Abstract
The possibility of high-temperature solid-phase synthesis of Ni-doped bismuth tantalate pyrochlore, not only from the oxide compounds BiTaO4 and NiO but also using nickel chloride NiCl2 as a precursor, was shown for the first time. The concentration range of the formation of nickel pyrochlore Bi2NixTa2O9−δ (0.85 ≤ x ≤ 1.0) at an equal molar ratio of bismuth(III)/tantalum(V) ions was determined. This indicates that the pyrochlore structure may be stable at 20–33% vacant bismuth sublattice. The influence of non-stoichiometric composition relative to bismuth and tantalum, by the example of Bi2±xNiTa2O9−δ and Bi2NiTa2−xO9−δ (x ≤ 0.5) compositions, on the phase composition of ceramics was studied. X-ray powder diffraction phase analysis of Bi2Ni1+yTa1.75−yO9−δ samples made it possible to determine the solubility limit of nickel ions in the tantalum sublattice. It was shown that the molar ratio of metal atoms (Ta, Ni) in the B2O6 (B-Ni(II), Ta(V)) octahedral sublattice of mixed pyrochlores is also limited.
1. Introduction
The crystal structure of pyrochlore is represented by the general formula A2B2O7, in which two interpenetrating weakly interacting sublattices A2O and B2O6 are distinguished [1]. The octahedra of the B2O6 basis structure, arranged around the diamond grid points, form a tetrahedral framework connected at the vertices. Two large A cations and one additional O atom per each formula unit of the (BO3)2 framework are placed in its cavities. Relatively small B cations (Ru(IV), Ta(V), Ti(IV), Zr(IV), Nb(V)) are arranged in octahedra, and large A cations (Bi(III), Pb(II), Sm(III)) are arranged in a distorted octahedron formed by oxygen atoms of A2O and B2O6 sublattices. Due to the structural flexibility, pyrochlores correspond to a wide range of cation substitutions in the crystallographic positions A and B, which contributes to the formation of hundreds of compositions with a certain set of practically useful properties [2,3,4,5,6,7,8]. Materials based on pyrochlores are used in solid-state devices as thermistors, thick-film resistors, and communication elements, and also as components of ceramic forms for radioactive waste. Materials based on bismuth pyrochlores are promising for multilayer ceramic capacitors and tunable microwave dielectric components due to their excellent dielectric properties, possibility of dielectric properties regulation by electric field, low sintering temperature, and chemical compatibility with fusible Ag, Cu conductors [9,10,11,12].
Traditionally, the optimization of the physicochemical properties of ceramics is performed by modifying their chemical composition. Nickel doping of bismuth pyrochlores positively affects the dielectric tunability and thermal dielectric constant [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] (−57 ppm/°C for Bi1.5NiNb1.5O7 [19]). Nickel pyrochlores based on bismuth niobate Bi1.6Ni2/3−yNb4/3+yO6.4+3y/2 exhibit high dielectric permittivity values of 127–168 while varying the y coefficient from −0.1 to 0.1 [14]. Bi2Ni2/3Nb4/3O7 ceramics show close values of dielectric permittivity 122 and dielectric loss tangent tanδ = 0.001 at 1 MHz [30]. Nickel-containing bismuth tantalate pyrochlores have lower dielectric permittivity compared to niobium ones, which is associated with a highly porous ceramic microstructure and decreased overall polarizability when replacing Nb(V) by Ta(V). For example, the dielectric permittivity value for Bi3Ni1.4Ta3O13.4 is 44.85 (RT, 105 Hz) [18] and for Bi2NiTa2O9 it is 32 for 30 °C and 1 MHz [16]. Meanwhile, nickel pyrochlores based on bismuth tantalate, which have a highly porous microstructure, are characterized by low values of the band gap width and are promising as photocatalysts [31]. Despite the prospects for use of nickel pyrochlores, the concentration stability region of bismuth tantalate-based nickel pyrochlores, in contrast to niobium pyrochlores [13], has not been studied in detail. In addition to the study of pyrochlore composition Bi2NiTa2O9 [16], a number of compositions Bi3Ni2−xTa3O14−x (−0.2 ≤ x ≤ 1.0) were systematically investigated [18]. Only one composition at x = 0.6 was referred to phase-pure pyrochlores. In our study, we determined the concentration range of formation of the nickel pyrochlore Bi2NixTa2O9−δ and the solubility limit of nickel ions in the tantalum sublattice. The samples with vacancies in the bismuth and tantalum sublattices were also studied. In addition, the possibility of solid-phase synthesis of Ni-doped bismuth tantalate pyrochlore, not only from the oxide compounds BiTaO4 and NiO but also using nickel chloride NiCl2 as a precursor, was demonstrated.
2. Experimental
Bi2NiTa2O9 pyrochlore was synthesized by solid-state reaction from bismuth orthoniobate BiTaO4 and nickel(II) oxide. The stoichiometric precursor mixture was homogenized in an agate mortar for one hour. Then, the obtained mixture was compacted in the form of disks for close contact of ceramic grains. The samples were heat-treated in two ways. In the first case, the sample was calcined once at 1050 °C for 15 h. In the second, calcination was carried out in two stages of 15 h at 950 and 1050 °C [32].
In another case, in addition to nickel(II) chloride, bismuth(III) and tantalum(V) oxides were used for the solid-state synthesis of Bi2NiTa2O9. The stoichiometric mixture of precursors was thoroughly ground in an agate mortar for one hour and then pressed in the form of disks. The samples were calcined in four 15 h steps at 650, 850, 950, and 1050 °C [16,17]. After calcination, the samples were homogenized and pressed again. In the traditional version of the ceramic synthesis for samples including Bi2NixTa2O9−δ, the synthesis conditions remained unchanged, but nickel(II) oxide was used instead of nickel chloride.
X-ray powder diffraction data were collected using a Shimadzu 6000 X-ray diffractometer (CuKα radiation; 2θ = 10–80°; scanning speed 2.0 deg/min). A vertical type of goniometer was used. The scanning mode was ϴ/2ϴ; the scanning radius was 185 mm. A scintillation counter was used as a detector. The scintillator is NaI. The irradiation zone of the sample is 1 cm2. Soller slots with parameters DS = 1, SS = 1, RS = 0.15 were used. The cell parameters were calculated using the CSD software package. Investigations of surface morphology and local quantitative elemental analysis were performed by scanning electron microscopy and energy dispersive X-ray spectroscopy (electron scanning microscope Tescan VEGA 3LMN, energy dispersion spectrometer INCA Energy 450). The optimum working distance was fixed within 15 mm with operating voltage at 10–15 kV. The spectral resolution was 0.01 keV. The content of chloride ions in the sample (Bi2NiTa2O8Cl2) was determined by atomic emission spectrometry using an optical emission ICP spectrometer SPECTRO CIROSCCD. The detection limit of chloride ions in the solution was 1.6 mg/L. For the chemical analysis, several sample weights (~7–8 mg) were taken. The volume of the solution prepared by dissolving the sample is 25 cm3.
3. Results and Discussion
3.1. Ceramic Synthesis
Previously, in a study of pyrochlore phase formation, it was found that bismuth orthotantalate is a precursor of pyrochlore phase. In this case, BiTaO4 cannot transform into pyrochlore alone because it has a large ratio of ionic radii r(Bi3+)/r(Ta5+) ~1.83 at r(Bi3+) = 1.17 Å (c.n. = 8) and r(Ta5+) = 0.64 Å (c.n. = 6) [31]. According to [32], the pyrochlore phase is actively synthesized at temperatures above 850 °C during the interaction of orthorhombic bismuth orthotantalate (α-BiTaO4) with nickel oxide. This gives a potential possibility for high-temperature synthesis of pyrochlore from these precursors. Initially, pyrochlore was synthesized by calcination at 1050 °C of the sample obtained from a homogeneous stoichiometric mixture of bismuth orthotantalate and nickel(II) oxide. According to X-ray powder diffraction phase analysis (Figure 1a), the sample contains the main pyrochlore phase and the impurity phase of nickel oxide. The pyrochlore unit cell parameter is 10.5547(4) Å. The presence of nickel oxide admixture indicates that the synthesis of nickel pyrochlore is a kinetically inhibited process and a single 15 h calcination was not enough. In the case of double 15 h calcination at 950 and 1050 °C (Figure 1b), the minimal amount of bismuth orthotantalate impurity was observed in X-ray powder diffraction patterns. The unit cell parameter of pyrochlore is 10.5224(8) Å. As can be seen, the unit cell parameter decreases during the formation of stoichiometric pyrochlore. It should be noted that the value of 10.5224(8) is comparable with the unit cell parameter of phase-pure Bi2NiTa2O9. Meanwhile, this study shows that repeated calcination with intermediate homogenization of the sample is necessary to obtain phase-pure pyrochlore, even from BiTaO4 and NiO.
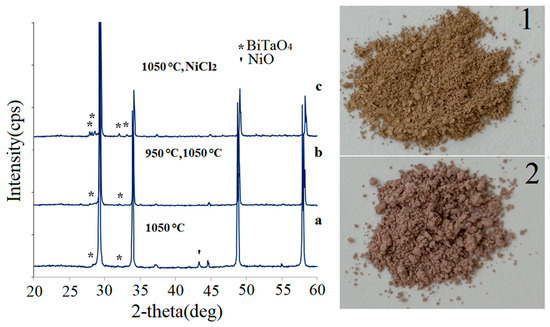
Figure 1.
X-ray powder diffraction patterns of Bi2NiTa2O9 samples synthesized from BiTaO4 and NiO in one stage at 1050 °C (a) and in two stages at 950 and 1050 °C (b), using the NiCl2 precursor (c); photographs of Bi2NiTa2O9 samples synthesized from (1) NiCl2 and (2) oxides.
Nickel(II) chloride was used as a precursor in order to clarify the possibility of synthesizing nickel pyrochlore with a mixed anionic sublattice consisting of chlorine and oxygen ions. Synthesis conditions were identical to those for pyrochlore synthesis from oxides. The sample was calcined four times at 650, 850, 950, and 1050 °C with intermediate homogenization. X-ray powder diffraction phase analysis showed the presence of bismuth orthotantalate triclinic impurity in the sample in the amount of 11 wt. % (Figure 1c). The unit cell parameter of pyrochlore is 10.4884(8) Å. The color of the sample is browner compared to the lilac color for nickel pyrochlore synthesized from oxides (Figure 2). Apparently, there are several reasons for the color change of the sample. Among these are the replacement of a part of oxygen ions in the octahedral environment of nickel(II) ions, change in the symmetry of the coordination environment or the oxidation degree of nickel ions, and influence of impurity substances. Since chloride ions are weak-field ligands, this substitution can lead to a decrease in the energy of splitting by the crystal field. A change in the splitting energy will affect the energy of the absorbed radiation and it will decrease. For this reason, the wavelength of the absorbed radiation will shift to the long wavelength region. As is known, the color of the compound is complementary to the color of the absorbed radiation. In this case, the color of the sample should be a shorter wavelength, which is not consistent with the experiment. Oxidation of nickel(II) ions to nickel(III) (Jahn–Teller ion) can lead to an increase in the energy of splitting by the crystal field and a decrease in the wavelength of absorbed radiation, which will lead to an increase in the wavelength of the complementary color. We observe a similar effect on our sample. The influence of the impurity on the color of nickel pyrochlore is unlikely because triclinic bismuth orthotantalate does not absorb visible radiation and its content in the sample is low. The effect of a change in the symmetry of the coordination of polyhedron of nickel ions on the color of the sample due to the imperfection of the anion sublattice caused by the partial replacement of oxygen ions by chloride ions or anion vacancies cannot be excluded.
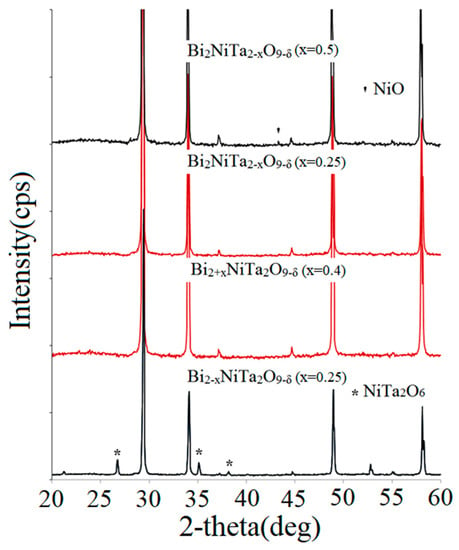
Figure 2.
X-ray powder diffraction patterns of Bi2−xNiTa2O9−δ, Bi2+xNiTa2O9+δ, and Bi2NiTa2−xO9−δ samples at different x values.
Energy dispersion spectroscopy analysis did not reveal a reliable content of chloride ions in the sample, either due to trace amounts or due to overlapping of chloride absorption lines (L and K series in the region of 0.183 eV and 2.62, 2.82 eV) with bismuth M-series lines in the 2.42–2.74 eV region. Atomic emission spectroscopy analysis of the preparation showed that chloride ion content in the sample was less than the limit of device sensitivity (1.6 mg/L). If one conventionally assumes that the content of chloride ions in the prepared solution is equal to the detection limit, then the chemical composition of the preparation corresponds to the formula Bi2NiTa2O8+δCl≤0.15 instead of the expected Bi2NiTa2O8Cl2 composition. From this result, we can conclude that high-temperature calcination (1050 °C) of the chloride-containing sample in air leads to the chlorine loss and violation of the compound stoichiometry.
3.2. Study of Pyrochlore Stability Area
Previously, we reported [16,18] on the preparation of phase-pure nickel-containing pyrochlore of the composition Bi2NiTa2O9 and Bi3Ni1.4Ta3O14−δ. Considering that nickel(II) ions occupy predominantly octahedral Ta(V) positions [16], it was assumed that the bismuth sublattice may be ~33% vacant compared to the tantalum one (Bi2NiTa2O9), while the tantalum(V) sublattice is 1/3 filled with nickel(II) ions. Therefore, the aim was to determine experimentally the maximum and minimum vacancy levels of the bismuth(III) sublattice and the degree of substitution of tantalum(V) ions by nickel(II) ions at which the pyrochlore structure remains stable. In order to determine the concentration range of nickel pyrochlore formation in the Bi2O3-NiO-Ta2O5 system, several series of compositions were investigated.
First of all, the influence of bismuth and tantalum composition non-stoichiometry on the phase composition of ceramics was investigated on the examples of Bi2−xNiTa2O9−δ, Bi2+xNiTa2O9+δ, and Bi2NiTa2−xO9−δ (x ≤ 0.5) compounds. X-ray powder diffraction patterns of the samples are shown in Figure 2. According to the X-ray powder diffraction phase analysis data, the Bi2−xNiTa2O9−δ samples are non-phase pure even at x = 0.25. The X-ray powder diffraction patterns of the samples demonstrate reflexes of a single impurity NiTa2O6 (sp. gr. P-1). The formation of the nickel tantalate impurity can be explained by the fact that the bismuth sublattice is significantly unfilled, by 42% and 50%, respectively. These values, as will be shown later, are above the bismuth sublattice defectivity limit for pyrochlore stabilization. In the case of Bi2+xNiTa2O9+δ compositions, the sample at x = 0.4 is single-phase. Based on this, one can conclude that the minimum vacancy limit of the bismuth sublattice is likely to be 20%. Thus, the allowable range of bismuth sublattice vacancy is about 20–33%.
X-ray powder diffraction phase analysis of Bi2NiTa2−xO9−δ (x = 0.25; 0.5) samples showed that the Bi2NiTa2−xO9−δ (x = 0.25) sample is single-phase. In the X-ray powder diffraction pattern of the composition at x = 0.5, the NiO impurity phase is determined in trace amounts. The degree of bismuth sublattice defectiveness for both compositions is acceptable and is equal to 27 and 20%, respectively. As will be shown below, the presence of a nickel oxide impurity is associated with an excess of the degree of substitution of Ta(V) ions. Apparently, for the stability of the octahedral tantalum sublattice, it is important to keep the strict molar ratio of n(Ni(II)/n(Ta(V)). In other words, there should be a limit for the substitution of sublattice B ions by heterovalent ions in mixed pyrochlores for the octahedral framework to remain stable. This limit should probably depend on the nature of the substituting atoms, as in the case of Ni(II) and Fe(III) (3d cations) and their ability to distribute over the bismuth(III) and tantalum(V) ion sublattices.
The degree of substitution of Ta(V) ions by Ni(II) ions for the considered compositions was calculated. For the Bi2NiTa2−xO9−δ samples (x = 0.25; 0.5), it was 36.4% and 40%, respectively. Since the Bi2NiTa2−xO9−δ sample (x = 0.5) is multiphase, the value of ~36% may be the limit in the case of nickel(II) ions. In order to estimate more precisely the limit of substitution of tantalum(V) ions by nickel(II) ions and to find out whether this number depends on the number of vacancies in the bismuth sublattice, two series of samples with different degrees of bismuth sublattice defects (indicated in percentages and given in parentheses) were considered. To solve this problem, Bi2Ni1+yTa1.75−yO9−δ (y = 0.1) (28%) and Bi2Ni0.85+yTa1.75−yO9−δ (y = 0.29) (30%) samples were prepared. The ratio of pyrochlore sublattice atoms A and B is variable (this ratio is in the range 27–33%), but the ratio of octahedral sublattice ions n(Ni(II)/n(Ta(V)) is fixed (degree of substitution 40%). The X-ray powder diffraction patterns of the studied samples show an impurity of NiO, regardless of the vacancy percentage of the bismuth sublattice. This result may indicate that the maximum degree of substitution of Ta(V) ions by nickel(II) ions is not affected by the degree of bismuth(III) sublattice defectiveness.
The X-ray powder diffraction phase analysis of the samples of Bi2NixTa2O9−δ series (0.4 ≤ x ≤ 1.2) with the equal molar ratio of bismuth(III)/tantalum(V) ions allowed us to establish the minimum and maximum degree of substitution of nickel(II) and Ta(V) ions to preserve the stability of the tantalum(V) octahedral sublattice. X-ray powder diffraction patterns of the synthesized samples are shown in Figure 3. The compositions at 0.85 ≤ x ≤ 1.0 are referred to as single-phase samples. The impurity composition of the multiphase samples is different. The samples at 0.4 ≤ x ≤ 0.8 exhibit β-BiTaO4 as an impurity [33], and the nickel tantalate (NiTa2O6) and NiO as an impurity are found at x > 1.0. This is confirmed by the results of [18]. This work is devoted to the investigation of Bi3Ni2−tTa3O14−x samples (–0.2 ≤ t ≤ 1.0). Among these samples, the authors detected one phase-pure pyrochlore at t = 0.6, which fits into the specified interval 0.85 ≤ x ≤ 1.0. Apparently, the formation of the NiTa2O6, NiO impurity is caused by exceeding the substitution limit of the octahedral sublattice, and the formation of bismuth orthotantalate indicates an insufficient ratio of Ni(II)/Ta(V) ions. Assuming that nickel(II) ions predominantly occupy the octahedral positions [16], it may be concluded that the octahedral framework, including the pyrochlore structure, is potentially stable if the octahedral positions are replaced in the range of 30–36%. It should be noted that 33% corresponds to the substitution of 1/3 of all octahedral positions. If one assumes that 2/3 of the positions are occupied by Ta(V) ions and 1/3 of the positions are occupied by Ni(II) ions, then the average oxidation degree of the octahedral sublattice B ions is +4. This conditional charge of the B ions corresponds to the traditional stoichiometry of the oxide pyrochlore A+32B+42O7, provided that the A ions are three-charged cations, such as Bi+3 [1,34].
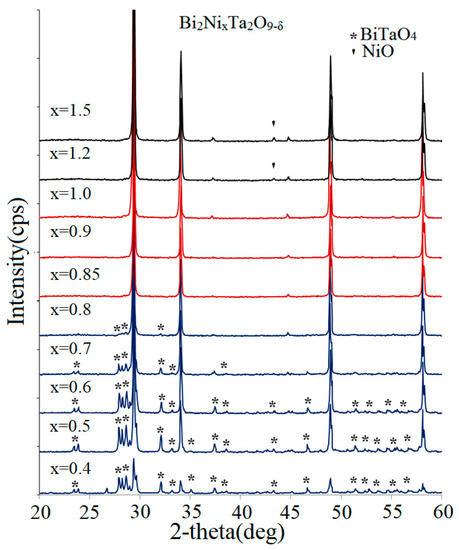
Figure 3.
X-ray powder diffraction patterns of Bi2NixTa2O9−δ (0.4 ≤ x ≤ 1.2) samples.
Based on the results of the analysis, one can conclude that the molar ratio of metal atoms (Ta, Ni) in the octahedral sublattice of B2O6 (B-Ni(II),Ta(V)) mixed pyrochlores is also limited. To preserve the stability of the Ta2O6 octahedral sublattice, the degree of substitution of tantalum(V) ions by nickel(II) ions cannot be less than 30% or greater than 36% (33 ± 3%). According to X-ray powder diffraction phase analysis, these conditions are crucial for the formation of pyrochlore structure. As shown above, the ultimate degree of substitution of Ta(V) ions does not depend on the number of vacancies in the bismuth sublattice. It should be noted that the established stability criteria for mixed pyrochlores do not contradict the traditional pyrochlore stability coefficient [1] but are only an addition that is true for mixed pyrochlores containing divalent nickel cations. According to [1], to describe the stability of oxide pyrochlores, the notion of the “pyrochlore stability field” based on the ratio of cationic radii is used. The r(A)/r(B) = 1.46–1.80 and 1.40–2.20 values range limits the stability of pyrochlores having A3+2B4+2O7 and A2+2B5+2O7 composition, respectively. For instance, for nickel pyrochlore of Bi2NiTa2O9 composition, it is equal to 1.78 and belongs to the specified intervals. Thus, this paper demonstrates that the degree of substitution of the octahedral coordination ions and the bismuth sublattice vacancy are important for the formation of phase-pure mixed bismuth pyrochlores.
4. Conclusions
The possibility of the ceramic high-temperature synthesis of the nickel pyrochlore based on the bismuth tantalate from BiTaO4 and NiO precursors is shown. It has been established that to obtain phase-clean pyrochlore, repeated heat treatment with intermediate homogenization of the sample is required. Using ISP atomic emission spectroscopy and EDS analysis, it has been shown that in the course of high-temperature (1050 °C) solid-state synthesis of the nickel pyrochlore using the NiCl2 precursor, the content of chloride ions in the synthesized pyrochlore is significantly lower than the target amount. In order to determine the concentration range of the stability of Ni-doped bismuth tantalate pyrochlores, the phase composition of several series of nickel-containing samples was studied. It was found that phase-pure nickel-doped bismuth tantalate pyrochlores are formed with a partially vacant bismuth sublattice and almost 1/3 of the Ta(V) positions are occupied by nickel(II) ions. It is shown that the most important condition for the formation of the pyrochlore structure is the requirement for an optimal degree of substitution of tantalum sites. The revealed conditions for the formation of nickel pyrochlores will make it possible to theoretically predict the stoichiometric composition of preparations for the synthesis of phase-pure pyrochlores.
Author Contributions
Conceptualization, N.A.Z.; Formal analysis, D.V.S.; Investigation, V.A.M., M.G.K., B.A.M., A.N.N. and N.A.Z.; Resources, S.V.N. and V.N.S.; Writing—original draft, N.A.Z.; Visualization, V.A.M. and B.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Higher Education of Russia grant number 075-15-2021-1351.
Data Availability Statement
Not applicable.
Acknowledgments
The Centre for X-ray Diffraction Studies at the Research Park of St. Petersburg State University (XRD Center SPbU) for providing instrumental and computational resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Arakawa, H. Preparation, structural and optical properties of a new class of compounds, Bi2MNbO7 (M = Al, Ga, In). Mater. Sci. Eng. B 2001, 79, 83–85. [Google Scholar] [CrossRef]
- Valant, M.; Babu, G.S.; Vrcon, M.; Kolodiazhnyi, T.; Axelsson, A.-K. Pyrochlore Range from Bi2O3–Fe2O3–TeO3 System for LTCC and Photocatalysis and the Crystal Structure of New Bi3(Fe0.56Te0.44)3O11. J. Am. Ceram. Soc. 2011, 95, 644–650. [Google Scholar] [CrossRef]
- Vanderah, T.A.; Siegrist, T.; Lufaso, M.W.; Yeager, M.C.; Roth, R.S.; Nino, J.C.; Yates, S. Phase Formation and Properties in the System Bi2O3:2CoO1+x:Nb2O5. Eur. J. Inorgan. Chem. 2006, 23, 4908–4914. [Google Scholar] [CrossRef]
- Miles, G.C.; West, A.R. Pyrochlore Phases in the System ZnO-Bi2O3-Sb2O5: I. Stoichiometries and Phase Equilibria. J. Am. Ceram. Soc. 2006, 89, 1042–1046. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Ellert, O.G.; Maksimov, Y.V.; Volodin, V.D.; Efimov, N.N.; Novotortsev, V.M. Subsolidus phase equilibria and magnetic characterization of the pyrochlore in the Bi2O3–Fe2O3–Sb2Ox system. J. Alloys Compd. 2013, 579, 311–314. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Lebedev, A.M.; Chumakov, R.G.; Makeev, B.A.; Kharton, V.V.; et al. Spectroscopic characterization of cobalt doped bismuth tantalate pyrochlore. Solid State Sci. 2022, 125, 106820. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Sekushin, N.A.; Semenov, V.G.; Fedorova, A.V.; Selyutin, A.A.; Krzhizhanovskaya, M.G.; Lutoev, V.P.; Makeev, B.A.; Kharton, V.V.; Sivkov, D.N.; et al. Dielectric properties, Mössbauer study, ESR spectra of Bi2FeTa2O9.5 with pyrochlore structure. J. Alloys Compd. 2022, 903, 163928. [Google Scholar] [CrossRef]
- Giampaoli, G.; Siritanon, T.; Day, B.; Li, J.; Subramanian, M.A. Temperature in-dependent low loss dielectrics based on quaternary pyrochlore oxides. Prog. Solid State Chem. 2018, 50, 16–23. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zheng, H. BMN-based transparent capacitors with high dielectric tunability. J. Alloys Compd. 2017, 699, 68–72. [Google Scholar] [CrossRef]
- Du, H.; Yao, X. Structural trends and dielectric properties of Bi-based pyrochlores. J. Mater. Sci. Mater. Electron. 2004, 15, 613–616. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Yu, S.; Sun, Z.; Zheng, H.; Li, J.; Luo, W. Temperature–stable dielectrics based on Cu–doped Bi2Mg2/3Nb4/3O7 pyrochlore ceramics for LTCC. Ceram. Int. 2018, 44, 333–338. [Google Scholar] [CrossRef]
- Valant, M.; Suvorov, D. The Bi2O3–Nb2O5–NiO Phase Diagram. J. Am. Ceram. Soc. 2005, 88, 2540–2543. [Google Scholar] [CrossRef]
- Valant, M. Dielectric Relaxations in Bi2O3–Nb2O5–NiO Cubic Pyrochlores. J. Am. Ceram. Soc. 2009, 92, 955–958. [Google Scholar] [CrossRef]
- Jin, Y.X.; Li, L.X.; Dong, H.L.; Yu, S.H.; Xu, D. Structures, phase transformations, and dielectric properties of (1−x)Bi2Zn2/3Nb4/3O7–xBi1.5NiNb1.5O7 pyrochlore ceramics prepared by aqueous sol–gel method. J. Alloys Compd. 2015, 622, 200–205. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Nekipelov, S.V.; Kharton, V.V.; Sekushin, N.A. Thermal Expansion, XPS Spectra, and Structural and Electrical Properties of a New Bi2NiTa2O9 Pyrochlore. Inorg. Chem. 2021, 60, 4924–4934. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Kharton, V.V.; Koroleva, A.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Makeev, B.A.; Lebedev, A.M.; et al. Novel Ni-Doped Bismuth–Magnesium Tantalate Pyrochlores: Structural and Electrical Properties, Thermal Expansion, X-ray Photoelectron Spectroscopy, and Near-Edge X-ray Absorption Fine Structure Spectra. ACS Omega 2021, 6, 23262–23273. [Google Scholar] [CrossRef]
- Abdullah, A.; Khalid, W.E.F.W.; Abdullah, S.Z. Synthesis and Characterization of Bismuth Nickel Tantalate Pyrochlore. Appl. Mech. Mater. 2015, 749, 30–35. [Google Scholar] [CrossRef]
- Liang, K.; Gao, L.; Fang, Z.; Liu, Z.; Guan, Z.; Chen, H.; Zhang, J. Effects of Ni2+ substitution on the structure and dielectric properties of Bi1.5MgNb1.5O7 cubic pyrochlores. J. Eur. Ceram. Soc. 2020, 41, 3425–3431. [Google Scholar] [CrossRef]
- Ning, P.; Li, L.; Zhang, X.; Wang, M.; Xia, W. Enhanced tunability of Bi3/2MNb3/2O7 (M = Zn, Mg, Ni) thin films. Mater. Lett. 2012, 87, 5–8. [Google Scholar] [CrossRef]
- Qasrawi, A.F.; Nazzal, E.M.; Mergen, A. Structural, optical, electrical and dielectric properties of Bi1.5 Zn0.92Nb1.5−xNxO6.92−3x/2 solid solution. Adv. Appl. Ceram. 2012, 111, 165–170. [Google Scholar] [CrossRef]
- Yee, K.A.; Han, K.R.; Kimp, H.T. The effect of V2O5 on the sintability and physical properties of Bi2O3–NiO–Nb2O5 and Bi2O3–ZnO–Nb2O5 temperature-stable dielectrics. J. Mater. Sci. 1999, 34, 4699–4704. [Google Scholar] [CrossRef]
- Nguyen, B.; Liu, Y.; Withers, R.L. The local crystal chemistry and dielectric properties of the cubic pyrochlore phase in the Bi2O3–M2+O–Nb2O5 (M2+ = Ni2+ and Mg2+) systems. J. Solid State Chem. 2007, 180, 549–557. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Norén, L.; Liu, Y.; Withers, R.L.; Wei, X.; Elcombe, M.M. The disordered structures and low temperature dielectric relaxation properties of two misplaced-displacive cubic pyrochlores found in the Bi2O3–MIIO–Nb2O5 (M = Mg, Ni) systems. J. Solid State Chem. 2007, 180, 2558–2565. [Google Scholar] [CrossRef]
- Gao, L.; Liang, K.; Guan, Z.; Liu, Z.; Fang, Z.; Chen, H.; Zhang, J. Disordered Structures and Dielectric Properties of Ni-Doped Bismuth Magnesium Niobate Pyrochlores. J. Phys. Chem. C 2021, 125, 27793–27799. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, L.; Chen, H.; Liang, K.; Liu, Z.; Guan, Z.; Zhang, J. XPS study of probing evidence for displacive disorder in Ni-doped bismuth magnesium niobate pyrochlore. Mater. Sci. Eng. B 2020, 259, 114601. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Ellert, O.G.; Zubavichus, Y.V.; Gajtko, O.M.; Efimov, N.N.; Svetogorov, R.D.; Murzin, V.Y. New complex bismuth oxides in the Bi2O3–NiO–Sb2O5 system and their properties. J. Solid State Chem. 2015, 225, 97–104. [Google Scholar] [CrossRef]
- Koroleva, M.S.; Piir, I.V.; Istomina, E.I. Synthesis, structure and electrical properties of Mg-, Ni-codoped bismuth niobates. Chim. Techno Acta 2017, 4, 231–241. [Google Scholar] [CrossRef]
- Sirotinkin, V.P.; Bush, A.A. Preparation and Dielectric Properties of Bi1.5MNb1.5O7 (M = Cu, Mg, Mn, Ni, Zn) Pyrochlore Oxides. Inorg. Mater. 2003, 39, 974–977. [Google Scholar] [CrossRef]
- Cann, D.P.; Randall, C.A.; Shrout, T.R. Investigation of the Dielectric Properties of Bismuth Pyrochlore. Solid State Commun. 1996, 100, 529–534. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- VMuraviev, V.A.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Korolev, R.I.; Zhuk, N.A. Synthesis of Bi2NiTa2O9 with a pyrochlore-type structure. Glass Ceram. 2022, 95, 40–46. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Belyy, V.A.; Kharton, V.V.; Chichineva, A.I. Phase transformations and thermal expansion of α- and β-BiTaO4 and the high-temperature modification γ-BiTaO4. Chem. Mater. 2020, 32, 5493–5501. [Google Scholar] [CrossRef]
- McCauley, R.A. Structural Characteristics of Pyrochlore Formation. J. Appl. Phys. 1980, 51, 290–294. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).