Abstract
In this work, first-principle calculations based on density functional theory are employed to investigate how chlorine doping influences the elastic moduli, ductility, and lattice thermal conductivity of Bi2O2Se, aiming to explore an effective method to improve its mechanical properties for its applications under thermal stress. Our findings reveal that chlorine(Cl) doping significantly affects the electronic structure and mechanical properties of Bi2O2Se. The electrons are distributed on the Fermi level, and the Cl-doped Bi2O2Se exhibits metal-like properties. In addition, Cl doping enhances the ductility and toughness of Bi2O2Se and reduces its lattice thermal conductivity. These results suggest that Cl doping is an effective approach for tuning the mechanical properties of Bi2O2Se.
1. Introduction
Presently, in the face of the escalating global energy crisis and increasing environmental pollution, it is imperative to develop green and sustainable energy resources [1,2]. Thermoelectric power generation (TEG) has become one of the hot research topics due to its advantages of being green and efficient [3]. The assembly, manufacturing process, and reliable operation of TEG devices necessitate thermoelectric materials with excellent mechanical performance. Inferior mechanical properties can lead to crack formation and subsequent performance degradation under thermal stress, especially considering the operational conditions involving cyclic temperature gradients [4,5]. Therefore, it is essential for materials to possess favorable mechanical properties in order to meet the requirements of practical applications.
In recent years, Bismuth oxyselenide (Bi2O2Se), in which weak electrostatic interactions exist between adjacent layers, has attracted widespread attention and become an emerging structural material with great development potential in high-performance electronic, optoelectronic, and flexible devices due to its high electron mobility and excellent air stability [6,7]. So far, experimental and theoretical investigations on the mechanical properties of Bi2O2Se are still in their infancy stage. Theoretically, Liu et al. [8] investigated the electronic structure, bulk modulus, shear modulus, and thermal conductivity of Bi2O2Se using density functional theory (DFT), and revealed its high anisotropy and potential as a mechanical material. Pang et al. [9] studied the mechanical response of Bi2O2Se under uniaxial and biaxial tension using first-principles calculations. They found that the band gap of Bi2O2Se decreases with increasing tensile strain, and a phase transition from semiconductor to metal occurs eventually, due to the evolution of electron localization function. Wang et al. [10] studied the mechanical properties of Bi2O2Se under a pressure of 50 GPa using first-principles calculations and found that the Bi2O2Se exhibits mechanical stability at least below 50 GPa. Furthermore, the Bi2O2Se exhibits anisotropic and malleable properties within the pressure range, with the anisotropy becoming increasingly significant as the pressure increases. Zhang et al. [11] studied the elastic properties of Bi2O2Se1−xTex by the first-principles calculations and proposed that substituting Se for Te in Bi2O2Se can optimize its mechanical properties. Despite these efforts, further improvements on the mechanical properties of Bi2O2Se are necessary in order to meet the requirements of thermoelectric generator device assembly and manufacturing processes, as well as to ensure reliable operation.
Experimentally, the halogen element Cl has been widely employed to enhance the mechanical properties of materials. Piriz et al. [12] investigated the mechanical properties of Cl-doped edge zigzag graphene nanoribbons (ZGNRs) and found that Cl-doped ZGNRs exhibit better mechanical properties as compared to pure graphene. Zhang et al. [13] prepared Cl-doped carbon nanotube sheets via floating catalyst chemical vapor deposition and studied the effects of Cl doping on the mechanical properties of carbon nanotube sheets. They found that the doped sandwich composite film displays excellent mechanical properties, with a tensile strength of 90 MPa. Theoretically, Park et al. [14] investigated the effect of Cl doping on the mechanical properties of amorphous carbon films using DFT and revealed that the introduction of Cl atoms into amorphous carbon films could significantly reduce the bulk modulus, thereby softening the films. These experimental and computational studies demonstrate that Cl doping is an effective approach for improving the mechanical properties of materials.
However, the research on the mechanical properties of Cl-doped Bi2O2Se has not been reported thus far. Therefore, in this work, we employ first-principles methods to investigate the effects of Cl doping on the geometrical, electronic, and mechanical properties of Bi2O2Se. The geometrical structure, density of state distributions, bulk modulus B, Young’s modulus E, shear modulus G, Debye temperature , and lattice thermal conductivity are all determined for Bi2O2Se before and after Cl doping. It is shown that Cl doping results in redistribution of electrons in Bi2O2Se, and the Cl-doped Bi2O2Se exhibits metallic properties. In addition, Cl doping affects the mechanical strength, toughness, ductility, and lattice thermal conductivity of Bi2O2Se considerably. As compared with the pristine Bi2O2Se, the Cl-doped system exhibits better ductility, better toughness, and reduced lattice thermal conductivity, which is beneficial to restrain heat transfer and reduce the heat loss of energy. This study, thus, provides a new method to tune the physical properties of Bi2O2Se and has important implications for promoting related investigations.
2. Computational Details
Our calculations are implemented in the Ab initio Simulation Package (VASP) code (Vienna, Austria) within the framework of DFT [15]. The interaction between electrons and ions is described by the projector augmented-wave (PAW) pseudopotential approximation [16]. In addition, the Perdew–Burke–Ernzerhof (PBE) functional within the generalized gradient approximation (GGA) [17] and the Heyd–Scuseria–Ernzerhof (HSE06) hybrid functional [18] are used to deal with the exchange-correlation potential between electrons. The Monkhorst-package scheme [19] with a 6 × 6 × 6 k-point sampling for the Brillouin-zone is used to perform all the calculations. Besides, the cut-off energy for the plane wave basis sets is 500 eV. In this work, the considered systems are Bi2O2Se and Bi2O2Se0.875Cl0.125. The elastic constants of the material are calculated by using the stress-strain method, in which six finite distortions of the lattice are performed, and the relationship between the stress component (i = 1–6) and the applied strain j (j = 1–6) at a small deformation is described as [20]. In the calculation, the energy and force convergence criteria are set to be 1 × 10−5 eV/atom and 1 × 10−2 eV/Å, respectively. The specific flowchart of the calculation process is shown in Figure 1.
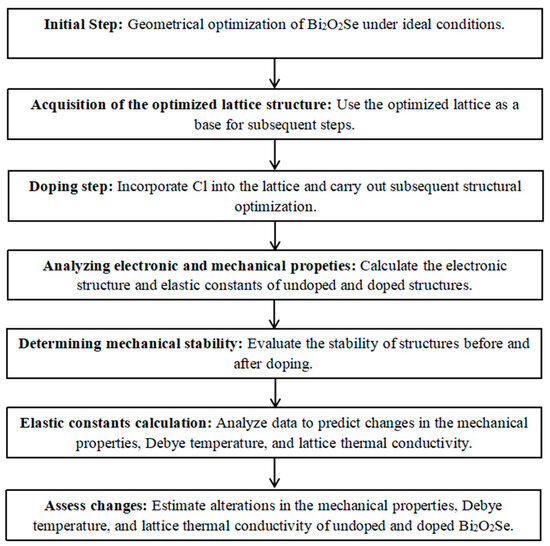
Figure 1.
Flowchart of the calculation process for undoped and doped Bi2O2Se.
3. Results and Discussions
3.1. Structural Properties of Bi2O2Se and Bi2O2Se0.875Cl0.125
The bulk phase of Bi2O2Se exhibits a tetragonal I4/mmm structure (No. 139) with a unit cell containing 10 atoms. Within Bi2O2Se, the curved [Bi2O2]2+ layers and [Se]2− layers stack alternately along the c-axis through weak electrostatic interactions [21]. Figure 2 presents the optimized geometrical structure of Bi2O2Se and Bi2O2Se0.875Cl0.125, both containing 40 atoms. The lattice constants, volumes, and bond lengths of Bi2O2Se and Bi2O2Se0.875Cl0.125 are presented in Table 1. For undoped Bi2O2Se, the calculated lattice constants are a0 = b0 = 3.917 Å and c0 = 12.357 Å, which show good agreements with other computational results of a0 = b0 = 3.90 Å and c0 = 12.39 Å [22], as well as experimental data of a0 = b0 = 3.88 Å and c0 = 12.16 Å [23]. Additionally, the <Bi-O> and <Bi-Se> bond lengths of Bi2O2Se are determined to be 2.337 Å and 3.311 Å, respectively. These values are comparable with the previously calculated results of 2.312 Å and 3.272 Å reported by Wu et al. [24]. As compared with Bi2O2Se, the calculated lattice constants a0 and b0 (3.946 Å) of Bi2O2Se0.875Cl0.125 are increased by 0.74%, while the c0 (12.287 Å) is decreased by 0.57%, resulting in a 0.91% volume expansion. Meanwhile, the calculated <Bi-O> bond length (2.339 Å) of Bi2O2Se0.875Cl0.125 is increased by 0.09% and <Bi-Se> bond length (3.336 Å) is increased by 0.76%. This is mainly because the following reactions occur after Cl doping [25]:
where extra electrons are produced, resulting in increased repulsive interaction between electrons and an expansion of volume.
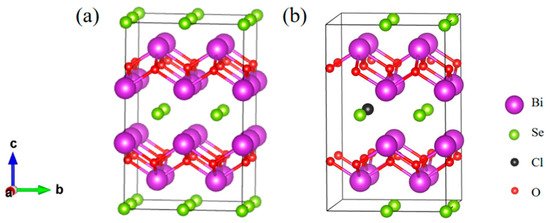
Figure 2.
The optimized geometrical structures of (a) Bi2O2Se and (b) Bi2O2Se0.875Cl0.125. The purple, green, black and red spheres denote Bi, Se, Cl and O atoms, respectively.

Table 1.
Comparison of lattice constants a0 (Å) and c0 (Å), volume (Å3), and bond length (Å) of Bi2O2Se and Bi2O2Se0.875Cl0.125 with experimental and other theoretical results.
3.2. The Influence of Cl Doping on the Electronic Structure of Bi2O2Se
We further explore the influence of Cl doping on the electronic structure of Bi2O2Se by investigating the density of state (DOS) distributions of Bi2O2Se and Bi2O2Se0.875Cl0.125. The total and projected DOS distributions around the Fermi level of Bi2O2Se and Bi2O2Se0.875Cl0.125, obtained by the standard DFT method, are plotted in Figure 3a,b, respectively. For Bi2O2Se, the valence bands ranging from 4 to 5.53 eV are predominantly dominated by Se 4p orbitals hybridized with O 2p and Bi 6s orbitals. The conduction bands ranging from 6.02 to 8 eV are mainly characterized by Bi 6p orbitals and O 2p orbitals. The obtained band gap between the valence band maxima (contributed by Bi 6s) and the conduction band minima (contributed by Bi 6p) of 0.49 eV for Bi2O2Se is consistent with previous theoretical values (0.41 eV [26], 0.43 eV [27], and 0.472 eV [28]). In addition, the strong hybridization between these orbitals will lead to higher stability in Bi2O2Se [29]. The DOS distributions of Bi2O2Se0.875Cl0.125 shown in Figure 3b indicate that the Fermi levels cross the valence bands and conduction bands, and many electrons are observed at the Fermi level. These features imply metallic properties for Bi2O2Se0.875Cl0.125, which differ from the results reported by Tan et al. [25], primarily because our doping concentration of 2.5% is higher than the dissolution limit of 1.5% in the literature.
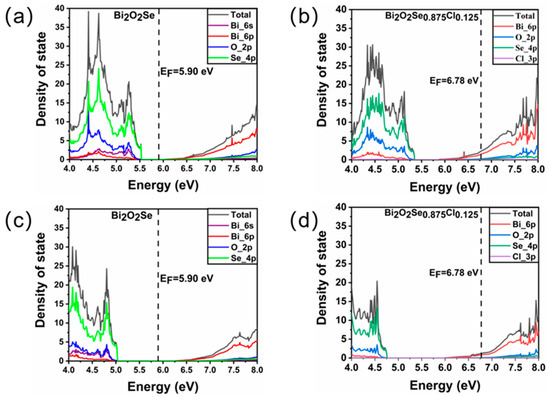
Figure 3.
The total and projected density of state distribution for Bi2O2Se and Bi2O2Se0.875Cl0.125 obtained by (a,b) standard DFT method and (c,d) hybrid DFT method. The EF denotes the Fermi energy.
Due to the underestimation of band gap values in standard DFT calculations, we further use the hybrid DFT method to calculate the total and projected DOS distributions around the Fermi level of Bi2O2Se and Bi2O2Se0.875Cl0.125, and the calculated results are displayed in Figure 3c,d. For Bi2O2Se, the calculated band gap between the valence band maxima (contributed by Bi 6s) and the conduction band minima (contributed by Bi 6p) is 1.05 eV, which is consistent with the previous computational values of 0.9 eV [30], 0.99 eV [8] and 1.01 eV [31]. Nonetheless, our hybrid DFT value is larger than the corresponding experimental value of 0.85 eV [24]. We note that due to the inclusion of a mixture of Hartree-Fock (HF) and DFT exchange terms, hybrid DFT calculations may overestimate or underestimate material band gaps [32,33], leading to higher or lower calculated values relative to their experimental counterparts [34]. The hybrid DFT, thus, represents a practical, although not perfect, solution to reproduce experimental band gaps. For Bi2O2Se0.875Cl0.125, it can be seen that the electron distribution obtained by the hybrid DFT method is more delocalized, as compared with the standard DFT results, and a certain number of electrons are still distributed at the Fermi level, i.e., the doped Bi2O2Se shows metal-like properties.
3.3. Mechanical Properties of Bi2O2Se and Bi2O2Se0.875Cl0.125
3.3.1. Elastic Constants of Bi2O2Se and Bi2O2Se0.875Cl0.125
The elastic constants () of Bi2O2Se and Bi2O2Se0.875Cl0.125 are first calculated based on the optimized geometrical structures. The Bi2O2Se possesses a tetragonal crystal structure, resulting in six independent elastic constants, namely C11, C12, C13, C33, C44 and C66 [8]. The calculated elastic constants are shown in Table 2 and compared with other literature values [8]. The calculated elastic constants for Bi2O2Se, i.e., C11 = 159.39 GPa, C12 = 73.55 GPa, C13 = 44.24 GPa, C33 = 121.28 GPa, C44 = 13.41 GPa, and C66 = 57.71 GPa, are in good agreement with previous computational results of C11 = 155.48 GPa, C12 = 71.56 GPa, C13 = 43.47 GPa, C33 = 119.01 GPa, C44 = 11.23 GPa, and C66 = 56.41 GPa [8]. Moreover, the calculated elastic constant C11 (159.39 GPa) is found to be larger than C33 (121.28 GPa), indicating that the Bi2O2Se exhibits higher resistance to deformation along the <100> direction as compared to the <001> direction. Moreover, the calculated value of C13 (44.24 GPa) is smaller than that of C12 (73.55 GPa), which indicates that the Bi2O2Se exhibits more significant contraction along the <001> direction as compared to the <010> direction when applying the same normal stress along the <100> direction. Furthermore, our results show that C66 (57.71 GPa) is larger than C44 (13.41 GPa), indicating that shear deformation along the (001) plane is more difficult to occur as compared to shear deformation along the (100) plane for Bi2O2Se [35,36].

Table 2.
The calculated elastic constants (C11, C12, C13, C33, C44 and C66 in GPa) for Bi2O2Se and Bi2O2Se0.875Cl0.125.
As compared with the values of Bi2O2Se, the calculated elastic constants C11 = 143.17 GPa, C12 = 63.39 GPa, C33 = 106.87 GPa, C44 = 11.96 Gpa, and C66 = 53.74 GPa for Bi2O2Se0.875Cl0.125 are reduced by 10.18%, 13.81%, 11.88%, 10.81%, and 6.88%, respectively, while the calculated value of C13 (46.38 GPa) is increased by 4.84%. These results suggest that Cl doping has significant effects on the mechanical properties of Bi2O2Se. Furthermore, the mechanical stability of Bi2O2Se and Bi2O2Se0.875Cl0.125 are determined by employing the following equation:
Our calculations demonstrate that both Bi2O2Se and Bi2O2Se0.875Cl0.125 satisfy the above mechanical stability criteria.
3.3.2. Elastic Moduli of Bi2O2Se and Bi2O2Se0.875Cl0.125
The bulk modulus (B), shear modulus (G) and Young’s modulus (E) of Bi2O2Se and Bi2O2Se0.875Cl0.125 can be calculated by using the Voigt–Reuss–Hill (VRH) approximation method [37]. In VRH approximation, the Voigt [38] and Reuss [39] approximations correspond to the upper and lower limits of the modulus, respectively. According to the Voigt approximation, the bulk modulus (BV) and shear modulus (GV) can be calculated by the following equations:
The bulk modulus (BR) and shear modulus (GR) can also be calculated by the Reuss approximation:
Then, the elastic modulus is the arithmetic average of the Voigt and Reuss approximations, which can be expressed as:
Further, the Young’s modulus (E) can be given by:
The calculated results are shown in Table 3, along with other results [8,40,41] for comparison. For Bi2O2Se, the calculated elastic moduli are B = 83.21 GPa, G = 29.59 Gpa, and E = 79.36 GPa, which agree well with the calculated results of B = 81.42 GPa, G = 27.38 GPa, and E = 73.56 GPa [8]. Besides, the calculated results of B = 83.21 GPa and E = 79.36 GPa are comparable to the experimental values of B = 71.5 GPa reported by Mahan et al. [41] and E = 66 GPa reported by Sagar et al. [40].

Table 3.
The calculated bulk modulus B (GPa), shear modulus G (GPa) and Young’s modulus E (GPa) for Bi2O2Se and Bi2O2Se0.875Cl0.125.
The B of Bi2O2Se0.875Cl0.125 is calculated to be 77.05 GPa, which is 7.40% lower than that of pure Bi2O2Se, indicating that the introduction of Cl dopant reduces the resistance of Bi2O2Se towards uniform compression. Moreover, Cl doping reduces the G of Bi2O2Se from 29.59 GPa to 26.20 GPa, indicating that the chemical bonds in Bi2O2Se0.875Cl0.125 are less strong and directional than those in Bi2O2Se, rendering it more susceptible to plastic deformation [42]. Furthermore, as compared with the calculated E of 79.36 GPa for Bi2O2Se, the calculated E = 70.59 GPa for Bi2O2Se0.875Cl0.125 is 11.05% smaller, i.e., the incorporation of Cl dopant in Bi2O2Se results in reduced tolerance to elastic deformation, thereby facilitating crack prevention under extreme temperature gradients [43]. In the literature, Zhang et al. [11] have reported that Te doping can reduce the elastic moduli of Bi2O2Se, which is similar to the phenomenon of Cl doping. In addition, the smaller B or E, the better the elastic compliance of materials. The presented results suggest that Cl doping enhances the elastic compliance of Bi2O2Se.
3.3.3. Ductility and Elastic Anisotropy of Bi2O2Se and Bi2O2Se0.875Cl0.125
Ductility is an important index to measure the mechanical properties of a material. It represents the ability of a material to exhibit significant plastic deformation without breaking under mechanical stress [44]. Based on the calculated elastic moduli, the B/G values and brittle-ductile behavior of Bi2O2Se and Bi2O2Se0.875Cl0.125 are further explored. The critical value for the brittle-ductile transition of the material is 1.75 [45,46]. When the B/G ratio is less than 1.75, the material exhibits brittleness; otherwise, it demonstrates ductility. The calculated B/G ratio for Bi2O2Se is 2.81, which is in close agreement with the value of 2.97 reported by MacIsaac et al. [47]. This result indicates that Bi2O2Se is a ductile material. For Bi2O2Se0.875Cl0.125, the calculated B/G value is 2.94, which is 4.63% higher than that of Bi2O2Se, suggesting that Cl doping enhances the ductility of Bi2O2Se, possibly due to the presence of metallic bonding in Bi2O2Se0.875Cl0.125.
Elastic anisotropy is another important mechanical property of materials, which is related to the appearance of microcracks [48,49]. The universal anisotropic index (AU) of Bi2O2Se and Bi2O2Se0.875Cl0.125 is calculated using the following formula [8]:
When the value of AU is not equal to 0, the material exhibits elastic anisotropy. For Bi2O2Se, the calculated AU value is 2.51, which indicates that Bi2O2Se is mechanically anisotropic. This finding is consistent with the conclusions drawn by Liu et al. [8]. Moreover, for Bi2O2Se0.875Cl0.125, the calculated AU value is 2.47, which is close to the AU value (2.51) of Bi2O2Se, indicating that Cl doping has slight impacts on the mechanical anisotropy of Bi2O2Se. The ELATE tool [50] can be utilized to generate directional plots of Young’s modulus for Bi2O2Se and Bi2O2Se0.875Cl0.125. A more isotropic modulus will resemble a sphere in shape. As illustrated in Figure 4, the plot of Bi2O2Se and Bi2O2Se0.875Cl0.125 deviates from sphere shape, indicating that the Young’s modulus of Bi2O2Se and Bi2O2Se0.875Cl0.125 is anisotropic.
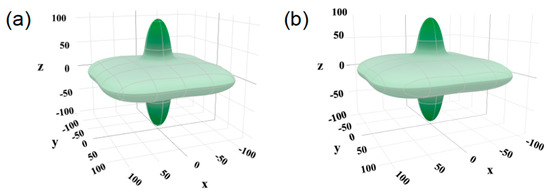
Figure 4.
The directional dependence of Young’s modulus E for (a) Bi2O2Se and (b) Bi2O2Se0.875Cl0.125. The unit is GPa, and each axis is the component of Young’s modulus along that axis.
3.3.4. Debye Temperature of Bi2O2Se and Bi2O2Se0.875Cl0.125
The Debye temperature is the characterization of binding force between atoms, and the specific formula is [51]:
where h and kB are the Planck constant and Boltzmann constant, respectively. The represents cell volume, n represents number of atoms in the cell, and is the average sound wave velocity. The can be calculated by [52,53]:
Here, the and are the longitudinal and transverse sound wave velocity, respectively, which can be calculated by [54]:
The calculated results are shown in Table 4, along with other results [8,10] for comparison. The calculated average sound velocity for Bi2O2Se is 2007 m/s, which is comparable to the value of 1939 m/s calculated by Liu et al. [8], but different from the calculated results of 1600 m/s reported by Wang et al. [10]. This is mainly because in the calculations of Wang et al. they used the CASTEP code with ultrasoft pseudopotentials, whereas we employ the VASP code with PAW pseudopotentials. Therefore, their calculated Debye temperature of 224.1 K is different from our calculated value of 181.0 K [10]. For Bi2O2Se0.875Cl0.125, our calculated Debye temperature (212.5 K) is 11.6 K smaller than that of pure Bi2O2Se (224.1 K), indicating that the atomic binding force in Bi2O2Se is reduced by Cl doping, due to the weakened covalency of chemical bonds [55], which will be beneficial to improve the toughness and processing performance of Bi2O2Se.

Table 4.
The calculated transverse wave velocity (m/s), longitudinal wave velocity (m/s), average wave velocity (m/s), and Debye temperature θ (K) for Bi2O2Se and Bi2O2Se0.875Cl0.125.
3.3.5. Lattice Thermal Conductivity of Bi2O2Se and Bi2O2Se0.875Cl0.125
The Slack equation [56] can be used to calculate the lattice thermal conductivity :
Here, A is a constant, and the calculation formula is given by:
In this equation, represents the average atomic mass, is the volume of each atom, γ is the Grüneisen parameter, Θ is the Debye temperature, and T is the absolute temperature. The Slack model has been demonstrated to provide lattice thermal conductivity results that agree well with experimental measurements, and has been extensively used in the calculation of lattice thermal conductivity of materials [30,57]. Figure 5 shows the temperature-dependent results of . It is observed that the lattice thermal conductivity of both Bi2O2Se and Bi2O2Se0.875Cl0.125 decreases with increasing temperature. For Bi2O2Se, the calculated at 300 K is 1.68 W/mK, which agrees well with the experimental data of = 1.8 W/mK and other calculated results of = 1.2 W/mK [31]. Furthermore, Cl doping reduces the lattice thermal conductivity of Bi2O2Se. For example, the calculated lattice thermal conductivity of Bi2O2Se0.875Cl0.125 at 300 K is 1.29 W/mK, which is 23.21% smaller than that of Bi2O2Se (1.68 W/mK). This is mainly due to the low elastic constants of Bi2O2Se0.875Cl0.125. The reduced lattice thermal conductivity will be beneficial for suppressing heat transfer and minimizing thermal energy loss. Bi2O2Se0.875Cl0.125 exhibits metallic character and electrons should also contribute to the thermal transport of Bi2O2Se. Considering that the Bi2O2Se is a semiconductor and its thermal conductivity is mainly contributed by phonons, and this work mainly focuses on the effect of Cl doping on the thermal transport properties of Bi2O2Se, the electronic thermal conductivity of Cl-doped Bi2O2Se, thus, is not considered in this work.
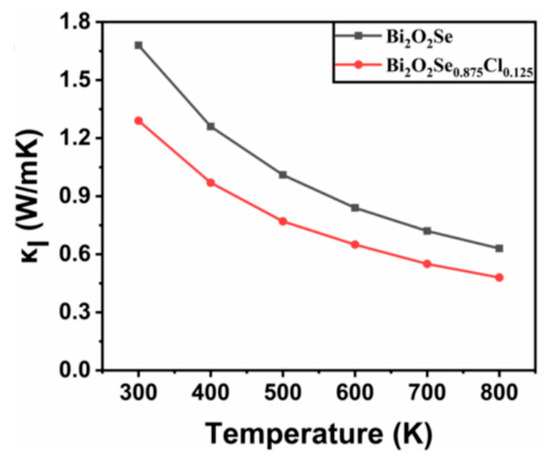
Figure 5.
The calculated lattice thermal conductivity κl of Bi2O2Se and Bi2O2Se0.875Cl0.125 as a function of temperature.
4. Conclusions
In this work, the effect of Cl doping on the structural, electronic, and mechanical properties of Bi2O2Se is investigated by using the first-principles calculations based on the DFT method. The main results are summarized as follows:
- The Cl doping results in an increase of 0.74% in the lattice constant a0 (3.946 Å) of Bi2O2Se, while c0 (12.287 Å) is decreased by 0.57% and the volume is expanded by 0.91%. At the same time, the calculated <Bi-O> bond lengths (2.339 Å) is increased by 0.09%, and the <Bi-Se> bond length (3.336 Å) is increased by 0.76%.
- The Cl doping has significant influences on the electronic structure of Bi2O2Se. Different from the semiconductor character of the pristine Bi2O2Se, the Bi2O2Se0.875Cl0.125 exhibits metallic properties, since a certain number of electrons are distributed at the Fermi level.
- As compared with the elastic constants of Bi2O2Se, the calculated elastic constants C11 = 143.17 GPa, C12 = 63.39 GPa, C33 = 106.87 GPa, C44 = 11.96 GPa and C66 = 53.74 GPa for Bi2O2Se0.875Cl0.125 are reduced by 10.18%, 13.81%, 11.88%, 10.81%, and 6.88%, respectively, while the calculated value of C13 (46.38 GPa) is increased by 4.84%. These results suggest that Cl doping has remarkable effects on the mechanical properties of Bi2O2Se. Both the doped and undoped Bi2O2Se satisfy the criteria of mechanical stability and exhibit elastic anisotropy.
- The Cl doping leads to a reduction of 7.40%, 11.46%, and 11.05% in the bulk modulus, shear modulus, and Young’s modulus of Bi2O2Se, respectively, suggesting that Cl doping leads to a more plastic deformation of Bi2O2Se, which could help to prevent cracking under extreme temperature gradients.
- The calculated B/G value of Bi2O2Se0.875Cl0.125 is 2.94, which is 4.63% higher than that of pure Bi2O2Se, indicating that Cl doping enhances the ductility of Bi2O2Se.
- The calculated Debye temperature θ of Bi2O2Se0.875Cl0.125 is 212.5 K, which is 11.6 K lower than that of pure Bi2O2Se (224.1 K). This lower Debye temperature helps to improve the toughness and processability of Bi2O2Se.
- The lattice thermal conductivity of both Bi2O2Se and Bi2O2Se0.875Cl0.125 decreases with increasing temperature. Cl doping reduces the lattice thermal conductivity of Bi2O2Se considerably. For example, the lattice thermal conductivity of Bi2O2Se0.875Cl0.125 at 300 K is 1.29 W/mK, which is 23.21% lower than that of pure Bi2O2Se (1.68 W/mK). The lower lattice thermal conductivity is beneficial in inhibiting heat transfer and minimizing thermal energy loss.
Generally, Cl doping leads to an improvement in the mechanical properties and a decrease in the lattice thermal conductivity of Bi2O2Se. Therefore, it is suggested that the Cl-doped Bi2O2Se can be used to develop TEG materials with good thermoelectric and mechanical properties.
Author Contributions
Conceptualization, B.L. and H.X.; methodology, H.X., H.Q., M.L. and X.Z.; software, H.X.; validation, B.L., H.Q. and M.L.; formal analysis, B.L. and H.Q.; investigation, B.L.; resources, X.Z., H.X. and L.Q.; data curation, B.L.; writing—original draft preparation, B.L.; writing—review and editing, H.X. and L.Q.; visualization, B.L.; supervision, H.Q., M.L. and H.X.; project administration, B.L.; funding acquisition, H.X. and L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
H. Y. Xiao was supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U1930120). L.Q. acknowledges the support of the National Natural Science Foundation of China (Grant No. 52072059). All the calculations in this paper have been carried out on Hefei advanced computing center.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, N.; Li, M.; Xiao, H.; Gao, Z.; Liu, Z.; Zu, X.; Li, S.; Qiao, L. Band degeneracy enhanced thermoelectric performance in layered oxyselenides by first-principles calculations. npj Computat. Mater. 2021, 7, 18. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.; Xiao, H.; Zu, X.; Qiao, L. Layered LaCuOSe: A Promising Anisotropic Thermoelectric Material. Phys. Rev. Appl. 2020, 13, 024038. [Google Scholar] [CrossRef]
- Liu, W.; Jie, Q.; Kim, H.S.; Ren, Z. Current progress and future challenges in thermoelectric power generation: From materials to devices. Acta Mater. 2015, 87, 357–376. [Google Scholar] [CrossRef]
- Yang, J.; Stabler, F.R. Automotive Applications of Thermoelectric Materials. J. Electron. Mater. 2009, 38, 1245–1251. [Google Scholar] [CrossRef]
- Wang, N.; Shen, C.; Sun, Z.; Xiao, H.; Zhang, H.; Yin, Z.; Qiao, L. High-Temperature Thermoelectric Monolayer Bi2TeSe2 with High Power Factor and Ultralow Thermal Conductivity. ACS Appl. Energy Mater. 2022, 5, 2564–2572. [Google Scholar] [CrossRef]
- Ren, D.S.; Feng, X.N.; Liu, L.S.; Hsu, H.J.; Lu, L.G.; Wang, L.; He, X.M.; Ouyang, M.G. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Hong, J.C.; Wang, Z.P.; Yao, Y.T. Fault prognosis of battery system based on accurate voltage abnormity prognosis using long short-term memory neural networks. Appl. Energy 2019, 251, 14. [Google Scholar] [CrossRef]
- Liu, J.; Tian, L.; Mou, Y.; Jia, W.; Zhang, L.; Liu, R. Electronic and mechanical property of high electron mobility semiconductor Bi2O2Se. J. Alloys Compd. 2018, 764, 674–678. [Google Scholar] [CrossRef]
- Pang, Z.; Li, T. Mechanics and strain engineering of bulk and monolayer Bi2O2Se. J. Mech. Phys. Solids 2021, 157, 104626. [Google Scholar] [CrossRef]
- Wang, Y.X.; Yan, Z.X.; Liu, W.; Zhou, G.L.; Gu, J.B. Ab initio study of the mechanical properties and thermal conductivity of Bi2O2X (X = Se, Te) under pressure. Solid State Sci. 2020, 106, 106299. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Zhang, W.; Yu, Z.; Yu, C.; Lu, H. Systematically investigate mechanical and electrical properties of Bi2O2Se by Te atom substitution and compare it with homologue Bi2O2Te from first-principles calculations. Mater. Today Commun. 2020, 24, 101182. [Google Scholar] [CrossRef]
- Piriz, S.; Fernandez-Werner, L.; Pardo, H.; Jasen, P.; Faccio, R.; Mombru, A.W. Mechanical properties and electronic structure of edge-doped graphene nanoribbons with F, O, and Cl atoms. Phys. Chem. Chem. Phys. 2017, 19, 21474–21480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, Y.; Wang, S.; Li, Q.; Li, M.; Zhang, Z. Enhanced dielectric and mechanical properties in chlorine-doped continuous CNT sheet reinforced sandwich polyvinylidene fluoride film. Carbon 2016, 107, 405–414. [Google Scholar] [CrossRef]
- Park, H.; Woo, D.; Lee, J.M.; Park, S.J.; Lee, S.; Kim, H.J.; Yoon, E.; Lee, G.D. First principles investigation on energetics, structure, and mechanical properties of amorphous carbon films doped with B, N, and Cl. Sci. Rep. 2019, 9, 18961. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, S.; Xiao, H.Y.; Singh, D.J.; Zhang, K.H.L.; Liu, Z.J.; Zu, X.T.; Li, S. Orbital controlled band gap engineering of tetragonal BiFeO3 for optoelectronic applications. J. Mater. Chem. C 2018, 6, 1239–1247. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Yao, H.; Ouyang, L.; Ching, W.-Y. Ab Initio Calculation of Elastic Constants of Ceramic Crystals. J. Am. Ceram. Soc. 2007, 90, 3194–3204. [Google Scholar] [CrossRef]
- Wu, J.; Tan, C.; Tan, Z.; Liu, Y.; Yin, J.; Dang, W.; Wang, M.; Peng, H. Controlled Synthesis of High-Mobility Atomically Thin Bismuth Oxyselenide Crystals. Nano Lett. 2017, 17, 3021–3026. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, P.; Qin, M.; Lou, Z.; Gong, L.; Xu, J.; Kong, J.; Yan, H.; Gao, F. Effect of La3+, Ag+ and Bi3+ doping on thermoelectric properties of SrTiO3: First-principles investigation. Ceram. Int. 2022, 48, 13803–13816. [Google Scholar] [CrossRef]
- Boiler, I. Die Kristallstruktur von Bi2O2Se. Monatsh. Chem. 1973, 104, 916–919. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Meng, M.; Chen, C.; Sun, Y.; Chen, Z.; Dang, W.; Tan, C.; Liu, Y.; Yin, J.; et al. High electron mobility and quantum oscillations in non-encapsulated ultrathin semiconducting Bi2O2Se. Nat. Commun. 2017, 12, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Lan, J.-L.; Ren, G.; Liu, Y.; Lin, Y.-H.; Nan, C.-W. Enhanced thermoelectric performance of n-type Bi2O2Se by Cl-doping at Se site. J. Am. Ceram. Soc. 2017, 100, 1494–1501. [Google Scholar] [CrossRef]
- Hu, K.; Han, J.; Xu, B.; Lin, Y.H. Thermoelectric power factor of doped Bi2O2Se: A computational study. Phys. Chem. Chem. Phys. 2020, 22, 27096–27104. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Cheng, C.; Duan, M.Y. The electronic and optical properties of multi-layer Bi2O2X (X = S, Se, Te) by first-principles calculations. Appl. Surf. Sci. 2023, 618, 156541. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, B.; Yu, G.; Zhang, J.; Ma, S.; Yuan, S.; Sun, T.; Wang, Y. Electronic structure and thermoelectric properties of Bi2O2Se with GGA and TB-mBJ potentials. Jpn. J. Appl. Phys. 2019, 58, 015501. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, M.; Wang, N.; Jiang, M.; Xiao, H.; Zhang, H.; Liu, Z.; Zu, X.; Qiao, L. Improved thermoelectric performance of bilayer Bi2O2Se by the band convergence approach. J. Mater. Chem. C 2019, 7, 11029–11039. [Google Scholar] [CrossRef]
- Zhu, X.L.; Liu, P.F.; Xie, G.; Wang, B.T. First-principles study of thermal transport properties in the two- and three-dimensional forms of Bi2O2Se. Phys. Chem. Chem. Phys. 2019, 21, 10931–10938. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Di Valentin, C.; Pacchioni, G. Electronic and Structural Properties of WO3: A Systematic Hybrid DFT Study. J. Phys. Chem. C 2011, 115, 8345–8353. [Google Scholar] [CrossRef]
- Garza, A.J.; Scuseria, G.E. Predicting Band Gaps with Hybrid Density Functionals. J. Phys. Chem. Lett. 2016, 7, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Franchini, C. Assessing the performance of self-consistent hybrid functional for band gap calculation in oxide semiconductors. J. Phys. Condens. Matter 2017, 29, 454004. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, J.; Gong, H.; Ren, Q.; Liao, Y.; Xiao, H.; Qiu, Q.; Feng, S.; Zu, X. First-principles study of point defects in U3Si2: Effects on the mechanical and electronic properties. Phys. Chem. Chem. Phys. 2022, 24, 4287–4297. [Google Scholar] [CrossRef]
- Koc, H.; Ozisik, H.; Deligoz, E.; Mamedov, A.M.; Ozbay, E. Mechanical, electronic, and optical properties of Bi(2)S(3) and Bi(2)Se(3) compounds: First principle investigations. J. Mol. Model. 2014, 20, 2180. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.H.; Buessem, W.R. The Voigt-Reuss-Hill (VRH) Approximation and the Elastic Moduli of Polycrystalline ZnO, TiO2 (Rutile), and α-Al2O3. J. Appl. Phys. 1968, 39, 2777–2782. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der Kristallphysik: Teubner-Leipzig; Macmillan: New York, NY, USA, 1928; p. 739. [Google Scholar]
- Reuss, A. Calculation of the flow limits of mixed crystals on the basis of the plasticity of monocrystals. Z. Angew. Math. Mech. 1929, 9, 49. [Google Scholar] [CrossRef]
- Sagar, R.U.R.; Khan, U.; Galluzzi, M.; Aslam, S.; Nairan, A.; Anwar, T.; Ahmad, W.; Zhang, M.; Liang, T. Transfer-Free Growth of Bi2O2Se on Silicon Dioxide via Chemical Vapor Deposition. ACS Appl. Electron. Mater. 2020, 2, 2123–2131. [Google Scholar] [CrossRef]
- Mahan, G.D. Figure of merit for thermoelectrics. J. Appl. Phys. 1989, 65, 1578–1583. [Google Scholar] [CrossRef]
- Majumder, R.; Hossain, M.M. First-principles study of structural, electronic, elastic, thermodynamic and optical properties of topological superconductor LuPtBi. Comput. Condens. Matter 2019, 21, e00402. [Google Scholar] [CrossRef]
- Nye, J.F.; Lindsay, R.B. Physical Properties of Crystals: Their Representation by Tensors and Matrices. Phys. Today 1957, 10, 26. [Google Scholar] [CrossRef]
- Shi, X.; Chen, H.; Hao, F.; Liu, R.; Wang, T.; Qiu, P.; Burkhardt, U.; Grin, Y.; Chen, L. Room-temperature ductile inorganic semiconductor. Nat. Mater. 2018, 17, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 45, 823–843. [Google Scholar] [CrossRef]
- Li, M.; Wang, N.; Zhang, S.; Hu, J.; Xiao, H.; Gong, H.; Liu, Z.; Qiao, L.; Zu, X. A review of the properties, synthesis and applications of lanthanum copper oxychalcogenides. J. Phys. D Appl. Phys. 2022, 55, 273002. [Google Scholar] [CrossRef]
- MacIsaac, D.; Kanner, G.; Anderson, G. Basic physics of the incandescent lamp (lightbulb). PhTea 1999, 37, 520–525. [Google Scholar] [CrossRef][Green Version]
- Tvergaard, V.H.; Hutchinsonm, J.W. Microcracking in Ceramics Induced by Thermal Expansion or Elastic Anisotropy. J. Am. Ceram. Soc. 1988, 71, 157–166. [Google Scholar] [CrossRef]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density functional theory for calculation of elastic properties of orthorhombic crystals: Application to TiSi2. J. Appl. Phys. 1998, 84, 4891–4904. [Google Scholar] [CrossRef]
- Gaillac, R.; Pullumbi, P.; Coudert, F.X. ELATE: An open-source online application for analysis and visualization of elastic tensors. J. Phys. Condens. Matter 2016, 28, 275201. [Google Scholar] [CrossRef] [PubMed]
- Debye, P. Zur theorie der spezifischen wärmen. Ann. Phys. 1912, 344, 789–839. [Google Scholar]
- Feng, J.; Xiao, B.; Wan, C.L.; Qu, Z.X.; Huang, Z.C.; Chen, J.C.; Zhou, R.; Pan, W. Electronic structure, mechanical properties and thermal conductivity of Ln2Zr2O7 (Ln = La, Pr, Nd, Sm, Eu and Gd) pyrochlore. Acta Mater. 2014, 72, 1742–1760. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Schreiber, E.; Anderson, O.L.; Soga, N.; Bell, J.F. Elastic constants and their measurement. J. Appl. Mech. 1975, 42, 747–748. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Y.; Hou, H.; Wang, B. The Effect of Alloying Elements on the Structural Stability, Mechanical Properties, and Debye Temperature of Al(3)Li: A First-Principles Study. Materials 2018, 11, 1471. [Google Scholar] [CrossRef] [PubMed]
- Morelli, D.T.; Slack, G.A. High Lattice Thermal Conductivity of Solids; Springer: New York, NY, USA, 2006; pp. 37–68. [Google Scholar]
- Li, Y.; Wang, J.; Wang, J. Approaching extremely low thermal conductivity by crystal structure engineering in Mg2Al4Si5O18. J. Mater. Res. 2015, 30, 3729–3739. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).