1. Introduction
Charcoal is a renewable resource, low-cost, and environmentally friendly material resulting from the carbonization of wood or plants by heat in the absence of air at a temperature above 300 °C. Various factors influence the final yield and quality of charcoal from biomass, including substrate composition, heating rate, and final pyrolysis temperature [
1,
2].
Feedstock characteristics affect the conversion process and product properties. Most biomass is composed of three organic compounds cellulose, hemicelluloses, and lignin. Due to their different behavior during heat treatment, biomass composition directly impacts the quality and yield of the product [
3,
4].
Furthermore, process conditions, including temperature and residence time, determine how much product can be obtained from pyrolysis of a given biomass and have to be defined depending on the desired properties of the char.
Between the process conditions, the heating rate is a very crucial parameter. The choice of pyrolysis time can enable the porosity and char particle size to be controlled, which is very important for potential applications [
5].
In recent years, charcoal has gained increasing attention because of its unique properties, such as its high carbon content and cation exchange capacity, large specific surface area, and stability. Among its many applications, it removes pollutants [
6], remediates soil [
7], reduces greenhouse gas emissions [
8], and significantly reduces formaldehyde emissions in particleboards composites [
9], constituting an ideal resource for environmental technologies due to its economic and ecological benefits [
10,
11].
Lately, considerable attention has been given to this material as a filler for polymer matrices to provide polymer composites with improved electrical and mechanical properties [
12,
13,
14]. In fact, it is well known the ability of carbon fillers, which include various types of carbon black and graphite, due to their favorable properties and the possibility of modification, to positively affect the improvement of features required [
15,
16,
17,
18,
19].
From an industrial standpoint, charcoal appears more convenient than the usual carbon fillers due to its affordability, renewable nature, and environmental friendliness.
Several articles have recently reported on the ability of charcoal to substitute less environmentally friendly carbon fillers, such as in epoxy resins, to improve electrical conductivity [
20] and mechanical properties [
21] or in PLA biocomposites whose properties can be modulated by selecting the content of carbon filler to govern the morphology as well as the rheological and mechanical behavior [
22].
More interestingly, the possibility to chemically modify this material allows for changing its chemical and physical properties, providing a suitable filler for the right polymer matrix. It is possible to modify charcoal’s properties by introducing functionalities on its surface, classified into oxygen, nitrogen, and sulfur-containing groups by using different approaches. As an example, physical activation (steam, plasma, etc.), acid or alkaline pretreatment, and chemical activation such as oxidation, nitrogenation, or sulfuration can insert the functional groups needed for a given application [
2].
For example, the treatment of charcoal with strong acids, such as sulfuric acid, nitric acid, and hydrochloric acid, can introduce carboxylic and phenolic groups onto the surface of the charcoal. These functional groups can enhance the hydrophilicity of charcoal and increase its adsorption capacity for pollutants, such as heavy metals and organic compounds [
23]. Similarly, treatment with amines can introduce amino groups onto the surface of the charcoal, which can enhance its adsorption capacity for cations, such as ammonium and phosphate ions. The resulting functionalized charcoal has also been used as a catalyst for various organic reactions, such as the Knoevenagel condensation [
24] and the Suzuki–Miyaura cross-coupling reaction [
25], due to the presence of the positively charged surface.
Further chemical modifications can be introduced by chemically bonding surface-specific molecules to the carbon in order to modulate the properties, the polarity, and the interactions with the polymer matrix [
26]. The chemical modification can be performed appropriately based on the nature of the functional groups. As part of this paper, we will focus on oxygen functional groups.
The surface modification can be realized through covalent or ionic functionalization, exploiting existing oxygen functionalities or by appropriate oxidation.
It has been reported that covalent functionalization can be used to provide modified biochar by grafting proper molecules. However, the process requires many steps and thermal treatment in order to ensure the successful obtainment of the required filler [
27,
28]. Conversely, the approach of ionic functionalization and exploiting the cation exchange allows us to easily obtain the adducts by a green procedure in a short reaction time.
We have recently described an eco-friendly procedure for preparing cationic drug adducts with charcoal and lidocaine, which could be suitable for cationic drug release [
29]. In particular, the adduct formation can be realized in an alkaline aqueous solution through a preliminary oxidation step that, assuring enhancing oxygen content, provides lidocaine uptake up to 30 wt% by ionic functionalization. It has to be noted that the oxidation step was crucial to provide high salt uptake, resulting in an efficient cationic exchange only with oxidized charcoal. Further confirmation of efficient cation exchange was obtained by UV spectra of neutral and acidic aqueous solutions, showing the ability to release the drug fast and completely in both neutral and acidic conditions.
In this study, charcoal is shown for the first time to bond ionically with quaternary phosphonium salts (QPS), tetraphenyl phosphonium bromide (TPPBr), and dodecyl triphenyl phosphonium bromide (DTPPBr) (
Figure 1a,b respectively) whose cationic nature enables them to attach to the surface of biochar through electrostatic interactions, forming a positively charged layer on the surface. With this sustainable procedure, it is possible to provide new interesting chemical-modified carbon fillers for potential application both as flame retardant agents [
30,
31] and antimicrobial material [
32,
33,
34,
35].
Concerning QPS used as a raw material in polymer matrices, enhanced flame retardant, and antimicrobial activity could be obtained for the adducts, as already observed using organic clays and intercalated graphite systems [
30,
36].
In fact, as a result of the intercalation of QPS with montmorillonite, the flame retardant filler was dispersed better in the polyamide polymer matrix, resulting in a more efficient network structure and leading to improved flame retardancy.
The action mechanism of quaternary phosphonium salt is different from the usual flame-retardant action of halide compounds; the flame-retardant function is both in the condensed and gas phase. Among the several factors determining the performance of phosphorus retardation of combustion in condensed and gas phases of a specific system, such as the polymers types, the elemental phosphorus or phosphorus present in the compound, the good dispersion and interaction with the polymer matrix represent an important parameter. Therefore, the use of intercalation compounds resulting in higher dispersibility could improve flame retardant properties [
37].
Additionally, graphite oxide compounds with different quaternary phosphonium salts were described with pH-dependent releases of phosphonium ions in aqueous solutions. Since phosphonium ions have poor dispersion in water, the ionic functionalization represents a significant improvement for their application in water/polar media.
Hence, chemical modification by cation exchange appears to be a simple and powerful method not only for increasing the chemical properties of molecules but also for increasing their dispersibility in various polymer matrices.
Based on the interesting results previously published [
30], we report the synthesis of the new adducts. using a straightforward green procedure base-assisted in a water solution that in reduced time, assures the high uptake of QPS. The reaction can be performed after a step of oxidation with hydrogen peroxide and subsequent basification in a water solution to provide new functionalized carbon materials via cation exchange in the presence of the phosphonium salts.
The products obtained have been characterized by elemental analysis, wide-angle X-ray diffraction, infrared spectroscopy, and thermogravimetry.
2. Materials and Methods
2.1. Materials, Oxidation Procedures, and Preparation of Compounds
Charcoal (Carbo lignis pulveratus) (C) was purchased from Caesar and Loretz GmbH in Hilden, Germany). Based on elemental analysis, its Oxygen/Carbon weight ratio was high (O/C = 0.22). Charcoal was oxidized by hydrogen peroxide at 60 °C; 1 mL of H2O2 30 wt.% was used for 2 mg of Charcoal in a typical procedure to give oxidized charcoal (oC). As reported, the results are for charcoal that has been oxidized for 24 h.
It was necessary to basify the oxidized charcoal before preparing its compounds with the cationic drug. The powder was dispersed and stirred for 15 min in 0.05 M NaOH solution (20 mL). TPP aqueous solutions (100 mL) were added to this dispersion and the reaction mixture was stirred at room temperature.
The reactions were stopped after 1 h, and the products were extensively washed with water to remove the residual excess salts. After drying the powders for 12 h at 60 °C, the mass yield was 80% for both adducts as evaluated on the total amount of oC used in the reactions.
The results presented pertain to the Charcoal/TPP adduct with a weight ratio of 1/3. The same procedure was utilized for the preparation of oC/DTPP adducts. To determine the salt uptake of TPP and DTPP at 20 wt% and 28 wt%, respectively, TGA analysis was conducted. Using an excess of TPP and DTPP resulted in lower uptake for both adducts. All reagents were utilized as received and were not purified.
2.2. Characterization Methods
Thermo FlashEA 1112 Series CHNS-O analyzer from Thermo Fisher Scientific Inc. (Waltham, MA, USA) was utilized to conduct elemental analyses on pretreated samples, which were oven-dried at 100 °C for 12 h. Surface areas of the carbon materials utilized were measured through nitrogen adsorption at liquid nitrogen temperature (77 K) using a Nova Quantachrome 4200e instrument by Quantachrome Instruments (Boynton Beach, FL, USA). Before adsorption measurements, samples were vacuum-degassed at 60 °C for 24 h. Surface area (SA-BET) values were obtained using an 11-point Brunauer–Emmett–Teller (BET) analysis.
The thermogravimetric (TGA) analysis was carried out using a Q500 manufactured by TA Instruments, from 10 to 800 °C with a heating rate of 10 °C under N2 below. Weight decreases below 100 °C were used to determine water content.
FTIR spectra were obtained with an FTIR (BRUKER Vertex70, Bruker, Karlsruhe, Germany) spectrometer equipped with a deuterated triglycine sulfate (DTGS) detector and a KBr beam splitter using KBr pellets. The spectra were obtained at a resolution of 2.0 cm−1 in the range of 3000–450 cm−1. The frequency scale was internally calibrated to 0.01 cm−1 using a He-Ne laser. The noise was reduced by signal-averaging 32 scans. The KBr pellets were prepared according to the following procedure: for solid compounds, ~1–2 mg of the sample were ground in the special mortar and pestle. Then ~80 mg of anhydrous KBr, treated at approximately 120 °C for two to three hours were mixed quickly with the sample; ~30–40 mg of the ground mixture were placed in a press under a pressure of 5000 PSI for 30 s before carefully removing one of the bolts. The obtained KBr pellet was carefully placed in the IR sample holder to obtain the IR spectrum.
Wide-angle X-ray diffraction (WAXD) patterns were obtained using an automatic Bruker D2 phaser diffractometer in reflection, with nickel-filtered Cu Kα radiation (1.5418 Å) at 35 kV and 40 mA. The d-spacings were calculated using Bragg’s law, and the observed integral breadths (βobs) were determined by fitting the intensity-corrected diffraction patterns with a Lorentzian function. The instrumental broadening (βinst) was determined by fitting a Lorentzian function to line profiles of a standard silicon powder 325 mesh (99%). For each observed reflection, the corrected integral breadths were determined by subtracting the instrumental broadening of the closest silicon reflection from the observed integral breadths (
β = βobs − βinst). The correlation lengths (
D) were determined using Scherrer’s equation, where
λ is the wavelength of the incident X-rays and q is the diffraction angle, assuming the Scherrer constant
K = 1.
Dispersibility tests were performed by using 5 mg/mL of oC/, oC/TPP, and oC/DTPP suspension in water, acetonitrile, and ethyl acetate. The solutions were sonicated for 5 min and the dispersibility was evaluated after 30 min of storage.
3. Results
Charcoal (C), exhibiting a BET surface area of 50 m2/g and an O/C weight ratio of 0.21, has been used as starting material. After the oxidation step performed at 60 °C by hydrogen peroxide for 24 h, the oxidized charcoal (oC) has been obtained with a 0.45 O/C weight ratio as determined by elemental analysis.
As revealed by FTIR (
Figure 2A), charcoal oxidation provides increasing oxygen functionalities on the surface. Specifically, an enhancement of the stretching vibration at 1160 cm
−1 related to secondary alcohol and the appearance of bands in the region 1270–1120 cm
−1 due to the ether functionalities were observed. Moreover, a significant increase in the 1700 cm
−1 stretching was detected due to unsaturated ketones or lactones. The TGA also confirms the successful oxidation by showing less thermostability for oC (
Figure 2B). Due to the amorphous nature of the starting materials, no change in the RX diffraction pattern after oxidation can be revealed.
After the oxidation, the resulting oC was used as a starting material for the following functionalization step. To evaluate the ability of oC to be ionically functionalized by TPP and DTPP, the reactions were both performed using the procedure base-assisted previously reported in an aqueous solution [
30] in the presence of a significant excess of the organic salts (oC/TPP and oC/DTPP weight ratio of 1/3).
It is worth noting that the same reactions performed in the presence of C only provided small uptake of cations (less than 5%), indicating the need for the oxidation step.
As can be seen from FTIR and TGA (
Figure 2), efficient functionalization was obtained for both the phosphonium salts.
More specifically, in the presence of TPP, 20 wt% of salt uptake was obtained as evaluated by the TGA for oC/TPP (
Figure 2B). Moreover, as shown by FTIR (
Figure 2A), the peaks at 529, 694, 725, 764, 995, 1105, and 1435 cm
−1 related to TPP are clearly observed in the adduct confirming the occurred functionalization.
Higher salt uptake up to 28 wt% was instead observed for oC/DTPP, as evaluated from TGA (
Figure 2C).
Again the FTIR shows the peaks at 499, 535, 691, 721, 749, 994, 1111, 1435, 2855, 2923, and 2954 cm−1 related to DTPP confirming the oC/DTPP adduct formation.
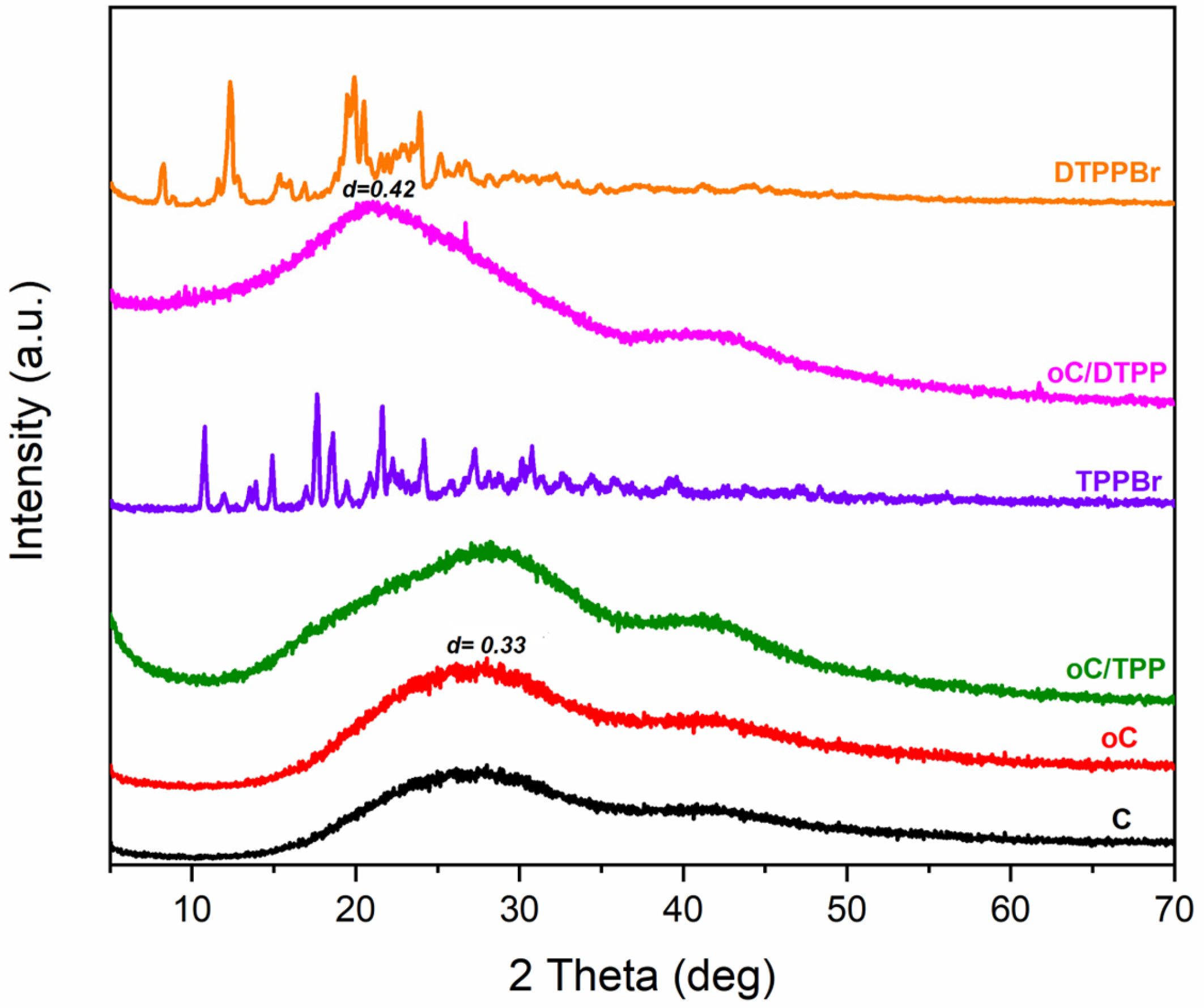
The RX diffraction pattern of oC/TPP and oC/DTPP are shown in
Figure 3.
The diffraction pattern of the oC/TPP shows only a broadening of the amorphous halo due to the charcoal’s amorphous structure, while the TPP salt’s peaks are missing because its crystallinity was completely lost as a consequence of the adduct formation.
Conversely, a shift of the amorphous halo centered at 2θ = 21° (d = 0.42 nm) has been detected for oC/DTPP, possibly due to DTTP’s significant uptake in the charcoal structure.
The lack of crystallinity of both salts in the adducts not only confirms the formation of the adducts but also the absence of a residual excess of salts in powders.
A further evaluation of the functionalization degree of oC was obtained by elemental analysis, as reported in
Table 1. For both the adducts, a reduction in the O/C ratio was detected concerning oC (0.38 and 0.35 for oC/TPP and oC/DTPP, respectively, versus 0.45 oC) as a consequence of the ionic functionalization.
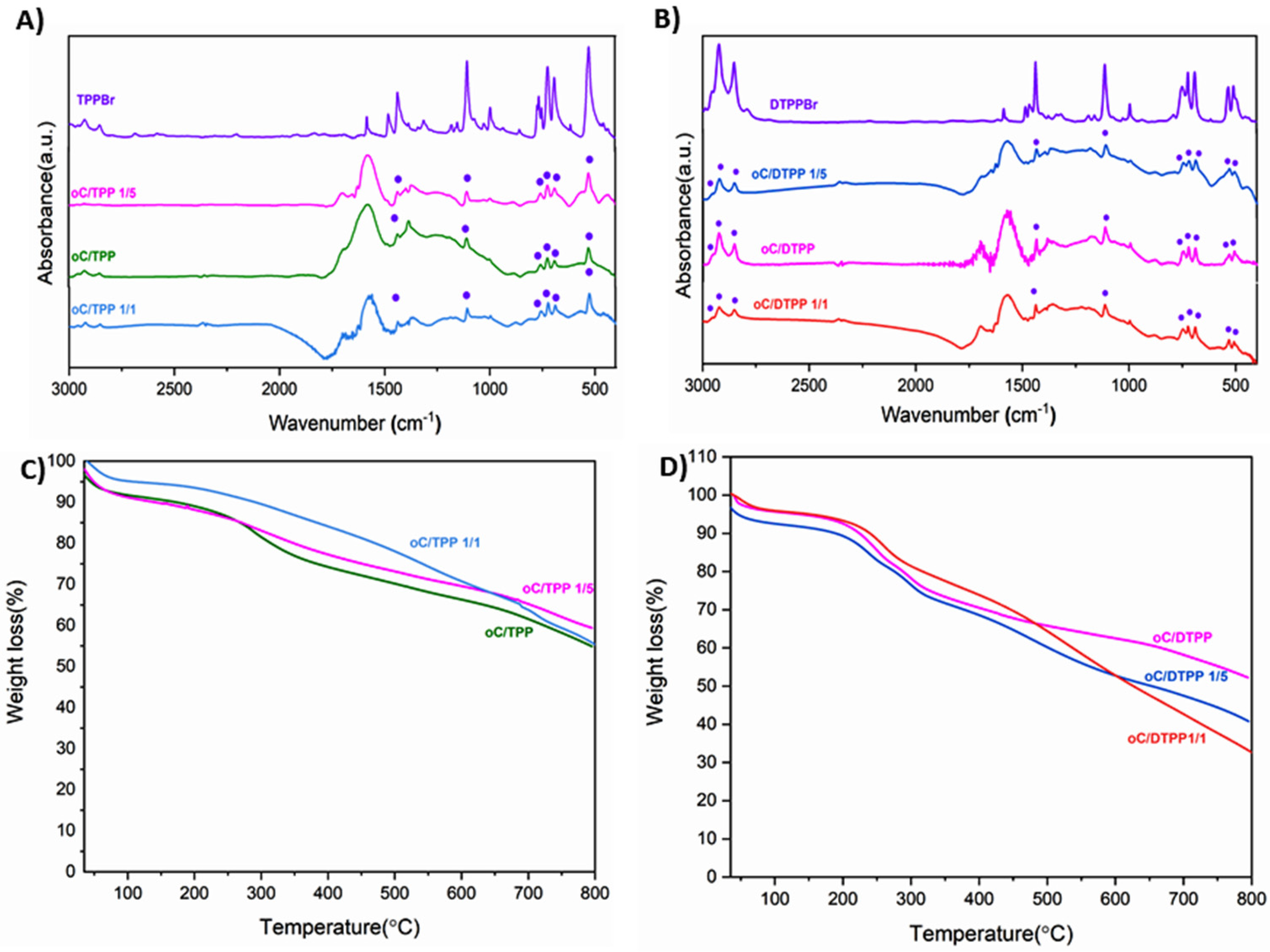
In an attempt to improve the procedure to provide higher salt uptake, a comparative study was performed, increasing and decreasing the weight ratio between the reagents for both the adducts to 1/1 and 1/5, respectively.
As can be seen, by the FTIR analyses (
Figure 4A,B), the occurrence of the formation of the adducts is still possible by reducing the amount of salts. Still, it results in less uptake for TPP and DTPP, respectively, as clearly observed by TGA analyses (
Figure 4C,D).
Conversely, no increase in salt uptake was achieved as the weight ratio between the reagents grew up to 1/5 as clearly shown in
Figure 4C,D for oC/TPP and oC/DTPP, respectively.
More interestingly, the oC/TPP obtained from different weight ratios of reagents have similar FTIR profiles, but the significantly different TGA degradations indicate that the 1/3 weight ratio was optimal.
In contrast, the oC/DTPP adduct from weight reagents ratio 1/3 is clearly the best option, as shown by FTIR, which reveals a high intensity of alkyl stretching vibration between 3000 and 2750 cm−1, as well as a comparable uptake with 1/5 by TGA.
Further confirmation of the best degree of functionalization for the 1/3 reagent ratio was obtained by comparing the elemental analyses for the different compounds (see
Table 1). In all cases, higher O/C reduction was obtained due to higher salt uptake with a 1/3 reagent ratio. Diffraction patterns from X-rays did not provide any additional information in either case.
The chemical modification introduced by ionic functionalization is able to affect the polarity of the carbon materials. Therefore, after choosing the best reaction condition to provide efficient functionalization, dispersibility tests were performed in water, acetonitrile and ethyl acetate were selected as organic solvents with the starting materials oC and the adducts oC/TPP and oC/DTTP, respectively.
As can be seen in
Figure 5, the usual oC dispersibility in water is lost as a consequence of the functionalization with both the phosphonium salts. More interestingly, the corresponding adducts oC/TPP and oC/DTPP, are dispersible in acetonitrile and more in ethyl acetate after 5 min of sonication and 30 min of storage, while they are completely not dispersible in water. Considering the polarity index for the solvents chosen [
38], (10 for water, 5.8 for acetonitrile, and 4.4 for Ethyl acetate) the functionalization with TPP and DTPP reduces the polarity of oC.
Based on these results, it is quite evident that the polarity modulation of the carbon materials can be realized by using an appropriate counterion. Changing the polarity of the starting material, it is possible to improve the interaction with the polymer matrices and the filler dispersibility, resulting in an enhancement of the properties, for example, the flame retardant and antimicrobial properties of the phosphonium salts described in this paper.
4. Conclusions
This paper presents an eco-friendly procedure to provide new charcoal-based fillers with possible antimicrobial or flame-retardant molecules.
The procedure includes an oxidation step performed in green conditions by using an H2O2 solution to increase the O/C ratio from 0.22 to 0.45, followed by the preparation of oxidized charcoal adducts with quaternary phosphonium salts, tetraphenyl phosphonium bromide (TPPBr) and dodecyl triphenyl phosphonium bromide (DTPPBr) in alkaline solution.
The reaction assures the obtainment of the oC/TPP and oC/DTPP adducts with a salt content of 20 and 28 wt%, respectively.
In addition, a study of changing the weight ratio between the reagents for both adducts was performed to improve salt uptake and optimize cationic exchange conditions. According to the investigation, the highest uptake is obtained by combining oC and QPS at a weight ratio of 1/3.
Dispersibility tests were performed in different media, water, acetonitrile, and ethyl acetate, with the starting material and both the adducts, showing the ability of functionalization to deeply change the polarities of the carbon material. As a result, a higher dispersibility can positively affect the possible interactions and improve the compatibility in other media, such as polymer matrices with different polarities.