017) developed an ultrathin layered hydroxide cobalt acetate (LHCA)-reduced graphene oxide nanocomposite that delivered > 1000 mAh g−1 at 1 A g−1 and ~780 mAh g−1 at 4 A g−1 after 300 cycles that are well above the theoretical limit of cobalt-based conversion anodes [118]. The exceptional performance was attributed to a surface-driven redox process involving reversible formation/decomposition of LiOH and LiAc, catalyzed by Co2+ centers and surface OH groups. During discharge, LHCA first undergoes lithiation and decomposition at ~1.5 V to generate metallic Co, LiOH, and LiAc. In the subsequent low-voltage region (~0.7 V), the freshly formed Co nanoparticles catalyze further reactions in which LiOH is reduced to Li2O and LiH, while LiAc is transformed into acetaldehyde, providing additional lithium storage well beyond the expected conversion capacity. Upon charging, these species are reversibly decomposed, regenerating hydroxyl and acetate groups and releasing Li+ back into the electrolyte. This LiOH-mediated mechanism, distinct from bulk conversion or interfacial storage, highlights the potential of engineered catalytic surfaces to expand the capacity of conversion-type anodes [118].
4. Future Perspectives
The discovery of extra capacity in TMO anodes, exceeding the theoretical limits set by conventional conversion reactions, has opened a promising new frontier in LIB research. This extra capacity has been attributed to various mechanisms, including interfacial lithium storage, pseudocapacitive behavior, redox-active SEI layers, and the formation of lithium-rich phases. Each of these mechanisms offers a unique opportunity to increase energy density without introducing entirely new chemistries, an appealing prospect for improving battery performance while leveraging existing manufacturing platforms.
However, capitalizing on this promise comes with several challenges. The exact origins and contributions of each mechanism remain only partially understood. Their influence often varies with factors such as material composition, morphology, particle size, cycling rate, and electrolyte chemistry. These uncertainties, coupled with practical issues such as low initial coulombic efficiency, structural instability from large volume changes, and capacity fading from unstable SEI layers, limit the translation of extra capacity into high-performance batteries. Moreover, some of these mechanisms may operate simultaneously, further complicating interpretation. Moving forward, one of the most critical challenges will be to systematically decouple and quantify the individual contributions of these mechanisms. This will require the application of advanced in situ and operando techniques, such as synchrotron-based X-ray absorption spectroscopy and X-ray diffraction, transmission electron microscopy, nuclear magnetic resonance, and operando Raman spectroscopy, combined with complementary ex situ characterizations. Coupling these methods with multiscale computational modeling, including density functional theory and machine-learning-assisted simulations, will provide essential insights into the energetics, kinetics, and reversibility of these processes.
To effectively harness extra capacity, tailoring electrode design at the nanoscale offers a promising strategy to amplify and stabilize extra capacity. Engineering the surface chemistry, tuning defect structures, and creating core–shell or heterostructure architectures can facilitate interfacial redox reactions and buffer volume changes. Additionally, the role of the electrolyte is increasingly recognized as a critical factor. Electrolyte formulations that support the formation of reversible SEI layers or stabilize metastable lithium-rich phases will be essential for harnessing these effects in practical batteries. Additives such as FEC and VC may also play key roles in modulating interfacial behavior and SEI composition.
In future research, the integration of these design principles into scalable and manufacturable electrode systems could enable the development of next-generation LIBs with dramatically improved energy density, fast-charging capability, and long cycle life. To realize this potential, interdisciplinary efforts involving materials synthesis, electrochemical analysis, interface engineering, and modeling will be necessary. A deeper mechanistic understanding and targeted exploitation of these extra capacity mechanisms will not only advance the performance of TMO anodes but could also inform the design of novel electrode materials for other battery chemistries, including sodium-ion and potassium-ion systems. In addition, to translate these advances into practice, it will be important to address the practical compatibility of TMO anodes with extra capacity with industrial electrode processing workflows. Techniques such as slurry casting, commonly used in commercial LIB fabrication, require optimization of binder systems, conductive additives, electrode thickness, and mass loading to accommodate the structural and electrochemical characteristics of TMOs. Furthermore, evaluating the long-term cycling stability, rate capability, and safety of these materials under realistic conditions, such as high areal capacity, limited electrolyte volume, and elevated temperature, is critical for bridging the gap between laboratory research and large-scale applications. Such considerations will be essential for translating the promising extra capacity of TMO anodes into commercial applications.
5. Conclusions
The observation of extra capacity beyond conversion reaction prediction in TMO anodes has expanded our understanding of lithium storage mechanisms in LIBs. This review highlights a range of contributing mechanisms, including interfacial lithium storage, reversible SEI formation, gel-like/polymeric films, the generation of LiOH and its subsequent reversible reaction with lithium to form Li2O and LiH, and the formation of lithium-rich phases, as illustrated in Figure 8. These mechanisms, while complex and system-dependent, offer a promising route to enhance energy density without the need for entirely new chemistries. Despite considerable progress, the precise origins and interplay of these mechanisms remain only partially understood. Their contributions vary significantly with material composition, nanostructure, cycling history, and electrolyte environment. Advanced in situ and operando characterization techniques, alongside theoretical modeling, are essential to disentangle and quantify the individual roles of each mechanism. Moreover, strategies such as nanoscale engineering, surface/interface optimization, and electrolyte formulation hold great potential to amplify and stabilize these extra capacity effects. A deeper mechanistic understanding and rational design of TMO-based electrodes could enable the development of high-performance LIBs that combine high capacity, fast-charging capability, and long-term cycling stability.
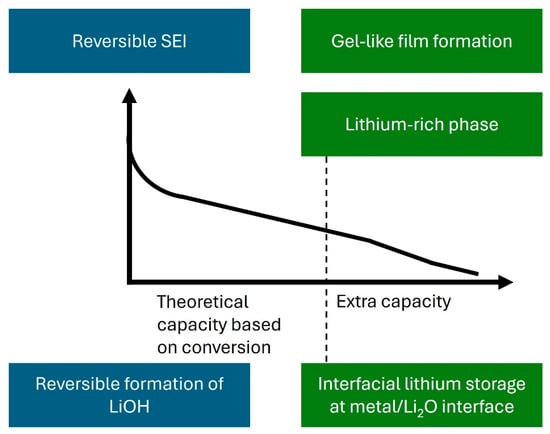
Figure 8.
Schematic illustration summarizing the mechanisms contributing to extra capacity.
Author Contributions
M.B., Conceptualization, writing, reviewing, Visualization and editing. R.R., Conceptualization, writing, reviewing, and editing. X.J., reviewing, and editing. A.M.H., writing, reviewing, and editing. L.Z., Conceptualization, writing, reviewing, Visualization and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that they have no known conflicts of interest or competing financial interests or personal relationships that could have appeared to influence the work reported in this paper “Beyond Theoretical Limits: Extra Capacity in Conversion Reaction of Transition Metal Oxide Anodes for Lithium-Ion Batteries”.
References
- Chee, W.; Lim, H.; Harrison, I.; Chong, K.; Zainal, Z.; Ng, C.; Huang, N. Performance of Flexible and Binderless Polypyrrole/Graphene Oxide/Zinc Oxide Supercapacitor Electrode in a Symmetrical Two-Electrode Configuration. Electrochim. Acta 2015, 157, 88–94. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Han, L.; Zhang, W.; Cai, J.; Zhong, M.; Zhao, Z. Electrochemical Performance of Bamboo Porous C@SiO2 Anode Composites. Int. J. Electrochem. Sci. 2022, 17, 22092. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Manfo, T.A.; Şahin, M.E. Intercalation reaction in lithium-ion battery: Effect on cell characteristics. Int. J. Mater. Eng. Technol. 2023, 6, 70–78. [Google Scholar]
- Xiang, J.; Wei, Y.; Zhong, Y.; Yang, Y.; Cheng, H.; Yuan, L.; Xu, H.; Huang, Y. Building practical high-voltage cathode materials for lithium-ion batteries. Adv. Mater. 2022, 34, 2200912. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, C.; Wu, H.; Li, L.; Zhang, C. Progress, challenge and perspective of graphite-based anode materials for lithium batteries: A review. J. Energy Storage 2024, 81, 110409. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Q.; Behzadnia, M.; Yang, Z.; Liu, Y. Multinonmetal-Doped V2O5 Nanocomposites for Lithium-Ion Battery Cathodes. ACS Appl. Energy Mater. 2024, 7, 11031–11037. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, X.; Chen, L.; Wu, Z.; Liang, Y. A high capacity nano Si composite anode material for lithium rechargeable batteries. Electrochem. Solid-State Lett. 1999, 2, 547. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhou, X.; Liu, Y.; Zhu, L. Crack-free silicon monoxide as anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 57141–57145. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, T.; Sow, C.H.; Shen, Z.X.; Lim, C.T.; Rao, G.V.S.; Chowdari, B.V.R. Alpha-Fe2O3 nanoflakes as an anode material for Li-ion batteries. Adv. Funct. Mater. 2007, 17, 2792–2799. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Lou, X.W. Iron-Oxide-Based Advanced Anode Materials for LithiumIon Batteries. Adv. Energy Mater. 2014, 4, 1300958. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. A critical review-promises and barriers of conversion electrodes for Li-ion batteries. J. Solid State Electrochem. 2017, 21, 1907–1923. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Lou, X.W. Nanostructured Conversion-type Anode Materials for Advanced Lithium-Ion Batteries. Chem 2018, 4, 972–996. [Google Scholar] [CrossRef]
- Jia, C.; Cao, B.; Zhang, W. Electrochemical properties of anatase TiO2 nanotubes as an anode material for lithium-ion batteries. Electrochim. Acta 2007, 52, 8044–8047. [Google Scholar]
- Paul, S.; Rahman, M.A.; Sharif, S.B.; Kim, J.-H.; Siddiqui, S.-E.-T.; Hossain, M.A.M. TiO2 as an Anode of high-performance lithium-ion batteries: A Comprehensive Review towards Practical Application. Nanomaterials 2022, 12, 2034. [Google Scholar]
- Das, S.; Bhowmik, K.C.; Rahman, A.; Barua, S. Anatase TiO2 as Anode of Lithium-Ion Batteries: A Comprehensive Review on Sustainable Synthesis and Electrochemical Properties. Adv. Energy Sustain. Res. 2025, 2500190. [Google Scholar] [CrossRef]
- Laruelle, S.; Grugeon, S.; Poizot, P.; Dollé, M.; Dupont, L.; Tarascon, J.-M. On the Origin of the Extra Electrochemical Capacity Displayed by MO/Li Cells at Low Potential. J. Electrochem. Soc. 2002, 149, A627. [Google Scholar] [CrossRef]
- Zheng, X.; Li, J. A review of research on hematite as anode material for lithium-ion batteries. Ionics 2014, 20, 1651–1663. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, D.; Li, Y.; Yuan, T.; Bahlawane, N.; Liang, C.; Sun, W.; Lu, Y.; Yan, M. Amorphous Fe2O3 as a high-rate and long-life anode material for lithium ion batteries. Nano Energy 2014, 4, 23–30. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Self-assembled hierarchical MoO2/graphene nanoarchitectures and their application as a high-performance anode material for lithium-ion batteries. ACS Nano 2011, 5, 7100–7107. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Hashem, A.M.; Abdel-Ghany, A.E.; El-Tawil, R.S.; Abuzeid, H.M.; Coughlin, A.; Chang, K.; Zhang, S.; El-Mounayri, H.; et al. Nanostructured molybdenum-oxide anodes for lithium-ion batteries: An outstanding increase in capacity. Nanomaterials 2021, 12, 13. [Google Scholar] [CrossRef]
- Keppeler, M.; Srinivasan, M. Interfacial Phenomena/Capacities Beyond Conversion Reaction Occurring in Nano-sized Transition-Metal-Oxide-Based Negative Electrodes in Lithium-Ion Batteries: A Review. ChemElectroChem 2017, 4, 2727–2754. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.; Yoon, J.; Um, J.H.; Lee, W.; Kim, J.; Cabana, J.; Yoon, W.-S. Exploring anomalous charge storage in anode materials for next-generation Li rechargeable batteries. Chem. Rev. 2020, 120, 6934–6976. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Kim, H.-S.; Kim, W.-S.; Wang, G. Synthesis of tuneable porous hematites (α-Fe2O3) for gas sensing and lithium storage in lithium ion batteries. Microporous Mesoporous Mater. 2012, 149, 36–45. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Nanocrystallinity effects in lithium battery materials Aspects of nano-ionics. Part IV. Phys. Chem. Chem. Phys. 2003, 5, 5215–5220. [Google Scholar] [CrossRef]
- Zhukovskii, Y.F.; Balaya, P.; Dolle, M.; Kotomin, E.A.; Maier, J. Enhanced lithium storage and chemical diffusion in metal-LiF nanocomposites: Experimental and theoretical results. Phys. Rev. B 2007, 76, 235414. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Liu, Z.; Nam, K.-W.; Borkiewicz, O.J.; Cheng, J.; Hua, X.; Dunstan, M.T.; Yu, X.; Wiaderek, K.M.; Du, L.-S.; et al. Origin of additional capacities in metal oxide lithium-ion battery electrodes. Nat. Mater. 2013, 12, 1130–1136. [Google Scholar] [CrossRef]
- Shon, J.K.; Lee, H.S.; Park, G.O.; Yoon, J.; Park, E.; Park, G.S.; Kong, S.S.; Jin, M.; Choi, J.-M.; Chang, H.; et al. Discovery of abnormal lithium-storage sites in molybdenum dioxide electrodes. Nat. Commun. 2016, 7, 11049. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, Y. Microwave-assisted synthesis of a Co3O4–graphene sheet-on-sheet nanocomposite as a superior anode material for Li-ion batteries. J. Mater. Chem. 2010, 20, 9735–9739. [Google Scholar] [CrossRef]
- Zhai, Y.; Ma, X.; Mao, H.; Shao, W.; Xu, L.; He, Y.; Qian, Y. Mn-Doped α-FeOOH Nanorods and α-Fe2O3 Mesoporous Nanorods: Facile Synthesis and Applications as High Performance Anodes for LIBs. Adv. Electron. Mater. 2015, 1, 1400057. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Xu, H.; Liu, H.; Kang, H.; Zhao, Y.; Liu, Y.; Wang, Y.; Yuan, H. Reconstruction of mini-hollow polyhedron Mn2O3 derived from MOFs as a high-performance lithium anode material. Adv. Sci. 2016, 3, 1500185. [Google Scholar] [CrossRef]
- Deng, Y.; Wan, L.; Xie, Y.; Qin, X.; Chen, G. Recent advances in Mn-based oxides as anode materials for lithium ion batteries. RSC Adv. 2014, 4, 23914–23935. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Zhao, Y.; Jia, B.; Yu, B. A review on the synthesis of manganese oxide nanomaterials and their applications on lithium-ion batteries. J. Nanomater. 2013, 2013, 736375. [Google Scholar] [CrossRef]
- Koo, B.; Xiong, H.; Slater, M.D.; Prakapenka, V.B.; Balasubramanian, M.; Podsiadlo, P.; Johnson, C.S.; Rajh, T.; Shevchenko, E.V. Hollow iron oxide nanoparticles for application in lithium ion batteries. Nano Lett. 2012, 12, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, Y.; Konstantinov, K.; Yao, J.; Ahn, J.-H.; Liu, H.; Dou, S. Nanosize cobalt oxides as anode materials for lithium-ion batteries. J. Alloys Compd. 2002, 340, L5–L10. [Google Scholar] [CrossRef]
- Wu, J.; Lau, W.-M.; Geng, D.-S. Recent progress in cobalt-based compounds as high-performance anode materials for lithium ion batteries. Rare Met. 2017, 36, 307–320. [Google Scholar] [CrossRef]
- Hou, C.; Wang, B.; Murugadoss, V.; Vupputuri, S.; Chao, Y.; Guo, Z.; Wang, C.; Du, W. Recent advances in Co3O4 as anode materials for high-performance lithium-ion batteries. Eng. Sci. 2020, 11, 19–30. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Konstantinov, K.; Lindsay, M.; Liu, H.; Dou, S. Investigation of cobalt oxides as anode materials for Li-ion batteries. J. Power Sources 2002, 109, 142–147. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Liu, Y.; Liu, H.; Wang, Y.; Yuan, H. Ultra-high capacity lithium-ion batteries with hierarchical CoO nanowire clusters as binder free electrodes. Adv. Funct. Mater. 2015, 25, 1082–1089. [Google Scholar]
- Ortiz, M.G.; Visintin, A.; Real, S.G. Synthesis and electrochemical properties of nickel oxide as anodes for lithium-ion batteries. J. Electroanal. Chem. 2021, 883, 114875. [Google Scholar] [CrossRef]
- Zhou, G.; Ding, W.; Guan, Y.; Wang, T.; Liu, C.; Zhang, L.; Yin, J.; Fu, Y. Progress of NiO-Based Anodes for High-Performance Li-Ion Batteries. Chem. Rec. 2022, 22, e202200111. [Google Scholar]
- Delmer, O.; Balaya, P.; Kienle, L.; Maier, J. Enhanced potential of amorphous electrode materials: Case study of RuO2. Adv. Mater. 2008, 20, 501–505. [Google Scholar] [CrossRef]
- Gregorczyk, K.E.; Liu, Y.; Sullivan, J.P.; Rubloff, G.W. In situ transmission electron microscopy study of electrochemical lithiation and delithiation cycling of the conversion anode RuO2. Acs Nano 2013, 7, 6354–6360. [Google Scholar] [CrossRef]
- Wang, H.; Hao, W.; Li, T.; Li, X.; Chang, K.; Zhou, X.; Hou, D.; Hashem, A.M.; Hwang, G.S.; Liu, Y.; et al. Elucidating the Mechanism Underlying the Augmented Capacity of MoO2 as an Anode Material in Li-Ion Batteries. J. Mater. Chem. A 2023, 11, 23012–23025. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Xie, Z.; Song, W.; Wang, L.; Wu, X.; Qu, F.; Chen, D.; Shen, G. High-performance energy-storage devices based on WO3 nanowire arrays/carbon cloth integrated electrodes. J. Mater. Chem. A 2013, 1, 7167–7173. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, H.; Hu, Q.; Zheng, S.; Li, L.; Xu, J.; Pang, H. Tungsten-based materials for lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1707500. [Google Scholar] [CrossRef]
- Shinde, P.A.; Jun, S.C. Review on recent progress in the development of tungsten oxide based electrodes for electrochemical energy storage. ChemSusChem 2020, 13, 11–38. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, M.; Cao, M. Developing WO3 as high-performance anode material for lithium-ion batteries. Mater. Lett. 2021, 285, 129129. [Google Scholar] [CrossRef]
- Yu, S.; Lee, S.H.; Lee, D.J.; Sung, Y.; Hyeon, T. Conversion reaction-based oxide nanomaterials for lithium ion battery anodes. Small 2016, 12, 2146–2172. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, L.; Lou, X.W. Metal oxide hollow nanostructures for lithium-ion batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar] [CrossRef]
- Xu, S.; Hessel, C.M.; Ren, H.; Yu, R.; Jin, Q.; Yang, M.; Zhao, H.; Wang, D. α-Fe2O3 multi-shelled hollow microspheres for lithium ion battery anodes with superior capacity and charge retention. Energy Environ. Sci. 2014, 7, 632–637. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Lu, S.; Li, L.; Sun, H. Micro-/Nanostructured Co3O4 Anode with Enhanced Rate Capability for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 7236–7243. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A review on design strategies for carbon based metal oxides and sulfides nanocomposites for high performance Li and Na ion battery anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar] [CrossRef]
- Chen, L.; Qu, Y.; Ma, Y. Carbon-assisted conversion reaction-based oxide nanomaterials for lithium-ion batteries. Sustain. Energy Fuels 2018, 2, 1124–1140. [Google Scholar]
- Deng, X.; Chen, Z.; Cao, Y. Transition metal oxides based on conversion reaction for sodium-ion battery anodes. Mater. Today Chem. 2018, 9, 114–132. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Tan, X.; An, Q.; Mai, L. Nanostructured conversion-type negative electrode materials for low-cost and high-performance sodium-ion batteries. Adv. Funct. Mater. 2018, 28, 1804458. [Google Scholar] [CrossRef]
- Dou, S.X.; Yu, Y. The state and challenges of anode materials based on conversion reactions for sodium storage. Small 2018, 14, 1703671. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Leung, K. Two-electron reduction of ethylene carbonate: A quantum chemistry re-examination of mechanisms. Chem. Phys. Lett. 2013, 568–569, 1–8. [Google Scholar] [CrossRef]
- Horstmann, B.; Single, F.; Latz, A. Review on multi-scale models of solid-electrolyte interphase formation. Curr. Opin. Electrochem. 2019, 13, 61–69. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.-B.; Zhao, C.-Z.; Huang, J.-Q.; Yang, S.-T.; Zhang, Q. Lithium metal protection through in-situ formed solid electrolyte interphase in lithium-sulfur batteries: The role of polysulfides on lithium anode. J. Power Sources 2016, 327, 212–220. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703. [Google Scholar] [CrossRef]
- Cheng, M.-Y.; Ye, Y.-S.; Chiu, T.-M.; Pan, C.-J.; Hwang, B.-J. Size effect of nickel oxide for lithium ion battery anode. J. Power Sources 2014, 253, 27–34. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Stavrinadis, A.; Gupta, S.; Christodoulou, S.; Konstantatos, G. Breaking the Open-Circuit Voltage Deficit Floor in PbS Quantum Dot Solar Cells through Synergistic Ligand and Architecture Engineering. ACS Energy Lett. 2017, 2, 1444–1449. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th Anniversary Article: Understanding the Lithiation of Silicon and Other Alloying Anodes for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Javad, R.S.; Roberto, G.; Agnieszka, W.; Franziska, M.; Marta, P.; Francesco, N.; Stefano, P.; Di, C.A. Is the Solid Electrolyte Interphase an Extra-Charge Reservoir in Li-Ion Batteries? ACS Appl. Mater. Interfaces 2017, 9, 4570–4576. [Google Scholar]
- Sun, H.; Xin, G.; Hu, T.; Yu, M.; Shao, D.; Sun, X.; Lian, J. High-rate lithiation-induced reactivation of mesoporous hollow spheres for long-lived lithium-ion batteries. Nat. Commun. 2014, 5, 4526. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Qin, L.; Zhang, Z.; Zhang, Z.; Zheng, Y.-Z.; Qu, Y. A bottom-up synthesis of α-Fe2O3 nanoaggregates and their composites with graphene as high performance anodes in lithium-ion batteries. J. Mater. Chem. A 2015, 3, 2158–2165. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Aurbach, D.; Ein-Eli, Y.; Markovsky, B.; Zaban, A.; Luski, S.; Carmeli, Y.; Yamin, H. The Study of Electrolyte Solutions Based on Ethylene and Diethyl Carbonates for Rechargeable Li Batteries: II. Graphite Electrodes. J. Electrochem. Soc. 1995, 142, 2882. [Google Scholar] [CrossRef]
- Yao, X.; Tang, C.; Yuan, G.; Cui, P.; Xu, X.; Liu, Z. Porous hematite (α-Fe2O3) nanorods as an anode material with enhanced rate capability in lithium-ion batteries. Electrochem. Commun. 2011, 13, 1439–1442. [Google Scholar] [CrossRef]
- Chen, D.; Quan, H.; Liang, J.; Guo, L. One-pot synthesis of hematite@graphene core@shell nanostructures for superior lithium storage. Nanoscale 2013, 5, 9684–9689. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.B.; Dwivedi, P.K.; Nalawade, A.C.; Qureshi, M.S.; Shelke, M.V. Highly durable Li-ion battery anode from Fe3O4 nanoparticles embedded in nitrogen-doped porous carbon with improved rate capabilities. J. Mater. Sci. 2020, 55, 15667–15680. [Google Scholar] [CrossRef]
- Tripathy, D.; Sampath, S. Understanding the high capacity contributions of Cu3PS4 towards lithium storage. J. Power Sources 2020, 478, 229066. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Zhang, F.; Chen, Q. CuO/Cu2O composite hollow polyhedrons fabricated from metal–organic framework templates for lithium-ion battery anodes with a long cycling life. Nanoscale 2013, 5, 4186–4190. [Google Scholar] [CrossRef]
- Chu, L.; Shi, Y.; Li, Z.; Sun, C.; Yan, H.; Ma, J.; Li, X.; Liu, C.; Gu, J.; Liu, K.; et al. Solid electrolyte interphase on anodes in rechargeable lithium batteries. Nano Res. 2023, 16, 11589–11603. [Google Scholar] [CrossRef]
- Huang, J.; Hu, L.; Xu, H.; Yang, Z.; Li, J.; Wang, P. Flexible g-C3N4 Enhancing Superior Cycling Stability of ZnFe2O4-Fe2O3 Nanosheet Composites as High-Capacity Anode Materials for Lithium-Ion Batteries. ChemElectroChem 2023, 10, e202300248. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, M.; Chen, Z.; Liu, X.-X. The construction of a sandwich structured Co3O4@C@PPy electrode for improving pseudocapacitive storage. RSC Adv. 2018, 8, 33374–33382. [Google Scholar] [CrossRef]
- Balaya, P.; Li, H.; Duppel, V.; Kienle, L.; Maier, J. High capacity and coulombic efficiency of RuO2 as electrode material for rechargeable lithium batteries. J. Electrochem. Soc. 2001, 148, A1266–A1270. [Google Scholar]
- Zhou, X.; Wen, J.; Shen, D.; He, H.; Liao, M.; Wang, Y.; Li, Y.; Shi, H.; Qiu, S.; Jiang, C.; et al. Nucleation-density-regulated dimensional evolution of growth unit from 2D nanosheets to 1D nanoneedles in self-assembled hierarchical NiCo2O4 for enhanced lithium storage. J. Mater. Chem. A 2025, 13, 11834–11847. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, Y.; Wang, L.; Du, C.; Chang, Z.; Geng, Q.; Li, Q.; Du, C.; Zhang, J. Research advances of amorphous metal oxides with the feature of Pseudocapacitance behavior in Lithium ion battery. J. Colloid Interface Sci. 2025, 700, 138331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Y.; Li, Q. Space Charge Storage Mechanism in Conversion-Type Electrode Materials. ChemSusChem 2025, 18, e2501139. [Google Scholar] [CrossRef]
- Li, H.; Balaya, P.; Maier, J. Li-Storage via Heterogeneous Reaction in Selected Binary Metal Fluorides and Oxides. J. Electrochem. Soc. 2004, 151, A1878. [Google Scholar] [CrossRef]
- Maier, J. Mass storage in space charge regions of nano-sized systems (Nano-ionics. Part V). Faraday Discuss. 2007, 134, 51–66. [Google Scholar] [CrossRef]
- Zhukovskii, Y.F.; Balaya, P.; Kotomin, E.A.; Maier, J. Evidence for Interfacial-Storage Anomaly in Nanocomposites for Lithium Batteries from First-Principles Simulations. Phys. Rev. Lett. 2006, 96, 058302. [Google Scholar] [CrossRef]
- Cherian, C.T.; Sundaramurthy, J.; Kalaivani, M.; Ragupathy, P.; Kumar, P.S.; Thavasi, V.; Reddy, M.V.; Sow, C.H.; Mhaisalkar, S.G.; Ramakrishna, S.; et al. Electrospun α-Fe2O3 nanorods as a stable, high capacity anode material for Li-ion batteries. J. Mater. Chem. 2012, 22, 12198–12204. [Google Scholar] [CrossRef]
- Chaudhari, S.; Srinivasan, M. 1D hollow α-Fe2O3 electrospun nanofibers as high performance anode material for lithium ion batteries. J. Mater. Chem. 2012, 22, 23049–23056. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wei, C.; Huang, X.; Zhang, X.; Wen, G. MoS2-based anode materials for lithium-ion batteries: Developments and perspectives. Particuology 2024, 87, 240–270. [Google Scholar] [CrossRef]
- Ahn, J.; Sasikala, S.P.; Jeong, Y.; Kim, J.G.; Ha, J.-H.; Hwang, S.H.; Jeon, S.; Choi, J.; Kang, B.-H.; Ahn, J.; et al. High-Energy–Density Fiber Supercapacitors Based on Transition Metal Oxide Nanoribbon Yarns for Comprehensive Wearable Electronics. Adv. Fiber Mater. 2024, 6, 1927–1941. [Google Scholar] [CrossRef]
- Li, K.; Xue, S.; Hu, Y.; Zheng, J.; Zhang, M.; Shen, Z. Size effect on interfacial pseudocapacitive contributions to lithium-ion storage in microscale carbon/TiO2 nanosheet composite. Mater. Today Sustain. 2022, 18, 100112. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, K.; Li, J.; Yuan, M.; Wang, J.; Wang, Q.; Lai, A.; Jiang, Y.; Yan, X.; Zhang, G.; et al. Beyond LiF: Tailoring Li2O-Dominated Solid Electrolyte Interphase for Stable Lithium Metal Batteries. ACS Nano 2024, 18, 1969–1981. [Google Scholar] [CrossRef]
- Sun, J.-X.; Liu, S.-H.; Chen, L.-Y.; Zhu, D.-D.; Yu, H.-X.; Niu, Y.-Z.; Zhang, L.-Q.; Li, Q.-H.; He, Y.; Miao, G.-X.; et al. Accelerating charging and elevating capacity of TiO2 by interface space charge storage. Rare Met. 2025, 44, 5404–5411. [Google Scholar] [CrossRef]
- Li, H.; Xia, Q.; Hu, Z.; Zhu, Y.; Yan, S.; Ge, C.; Zhang, Q.; Wang, X.; Shang, X.; Fan, S.; et al. Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry. Nat. Mater. 2021, 20, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xia, Q.; Zhang, H.; Li, Z.; Wang, H.; Li, X.; Zuo, F.; Zhang, F.; Wang, X.; Ye, W.; et al. Operando Magnetometry Probing the Charge Storage Mechanism of CoO Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2006629. [Google Scholar] [CrossRef]
- Bock, D.C.; Pelliccione, C.J.; Zhang, W.; Timoshenko, J.; Knehr, K.W.; West, A.C.; Wang, F.; Li, Y.; Frenkel, A.I.; Takeuchi, E.S.; et al. Size dependent behavior of Fe3O4 crystals during electrochemical (de)lithiation: An in situ X-ray diffraction, ex situ X-ray absorption spectroscopy, transmission electron microscopy and theoretical investigation. Phys. Chem. Chem. Phys. 2017, 19, 20867–20880. [Google Scholar] [CrossRef]
- Xia, H.; Hu, Y.; Li, Z.; Lan, H.; Zhang, J. Electron Spin Polarization in Rechargeable Batteries: Theoretical Foundation and Practical Applications. Adv. Funct. Mater. 2024, 35, 2413491. [Google Scholar] [CrossRef]
- Zuo, F.; Zhang, H.; Ding, Y.; Liu, Y.; Li, Y.; Liu, H.; Gu, F.; Li, Q.; Wang, Y.; Zhu, Y.; et al. Electrochemical interfacial catalysis in Co-based battery electrodes involving spin-polarized electron transfer. Proc. Natl. Acad. Sci. USA 2023, 120, e2314362120. [Google Scholar] [CrossRef]
- Kang, S.K.; Kim, M.; Shin, H.H.; Yoon, W.; Lee, S.; Jang, D.; Choi, J.; Park, G.H.; Park, J.; Kim, W.B. Exceeding Theoretical Capacity in Exfoliated Ultrathin Manganese Ferrite Nanosheets via Galvanic Replacement-Derived Self-Hybridization for Fast Rechargeable Lithium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2300143. [Google Scholar] [CrossRef]
- Wang, F.; Wen, X.; Mittal, U.; Nekouei, R.K.; Foller, T.; Shang, Y.; Bhadra, A.; Chu, D.; Sharma, N.; Kundu, D.; et al. Structure-dependent lithium storage characteristics of Fe3O4/rGO aerogels. Carbon 2024, 222, 119003. [Google Scholar] [CrossRef]
- Teng, X.; Li, X.; Yang, H.; Guan, L.; Li, Y.; Yun, H.; Li, Z.; Li, Q.; Hu, H.; Wang, Z.; et al. Uncovering the origin of the anomalously high capacity of a 3d anode via in situ magnetometry. Chem. Sci. 2023, 14, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, M.; Du, X.; Gao, X.; Feng, K.; Liu, Y.; Yang, X.; Sun, R.; Luo, D.; Chen, Z. Li2O-Enhanced Solid Electrolyte Interphase Surpassing LiF-Only SEI for High-Performance All-Solid-State Li Batteries. Adv. Energy Mater. 2025, 15, 2502589. [Google Scholar] [CrossRef]
- Ren, Y.-J.; Guan, H.-B.; Hou, Y.-L.; Zhang, B.-H.; Tian, K.-K.; Xiong, B.-Q.; Chen, J.-Z.; Zhao, D.-L. Enhancing Rapid Li+/Na+ Storage Performance via Interface Engineering of Reduced Graphene Oxide-Wrapped Bimetallic Sulfide Nanocages. ACS Appl. Mater. Interfaces 2024, 16, 45619–45631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, M.; Yu, P.; Yu, J. Spin-Polarized Surface Capacitance Effects Enable Fe3O4 Anode Superior Wide Operation-Temperature Sodium Storage. Adv. Sci. 2024, 11, 2306992. [Google Scholar] [CrossRef]
- Zheng, K.; Mu, Y.; Han, M.; Liu, J.; Zou, Z.; Hu, H.; Chu, Y.; Yu, F.; Li, W.; Wei, L.; et al. Investigations of Mechanisms Leading to Capacity Differences in Li/Na/K-Ion Batteries with Conversion-Type Transition-Metal Sulfides Anodes. Adv. Sci. 2024, 11, 2410653. [Google Scholar] [CrossRef]
- Xia, C.; Zhou, Y.; Velusamy, D.B.; Farah, A.A.; Li, P.; Jiang, Q.; Odeh, I.N.; Wang, Z.; Zhang, X.; Alshareef, H.N. Anomalous Li Storage Capability in Atomically Thin Two-Dimensional Sheets of Nonlayered MoO2. Nano Lett. 2018, 18, 1506–1515. [Google Scholar] [CrossRef]
- Li, X.; Hao, W.; Wang, H.; Li, T.; Trikkaliotis, D.; Zhou, X.; Hou, D.; Chang, K.; Hashem, A.M.; Liu, Y. Lithium storage mechanisms and electrochemical behavior of a molybdenum disulfide nanoparticle anode. Energy Environ. Mater. 2025, 8, e12855. [Google Scholar] [CrossRef]
- Su, L.; Hei, J.; Wu, X.; Wang, L.; Zhou, Z. Ultrathin Layered Hydroxide Cobalt Acetate Nanoplates Face-to-Face Anchored to Graphene Nanosheets for High-Efficiency Lithium Storage. Adv. Funct. Mater. 2017, 27, 1605544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).