Abstract
The effect of synthesis conditions on the features of the long- and short-range order of Ln(WO) (Ln = Gd, Dy, Ho, Yb) powders synthesized via coprecipitation of salts has been studied by a complex of physico-chemical techniques including synchrotron X-ray powder diffraction, X-ray absorption spectroscopy, Raman and infrared spectroscopy, and simultaneous thermal analysis. It was found that crystallization of amorphous precursors begins at 600 °C/3 h and leads to the formation of the monoclinic structure with sp. gr. C12/c1(15) for Ln(WO) (Ln = Gd, Dy) and with sp. gr. P12/a1(14) for Ln = Yb, whereas crystallization of Ho precursor requires even higher temperature. After annealing at 1000 °C, the P12/a1(14) phase becomes the dominant phase component for all heavy lanthanoid types except for Ln = Gd. It was shown that the Ln (Ln = Dy, Ho, and Yb) tungstates with the P12/a1(14) monoclinic structure correspond to trihydrates Ln(WO)·3HO formed due to a rapid spontaneous hydration under ambient conditions. It was concluded that the proneness to hydration is due to a specific structure of the P12/a1(14) phase with large voids available to water molecules. Modifications in the local structure of Ln-O coordination shell accompanying the structure type change and hydration are monitored using EXAFS spectroscopy.
1. Introduction
The lanthanoid (Ln) tungstates present a wide group of inorganic complex oxides of transition metal and rare earth elements, which attract great interest both for basic science (great chemical flexibility, rich polymorphism and phase transformations, high ionic conductivity, etc.), and for practical uses (from electronics to biology) due to their multifunctional properties (optical, electronic, luminescence and so on) [1,2,3,4]. The most typical Ln tritungstates Ln(WO) with the 2:3 lanthanoid:tungsten atomic ratio have been known for many decades [5,6,7]. In particular, a considerable interest in the Ln(WO) tungstates is observed in the past few decades because these compounds exhibit negative thermal expansion (NTE) [8,9,10,11].
Nowadays, it is commonly accepted that a crystalline structure of Ln(WO) is governed by the ionic radius of the Ln cation [7,9]. Light Ln (Ln = La–Eu) tungstates from the first half of the rare-earth series at room temperature are characterized by a monoclinic structure (space group C12/c1) in which the Ln cation is eight-coordinated by the oxygen atoms. Meanwhile, heavier Ln (Ln = Gd–Dy) tungstates from the mid of the series adopt at room temperature a monoclinic structure. These tungstates upon heating transform into an orthorhombic structure, which is reported to be intrinsically hygroscopic at room temperature. The heaviest Ln (Ln = Ho–Lu) tungstates with smallest ionic radii are highly hygroscopic and form a stable orthorhombic trihydrate structure (sp. gr. Pnca) at room temperature in which the Ln cation is six-coordinated with the oxygen atoms [9].
However, some issues related to phase preferences of the Ln tungstates as well as exact boundary conditions of mutual phase transitions between them remain a subject of debate so far. The formation of light lanthanoid tungstates from La to Eu in their high-temperature modification was reported in [7]. This result contradicts to another report [9], where no high-temperature phase was found. According to numerous reports, the RE(WO) (RE = La–Ho) family has modulated scheelite structure (-Eu(WO)-structure of sp. gr. C12/c1) at room conditions [12,13,14,15,16,17,18]. In the case of Ho(WO) there were some small peaks in its diffraction pattern, which belonged to the -Sc(WO)—phase (hydrated at room conditions) [18]. It is generally accepted [19] that 1/3 of all sites within the Ln sublattice in the Ln (Ln = La–Ho) tungstates with the imperfect scheelite-type structure remain unoccupied. The Ln vacancies tend to form an ordered superstructure at low temperatures, while statistically disordered structures tend to form upon heating. Xiao et al. [20] have shown that Ho(WO) has a monoclinic structure and is not hygroscopic. However, Lahoz et al. [21] reported the presence of a small fraction of the hydrated -phase in the structure of Ho(WO) crystals.
Neither lattice parameters nor atomic coordinates within the crystal structure of hygroscopic stable trihydrate Ln tungstates have been unequivocally determined so far. According to [9] the heavy Ln (Ln = Y, Er and Yb) tungstates form the trihydrate orthorhombic Ln(WO)3HO at room temperature. On the other hand it was reported that Y(WO)3HO trihydrated phase can be indexed with a monoclinic cell (P2/m) [8,22,23]. This monoclinic cell can be converted to a pseudo-orthorhombic phase, which is comparable to unhydrated orthorhombic phase at room temperature [8]. However, the mechanism responsible for the phase transition observed remains essentially unclear.
A detailed analysis of the literature data on the crystal structures and lattice parameters of heavy Ln tungstates is presented in Table S1.
An important information about the local structure features around W sites in Ln tungstates can be obtained with X-ray absorption fine structure (XANES and EXAFS) spectroscopy through the analysis of precise positions and relative intensities of W -pre-edge peak and -main edge [24,25] and non-linear curve fitting of coordination shells in EXAFS Fourier Transforms.
A lot of publications available in the literature describe properties of well-crystallized Ln tungstates prepared by the solid-state synthesis method [6,7,9,19,22]. This method requires the use of high calcination temperatures (usually ≥1000 °C) and long processing times (usually few tens hours) and cannot be used to monitor changes with the crystal structure during its emergence. In the present study, we apply another synthesis strategy, based on the calcination of precursor powders synthesized via the coprecipitation of respective salt mixtures [5,23,26,27]. This method is especially suitable to elucidate the stepwise evolution of overall crystallinity and phase transitions upon the emergence of well-crystallized material. It is realized by appropriate high-temperature treatments of the initially amorphous precursors at variable temperatures [28,29,30].
The aim of this work is thus to elucidate the influence exerted by synthesis conditions (including the type of the Ln cation, calcination temperature, conditions of cooling and subsequent storage time in air) on the long- and short-range order within Ln tungstates Ln(WO) (Ln = Gd, Dy, Ho, Yb) synthesized via the coprecipitation followed by specific high-temperature treatments of the precipitated hydrated precursors. We apply a complex of physico-chemical methods, including synchrotron X-ray powder diffraction, XAFS spectroscopy, Raman and Fourier transform infrared (FT-IR) spectroscopies, supplemented by simultaneous thermal analysis. Earlier, we have successfully shown the potency of such multi-technique structural analysis of lanthanoid hafnates [28], zirconates [29,30], titanates [30], molybdates [31], light and intermediate Ln tungstates [32].
2. Experimental
2.1. Synthesis
The lanthanoid tungstates powders Ln(WO) were prepared by a reverse coprecipitation method similar to that reported in [23]. The starting chemicals Ln(NO)·xHO (Ln = Gd, Dy, Ho, Yb) (the purity not lower than 99.95–99.99% and x = 4–6 depending of Ln type) and NaWO·2HO were purchased from CHIMMED (Moscow, Russia). The Ln(NO) aqueous solutions (0.1 mol/L) were dropwise poured into the NaWO aqueous solution (0.15 mol/L) at a rate of 9 mL/min at continuous intense stirring. The solutions of salt precursors were mixed in exact ratios required by stoichiometry, i.e., Ln:W equals to 1:1.5. The aqueous suspensions obtained were characterized by a nearly neutral pH of 7–8. They were aged for an hour at room temperature to guarantee that the reaction is complete. The precipitates formed were filtered off, washed for several times with distilled water and then dried for 6 h at 80 °C. The dried precursors were then ground in an agate mortar to a fine powder and loaded into a muffle furnace LHT 02/16 (Nabertherm GmbH, Lilienthal, Germany). The powders were heated to specific temperatures in the range 500–1000 °C in air at a temperature ramp rate of 10 °C/min and kept at a constant temperature for 3 h. The annealed samples were either allowed to slowly cool to room temperature by unplugging the furnace or were quickly cooled (quenched) to room temperature by reloading from the hot furnace to the open air or a desiccator. The full list of samples studied is given in Table S2.
We adopted the following naming convention to denote samples under study. The first part of the codename corresponds to the chemical composition (LnWO, where Ln = Gd, Dy, Ho, Yb). The second term indicates the calcination temperature used for the sample preparation (ranged from 500 °C to 1000 °C). Auxiliary conditions describing the preparation protocol are given in the third coding term (desic or repeat). Since the heavy Ln tungstates are intrinsically hygroscopic, the fourth coding term indicates the duration of their storage in air (from few minutes to 1.5 year). A detailed description of sample preparation and respective coding is given in Table 1.

Table 1.
Synthesis conditions and symbols for Ln(WO) samples.
2.2. Characterization
The atomic emission spectroscopy with inductively coupled plasma (ICP-AES) was applied to quantify atomic ratios of metal cations in the freshly prepared precursors. ICP-AES measurements were performed using a Vista-PRO spectrometer (Varian Inc., Palo Alto, CA, USA).
The simultaneous thermal analysis (STA) of the powders, which implied thermogravimetry (TG) and differential scanning calorimetry (DSC) was performed with a SDT Q600 (TA Instruments, New Castle, DE, USA) analyzer over the range of temperatures of 30–1000 °C at a temperature ramp rate of 10 °C/min in the air atmosphere. To determine the water absorption rate all analyzed powders immediately prior to measurements were dried in a desiccator at 200 °C for 1 h. The kinetics experiments were conducted in air at a temperature of 25 ± 1 °C and relative humidity of (60 ± 5)%. Changes in the mass of the samples were monitored with an analytical balance XS205DV (Mettler Toledo LLC, Columbus, OH, USA) with a nominal accuracy of 1·10 g.
The preliminary crystallographic analysis of the synthesized powders was done by X-ray powder diffraction (XRD) using a MiniFlex 600 in-lab diffractometer (Rigaku, Tokyo, Japan) with Cu K-radiation ( = 1.5406 Å). More accurate structural information was gathered using synchrotron radiation-based X-ray powder diffraction (s-XRD) at the X-ray Structural Analysis beamline (Belok/XSA) of the Kurchatov Synchrotron Radiation Source (NRC Kurchatov Institute, Moscow, Russia) (the electron storage ring with the electron energy of 2.5 GeV and mean current stored of 100 mA). The incident radiation was monochromated to 0.8 Å (the photon energy 15,498 eV) using a Si(111) double-crystal monochromator. The X-ray beam spot size was about 400 m [33]. The synchrotron data acquisition was performed at room temperature in the transmission geometry using a SX165 CCD detector (Rayonix LLC, Evanston, IL, USA). The sample-to-detector distance was 150 mm. To extend the scattering angle range, the detector plane was tilted to 29.5°. The exposure time was 5 min. The polycrystalline reference powder LaB NIST SRM 660a was used for the angular scale calibration. The Rietveld full-profile analysis was carried out using the JANA2006 software [34].
W L-edge (12,099.8 eV), L-edge (10,206.8 eV) and LnL-edges (from 7242.8 eV for Gd to 8943.6 eV for Yb) XAFS spectra were measured at the Structural Materials Science beamline at the Kurchatov Synchrotron Radiation Source (NRC Kurchatov Institute, Moscow, Russia) [35]. The measurements were done in the transmission geometry at room temperature. A Si(111) channel-cut monochromator was used for the energy scanning, which provided an apparent energy resolution E/E∼10. Intensities of the X-ray beams before (I) and after (I) the samples were measured with ion chambers with appropriate N/Ar mixtures. The weighted normalized EXAFS function was Fourier transformed over the k range of 2–14.5 Å (W L-edge) or 2–11 Å (LnL-edges). X-ray Absorption Near-Edge Structure (XANES) spectra were processed using the ATHENA software from the IFFEFIT data analysis package [36,37]. Extended X-ray Absorption Fine Structure (EXAFS) spectra were analyzed by means of non-linear curve fitting in the k-space using standard procedure [38] using the IFFEFIT and VIPER [39] data analysis packages. Photoelectron backscattering amplitudes and phase functions were determined ab initio with the FEFF9 code [40].
The Raman spectra were acquired with an inVia Qontor confocal Raman microscope (Renishaw plc, Wotton-Under-Edge, UK) at = 532 nm and = 785 nm over a wavenumber range of 50–2700 cm at a spectral resolution of 1 cm. A part of measurements was performed with a Nicolet iS50 FT-IR spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) with an auxiliary Thermo Scientific iS50 Raman unit ( = 1064 nm) at room temperature over a wavenumber interval of 100–3700 cm at a spectral resolution of 4 cm.
FT-IR spectra were recorded on a Nicolet iS50 FT-IR spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with a single reflection diamond module (ATR). IR spectra were collected over a wavenumber range of 400–4000 cm at a 4 cm resolution.
3. Results and Discussion
3.1. ICP-AES Study
According to the synthesis protocol, the precipitates immediately formed as a result of the reaction between Ln cations and tungstate anions (WO) as soon as reactants were mixed. ICP-AES measurements confirmed that the Ln:W atomic ratio was close to 1:1.5 in the as synthesized precursors in an agreement with the synthesis strategy. The residual concentration of sodium from reactants after careful washing did not exceed 0.07 wt.%. Thus, the as synthesized precursor powders corresponded to stoichiometry Ln(WO)HO and the potential formation of sodium-containing double tungstates NaLn(WO) can be decisively ruled out.
3.2. STA Study
The freshly synthesized precursor powders were characterized using STA to address their thermal stability and determine characteristic temperatures of major structure formation trends therein. According to the TG data, the most significant mass loss (∼80% of the total loss) is observed below ∼200–220 °C (Figure 1a).
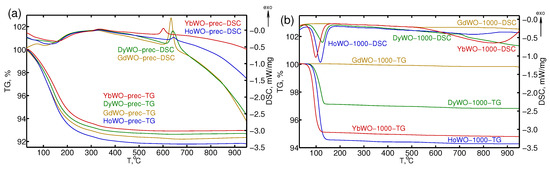
Figure 1.
STA curves for Ln tungstates precursors LnWO-prec (a) and LnWO-1000-1.5y (Ln = Gd, Dy, Ho, Yb) powders (b).
Some distinct sections are clearly seen in the TG curves, which are characterized by different slopes and thus to different mass loss rates (m/T). The first section occurs in a range from 50 °C and 90–120 °C (m/T∼0.03%/°C), whereas the second region lies in a range from 90 to 120 °C to 155–170 °C (m/T∼0.05%/°C), and the third one—from 155 to 170 °C up to 200–220 °C (m/T∼0.02%/°C). The corresponding DSC curves also reveal two weak broadened endothermic peaks at temperaturea around 50–60 °C and 140–150 °C, slightly varying for precursors bearing different Ln types. This clearly implies that the loss of water from precursors proceeds via two distinct stages, which in turn, assumes that the samples bear two types of non-structural occluded HO. A heating to higher temperature of 550–600 °C removes the residual structural more strongly bonded water. This is in accord with earlier results reported elsewhere [41], claiming that the dehydration of Ln tungstates and molybdates is divided into several distinct stages: the adsorbed water gets desorbed at 50–70 °C, the occluded water is removed—at 50–150 °C, the hydration water is removed—at 150–250 °C, and the water of OH-groups are finally removed at as high as 500 °C. The total mass loss of the samples for the series of precursors under study lies in the range from 7.14% to 8.23% (Table 2). The nominal water content in each sample, which can be formulated as Ln(WO)(4.7–5.5)HO (depending on the Ln type), is given in Table 2.

Table 2.
STA results of as-prepared lanthanoid tungstates precursors.
Upon a further increase in temperature to ∼600 °C all precursors demonstrate a peak of exotermic processes in the DSC curves (Figure 1a, Table 2).The exact temperature corresponding to the peak maximum increases along the series from Gd to Ho but starts to decrease again on going to Yb (600.9 °C). These exothermic processes correlated with the XRD data (see Section 3.3 below) and are due to the initiation of the crystallization of the precursor powders giving rise to Ln(WO). It is of note that Gd and Dy tungstates are characterized by higher crystallization enthalpies (116 J/g and 91 J/g, respectively) with respect to analogous Ho and Yb derivatives (17 J/g and 24 J/g, respectively) (Table 2).
Experimental STA curves for well-crystallized samples stored in air for 1.5 year coded as LnWO-1000-1.5y (Ln = Gd, Dy, Ho, Yb) are shown in Figure 1b. As it can be seen from Figure 1b, the sample GdWO-1000-1.5y contains the minimum amount of absorbed water. Heavier Ln (Ln = Dy, Ho, Yb) tungstates demonstrate higher hydration degrees. Furthermore, their dominant mass loss (up to 90–95%) occurs in a temperature range of 60–180 °C. So that a hydration degree (nHO) (i.e., the water amount per 1 mol Ln(WO)) was calculated based on mass losses at 200 °C, observed from thermogravimetric curves, obtained under the same conditions described for STA of the precursors. The apparent effects of the lanthanoid cation type and duration of storage in air on the hydration degrees of the samples are given in Table S3.
As it can be judged from Table S3, the maximum content of absorbed water is observed for the Ho and Yb tungstates, and their stoichiometries thus nearly correspond to trihydrates. Our experimental values for the hydration degrees of these samples (Table S3) are somewhat larger than the ones previously reported [9,27]. This discrepancy could be due to the fact that the hydration degrees reported in [9,27] were calculated as mass losses in a temperature range between 60 °C and 120 °C and thus the dehydration could be incomplete in those cases. The Dy tungstate occupies an intermediate position between Gd and Ho, Yb derivatives.
We observe that Ln (Ln = Dy, Ho, Yb) tungstates exposed to air for one day contained lower amounts of absorbed water with respect to their analogues experienced longer air storage durations from 7 to 9 days to 1.5 year. Moreover, air aging resulted in an apparent shift in the dehydration temperature towards higher values, which could indirectly indicate progressive conversion of weakly bound occluded water molecules to hydration water incorporated into the crystal structure of initially unhydrated rare-earth tungstates. This observation is in full accord with ab initio calculation results [42], which predict that the O end of the inserted HO molecules prefer to bind to Ln or W cations, whereas the two H atoms of each HO molecule tend to point outward to the nearby bridge O atoms shared by LnO octahedra and WO tetrahedra, and form hydrogen bonds, which play a key role in the binding between HO and the materials.
The observation of Ho and Yb tungstates trihydrates in samples exposed to air for few days indicate that the water absorption proceeds relatively fast. To learn more details, we studied kinetics of the hydration. According to results obtained, fresh non-hydrated LnWO-1000 (Ln = Dy, Ho, Yb) powders accumulate 80–85% of hydration theoretical maximum upon exposure to air within 1–1.5 h (Figure 2a). This is in a good agreement with the literature data [43]. They reported that water absorption by (LuEu)(WO) tungstate is completed for 3–4 h. As it can be seen from Figure 2a, Dy(WO)-1000 °C is characterized by the maximum water absorption rate. The resultant descending order of hydration rates looks as follows: Dy (0.912 Å; CN = 6) → Ho (0.901 Å; CN = 6) → Yb (0.868 Å; CN = 6). This regularity can be rationalized by a balance of kinetics- and energy-related factors. The absolute value of water absorption energy in Ln tungstates, according to [42], is expected to decrease as the ionic radius of the Ln cation decreases.
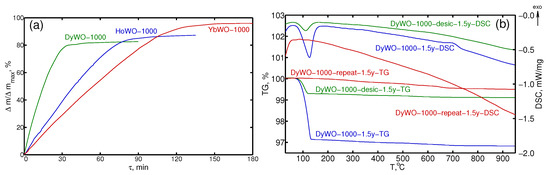
Figure 2.
Hydration kinetics for LnWO-1000 (a) and STA curves for differently treated Dy tungstate samples (b): (1) DyWO-1000-1.5y; (2) DyWO-1000-desic-1.5y; (3) DyWO-1000-repeat-1.5y.
It is of note that DyWO-1000 powders demonstrated the most peculiar behavior among the series under study. Two samples quenched in air (DyWO-1000-1.5y) and in a desiccator (DyWO-1000-desic-1.5y) revealed significantly different hydration degrees after prolonged storage in air for 1.5 year (Table S3, Figure 2b). Furthermore, both powders lost their hygroscopicity after repeated heating to 1000 °C (DyWO-1000-repeat-1.5y). This is in contrast with the behavior of Ho and Yb tungstates, which retained their hygroscopicity by quickly absorbing water after repeated heating (Table S3). The DSC curve of DyWO-1000-1.5y sample quenched in air and then stored for 1.5 year reveals an exothermic peak at 700 °C. However, similar peak is observed neither for the Dy(WO)-1000 °C sample cooled in a desiccator (DyWO-1000-desic-1.5y) nor for the sample after a repeated heating up to 1000 °C (DyWO-1000-repeat-1.5y).
These features of the thermal behavior related to the hydration degrees in Ln tungstates under study are evidently determined by differences in their crystal structures, which is discussed in more detail below in Section 3.3.
3.3. XRD Study
The as prepared precursors were essentially X-ray amorphous for all Ln cations. Their experimental diffraction patterns revealed however a broad but distinct peak indicative of the emergence of specific short-range order therein. The huge width of the diffraction peak for the precursors prevented us from an accurate evaluation of crystallite sizes by the Scherrer formula. We thus believe that the crystallite sizes for these samples do not exceed ∼1 nm. The precursor crystallization process started at ∼600 °C (Figure 3a). As it can be easily followed by Figure 3a, GdWO-600-6d and DyWO-600-6d powders were characterized by identical monoclinic structures (sp. gr. C12/c1(15)). Meanwhile, a residual amorphous halo was apparent in patterns of DyWO-600-6d and HoWO-600-6d, which is indicative of the partial preservation of amorphous fraction. The maximum residual amorphous phase fraction was observed for HoWO-600-6d. For the heavier Yb tungstate YbWO-600-6d, however, only very low amorphous fraction was observed. Furthermore, this powder can be regarded as nanocrystalline with an effective crystallite size of 26(1) nm characterized by an alternative monoclinic structure (sp. gr. P12/a1(14)). Crystallization trends observed by X-ray diffraction are in accord with crystallization temperatures estimated from DSC curves measured for precursors (see Figure 1a, Table 2).
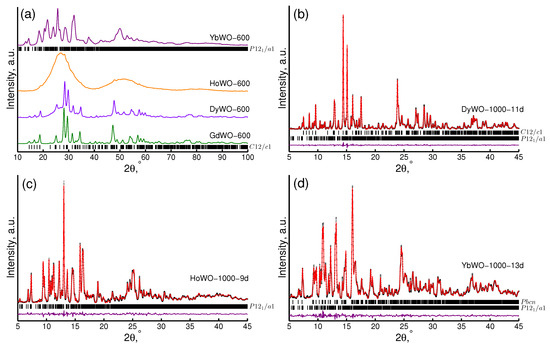
Figure 3.
XRD patterns of LnWO-600-6d (Cu K) (a) and s-XRD patterns ( = 0.8 Å) of DyWO-1000-11d (b), HoWO-1000-9d (c), YbWO-1000-13d (d).
Calcination of the samples at a higher temperature of 1000 °C gave rise to further increase in the crystallite size (narrowing of the diffraction peaks). Moreover, the phase composition of the samples also changed (Figure 3b–d). According to our analysis, the exact phase composition of a sample depends strongly both on the type of the lanthanoid cation therein and its thermal history (Table S4).
All hydrated heavy Ln tungstates (Ln = Dy, Ho, Yb) powders are characterized by the monoclinic structure (sp. gr. P12/a1(14)) similar to that identified and studied in detail for In(WO) [44]. However, this conclusion contradicts to other literature data claiming that trihydrated form of heavy Ln tungstates Ln(WO)3HO has either orthorhombic [9] or monoclinic structure (sp. gr. P2/m) [8,22,23]. It is worth noting that the cell parameters we obtained (Table S4) agree well with data reported by other authors [8,23].
As it can be seen from Table S4, the annealing of precursors at 1000 °C gives rise to the formation of single-phase samples for Gd(WO) and Ho(WO), but two-phase mixtures are afforded for Dy(WO) and Yb(WO).
In the case of Gd(WO), both the freshly prepared sample and the one after a prolonged storage in air (1.5 year) contain only the monoclinic structure (sp. gr. C12/c1) with only minute modifications related to the air storage expressed as a some decrease in the apparent crystallite size. The best-fit atomic coordinates and isothermal temperature factors for the monoclinic structure (sp. gr. C12/c1) of well-crystallized Gd(WO) and Dy(WO) prepared by calcination at 1000 °C from Rietveld refinements were reported by us earlier [32]. A sketch of the monoclinic crystal structure (sp. gr. C12/c1) in a polyhedral representation is shown in Figure 4a. This crystal structure type is dominant for light lanthanoid tungstates [12,13,14,15,16,17,18].
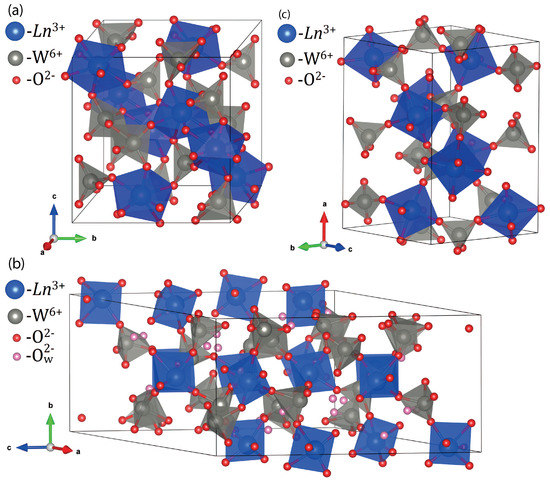
Figure 4.
(a) Polyhedral representation of the monoclinic structure (sp. gr. C12/c1) encountered as the main phase in lanthanoid tungstates Ln(WO) (Ln = Gd, Dy). The lanthanoid Ln cations are eight-fold coordinated (shown in blue). The W cations form WO tetrahedra (shown in grey). All the oxide O anions are shown in red; (b) Polyhedral representation of the monoclinic structure (sp. gr. P12/a1) encountered as the main phase in lanthanoid tungstates Ln(WO) (Ln = Ho, Yb). The lanthanoid Ln cations are six-fold coordinated (shown in blue). The W cations form WO tetrahedra (shown in grey). All the oxide O anions are shown in red. Additional oxygen atoms of hydration HO molecules are shown in pink; (c) Polyhedral representation of the orthorhombic structure (sp. gr. Pbcn) encountered as an admixture phase in Yb(WO). The Yb cations are six-fold coordinated (shown in blue). The W cations form WO tetrahedra (shown in grey). The oxide O anions are shown in red.
A complex evolution of crystal structures was observed for the two-phase samples. Dy(WO) can be regarded as a boundary compounds demonstrating the C12/c1(15) → P12/a1(14) phase transition (Figure S1). As it can be seen from Table S4, the fraction of the monoclinic structure (sp. gr. P12/a1(14)) increases in the course of the air storage. Furthermore, the sample DyWO-1000-1.5y prepared by quenching in air contained the monoclinic phase (sp. gr. P12/a1(14)) in an amount several fold higher than that in the DyWO-1000-desic-1.5y sample quenched in a desiccator, despite identically long post-synthesis exposure to air.
The repeated annealing of both samples to 1000 °C gave rise to single-phase powders with the monoclinic structure (sp. gr. C12/c1(15)), i.e., initiated the P12/a1(14) → C12/c1(15) phase transition (Table S4). Therefore, we may associate the exothermic DSC peak around 700 °C for the sample DyWO-1000-1.5y with the P12/a1(14) → C12/c1(15) phase transition (Figure 2b). A combined analysis of X-ray diffraction (Table S4) and STA results (Table S3) indicates that it is the monoclinic structure (sp. gr. P12/a1(14)) that is exclusively responsible for the intrinsic hygroscopicity of the heavy lanthanoid tungstates. The apparent hydration degree for DyWO-1000-1.5y corrected for the experimental fraction of the P12/a1 phase is 3.4, which is reasonably close to respective values in Ho and Yb tungstate trihydrates (Table S3).
The Ho(WO) samples contain predominantly the monoclinic phase (sp. gr. P12/a1) corresponding to a hydrated holmium tungstate with approximately 3.0–3.5 hydration water molecules per formula unit. This was not altered to any significant extent by a prolonged storage in air (1.5 year). Though the storage resulted in a small decrease both in cell parameters and volume as well as in an apparent decrease in a crystallite size. Both quenching in air and in a desiccator afforded samples with the same phase compositions. The quenching, having no effect on the phase composition, led to some decrease in the cell parameters, its volume, and the crystallite size (Table S4).
We tried to refine the atomic coordinates in the monoclinic phase (sp. gr. P12/a1) of holmium tungstate using crystallographic parameters for anhydrous In(WO) [44]. Positions of the cations were allowed to vary with identical thermal parameters. Positions of the oxygen atoms were refined with geometrical constraints to yield reasonable Ho-O and W-O bond lengths. At last stages of the refinement, six extra oxygen atoms per asymmetric unit corresponding to hydration water molecules were placed into voids of the crystal structure. The list of best-fit coordinates is given in Table S5. Polyhedral representation of the monoclinic structure (sp. gr. P12/a1) encountered as the main phase in Ho(WO) is shown in Figure 4b.
In the case of Yb, a two-phase sample YbWO-1000-13d was afforded simultaneously containing monoclinic (sp. gr. P12/a1(14)) tungstate trihydrate and an orthorhombic phase (sp. gr. Pbcn(60)) typical for the unhydrated form of heavy Ln tungstates (Table S4). As it can be seen from Table S4, the fraction of the monoclinic structure (sp. gr. P12/a1(14)) increases in the course of air storage, which ultimately results in a complete disappearance of the orthorhombic phase (sp. gr. Pbcn(60)) (Figure S2). Powders quenched in air and in a desiccator and then aged in air were characterized by similar phase compositions and cell parameters (Table S4). Polyhedral representation of the orthorhombic structure (sp. gr. Pbcn) encountered as an admixture phase in Yb(WO) is shown in Figure 4c.
The powder diffraction results can be thus summarized as follows. Within the same crystal structure type (the same syngony and space group) unit cell parameters and volume regularly decrease with a nominal decrease in the Ln cation radius. For the two-phase samples, the fraction of the monoclinic phase (sp. gr. P12/a1(14)) increases as air exposure duration increases. At that the unit cell parameters and volume also tend to decrease. The decrease in the unit cell volume upon progressive hydration corresponds to earlier reported data [45]. Additionally, the aging is accompanied by a decrease in the apparent crystallite size (Table S4).
It is important to note that both orthorhombic structure (sp. gr. Pbcn) and monoclinic structure (sp. gr. P12/a1) are less dense as compared to the monoclinic structure (sp. gr. C12/c1) and contains some structural voids accessible for water molecules, which can be considered as a driving force of the hydration (see above Section 3.2 and below Section 3.5.2). The two former structures are closely related to each other. The monoclinic structure (sp. gr. P12/a1) can be regarded as a lower-symmetry superstructure of the parent orthorhombic structure (sp. gr. Pbcn) resulted from a loss of one two-fold symmetry axis. We speculate that the orthorhombic phase is observed for heavier lanthanoid tungsates at an intermediate stage of hydration, whereas the full hydration stabilizes the monoclinic structure (sp. gr. P12/a1).
3.4. X-ray Absorption Spectroscopy
The crystal structure as imaged by diffraction corresponds to an ensemble-averaged atomic configurations. Furthermore, the local structure probed by other techniques could differ drastically from the crystallographic data. We used local structure-sensitive X-ray absorption fine structure (XAFS) spectroscopy to elucidate the local atomic environment of W and Ln atoms within the Ln tungstates under study and compare them with diffraction-extracted crystallographic parameters.
3.4.1. W L- and L-Edges XAFS Study
The analysis of XAFS spectra at the W L- and L-edges is commonly applied to gather information on the local environment symmetry, coordination polyhedra, and oxidation state of the W species in various complex oxide compounds due to unique properties of this technique [24,25].
W L-Edge XANES
Figure 5a shows the W L-edge XANES spectra of hydrated well-crystallized Ln tungstate powders. All the spectra manifest a main-edge (the dipole-allowed 2 6p electron transitions) and a sharp pre-edge peak (the dipole forbidden 2 5d electron transitions) gathering a non-zero intensity due to the hybridization [24]. The exact position (12,102 eV) and intensity of the pre-edge peak reveal no statistically significant dependence on the Ln type. They are also similar to those in NaWO2HO (the experimental position is 12,103 eV), which is a reference sample with tetrahedral WO units [24]. This indicates the presence of undisturbed tetrahedral configurations WO in the studied Ln tungstates regardless of the Ln, i.e., their crystal structure type (see Table S4). As the Ln atomic number increases, the position of the main absorption maximum in the W L-edge spectra shifts towards higher energies (from 12,131.9 eV for Gd(WO) to 12,135.9 eV for Yb(WO)) (Figure 5a), which could be due to a shift in the exact position of the Fermi level for heavy Ln tungstates due to an increase in the number of 5d electrons localized in hybridized orbitals.
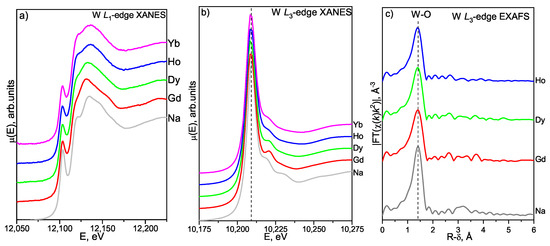
Figure 5.
W L-(a) and L-edge (b) XANES spectra and W L-edge EXAFS FT moduli (c) of hydrated LnWO-1000-1.5y powders and NaWO2HO (reference sample).
W L-Edge XANES
Figure 5b displays the W L-edge XANES spectra of hydrated well-crystallized Ln tungstate powders along with reference sample NaWO2HO. As it becomes evident from Figure 5b, the near-edge spectra reveal a common well-resolved narrow and intense asymmetric peak due to the dipole-allowed electron transitions 2 5d. The spectral parameters of the white line peak for all Ln(WO) samples under study are virtually identical to each other for all the Ln types in the study and are again close to those in the NaWO2HO reference (10,209 eV). This confirms the earlier suggestion that undisturbed WO tetrahedra predominate as major structural units in all studied Ln(WO) samples, irrespective of their crystal structure type (see Table S4). An evident weakening of the right-hand shoulder of the white line (at about 10,218 eV) for heavier lanthanoids (that is well pronounced on going from the spectrum of gadolinium compound to that of dysprosium) can be due to a change in the effective coordination geometry of the WO tetrahedral units accompanying the transformation of the Ln(WO) structure type from one monoclinic (sp. gr. C12/c1(15)) in Gd(WO) to another monoclinic phase (sp. gr. P12/a1(14)) in heavier tungstates Ln(WO) (Ln = Dy, Ho, Yb).
W L-Edge EXAFS
The Fourier transform moduli of W L-edge EXAFS spectra for hydrated well-crystallized LnWO-1000-1.5y samples are shown in Figure 5c and Figure S3. The W L-edge EXAFS FT moduli are dominated by a single intense peak at 1.4 Å, assigned to the W-O coordination shell (CS) formed by WO tetrahedra. Longer-distance CSs are characterized by negligible intensities. This assumes that the short-range atomic order of heavy metal ions around the W site is rather irregular in the studied LnWO-1000-1.5y samples. Positions of FT modulus peaks shown in Figure 5c are apparently shorter than real interatomic distances due to the phase shift associated with the photoelectron backscattering. Real bond lengths obtained from EXAFS non-linear curve-fitting are given in Table 3.

Table 3.
W L-edge EXAFS best-fit results for the W-O coordination shells for hydrated well-crystallized LnWO-1000-1.5y (Ln = Gd, Dy, Ho, Yb ) powders.
We applied two structural models to approximate the first W-O CS peak in EXAFS FTs. According to preliminary evaluations, we established that a set of a few W-O distances is required to achieve a reasonable fit, i.e., the W-O CS is split. The best-fit values of the local-structure parameters are compiled in Table 3. Despite W-O distance of 1.75–1.8 Å typical of tetrahedrally coordinated tungsten, an extra component corresponding to a W-O distance of 2.1–2.2 Å was necessary to reproduce the experimental curve. Furthermore, we performed the curve fitting with refinable coordination numbers (model 1) and coordination numbers fixed at idealized values “2+2+1”(model 2). The results obtained clearly indicate that the WO tetrahedra in the heavy lanthanoid tungstates are actually noticeably distorted. Unfortunately, due to the partial overlap of W L–edge and Yb L-edge spectra, the accuracy of local-structure parameters did not allow us to establish a reliable dependence of the W-O bond lengths on the lanthanoid cation type, although the trend of bond length shortening towards the end of the rare-earth series is evident.
3.4.2. Lanthanoid L-Edge XAFS Study
Ln L-Edge XANES
The Ln L-edge XANES spectra of hydrated well-crystallized LnWO-1000-1.5y samples are shown in Figure 6a. The narrow strong main-edge peaks at +2.5 eV of the scale (FWHM is approximately 6 eV) are due to the Ln 2 5d electronic transitions [46]; their positions and shapes lie within normal values typically observed for compounds containing trivalent lanthanoid ions. The principal spectral parameters in the Ln L-edge spectra demonstrate prominent similarity along the lanthanoid series, which strongly assumes trivalent Ln cations are exclusively present in the analyzed compounds. In the Ln L-edge XANES spectral of Dy, Ho, and Yb tungstates, the white line is followed by a plateau. Taking into account the s-XRD results discussed above (Table S4), we may associate this plateau with a change in the local symmetry of Ln atoms due to the transformation of one monoclinic structure C12/c1(15) into another one P12/a1(14).
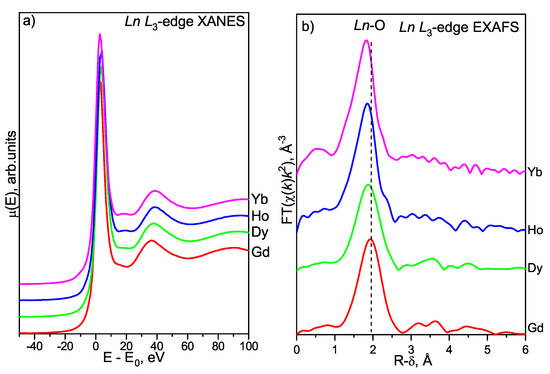
Figure 6.
Ln L-edge XANES spectra (a) and EXAFS FT moduli (b) of hydrated LnWO-1000-1.5y.
Ln L-Edge EXAFS
The FT moduli of Ln L-edge EXAFS-spectra of hydrated well-crystallized LnWO-1000-1.5y samples are displayed in Figure 6b and Figure S4. In all experimental curves, the first dominant peak appears around 1.8–1.9 Å; it can be assigned to the Ln-O CS. Other coordination shells are weak and smeared (similar to the case of W L-edge EXAFS FTs). This indicates a significant degree of local disorder around the Ln cations in Ln(WO). As it becomes clear from Figure 6b, the positions of lanthanoid-oxygen Ln-O and lanthanoid-metal Ln-M components move to shorter interatomic distances as the Ln cation radii decrease (or, equivalently, as the Ln atomic numbers increase).
Similarly to the case of the aforementioned W-O CS coordination shell, we started with a simple local-structure model with Ln-O interatomic distances R, corresponding Debye-Waller factors , and coordination numbers N were allowed to refine freely to achieve a best fit. According to fits in this simplest model with one Ln-O shell (Table 4, model 1), the effective Ln-O bond length shortens for Ln cations with nominally smaller ionic radii along the series Gd–Dy–Ho–Yb. As it can be seen fromTable 4, the best-fit coordination numbers N are close to 8 for the Gd and Dy tungstates that possess the monoclinic structure (sp. gr. C12/c1(15)). A switch of the structure type to another monoclinic lattice (sp. gr. P12/a1(14)) in Ho(WO) is accompanied by a decrease in the effective coordination number. The trend continues for Yb tungstate, in which N approaches a value of 6, which is typical for the structure of heavy lanthanoid tungstates described in the literature [9,23,41]. Our analysis indicated that the change in the effective N upon the C12/c1(15) → P12/a1(14) transformation is continuous rather than abrupt.

Table 4.
LnL-edge EXAFS best-fit results for Ln-O shells of hydrated well-crystallized LnWO-1000-1.5y powders (Ln = Gd, Dy, Ho, Yb).
A further analysis was carried out using a more complicated local structure model based on fixed values of N. The model postulated the Ln-O coordination shell split into two components with fixed coordination numbers of N = 6 and N = 2 (Table 4, model 2). The validity of this model was evaluated using the Fisher’s test [39]. As it can be seen from Table 4, the statistical probability of this model with the split Ln-O coordination shell (6 + 2) is 23% for Gd(WO). This criterion increases to 52.7% for Dy(WO), when an alternative monoclinic structure (sp. gr. P12/a1(14)) emerges according to X-ray diffraction data. In the case of Ho(WO), which contents strictly single-phase (sp. gr. P12/a1(14)), the model becomes statistically reliable with the Fisher’s criterion as high as 98%. For Yb(WO) with the even smaller ionic radius of the lanthanoid cation Yb, the basic local-structure model assumes that the Ln-O coordination shell is formed by one unsplit component with the coordination number of 6. In the full agreement with intuitive anticipation, the effective Ln–O bond lengths refined from EXAFS spectra (Table 4) decrease along the series Gd–Dy–Ho–Yb of descending ionic radii. It is also of note that the spread between the two components within a split shell increases as the Ln cation radius decreases in the series Gd (1.053 Å) > Dy (1.027 Å) > Ho (1.015 Å), where it applies.
Therefore, based on a combined analysis of s-XRD and XAFS spectroscopy results, we envisage the following mechanism of the structural rearrangement at local level associated with the monoclinic lattice transformations C12/c1(15) → P12/a1(14). The onset of the transformation falls onto Gd(WO) (Table S4 and Table 4). The Gd-O coordination shell at the local level tends to split into two component (6 + 2) at the dominance of the monoclinic structure (sp. gr. C12/c1(15)). With a further decrease in the Ln cation radius the statistical significance of the Ln-O CS splitting as well as of the distance spread between the components increases, which is accompanied by the appearance of the monoclinic structure (sp. gr. P12/a1(14)) in Dy(WO), which further becomes the dominant phase in Ho(WO). A further decrease in the Ln radius stabilizes the monoclinic structure of hydrated Yb(WO) (sp. gr. P12/a1(14)) with an unsplit Yb-O coordination shell with the coordination number of 6.
3.5. Vibrational Spectroscopy
Essential information concerning structural features of the sublattice formed by light oxygen atoms in the title heavy lanthanoid tungstates can be learned from vibrational spectroscopy, comprised of infrared absorption (IR) and Raman scattering spectroscopy. For nearly ideal tetrahedral WO moieties, 4 distinct normal vibrations are symmetrically allowed: stretching symmetric W–O () bond vibration , stretching bending W–O (E) bond vibration , stretching asymmetric () and bending asymmetric () W–O bond vibrations and , respectively, [43].
3.5.1. Raman Study
Figure 7a demonstrates the experimental Raman spectra of Gd(WO) sample prepared by thermal treatments of the respective precursor powders at different temperatures. The Raman spectra of amorphous samples, i.e., the freshly prepared precursor and the sample annealed at a low temperature of 500–550 °C, contain three broad vibrational bands around ∼345 cm, 825 cm, and 950 cm, which are assigned to bending modes, and stretching modes of the WO moieties. The start of crystalline phase formation in the initially amorphous structure in GdWO-600 yields the emergence of many new Raman bands (Figure 7a) corresponding to monoclinic structure of Ln tungstates [47]. A calcination at even higher temperature from 700 °C to 1000 °C induces no significant changes in the Raman spectra. This implies the the oxygen sublattice formation in Gd(WO) is virtually complete at ∼700 °C.
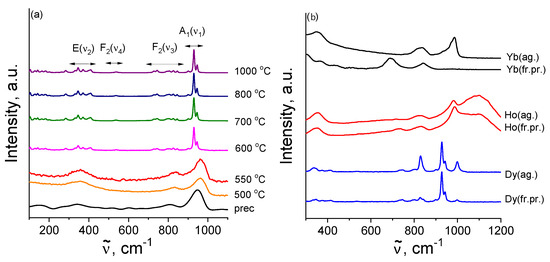
Figure 7.
Raman spectra of Gd(WO) samples synthesized by the annealing of the precursor at different temperatures (a); fresh prepared and cooled in a desiccator (fr.pr.) and hydrated Dy(WO), Ho(WO) and Yb(WO) well-crystallized powders aged in air 1.5 years (ag.) (b).
The internal (above 300 cm) vibrational bands observed in the spectrum of Gd(WO) ( point group symmetry) correspond to two slightly different WO tetrahedral units occured in the unit cell. More specifically, W(1)O units ( site symmetry, mildly distorted tetrahedral units) and W(2)O units ( site symmetry, highly distorted tetrahedral units), which is in accordance with the earlier results for the monoclinic La(WO) phase reported in [48].
The appearance of an additional monoclinic phase (sp. gr. P12/a1(14)) in Dy(WO) results in changing the shape of the Raman spectra (Figure 7b). The changes become cardinal upon a complete phase transition C12/c1(15) → P12/a1(14) in Ho(WO) and Yb(WO). Indeed, new Raman bands show up in the regions of 340–350 cm, 830–845 cm, and 985–1000 cm (for Ho(WO) also in the region of 1100 cm), and the modes at 929 cm and 948 cm completely disappear (Figure 7b). As can be seen from Figure 7b, the intensity of the Raman modes increased in the region of high wavenumbers (∼980–1100 cm) for all hygroscopic tungstates after storage in air compared to freshly obtained samples. This effect can be explained as follows. According to [9,45], the hydration of Ln tungstates decreases their unit-cell volume (7% for YWO [45]), which leads to smaller lattice parameters and shorter bond lengths. This moves the Raman shift towards higher wavenumbers and increases the intensity of the scattering (Figure 7b).
3.5.2. FT-IR Study
FT-IR analysis was applied to identify specific chemical bonds and fragments occurring in the synthesized samples. The freshly prepared amorphous precursor powders demonstrate very similar FT-IR spectra, with three broadened strong peaks around 824–832 cm, 1624–1627 cm, and 3340–3360 cm, together with several less intense bands at ∼440–450 cm, 620–630 cm, 920–930 cm, and 1400–1415 cm, and 1520–1530 cm (Figure 8a). The spectral features lying in the range of 400–1000 cm can be assigned to the bending and stretching modes of the WO moieties, and those in the range of 1623–1625 cm and 3340–3360 cm are due to the bending mode () of H–O–H vibrations and longitudinal stretching vibrations of the O–H groups ( and ) of the surface-absorbed water molecules, respectively, [26,49,50,51].
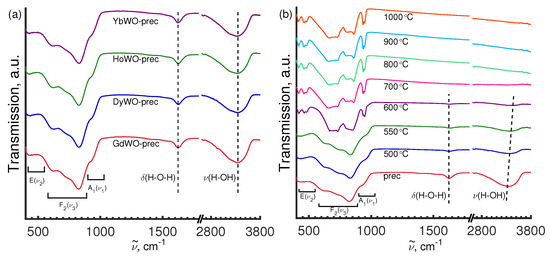
Figure 8.
FT-IR spectra of Ln tungstate precursors LnWO-prec (a), Gd(WO) samples prepared by the calcination of the precursor at different temperatures (b).
It is observed that the crystallization of GdWO-600 (monoclinic, sp. gr. C12/c1(15)) leads to the appearance in the FT-IR spectrum of intense bands assigned to vibrations of the WO groups observed at wavenumbers ranged range from 400 cm to 1000 cm. Furthermore, the O–H absorption bands get weaker and eventually disappear due to dehydration (Figure 8b). As it can be seen from Figure 8b, there are virtually no change in the FT-IR spectra of Gd(WO) for annealing temperatures raised from 700 °C to 1000 °C, which strongly implies that the oxygen sublattice is successfully formed already at 700 °C. The vibrational peaks at around 728 cm and 860 cm are assigned to the O–W–O stretching modes of the WO tetrahedron. The 418 cm and 460 cm bands can be attributed to the stretching vibrations of the W–O bonds [52]. It is of note that the FT-IR peaks revealed in the spectra of the well-crystallized Gd(WO)-1000 °C sample are assigned to the tetrahedral W(1)O and W(2)O units in La(WO), which crystallizes in the monoclinic structure (sp. gr. C12/c1(15)) as reported by other authors [48].
The FT-IR spectra of heavy Ln tungstates with the monoclinic structure (sp. gr. P12/a1(14)) were very different from the spectra of Gd(WO) (monoclinic, sp. gr. C12/c1(15)). Namely, the FT-IR spectra of Ho(WO) and Yb(WO) show a significant decrease in the number of peaks, as well as the presence of O–H absorption bands even for well-crystallized samples calcined at 1000 °C (Figure 9). As it can be seen from Figure 9a, the FT-IR spectrum of Dy(WO) is intermediate between the Gd and (Ho or Yb) tungstates. It contains a large number of absorption bands corresponding to the monoclinic structure, but also it shows O-H absorption bands. This is in a good agreement with STA and X-ray diffraction data that the synthesized sample is a mixture of two monoclinic phases (sp. gr. C12/c1(15) and (sp. gr. P12/a1(14)) (see Table S4).
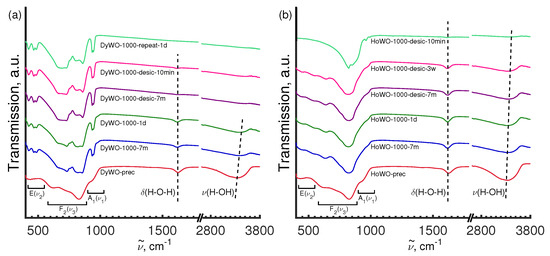
Figure 9.
FT-IR spectra of Dy(WO) (a) and Ho(WO) (b) powders prepared under different conditions.
Furthermore, we found that synthesized and quenched in a desiccator LnWO-1000-desic (Ln = Dy, Ho and Yb) powders reveal very weak vibrational peaks at 1613–1616 cm and 3380–3500 cm of the O-H bonds of the surface-absorbed water molecules (Figure 9 and Figure S5). The FT-IR spectra of Dy(WO) demonstrate only minute changes during storage in air in the region from 400 cm to 1000 cm, assigned to vibrational modes within the WO tetrahedra in the monoclinic structure (sp. gr. C12/c1(15). The intensity of the O-H absorption modes for sample DyWO-1000-desic is weaker as compared to sample DyWO-1000 which is evidently caused by the higher content of the hygroscopic phase (sp. gr. P12/a1) in the latter (see Table S4) For DyWO-1000, the intensity of the O-H absorption bands shows a dramatic growth upon air storage (Figure 9a).
The FT-IR spectrum of the as prepared and quenched in a desiccator HoWO-1000-desic sample manifests absorption bands at 823 cm, 850 cm (both asymmetric stretching vibration [43]), 962 cm and 993 cm (both symmetric stretching vibration [43]), 1614 cm (bending vibration of hydrogen bonded OH-group of the water molecule) and 3377 cm (stretching vibration of the OH-group) [26,49,50,51] (Figure 9b). The hydration of the sample upon storage in air leads to the disappearance of absorption bands at 850 cm, 962 cm and 993 cm, as well as to a shift in the position of the most intense peak from 823 cm to 819 cm. Disappearance of specific vibrational features in the range typical of internal stretching vibrations within the WO tetrahedron can be explained assuming that water molecules incorporated in the Ln(WO) framework, block the rocking-type motion of the coordination polyhedra and thus some vibrational bands become silent [9,43]. In addition, a significant increase in the intensity of the O-H absorption bands is observed at 1618 cm and 3377 cm, along with appearance of absorption band at 640 cm, characteristic for libration motion of water molecules [51] (Figure 9b). The FT-IR spectra of Yb(WO) (Figure S5) are similar to that of Ho(WO) and change in a similar way upon storage in air.
The observed differences in the water absorption capacity of Gd and heavy Ln (Ln = Dy, Ho, Yb) tungstates may be explained by the structural difference. Gd(WO) (monoclinic, sp. gr. C12/c1(15)) has a closely packed structure due to edge sharing GdO polyhedra. The heavier Ln tungstates (sp. gr. P12/a1(14)) have open structures with corner-sharing LnO and WO moieties (with the Ln–O–W bridges), which tend to accommodate additional HO molecules within empty intraframework cavities (Figure 4).
4. Conclusions
Effects of the lanthanoid cation type and annealing temperature on the phase preference, short- and long-range structures of Ln(WO) tungstates synthesized via the coprecipitation are elucidated in detail with a set of contemporary techniques, including synchrotron radiation-based X-ray diffraction, X-ray absorption spectroscopy, Raman and FT-IR spectroscopy, and simultaneous thermal analysis. The crystallization of initially amorphous precursors is initiated at 600–650 °C to afford crystalline powders with the monoclinic structures of either C12/c1(15) for Ln = Gd, and Dy or P12/a1(14) types for Yb(WO). For the Dy(WO) sample calcined at 1000 °C, an alternative monoclinic structure (sp. gr. P12/a1) emerges, which further becomes the dominant one for Ln = Ho, Yb. A repeated heating of Dy(WO) to 1000 °C induces an irreversible transformation P12/a1(14)→ C12/c1(15) therein. Meanwhile, holmium and ytterbium tungstates trihydrates Ho(WO)3HO and Yb(WO)3HO retain their crystal structures (sp. gr. P12/a1(14)).
The local structure of all well-crystallized compounds is composed of Ln lanthanoid cations and somewhat distorted WO anionic tetrahedra. The change in the space group preference of the monoclinic crystalline structure of Ln tungstates along the series Gd-Dy-Ho-Yb is governed by progressive changes in the Ln-O coordination shell. The high hygroscopicity of lanthanoid tungstates Ln(WO) (Ln = Dy, Ho, Yb) is rationalized on the basis of structural features of the monoclinic lattice (sp. gr. P12/a1).
Most essential features of phase preferences and hydration behavior found for heavy lanthanoid tungstates in the present study are summarized in Table 5.

Table 5.
Summary of structural features of heavy Ln tungstates under study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12070892/s1.
Author Contributions
Conceptualization, V.V.P.; methodology, V.V.P.; validation, V.V.P., A.P.M. and Y.V.Z.; formal analysis, A.A.Y., B.R.G., S.G.R. and E.V.K.; investigation, V.V.P., Y.V.Z., S.G.R., F.E.D., R.D.S., E.V.K., N.A.T., N.V.O. and I.V.S.; resources, V.V.P.; data curation, A.A.Y. and B.R.G.; writing—original draft preparation, V.V.P. and Y.V.Z.; writing—review and editing, V.V.P., Y.V.Z. and A.A.I.; visualization, A.A.Y., B.R.G., A.A.I., F.E.D. and E.V.K.; supervision, A.P.M.; project administration, A.P.M.; funding acquisition, A.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Federation represented by the Ministry of Science and Higher Education of the Russian Federation (Agreement No 075-15-2021-1352).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors thank O. N. Seregina, I. G. Rachenok, and K. V. Ponkratov for their help in experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Imanaka, N.; Köhler, J.; Tamura, S.; Adachi, G. Ion conducting behavior in (Lu1−xMx)2(WO4)3 solid solutions (M = Sm, Ho, Er) with the Sc2(WO4)3 type structure. Eur. J. Inorg. Chem. 2002, 1, 105–109. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, B. RE2(MO4)3:Ln3+ (RE = Y, La, Gd, Lu; M = W, Mo; Ln = Eu, Sm, Dy) microcrystals: Controlled synthesis, microstructure and tunable luminescence. CrystEngComm 2013, 15, 5694. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Deun, R.V. Rare earth tungstate and molybdate compounds—From 0D to 3D architectures. Chem. Soc. Rev. 2013, 42, 8835–8848. [Google Scholar] [CrossRef]
- Liu, J.; Kaczmarek, A.; Deun, R. Advances in tailoring luminescent rare-earth mixed inorganic materials. Chem. Soc. Rev. 2018, 47, 7225–7238. [Google Scholar] [CrossRef]
- Vickery, R.C. Studies of the rare-earth tungstates. J. Chem. Soc. 1949, 2501–2505. [Google Scholar] [CrossRef]
- Borchardt, H. Rare Earth Tungstates and 1:1 Oxytungstates. J. Chem. Phys. 1963, 39, 504–511. [Google Scholar] [CrossRef]
- Nassau, K.; Levinstein, H.J.; Loiacono, G.M. A comprehensive study of trivalent tungstates and molybdates of the type L2(MO4)3. J. Phys. Chem. Solids 1965, 26, 1805–1816. [Google Scholar] [CrossRef]
- Sleight, A.W. Negative Thermal Expansion. MRS Proc. 2002, 755, 106. [Google Scholar] [CrossRef]
- Sumithra, S.; Umarji, A.M. Role of crystal structure on the thermal expansion of Ln2W3O12 (Ln = La, Nd, Dy, Y, Er and Yb). Solid State Sci. 2004, 6, 1313–1319. [Google Scholar] [CrossRef]
- Liu, H.; Sun, W.; Zhang, Z.; Lovings, L.; Lind, C. Thermal Expansion Behavior in the A2M3O12 Family of Materials. Solids 2021, 2, 87–107. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Pontón, P.I.; Romao, C.P.; Moreira, T.; White, M.A. Negative and Near-Zero Thermal Expansion in A2M3O12 and Related Ceramic Families: A Review. Front. Mater. 2021, 8, 741560. [Google Scholar] [CrossRef]
- Templeton, D.H.; Zalkin, A. Crystal structure of europium tungstate. Acta Cryst. 1963, 16, 762–766. [Google Scholar] [CrossRef] [Green Version]
- Gärtner, M.; Abeln, D.; Pring, A.; Wilde, M.; Reller, A. Synthesis, Structure, and Reactivity of Novel Lanthanum Tungstates. J. Solid State Chem. 1994, 111, 128–133. [Google Scholar] [CrossRef]
- Gressling, T.; Müller-Buschbaum, H. Zur Kristallstruktur von Ce2(WO4)3/On the Crystal Structure of Ce2(WO4)3. Z. Naturforsch. B 1995, 50, 1513–1516. [Google Scholar] [CrossRef]
- Shen, R.; Wang, C.; Wang, T.; Dong, C.; Chen, X.; Liang, J. Crystal Structures of Dy2(WO4)3 and GdY(WO4)3. Rare Met. 2003, 22, 49–54. [Google Scholar]
- Weil, M.; Stöger, B.; Aleksandrov, L. Nd2(WO4)3. Acta Crystallogr. E 2009, 65, i45. [Google Scholar] [CrossRef] [Green Version]
- dos Passos, R.H.D.; de Souza, C.P.; Bernard-Nicod, C.; Leroux, C.; Arab, M. Structural and electrical properties of cerium tungstate: Application to methane conversion. Ceram. Int. 2020, 46, 8021–8030. [Google Scholar] [CrossRef]
- Sabalisck, N.P.; de Cos, G.G.; González-Silgo, C.; Guzmán-Afonso, C.; Lavín, V.; López-Solano, J.; Martín-Mateos, I.T.; Mestres, L.; Mujica, A.; Santamaría-Pérez, D.; et al. Role of rare earth sites and vacancies in the anomalous compression of modulated scheelite tungstates RE2(WO4)3. Phys. Rev. Mater. 2021, 5, 123601. [Google Scholar] [CrossRef]
- Pestereva, N.N.; Vyatkin, I.A.; Lopatin, D.A.; Guseva, A.F. Nature of ionic conductivity of lanthanoid tungstates with imperfect scheelite structure. Russ. J. Electrochem. 2016, 52, 1082–1089. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, Y.; Peng, J.; Wu, M.; Chen, D.; Hu, Z.; Kiyanagi, R.; Fieramosca, J.; Short, S.; Jorgensen, J. Thermal expansion properties of A2(MO4)3 (A = Ho and Tm; M = W and Mo). Solid State Sci. 2008, 10, 321–325. [Google Scholar] [CrossRef]
- Lahoz, F.; Sabalisck, N.P.; Cerdeiras, E.; Mestres, L. Nano-to millisecond lifetime luminescence properties in Ln2(WO4)3 (Ln = La, Ho, Tm and Eu) microcrystalline powders with different crystal structures. J. Alloys Compd. 2015, 649, 1253–1259. [Google Scholar] [CrossRef]
- Kol’tsova, T.N. X-ray diffraction study of Y2W3O12·3H2O. Inorg. Mater. 2001, 37, 1175–1177. [Google Scholar] [CrossRef]
- Pontón, P.I.; Prisco, L.P.; Dosen, A.; Faro, G.S.; de Abreu, M.A.; Marinkovic, B.A. Co-precipitation synthesis of Y2W3O12 submicronic powder. Ceram. Int. 2017, 43, 4222–4228. [Google Scholar] [CrossRef]
- Yamazoe, S.; Hitomi, Y.; Shishido, T.; Tanaka, T. XAFS Study of Tungsten L1- and L3-Edges: Structural Analysis of WO3 Species Loaded on TiO2 as a Catalyst for Photo-oxidation of NH3. J. Phys. Chem. C 2008, 112, 6869–6879. [Google Scholar] [CrossRef]
- Timoshenko, J.; Anspoks, A.; Kalinko, A.; Kuzmin, A. Local structure of nanosized tungstates revealed by evolutionary algorithm. Phys. Status Solidi A 2015, 212, 265–273. [Google Scholar] [CrossRef]
- Kuriakose, S.; H, H.; Jose, A.; John, M.; Varghese, T. Structural and optical characterization of lanthanum tungstate nanoparticles synthesized by chemical precipitation route and their photocatalytic activity. Opt. Mater. 2020, 99, 109571. [Google Scholar] [CrossRef]
- Meng, Q.; Hua, R.; Chen, B.; Tian, Y.; Lu, S.; Sun, L. Study on Luminescent Properties of Eu3+ Doped Gd2WO6, Gd2W2O9 and Gd2(WO4)3 Nanophosphors Prepared by Co-Precipitation. J. Nanosci. Nanotechnol. 2011, 11, 182–188. [Google Scholar] [CrossRef]
- Popov, V.V.; Menushenkov, A.P.; Yaroslavtsev, A.A.; Zubavichus, Y.V.; Gaynanov, B.R.; Yastrebtsev, A.A.; Leshchev, D.S.; Chernikov, R.V. Fluorite-pyrochlore phase transition in nanostructured Ln2Hf2O7 (Ln = La-Lu). J. Alloys Compd. 2016, 689, 669–679. [Google Scholar] [CrossRef]
- Popov, V.V.; Menushenkov, A.P.; Gaynanov, B.R.; Zubavichus, Y.V.; Svetogorov, R.D.; Yastrebtsev, A.A.; Pisarev, A.A.; Arzhatkina, L.A.; Ponkratov, K.V. Features of formation and evolution of crystal and local structures in nanocrystalline Ln2Zr2O7 (Ln = La–Tb). J. Phys. Conf. Ser. 2017, 941, 012079. [Google Scholar] [CrossRef] [Green Version]
- Popov, V.V.; Menushenkov, A.P.; Ivanov, A.A.; Gaynanov, B.R.; Yastrebtsev, A.A.; d’Acapito, F.; Puri, A.; Castro, G.R.; Shchetinin, I.V.; Zheleznyi, M.V.; et al. Comparative analysis of long- and short-range structures features in titanates Ln2Ti2O7 and zirconates Ln2Zr2O7 (Ln = Gd, Tb, Dy) upon the crystallization process. J. Phys. Chem. Solids 2019, 130, 144–153. [Google Scholar] [CrossRef]
- Popov, V.V.; Menushenkov, A.P.; Yastrebtsev, A.A.; Molokova, A.Y.; Rudakov, S.G.; Svetogorov, R.D.; Tsarenko, N.A.; Ponkratov, K.V.; Ognevskaya, N.V.; Seregina, O.N. The effect of the synthesis conditions on the structure and phase transitions in Ln2(MoO4)3. Solid State Sci. 2021, 112, 106518. [Google Scholar] [CrossRef]
- Popov, V.V.; Menushenkov, A.P.; Yastrebtsev, A.A.; Rudakov, S.G.; Ivanov, A.A.; Gaynanov, B.R.; Svetogorov, R.D.; Khramov, E.V.; Zubavichus, Y.V.; Molokova, A.Y.; et al. Multiscale study on the formation and evolution of the crystal and local structures in lanthanoid tungstates Ln2(WO)4)3. J. Alloys Compd. 2022, 910, 164922. [Google Scholar] [CrossRef]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Tech. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Chernyshov, A.; Veligzhanin, A.; Zubavichus, Y. Structural Materials Science end-station at the Kurchatov Synchrotron Radiation Source: Recent instrumentation upgrades and experimental results. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical approaches to X-ray absorption fine structure. Rev. Mod. Phys. 2000, 72, 621–654. [Google Scholar] [CrossRef]
- Klementev, K.V. Extraction of the fine structure from X–ray absorption spectra. J. Phys. D Appl. Phys. 2001, 34, 209–217. [Google Scholar] [CrossRef]
- Rehr, J.J.; Kas, J.J.; Vila, F.D.; Prange, M.P.; Jorissen, K. Parameter-free calculations of X-ray spectra with FEFF9. Phys. Chem. Chem. Phys. 2010, 12, 5503–5513. [Google Scholar] [CrossRef]
- Evdokimov, A.A.; Efremov, V.A.; Trunov, V.K.; Kleinman, I.A.; Dzurinskii, B.F. Rare Earth Elements Compounds. Molybdates, Tungstates; Nauka (Science): Moscow, Russia, 1991. (In Russian) [Google Scholar]
- Wu, M.Y.; Jia, Y.; Sun, Q. Effects of A3+ cations on hydration in A2M3O12 family materials: A first-principles study. Comput. Mater. Sci. 2016, 111, 28–33. [Google Scholar] [CrossRef]
- Shmurak, S.Z.; Kedrov, V.V.; Kiselev, A.P.; Fursova, T.N.; Zver’kova, I.I.; Khasanov, S.S. Spectral and Structural Characteristics of (Lu1-xEux)2(WO4)3 Tungstates. Phys. Solid State 2019, 61, 2117–2129. [Google Scholar] [CrossRef]
- Richard, A.P.; Edwards, D.D. Subsolidus phase relations and crystal structures of the mixed-oxide phases in the In2O3–WO3 system. J. Solid State Chem. 2004, 177, 2740–2748. [Google Scholar] [CrossRef]
- Sumithra, S.; Umarji, A.M. Hygroscopicity and bulk thermal expansion in Y2W3O12. Mater. Res. Bull. 2005, 40, 167–176. [Google Scholar] [CrossRef]
- Luo, Q.H.; Howell, R.C.; Dankova, M.; Bartis, J.; Williams, C.W.; Horrocks, W.D.; Young, V.G.; Rheingold, A.L.; Francesconi, L.C.; Antonio, M.R. Coordination of Rare-Earth Elements in Complexes with Monovacant Wells–Dawson Polyoxoanions. Inorg. Chem. 2001, 40, 1894–1901. [Google Scholar] [CrossRef]
- Sabalisck, N.P.; Lopez-Solano, J.; Guzman-Afonso, C.; Santamaria-Perez, D.; Gonzalez-Silgo, C.; Mujica, A.; Munoz, A.; Rodriguez-Hernandez, P.; Radescu, S.; Vendrell, X.; et al. Effect of pressure on La2(WO4)3 with a modulated scheelite-type structure. Phys. Rev. B 2014, 89, 174112. [Google Scholar] [CrossRef] [Green Version]
- Burcham, L.J.; Wachs, I.E. Vibrational analysis of the two non-equivalent, tetrahedral tungstate (WO4) units in Ce2(WO4)3 and La2(WO4)3. Spectrochim. Acta A 1998, 54, 1355–1368. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Rahimi-Nasrabadi, M.; Ganjali, M.; Sadeghpour, K.M.; Norouzi, P.; Faridbod, F. Facile and Effective Synthesis of Praseodymium Tungstate Nanoparticles through an Optimized Procedure and Investigation of Photocatalytic Activity. Open Chem. J. 2017, 15, 129–138. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Ganjali, M.R.; Banan, A.R.; Ahmadi, F. Synthesis procedure optimization and characterization of europium (III) tungstate nanoparticles. J. Mol. Struct. 2014, 1074, 85–91. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Yin, M.; Liu, Y.; Mei, L.; Molokeev, M.S.; Huang, Z.; Fang, M. Preparation, crystal structure and up-conversion luminescence of Er3+, Yb3+ co-doped Gd2(WO4)3. RSC Adv. 2015, 5, 73077–73082. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).