Controlled Synthesis and Growth Mechanism of Two-Dimensional Zinc Oxide by Surfactant-Assisted Ion-Layer Epitaxy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of 2D ZnO Nanosheets
2.2. Materials Characterization
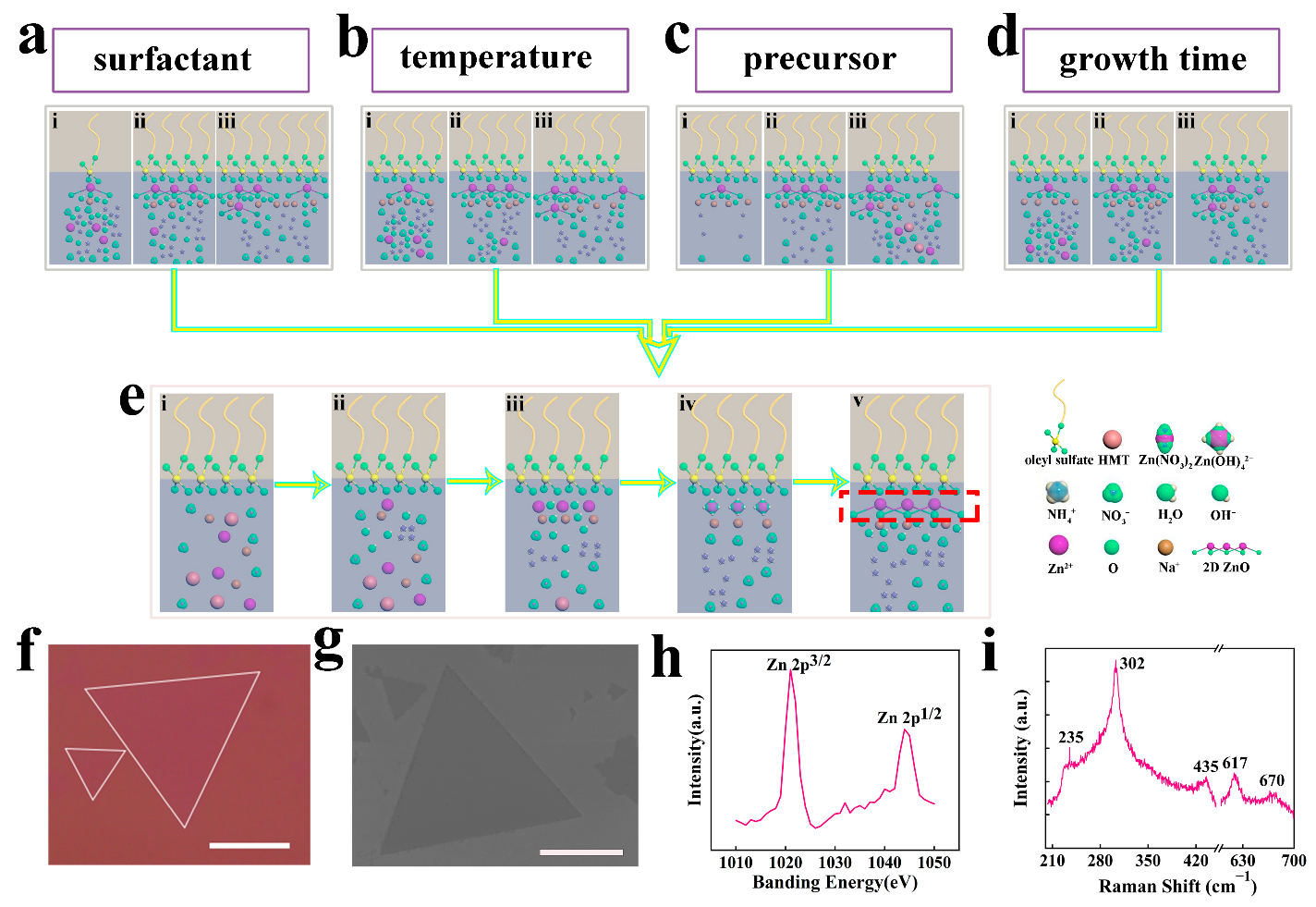
3. Results and Discussion
3.1. Effect of the Ratio of Sodium Oleyl Sulfate to on 2D ZnO Growth
3.2. Effect of Temperature on 2D ZnO Growth
3.3. Effect of Precursor Concentration on 2D ZnO Growth
3.4. Effect of Growth Time on 2D ZnO Growth
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Izaki, M.; Chizaki, R.; Saito, T.; Murata, K.; Sasano, J.; Shinagawa, T. Hybrid ZnO/Phthalocyanine Photovoltaic Device with Highly Resistive ZnO Intermediate Layer. ACS Appl. Mater. Interfaces 2013, 5, 9386–9395. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.J.; Wang, X.; Knuesel, R.J.; Jacobs, H.O. Patterned Growth and Transfer of ZnO Micro and Nanocrystals with Size and Location Control. Adv. Mater. 2008, 20, 1474–1478. [Google Scholar] [CrossRef]
- Lee, P.-C.; Hsiao, Y.-L.; Dutta, J.; Wang, R.-C.; Tseng, S.-W.; Liu, C.-P. Development of porous ZnO thin films for enhancing piezoelectric nanogenerators and force sensors. Nano Energy 2020, 82, 105702. [Google Scholar] [CrossRef]
- Hsu, Y.F.; Xi, Y.Y.; Tam, K.H.; Djurišić, A.B.; Luo, J.; Ling, C.C.; Cheung, C.K.; Ng, A.M.C.; Chan, W.K.; Deng, X.; et al. Undoped p-Type ZnO Nanorods Synthesized by a Hydrothermal Method. Adv. Funct. Mater. 2008, 18, 1020–1030. [Google Scholar] [CrossRef]
- Kang, Z.; Gu, Y.; Yan, X.; Bai, Z.; Liu, Y.; Liu, S.; Zhang, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Enhanced photoelectrochemical property of ZnO nanorods array synthesized on reduced graphene oxide for self-powered biosensing application. Biosens. Bioelectron. 2015, 64, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wu, L.; Liu, Y.; Yang, Y. Chemo-phototronic effect induced electricity for enhanced self-powered photodetector system based on ZnO nanowires. Nano Energy 2021, 89, 106449. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Ye, L.; Wu, M.; Xie, R.; Wang, X.; Chen, X.; Fan, Z.; Wang, J.; Hu, W. Ferroelectric Localized Field-Enhanced ZnO Nanosheet Ultraviolet Photodetector with High Sensitivity and Low Dark Current. Small 2018, 14, 1800492. [Google Scholar] [CrossRef]
- Meng, J.; Li, Q.; Huang, J.; Pan, C.; Li, Z. Self-powered photodetector for ultralow power density UV sensing. Nano Today 2022, 43, 101399. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, J.; Wang, X.; Ma, W.; Que, M.; Chen, Q.; Li, F.; Liu, X.; Gao, W.; Pan, C. Self-powered high-performance flexible GaN/ZnO heterostructure UV photodetectors with piezo-phototronic effect enhanced photoresponse. Nano Energy 2022, 94, 106945. [Google Scholar] [CrossRef]
- Hoffmann, M.W.G.; Mayrhofer, L.; Casals, O.; Caccamo, L.; Hernandez-Ramirez, F.; Lilienkamp, G.; Daum, W.; Moseler, M.; Waag, A.; Shen, H.; et al. A highly selective and self-powered gas sensor via organic surface functionalization of p-Si/n-ZnO diodes. Adv. Mater. 2014, 26, 8017–8022. [Google Scholar] [CrossRef]
- Shan, H.; Liu, C.; Liu, L.; Wang, L.; Zhang, X.; Chi, X.; Bo, X.; Wang, K. Excellent ethanol sensor based on multiwalled carbon nanotube-doped ZnO. Chin. Sci. Bull. 2014, 59, 374–378. [Google Scholar] [CrossRef]
- Yuan, H.; Aljneibi, S.A.A.A.; Yuan, J.; Wang, Y.; Liu, H.; Fang, J.; Tang, C.; Yan, X.; Cai, H.; Gu, Y.; et al. ZnO Nanosheets Abundant in Oxygen Vacancies Derived from Metal-Organic Frameworks for ppb-Level Gas Sensing. Adv. Mater. 2019, 31, e1807161. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Shi, J.; Zhang, Y.; Quan, W.; Chen, X.; Yang, J.; Zeng, M.; Zhou, Z.; Su, Y.; Wei, H.; et al. Fast and recoverable NO2 detection achieved by assembling ZnO on Ti3C2Tx MXene nanosheets under UV illumination at room temperature. Nanoscale 2022, 14, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Lu, J.F.; Zhang, X.J.; Peng, X.L.; Li, J.; Yang, Y.T.; Hong, B.; Wang, X.Q.; Jin, D.F.; Jin, H.X. Reversible switching from P- to N-Type NO2 sensing in ZnO Rods/rGO by changing the NO2 concentration, temperature, and doping ratio. J. Phys. Chem. C 2022, 126, 14470–14478. Available online: https://pubs.acs.org/doi/full/10.1021/acs.jpcc.2c03616 (accessed on 29 November 2022). [CrossRef]
- Park, S.J.; Das, G.S.; Schuett, F.; Adelung, R.; Mishra, Y.K.; Tripathi, K.M.; Kim, T. Visible-light photocatalysis by car-bon-nano-onion-functionalized ZnO tetrapods: Degradation of 2,4-dinitrophenol and a plant-model-based ecological assess-ment. NPG Asia Mater. 2019, 11. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, D.; Xu, Z.; Zheng, Z.; Zhang, L.; Shen, D. Monochromatic visible light-driven photocatalysis realized on 2D ZnO shell arrays. J. Mater. Chem. A 2013, 1, 9132–9137. [Google Scholar] [CrossRef]
- Song, S.; Song, H.; Li, L.; Wang, S.; Chu, W.; Peng, K.; Meng, X.; Wang, Q.; Deng, B.; Liu, Q.; et al. A selective Au-ZnO/TiO2 hybrid photocatalyst for oxidative coupling of methane to ethane with dioxygen. Nat. Catal. 2022, 5, 1032–1042. [Google Scholar] [CrossRef]
- Peng, F.; Lin, J.; Li, H.; Liu, Z.; Su, Q.; Wu, Z.; Xiao, Y.; Yu, H.; Zhang, M.; Wu, C.; et al. Design of piezoelectric ZnO based catalysts for ammonia production from N2 and H2O under ultrasound sonication. Nano Energy 2022, 95, 107020. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Liu, Y.; Dong, Z.; Luo, L. Surface Plane Effect of ZnO on the Catalytic Performance of Au/ZnO for the CO Oxidation Reaction. J. Phys. Chem. C 2022, 126, 14155–14162. [Google Scholar] [CrossRef]
- Gleißner, R.; Noei, H.; Chung, S.; Semione, G.D.L.; Beck, E.E.; Dippel, A.-C.; Gutowski, O.; Gizer, G.; Vonk, V.; Stierle, A. Copper Nanoparticles with High Index Facets on Basal and Vicinal ZnO Surfaces. J. Phys. Chem. C 2021, 125, 23561–23569. [Google Scholar] [CrossRef]
- Kim, H.; Yun, S.; Kim, K.; Kim, W.; Ryu, J.; Nam, H.G.; Han, S.M.; Jeon, S.; Hong, S. Breaking the elastic limit of piezoelectric ceramics using nanostructures: A case study using ZnO. Nano Energy 2020, 78, 105259. [Google Scholar] [CrossRef]
- Yin, B.; Qiu, Y.; Zhang, H.; Lei, J.; Chang, Y.; Ji, J.; Luo, Y.; Zhao, Y.; Hu, L. Piezoelectric performance enhancement of ZnO flexible nanogenerator by a NiO-ZnO p-n junction formation. Nano Energy 2015, 14, 95–101. [Google Scholar] [CrossRef]
- Park, J.; Ghosh, R.; Song, M.S.; Hwang, Y.; Tchoe, Y.; Saroj, R.K.; Ali, A.; Guha, P.; Kim, B.; Kim, S.-W.; et al. Individually addressable and flexible pressure sensor matrixes with ZnO nanotube arrays on graphene. NPG Asia Mater. 2022, 14, 40. [Google Scholar] [CrossRef]
- Yu, Q.; Ge, R.; Wen, J.; Du, T.; Zhai, J.; Liu, S.; Wang, L.; Qin, Y. Highly sensitive strain sensors based on piezotronic tunneling junction. Nat. Commun. 2022, 13, 778. [Google Scholar] [CrossRef]
- An, C.; Qi, H.; Wang, L.; Fu, X.; Wang, A.; Wang, Z.L.; Liu, J. Piezotronic and piezo-phototronic effects of atomically-thin ZnO nanosheets. Nano Energy 2020, 82, 105653. [Google Scholar] [CrossRef]
- Yalishev, V.; Yuldashev, S.U.; Kim, Y.S.; Park, B.H. The role of zinc vacancies in bipolar resistance switching of Ag/ZnO/Pt memory structures. Nanotechnology 2012, 23, 375201. [Google Scholar] [CrossRef]
- Wang, X.; Qian, H.; Guan, L.; Wang, W.; Xing, B.; Yan, X.; Zhang, S.; Sha, J.; Wang, Y. Influence of metal electrode on the performance of ZnO based resistance switching memories. J. Appl. Phys. 2017, 122, 154301. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Y.; Chang, T.; Zhang, P.; Li, J.; Xue, P.; Long, Y.; Shohet, J.L.; Voyles, P.M.; Ma, Z.; et al. Memristive Behavior Enabled by Amorphous–Crystalline 2D Oxide Heterostructure. Adv. Mater. 2020, 32, 2000801. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Gao, G.; Pang, Y.; Yin, X.; Feng, X.; Zhu, L.; Bai, Y.; Chen, L.; Xiao, T.; et al. Ultrathin Piezotronic Transistors with 2 nm Channel Lengths. ACS Nano 2018, 12, 4903–4908. [Google Scholar] [CrossRef]
- Jang, E.S.; Won, J.-H.; Hwang, S.-J.; Choy, J.-H. Fine tuning of the face orientation of ZnO crystals to optimize their photo-catalytic activity. Adv. Mater. 2006, 18, 3309–3312. [Google Scholar] [CrossRef]
- Gupta, S.P.; Pawbake, A.S.; Sathe, B.R.; Late, D.J.; Walke, P.S. Superior humidity sensor and photodetector of mesoporous ZnO nanosheets at room temperature. Sens. Actuators B Chem. 2019, 293, 83–92. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Zhang, Z.; Feng, X.; Zhu, L.; Guo, H.; Ding, W.; Chen, L.; Qin, Y.; Wang, Z.L. 2D piezotronics in atomically thin zinc oxide sheets: Interfacing gating and channel width gating. Nano Energy 2019, 60, 724–733. [Google Scholar] [CrossRef]
- Gao, J.; Meng, H.; Hu, Q.; Chang, J.; Feng, L. 2D ZIF-derived ZnO nanosheets—An example for improving semiconductor metal oxide detector performance in gas chromatography through material design strategy. Sens. Actuators B Chem. 2019, 307, 127580. [Google Scholar] [CrossRef]
- Ren, K.; Yu, J.; Tang, W. Two-dimensional ZnO/BSe van der waals heterostructure used as a promising photocatalyst for water splitting: A DFT study. J. Alloys Compd. 2019, 812, 152049. [Google Scholar] [CrossRef]
- Yu, H.; Liao, Q.; Kang, Z.; Wang, Z.; Liu, B.; Zhang, X.; Du, J.; Ou, Y.; Hong, M.; Xiao, J.; et al. Atomic-thin ZnO sheet for visible-blind ultraviolet photodetection. Small 2020, 16, 2005520. [Google Scholar] [CrossRef]
- Kumar, M.; Bhatt, V.; Kim, J.; Abhyankar, A.C.; Chung, H.-J.; Singh, K.; Bin Cho, Y.; Yun, Y.J.; Lim, K.S.; Yun, J.-H. Holey engineered 2D ZnO-nanosheets architecture for supersensitive ppm level H2 gas detection at room temperature. Sens. Actuators B Chem. 2021, 326, 128839. [Google Scholar] [CrossRef]
- Rafique, S.; Kasi, A.K.; Aminullah; Kasi, J.K.; Bokhari, M.; Shakoor, Z. Fabrication of Br doped ZnO nanosheets piezoelectric nanogenerator for pressure and position sensing applications. Curr. Appl. Phys. 2020, 21, 72–79. [Google Scholar] [CrossRef]
- Mahmood, N.; Khan, H.; Tran, K.; Kuppe, P.; Zavabeti, A.; Atkin, P.; Ghasemian, M.B.; Yang, J.; Xu, C.; Tawfik, S.A.; et al. Maximum piezoelectricity in a few unit-cell thick planar ZnO—A liquid metal-based synthesis approach. Mater. Today 2021, 44, 69–77. [Google Scholar] [CrossRef]
- Samavati, A.; Nur, H.; Ismail, A.F.; Othaman, Z. Radio frequency magnetron sputtered ZnO/SiO2/glass thin film: Role of ZnO thickness on structural and optical properties. J. Alloys Compd. 2016, 671, 170–176. [Google Scholar] [CrossRef]
- Akhtaruzzaman; Hossain, M.I.; Islam, M.A.; Shahiduzzaman; Muhammad, G.; Hasan, A.K.M.; Tsang, Y.H.; Sopian, K. Nanophotonic-structured front contact for high-performance perovskite solar cells. Sci. China Mater. 2022, 65, 1727–1740. [Google Scholar] [CrossRef]
- Walter, T.N.; Lee, S.; Zhang, X.; Chubarov, M.; Redwing, J.M.; Jackson, T.N.; Mohney, S.E. Atomic layer deposition of ZnO on MoS2 and WSe2. Appl. Surf. Sci. 2019, 480, 43–51. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, K.-M.; Kim, M.; Park, H.-H. Film thickness effect in c-axis oxygen vacancy-passivated ZnO prepared via atomic layer deposition by using H2O2. Appl. Surf. Sci. 2020, 529, 147095. [Google Scholar] [CrossRef]
- Lu, J.; Wang, W.; Liang, J.; Lan, J.; Lin, L.; Zhou, F.; Chen, K.; Zhang, G.; Shen, M.; Li, Y. Contact Resistance Reduction of Low Temperature Atomic Layer Deposition ZnO Thin Film Transistor Using Ar Plasma Surface Treatment. IEEE Electron Device Lett. 2022, 43, 890–893. [Google Scholar] [CrossRef]
- Parmar, D.H.; Pina, J.M.; Zhu, T.; Vafaie, M.; Atan, O.; Biondi, M.; Najjariyan, A.M.; Hoogland, S.; Sargent, E.H. Controlled crystal plane orientations in the ZnO transport layer enable high-responsivity, low-dark-current infrared photodetectors. Adv. Mater. 2022, 34, 2200321. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, A.; Engel, S.; Sangiorgi, N.; Sanson, A.; Bartolome, J.F.; Graef, S.; Mueller, F.A. ZnO thin films on single carbon fibres fabricated by Pulsed Laser Deposition (PLD). Appl. Surf. Sci. 2017, 399, 282–287. [Google Scholar] [CrossRef]
- Li, B.; Ding, L.; Gui, P.; Liu, N.; Yue, Y.; Chen, Z.; Song, Z.; Wen, J.; Lei, H.; Zhu, Z.; et al. Pulsed Laser Deposition Assisted van der Waals Epitaxial Large Area Quasi-2D ZnO Single-Crystal Plates on Fluorophlogopite Mica. Adv. Mater. Interfaces 2019, 6, 1901156. [Google Scholar] [CrossRef]
- Wang, F.; Seo, J.-H.; Luo, G.; Starr, M.B.; Li, Z.; Geng, D.; Yin, X.; Wang, S.; Fraser, D.G.; Morgan, D.; et al. Na-nometre-thick single-crystalline nanosheets grown at the water-air interface. Nat. Commun. 2016, 7, 10444. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, Y.; Jacobs, R.; Shi, Y.; Szlufarska, I.; Morgan, D.; Wang, X. Massive Vacancy Concentration Yields Strong Room-Temperature Ferromagnetism in Two-Dimensional ZnO. Nano Lett. 2019, 19, 7085–7092. [Google Scholar] [CrossRef]
- Yin, X.; Shi, Y.; Wei, Y.; Joo, Y.; Gopalan, P.; Szlufarska, I.; Wang, X. Unit Cell Level Thickness Control of Single-Crystalline Zinc Oxide Nanosheets Enabled by Electrical Double-Layer Confinement. Langmuir 2017, 33, 7708–7714. [Google Scholar] [CrossRef]
- Kim, S.; Iida, K.; Kuromiya, Y.; Seto, T.; Higashi, H.; Otani, Y. Effect of Nucleation Temperature on Detecting Molecular Ions and Charged Nanoparticles with a Diethylene Glycol-Based Particle Size Magnifier. Aerosol Sci. Technol. 2014, 49, 35–44. [Google Scholar] [CrossRef]
- Mao, J.; Li, J.-J.; Ling, T.; Liu, H.; Yang, J.; Du, X.-W. Facile synthesis of zinc hydroxide carbonate flowers on zinc oxide nanorods with attractive luminescent and optochemical performance. Nanotechnology 2011, 22, 245607. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, H.; Lu, B.; Wei, Y. Formation Mechanism of 1D ZnO Nanowhiskers in Aqueous Solution. J. Phys. Chem. C 2010, 114, 21132–21137. [Google Scholar] [CrossRef]
- Fang, M.; Liu, Z.W. Controllable size and photoluminescence of ZnO nanorod arrays on Si substrate prepared by micro-wave-assisted hydrothermal method. Ceram. Int. 2017, 43, 6955–6962. [Google Scholar] [CrossRef]
- Luo, C.-Q.; Ho, L.-P.; Ling, F.C.-C. The origin of additional modes in Raman spectra of ZnO:Sb films. Physica B 2020, 593, 412256. [Google Scholar] [CrossRef]
- Das, P.K.; Biswal, R.; Choudhary, R.J.; Sathe, V.; Ganesan, V.; Khan, S.A.; Mishra, N.C.; Mallick, P. Effect of 120 MeV Au9+ ion irradiation on the structure and surface morphology of ZnO/NiO heterojunction. Surf. Interface Anal. 2018, 50, 954–961. [Google Scholar] [CrossRef]
- Sharma, G.N.; Dutta, S.; Singh, S.K.; Chatterjee, R. Growth and optical properties of nano-textured (110) Pb(Zr0.52Ti0.48)O3/(001) ZnO hetero-structure on oxidized silicon substrate. J. Mater. Sci.-Mater. Electron. 2017, 28, 5058–5062. [Google Scholar] [CrossRef]
- Husain, S.; Alkhtaby, L.A.; Giorgetti, E.; Zoppi, A.; Miranda, M.M. Effect of Mn doping on structural and optical properties of sol gel derived ZnO nanoparticles. J. Lumin. 2013, 145, 132–137. [Google Scholar] [CrossRef]
- Zamiri, R.; Kaushal, A.; Rebelo, A.; Ferreira, J. Er doped ZnO nanoplates: Synthesis, optical and dielectric properties. Ceram. Int. 2014, 40, 1635–1639. [Google Scholar] [CrossRef]
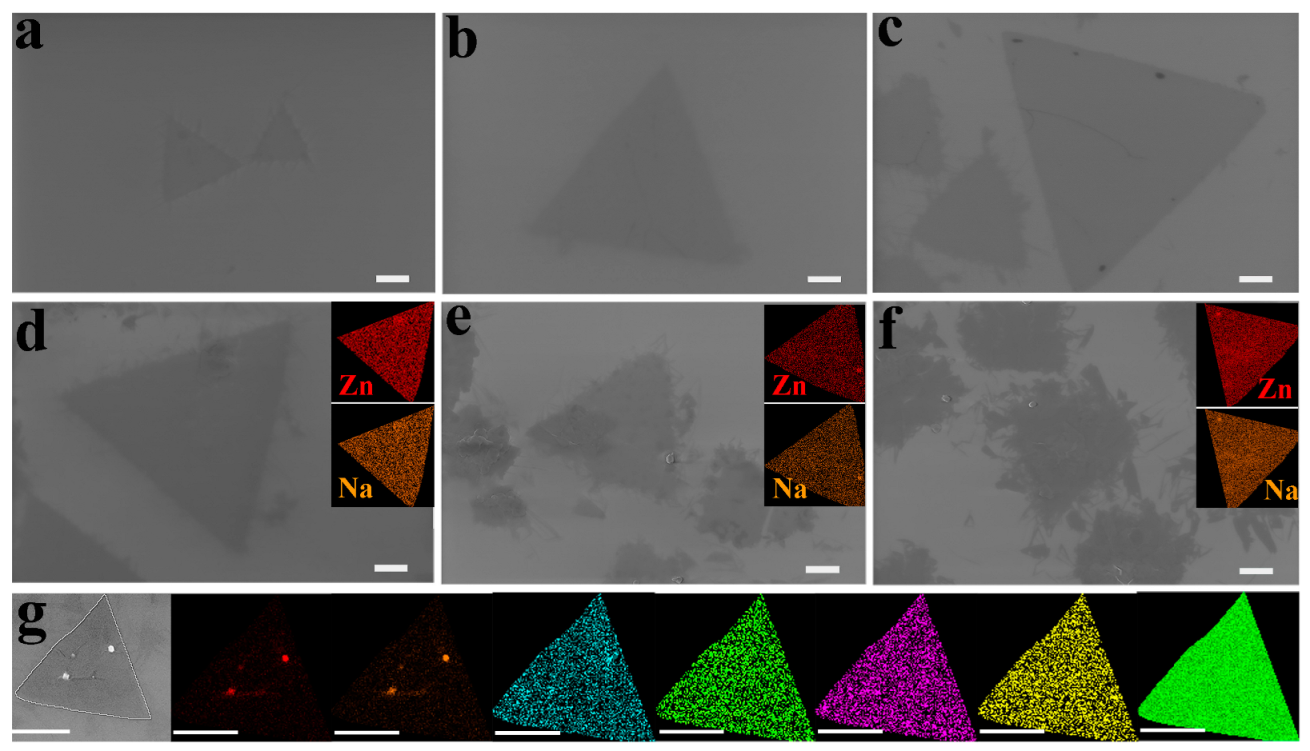
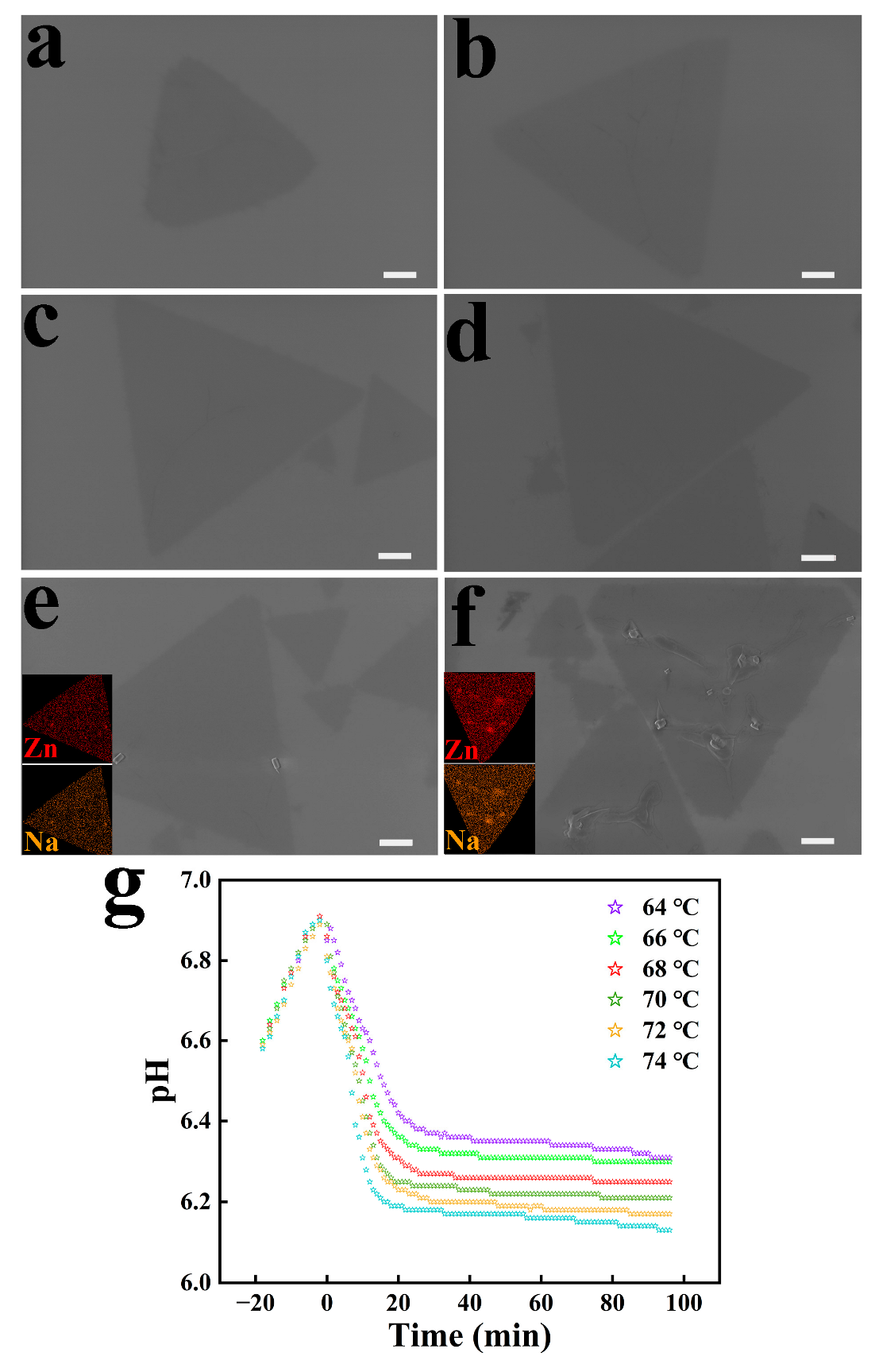
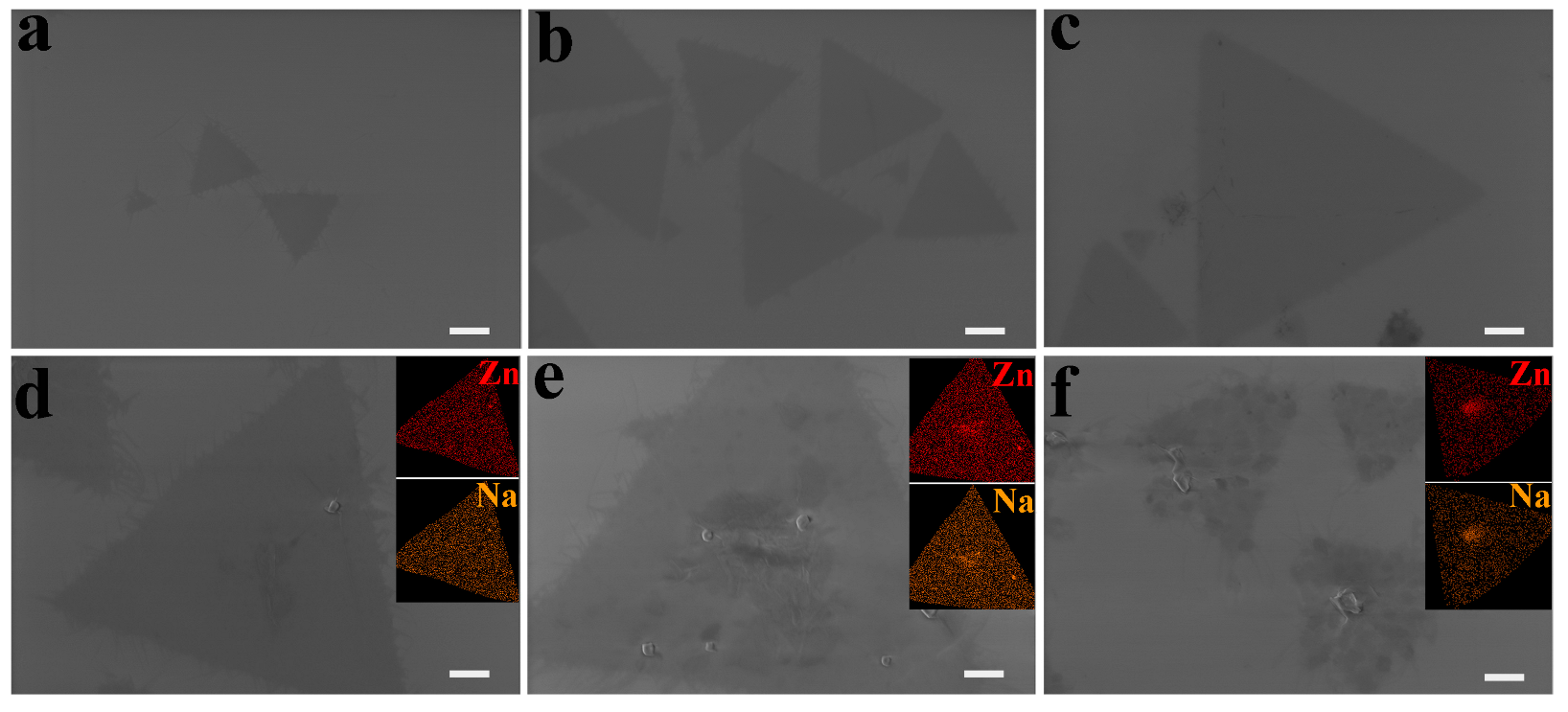
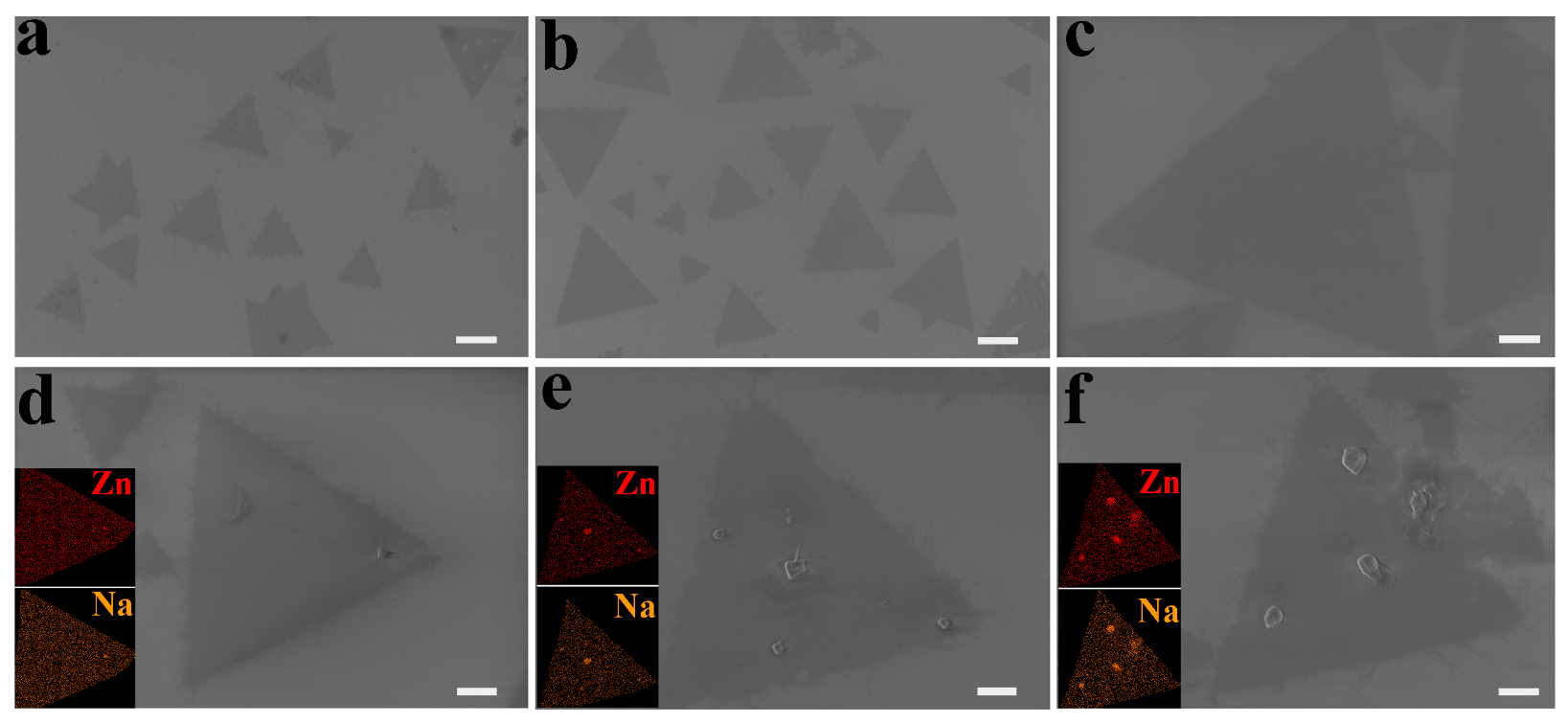
| Parameters | Value | Size (μm) | Morphology |
|---|---|---|---|
| The ratio of sodium oleyl sulfate to | 6 × 10−5 | 2.1 | triangle |
| 8 × 10−5 | 6.5 | triangle | |
| 10 × 10−5 | 8.4 | triangle | |
| 11 × 10−5 | 7.2 | triangle + rough | |
| 13 × 10−5 | 4.6 | triangle + rough | |
| 14 × 10−5 | 3.7 | triangle + rough | |
| Temperature (°C) | 64 | 5.2 | triangle |
| 66 | 7.5 | triangle | |
| 68 | 7.6 | triangle | |
| 70 | 8.5 | triangle | |
| 72 | 7.8 | triangle + rough | |
| 74 | 7.6 | truncated triangle + rough | |
| precursor concentration (mM) | 40 | 1.8 | triangle |
| 45 | 3.5 | triangle | |
| 50 | 8.6 | triangle | |
| 55 | 10.1 | triangle + rough | |
| 60 | 13 | triangle + rough | |
| 65 | 3.8 | triangle + rough | |
| Growth time (min) | 40 | 1.5 | triangle |
| 45 | 2.1 | triangle | |
| 50 | 8.9 | triangle | |
| 55 | 7.7 | triangle + rough | |
| 60 | 7.3 | triangle + rough | |
| 65 | 7.2 | triangle + rough |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Sun, Q.; Chen, Z.; Wen, D.; Tan, Z.; Lu, Y.; He, Y.; Chen, P. Controlled Synthesis and Growth Mechanism of Two-Dimensional Zinc Oxide by Surfactant-Assisted Ion-Layer Epitaxy. Crystals 2023, 13, 5. https://doi.org/10.3390/cryst13010005
Huang C, Sun Q, Chen Z, Wen D, Tan Z, Lu Y, He Y, Chen P. Controlled Synthesis and Growth Mechanism of Two-Dimensional Zinc Oxide by Surfactant-Assisted Ion-Layer Epitaxy. Crystals. 2023; 13(1):5. https://doi.org/10.3390/cryst13010005
Chicago/Turabian StyleHuang, Chunfeng, Qi Sun, Zhiling Chen, Dongping Wen, Zongqian Tan, Yaxian Lu, Yuelan He, and Ping Chen. 2023. "Controlled Synthesis and Growth Mechanism of Two-Dimensional Zinc Oxide by Surfactant-Assisted Ion-Layer Epitaxy" Crystals 13, no. 1: 5. https://doi.org/10.3390/cryst13010005
APA StyleHuang, C., Sun, Q., Chen, Z., Wen, D., Tan, Z., Lu, Y., He, Y., & Chen, P. (2023). Controlled Synthesis and Growth Mechanism of Two-Dimensional Zinc Oxide by Surfactant-Assisted Ion-Layer Epitaxy. Crystals, 13(1), 5. https://doi.org/10.3390/cryst13010005







