Rapid Aqueous-Phase Synthesis and Photoluminescence Properties of K0.3Bi0.7F2.4:Ln3+ (Ln = Eu, Tb, Pr, Nd, Sm, Dy) Nanocrystalline Particles
Abstract
:1. Introduction
2. Experimental Process
3. Results and Discussion
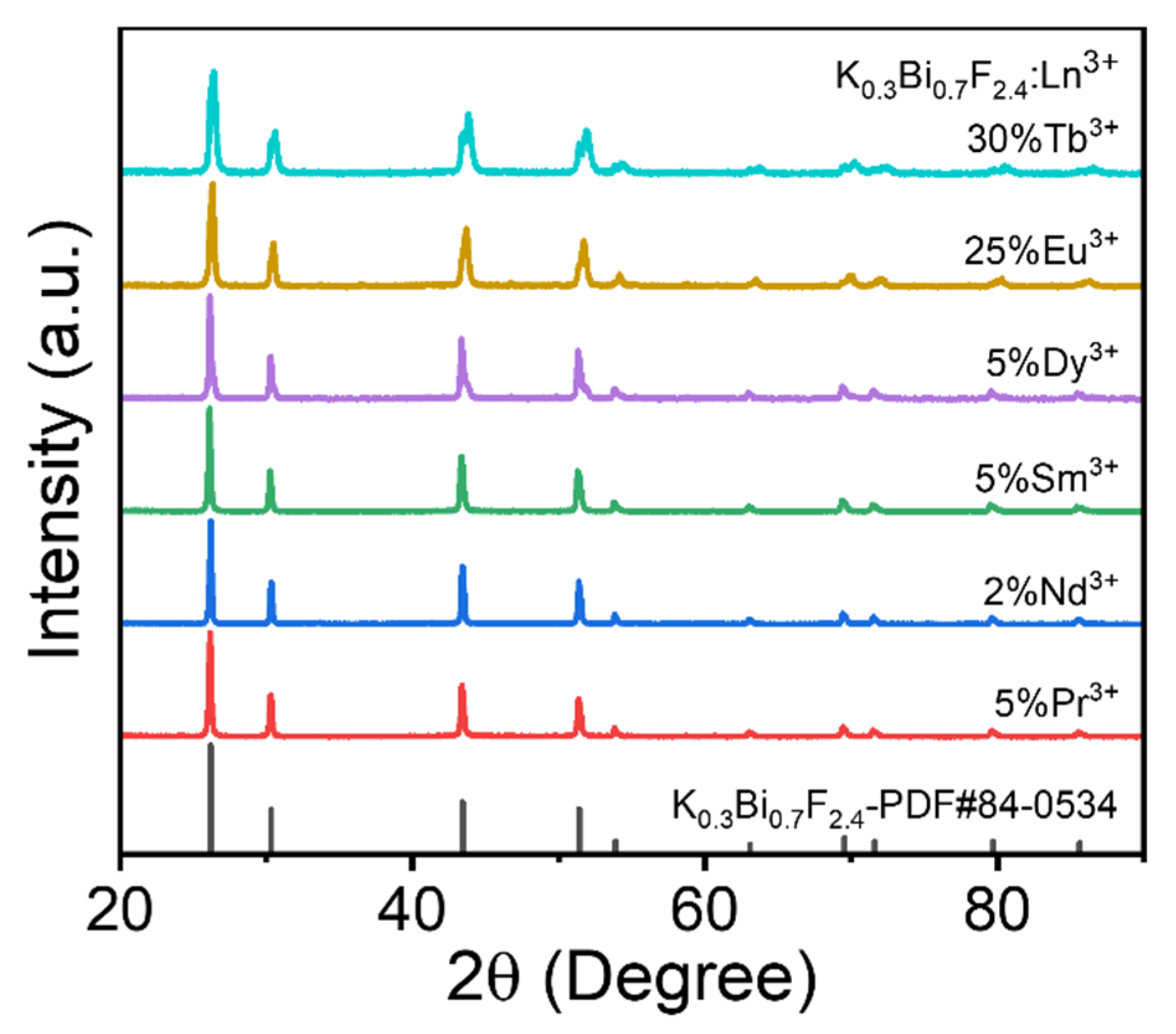
3.1. Phase Composition of the Pure and Doped KBF Nanocrystals
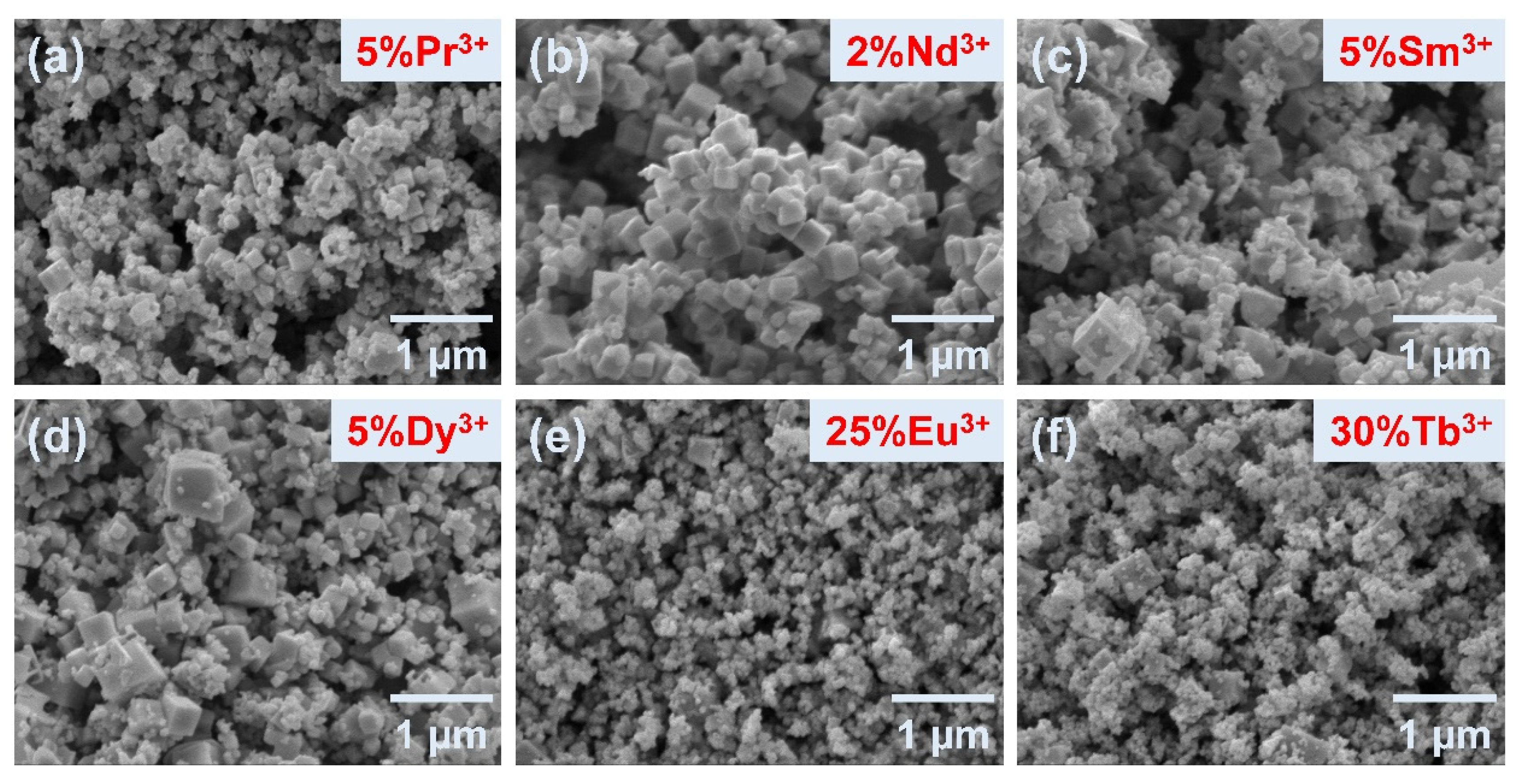
3.2. Morphology Characterization of Pure and Doped KBF Nanocrystalline Particles
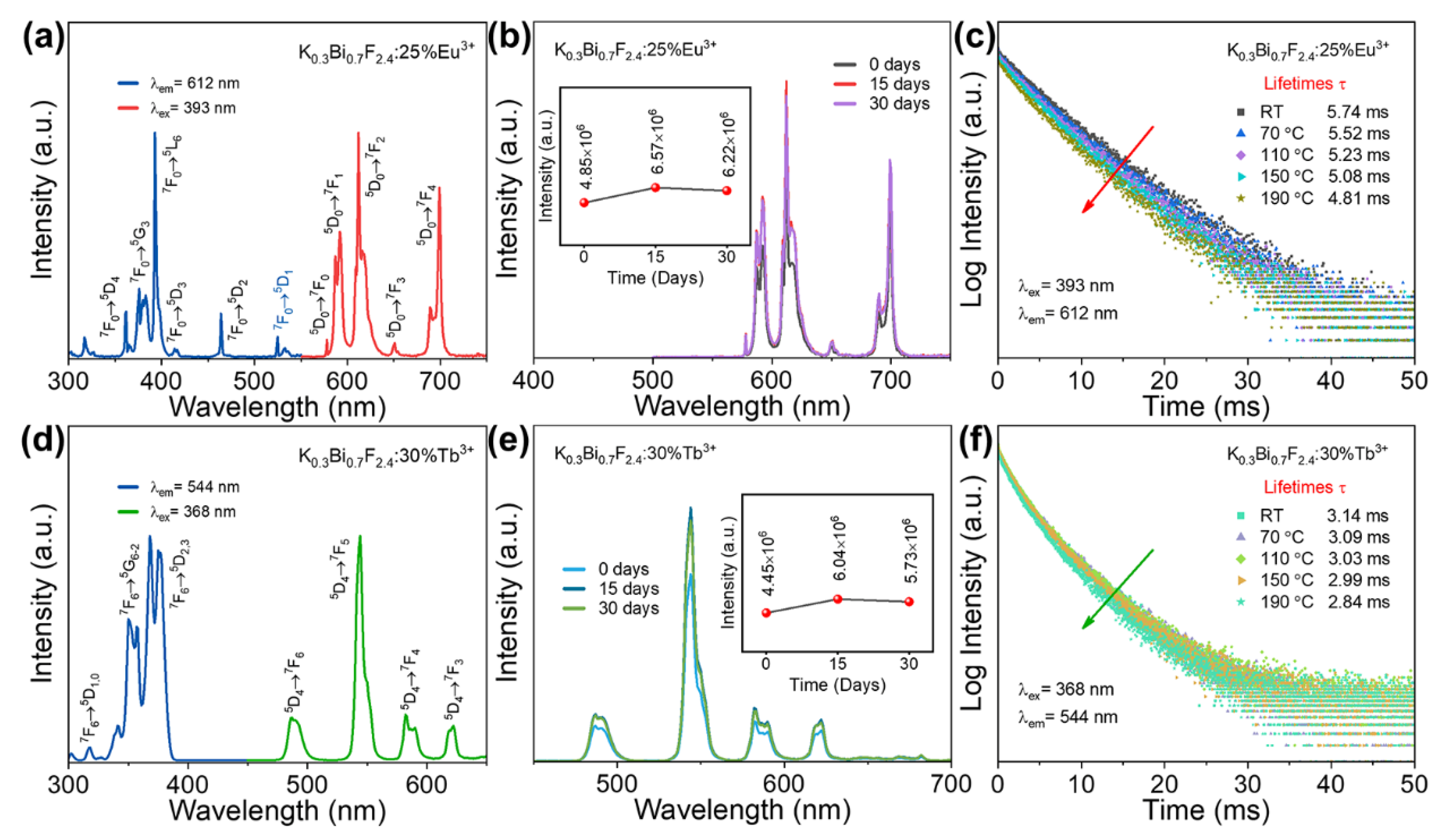
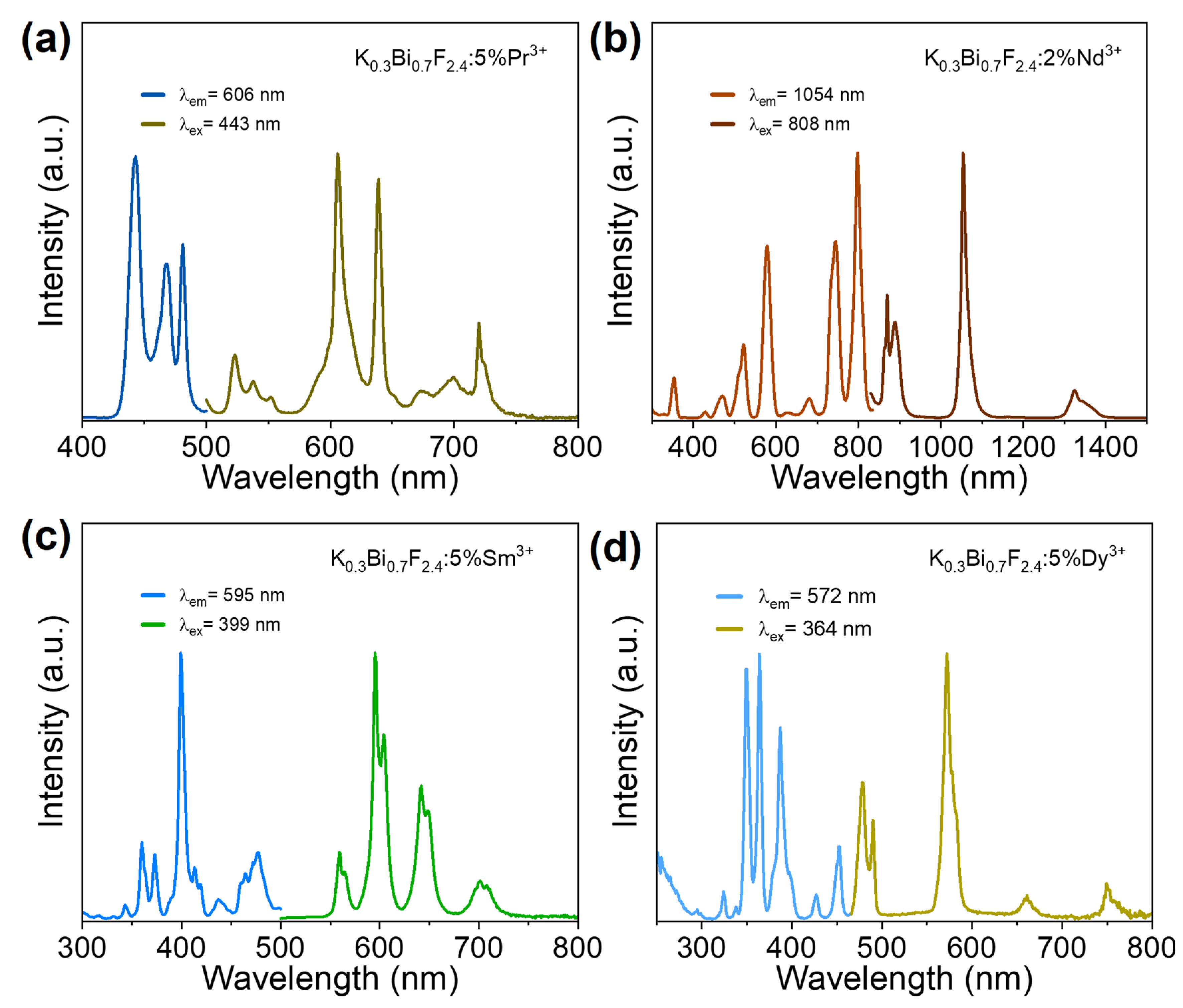
3.3. Photoluminescence Properties of KBF:Ln Nanophosphors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terraschke, H.; Wickleder, C. UV, blue, green, yellow, red, and small: Newest developments on Eu2+-doped nanophosphors. Chem. Rev. 2015, 115, 11352–11378. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Massey, M.; Rees, K.; Higgins, R.; Krause, K.D.; Darwish, G.H.; Peveler, W.J.; Xiao, Z.; Tsai, H.Y.; Gupta, R.; et al. Photoluminescent nanoparticles for chemical and biological analysis and imaging. Chem. Rev. 2021, 121, 9243–9358. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Lin, G.; Clarke, C.; Zhou, J.; Jin, D. Optical nanomaterials and enabling technologies for high-security-level anticounterfeiting. Adv. Mater. 2020, 32, 1901430. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W.; et al. Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv. Mater. 2018, 30, 1701460. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, L.; Liang, Y.; Wang, W.; Yan, S.; Bi, J.; Sun, K. Yolk–shell structured Bi2SiO5:Yb3+,Ln3+ (Ln = Er, Ho, Tm) upconversion nanophosphors for optical thermometry and solid-state lighting. CrystEngComm 2020, 22, 4438. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Multicolor tuning of lanthanide-doped nanoparticles by single wavelength excitation. Acc. Chem. Res. 2014, 47, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, B.; Wang, X.; Lu, L.; Lu, Q.; Sun, M.; Wu, T.; Ma, T.; Xu, J.; Xu, Y.; et al. Multimodal luminescent Yb3+/Er3+/Bi3+-doped perovskite single crystals for X-ray detection and anti-counterfeiting. Adv. Mater. 2020, 32, 2004506. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, Z.; Zhang, Y.; Guo, S.; Pendharkar, A.I.; Lu, M.; Huang, L.; Huang, W.; Han, G. Emerging ≈800 nm excited lanthanide-doped upconversion nanoparticles. Small 2017, 13, 1602843. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, J.; Liu, Y.; Lin, J.; Wang, S.; Huang, F.; Chen, D. Lanthanide-doped core@multishell nanoarchitectures: Multimodal excitable upconverting/downshifting luminescence and high-level anti-counterfeiting. Small 2020, 16, 2000708. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Zamarrón, A.; de la Fuente, A.J.; Sanz-Rodríguez, F.; Maestro, L.M.; Rodriguez, E.M.; Jaque, D.; Solé, J.G.; Capobianco, J.A. Temperature sensing using fluorescent nanothermometers. ACS Nano 2010, 4, 3254–3258. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, D.; Chen, W.; Zhang, W.; Su, X.; Deng, R.; An, Z.; Chen, H.; Xie, R.J. X-ray-charged bright persistent luminescence in NaYF4:Ln3+@NaYF4 nanoparticles for multidimensional optical information storage. Light Sci. Appl. 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.X.; Zhang, Y.W.; Si, R.; Yan, Z.G.; Sun, L.D.; You, L.P.; Yan, C.H. High-quality sodium rare-earth fluoride nanocrystals: Controlled synthesis and optical properties. J. Am. Chem. Soc. 2006, 128, 6426–6436. [Google Scholar] [CrossRef] [PubMed]
- Gnanasammandhan, M.K.; Idris, N.M.; Bansal, A.; Huang, K.; Zhang, Y. Near-IR photoactivation using mesoporous silica-coated NaYF4:Yb,Er/Tm upconversion nanoparticles. Nat. Protoc. 2016, 11, 688–713. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Synthesis of hexagonal-phase NaYF4:Yb,Er and NaYF4:Yb,Tm nanocrystals with efficient up-conversion fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Liu, D.; Xu, X.; Du, Y.; Qin, X.; Zhang, Y.; Ma, C.; Wen, S.; Ren, W.; Goldys, E.M.; Piper, J.A.; et al. Three-dimensional controlled growth of monodisperse sub-50 nm heterogeneous nanocrystals. Nat. Commun. 2016, 7, 10254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R. Green bismuth. Nat. Chem. 2010, 2, 336. [Google Scholar] [CrossRef] [PubMed]
- Swart, H.C.; Kroon, R.E. Ultraviolet and visible luminescence from bismuth doped materials. Opt. Mater. X 2019, 2, 100025. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Yi, X.; Ming, H.; Ma, Z.; Peng, M. Rechargeable and sunlight-activated Sr3Y2Ge3O12:Bi3+ UV–Visible-NIR persistent luminescence material for night-vision signage and optical information storage. Chem. Eng. J. 2021, 421, 127820. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, M.; Zhang, S.; Zhang, H.; Shi, X.; Ye, S.; Huang, B.; Du, Y.; Yan, C.H. Rare-earth-based perovskite Cs2AgScCl6:Bi for strong full visible spectrum emission. Adv. Funct. Mater. 2022, 2204780. [Google Scholar] [CrossRef]
- Lei, P.; An, R.; Yao, S.; Wang, Q.; Dong, L.; Xu, X.; Du, K.; Feng, J.; Zhang, H. Ultrafast synthesis of novel hexagonal phase NaBiF4 upconversion nanoparticles at room temperature. Adv. Mater. 2017, 29, 1700505. [Google Scholar] [CrossRef]
- Back, M.; Ueda, J.; Ambrosi, E.; Cassandro, L.; Cristofori, D.; Ottini, R.; Riello, P.; Sponchia, G.; Asami, K.; Tanabe, S.; et al. Lanthanide-doped bismuth-based fluoride nanocrystalline particles: Formation, spectroscopic investigation, and chemical stability. Chem. Mater. 2019, 31, 8504–8514. [Google Scholar] [CrossRef]
- Saraf, R.; Shivakumara, C.; Behera, S.; Dhananjaya, N.; Nagabhushana, H. Synthesis of Eu3+-activated BiOF and BiOBr phosphors: Photoluminescence, Judd–Ofelt analysis and photocatalytic properties. RSC Adv. 2015, 5, 9241–9254. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Y.; Seo, H.J. Eu3+-doped Bi7O5F11 microplates with simultaneous luminescence and improved photocatalysis. APL Mater. 2020, 8, 081109. [Google Scholar] [CrossRef]
- Du, P.; Huang, X.; Yu, J.S. Facile synthesis of bifunctional Eu3+-activated NaBiF4 red-emitting nanoparticles for simultaneous white light-emitting diodes and field emission displays. Chem. Eng. J. 2018, 337, 91–100. [Google Scholar] [CrossRef]
- Sarkar, S.; Dash, A.; Mahalingam, V. Strong stokes and upconversion luminescence from ultrasmall Ln3+-doped BiF3 (Ln = Eu3+, Yb3+/Er3+) nanoparticles confined in a polymer matrix. Chem. Asian J. 2014, 9, 447–451. [Google Scholar] [CrossRef]
- Zhao, S.; Tian, R.; Shao, B.; Feng, Y.; Yuan, S.; Dong, L.; Zhang, L.; Wang, Z.; You, H. One-pot synthesis of Ln3+-doped porous BiF3@PAA nanospheres for temperature sensing and pH-responsive drug delivery guided by CT imaging. Nanoscale 2020, 12, 695–702. [Google Scholar] [CrossRef]
- An, R.; Lei, P.; Zhang, P.; Xu, X.; Feng, J.; Zhang, H. Near-infrared optical and X-ray computed tomography dual-modal imaging probe based on novel lanthanide-doped K0.3Bi0.7F2.4 upconversion nanoparticles. Nanoscale 2018, 10, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, F.; Ju, D.; Zhou, A.; Khan, A.; Chen, Z.; Sang, X.; Feng, M.; Liu, L. Room-temperature ultrafast synthesis, morphology and upconversion luminescence of K0.3Bi0.7F2.4:Yb3+/Er3+ nanoparticles for temperature-sensing application. CrystEngComm 2020, 22, 7066–7074. [Google Scholar] [CrossRef]
- Gao, X.; Song, F.; Khan, A.; Chen, Z.; Ju, D.; Sang, X. Room temperature synthesis, Judd Ofelt analysis and photoluminescence properties of down-conversion K0.3Bi0.7F2.4:Eu3+ orange red phosphors. J. Lumin. 2021, 230, 117707. [Google Scholar] [CrossRef]
- Du, P.; Wan, X.; Luo, L.; Li, W.; Li, L. Thermally stable Tb3+/Eu3+-codoped K0.3Bi0.7F2.4 nanoparticles with multicolor luminescence for white-light-emitting diodes. ACS Appl. Nano Mater. 2021, 4, 7062–7071. [Google Scholar] [CrossRef]
- Chen, D.; Bi, J.; Wang, W.; Wang, X.; Zhang, Y.; Liang, Y. Rapid aqueous-phase synthesis of highly stable K0.3Bi0.7F2.4 upconversion nanocrystalline particles at low temperature. Inorg. Chem. Front. 2021, 8, 1039–1048. [Google Scholar] [CrossRef]
- Chen, D.; Miao, S.; Liang, Y.; Wang, W.; Yan, S.; Bi, J.; Sun, K. Controlled synthesis and photoluminescence properties of Bi2SiO5:Eu3+ core-shell nanospheres with an intense 5D0→7F4 transition. Opt. Mater. Express 2021, 11, 355–370. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Zhuang, Y.; Chen, W.; Long, H.; Chen, H.; Xie, R.J. NaMgF3:Tb3+@NaMgF3 nanoparticles containing deep traps for optical information storage. Adv. Opt. Mater. 2021, 9, 2100624. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, S.; Liang, Y.; Liang, C.; Chen, D.; Shan, Z.; Sun, K.; Wang, X.J. Blue LED-pumped intense short-wave infrared luminescence based on Cr3+-Yb3+-codoped phosphors. Light Sci. & Appl. 2022, 11, 136. [Google Scholar]
- Yuan, C.; Li, R.; Liu, Y.; Zhang, L.; Zhang, J.; Leniec, G.; Sun, P.; Liu, Z.; Luo, Z.; Dong, R.; et al. Efficient and broadband LiGaP2O7:Cr3+ phosphors for smart near-infrared light-emitting diodes. Laser Photonics Rev. 2021, 15, 2100227. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, F.; Chen, Y.; Wang, X.; Sun, K.; Pan, Z. Red/near-infrared/short-wave infrared multi-band persistent luminescence in Pr3+-doped persistent phosphors. Dalton Trans. 2017, 46, 11149–11153. [Google Scholar] [CrossRef]
- Wang, B.; Lin, H.; Xu, J.; Chen, H.; Lin, Z.; Huang, F.; Wang, Y. Design, preparation, and characterization of a novel red long-persistent perovskite phosphor: Ca3Ti2O7:Pr3+. Inorg. Chem. 2015, 54, 11299–11306. [Google Scholar] [CrossRef]
- Chen, D.; Liang, Y.; Miao, S.; Bi, J.; Sun, K. Nd3+-doped Bi2SiO5 nanospheres for stable ratiometric optical thermometry in the first biological window. J. Lumin. 2021, 234, 117967. [Google Scholar] [CrossRef]
- Li, Y.C.; Chang, Y.H.; Lin, Y.F.; Chang, Y.S.; Lin, Y.J. Synthesis and luminescent properties of Ln3+ (Eu3+, Sm3+, Dy3+)-doped lanthanum aluminum germanate LaAlGe2O7 phosphors. J. Alloys Compd. 2007, 439, 367–375. [Google Scholar] [CrossRef]
- Tian, T.; Feng, H.; Zhang, Y.; Zhou, D.; Shen, H.; Wang, H.; Xu, J. Crystal growth and luminescence properties of Dy3+ and Ge4+ co-doped Bi4Si3O12 single crystals for high power warm white LED. Crystals 2017, 7, 249. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Miao, S.; Chen, D.; Liang, Y. Rapid Aqueous-Phase Synthesis and Photoluminescence Properties of K0.3Bi0.7F2.4:Ln3+ (Ln = Eu, Tb, Pr, Nd, Sm, Dy) Nanocrystalline Particles. Crystals 2022, 12, 963. https://doi.org/10.3390/cryst12070963
Wang W, Miao S, Chen D, Liang Y. Rapid Aqueous-Phase Synthesis and Photoluminescence Properties of K0.3Bi0.7F2.4:Ln3+ (Ln = Eu, Tb, Pr, Nd, Sm, Dy) Nanocrystalline Particles. Crystals. 2022; 12(7):963. https://doi.org/10.3390/cryst12070963
Chicago/Turabian StyleWang, Weili, Shihai Miao, Dongxun Chen, and Yanjie Liang. 2022. "Rapid Aqueous-Phase Synthesis and Photoluminescence Properties of K0.3Bi0.7F2.4:Ln3+ (Ln = Eu, Tb, Pr, Nd, Sm, Dy) Nanocrystalline Particles" Crystals 12, no. 7: 963. https://doi.org/10.3390/cryst12070963
APA StyleWang, W., Miao, S., Chen, D., & Liang, Y. (2022). Rapid Aqueous-Phase Synthesis and Photoluminescence Properties of K0.3Bi0.7F2.4:Ln3+ (Ln = Eu, Tb, Pr, Nd, Sm, Dy) Nanocrystalline Particles. Crystals, 12(7), 963. https://doi.org/10.3390/cryst12070963





