A Novel Nanostructured Mullite Feedstock for Environmental Barrier Coatings via Atmosphere Plasma Spraying
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Process
2.3. Characterization Methods
3. Results and Discussion
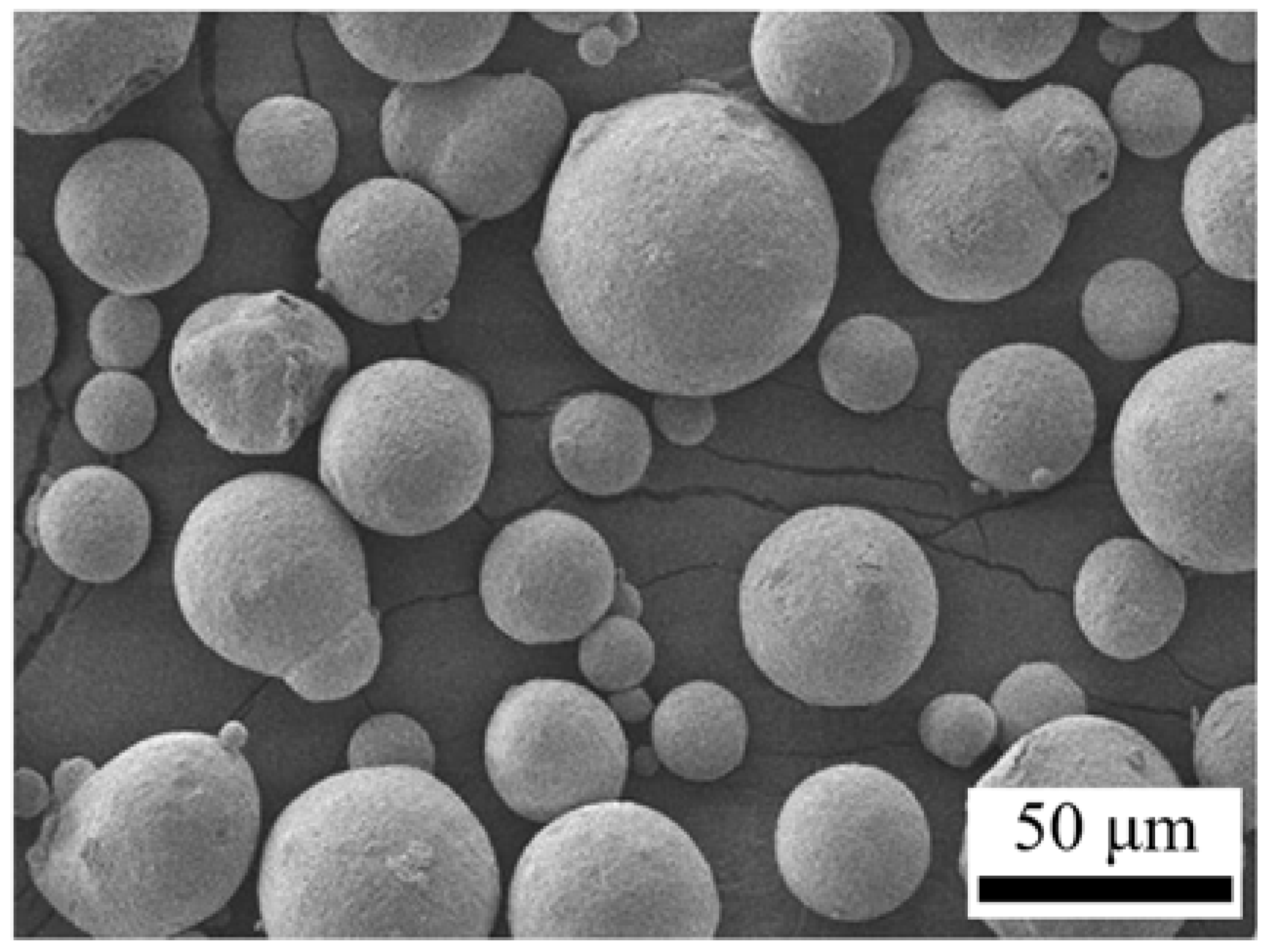
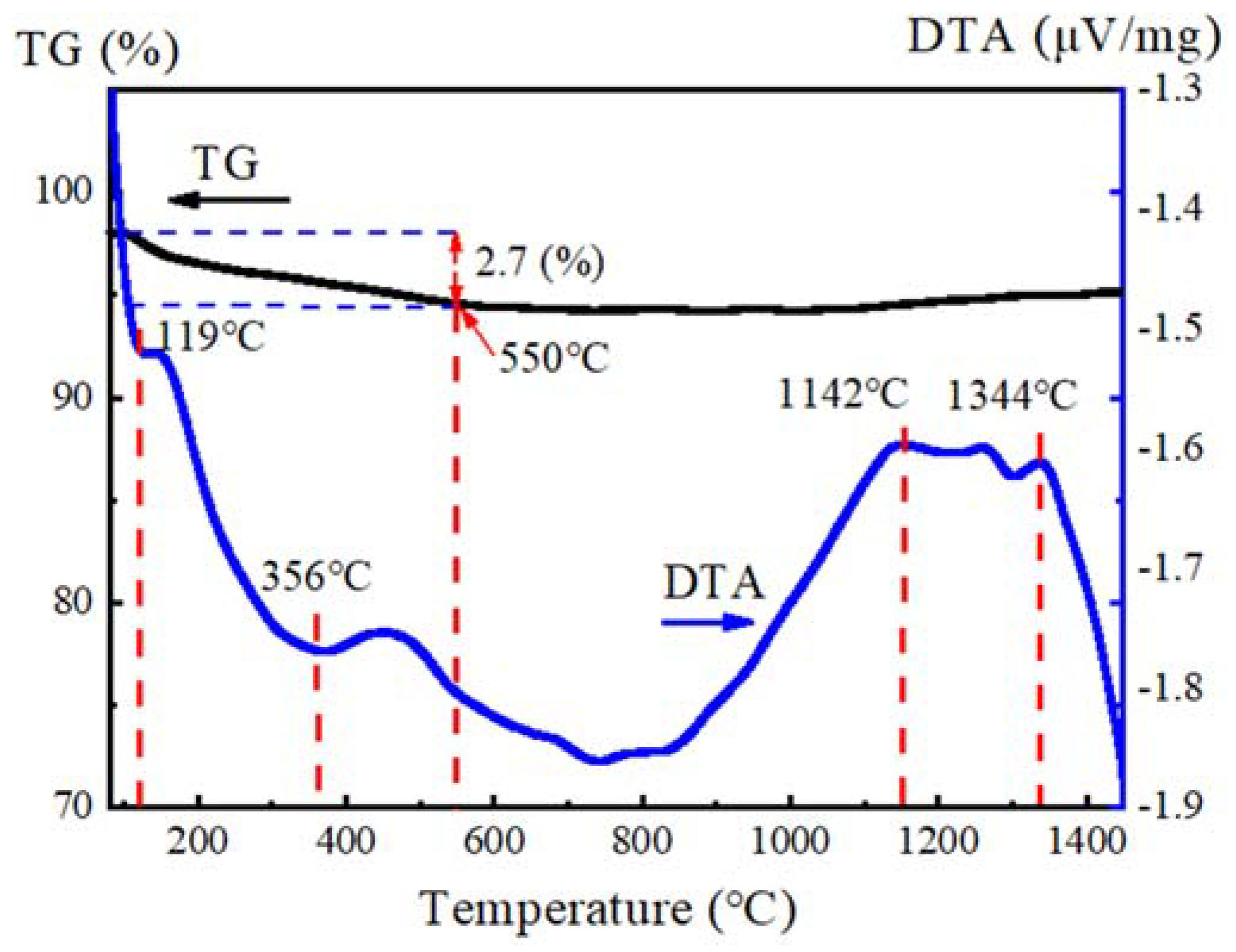
3.1. Characteristic of Spray-Dried Powders
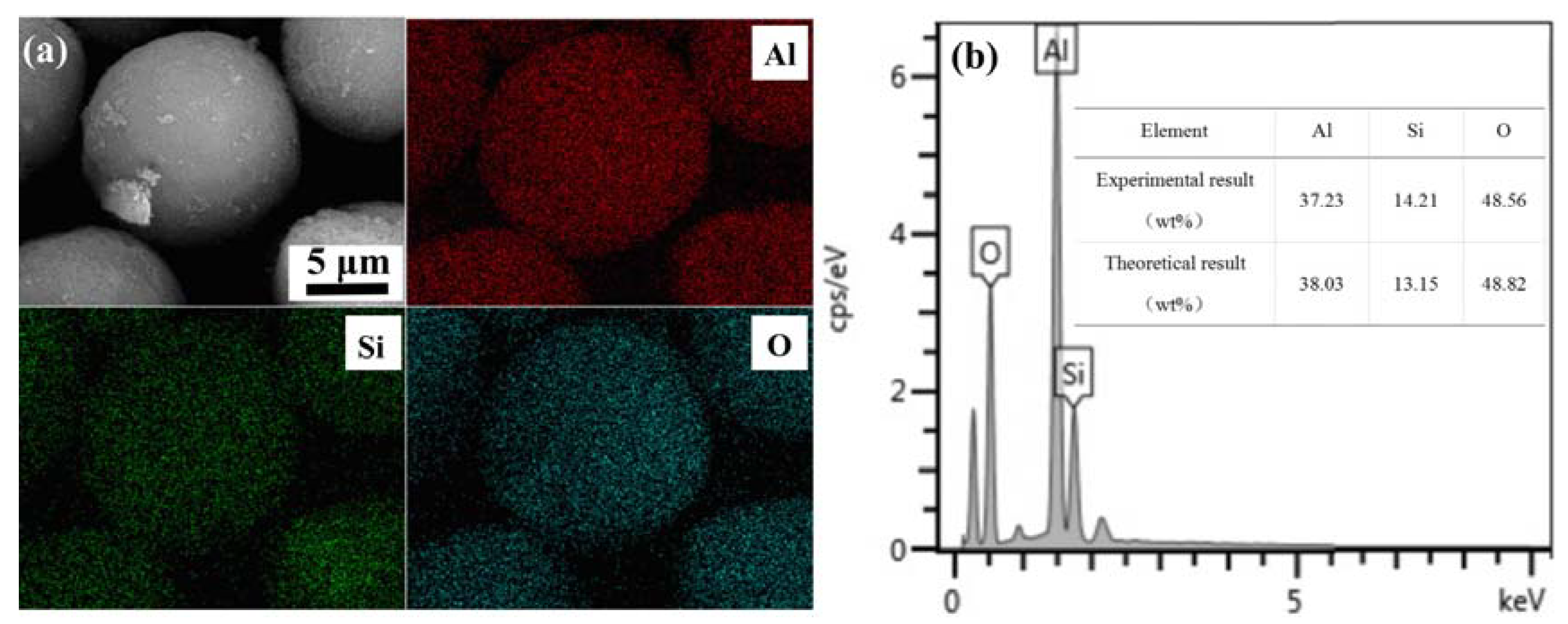
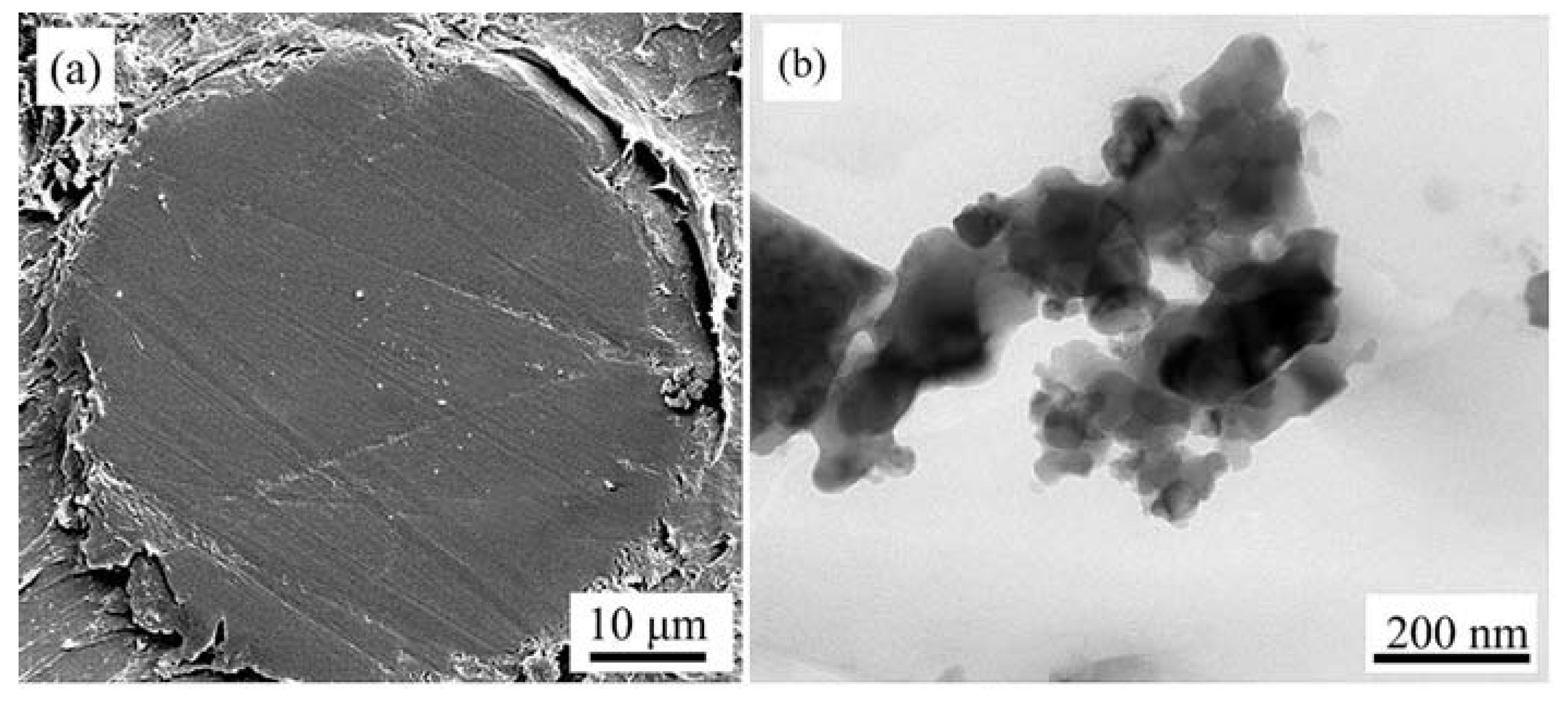
3.2. Characterization of Flame Spheroidized Powders
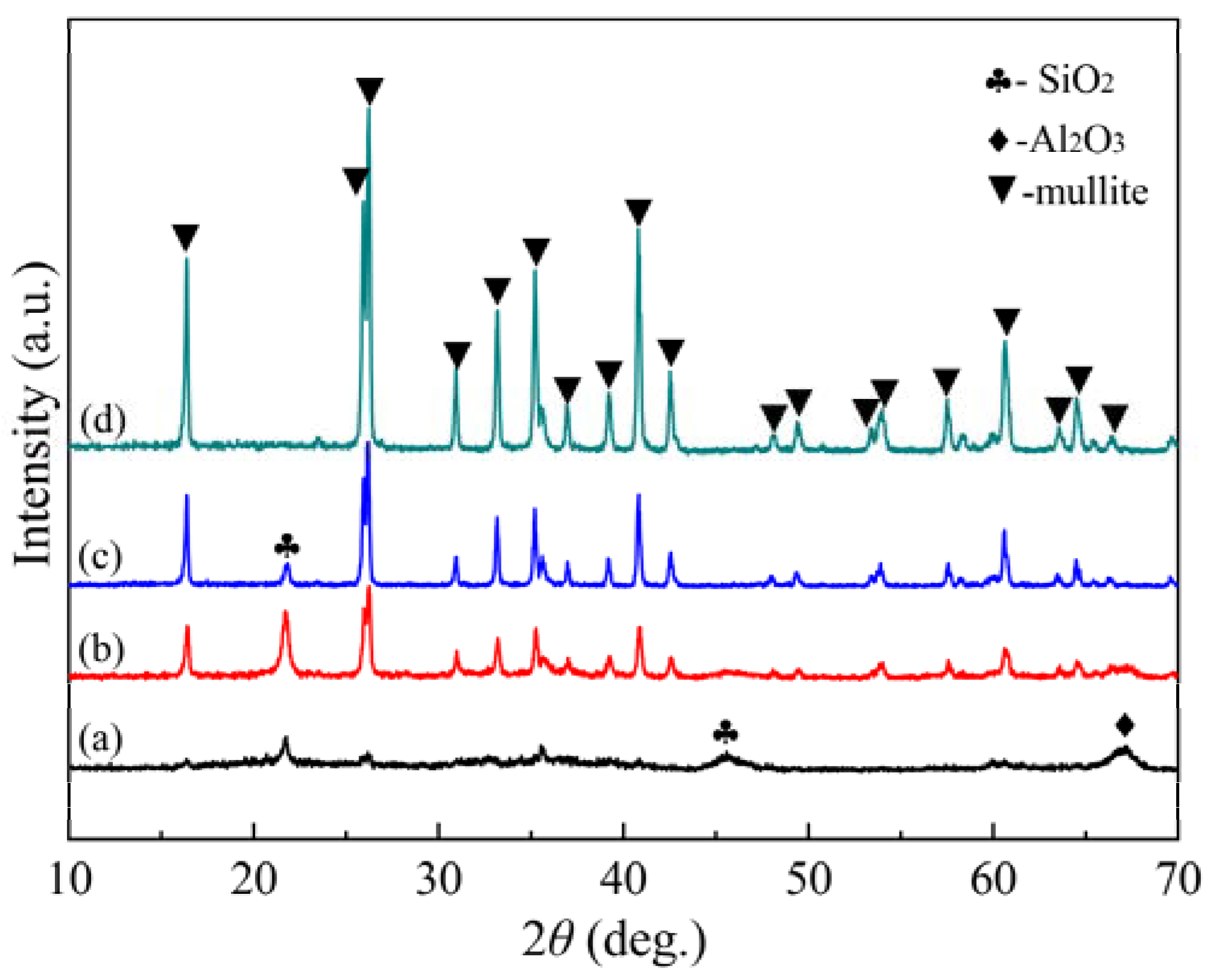
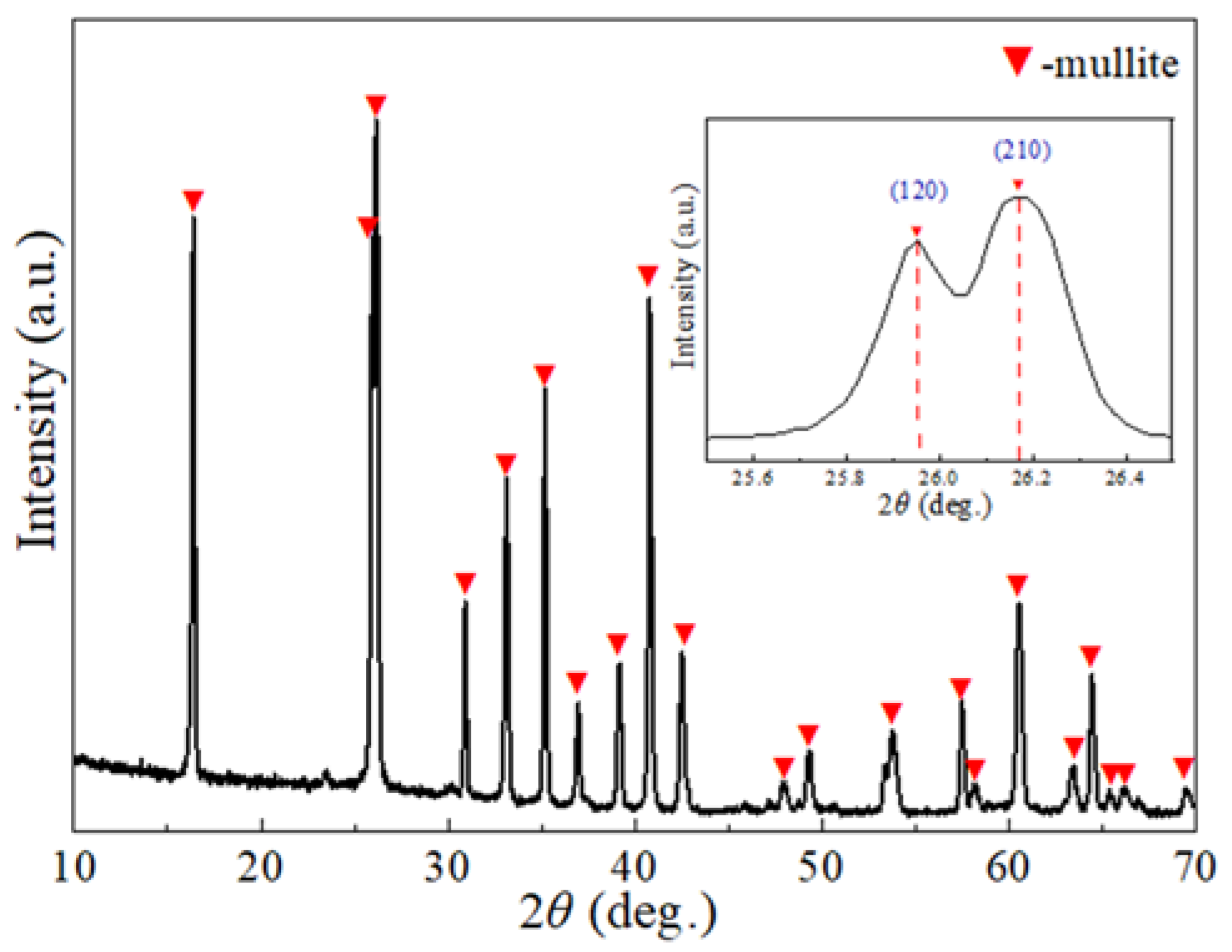
3.3. Phase Composition of Mullite Coating
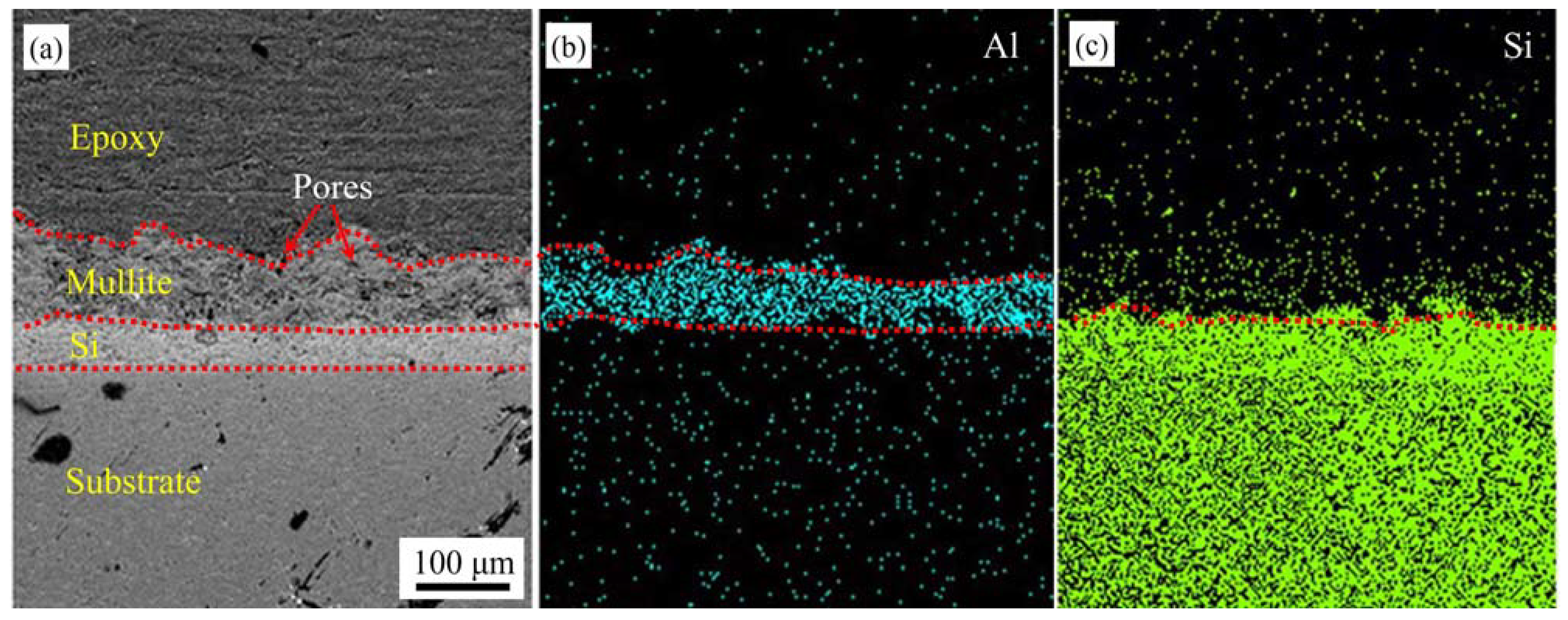
3.4. Morphology of Nanostructured Environmental Barrier Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.N. Current Status of Environmental Barrier Coatings for Si-Based Ceramics. Surf. Coat. Technol. 2000, 133–134, 1–7. [Google Scholar] [CrossRef]
- Basu, S.N.; Sarin, V.K. Thermal and Environmental Barrier Coatings for Si-Based Ceramics. Compr. Hard Mater. 2014, 2, 469–489. [Google Scholar]
- Ramasamy, S.; Tewari, S.N.; Lee, K.N.; Bhatt, R.T.; Fox, D.S. Slurry Based Multilayer Environmental Barrier Coatings for Silicon Carbide and Silicon Nitride Ceramics—I. Processing. Surf. Coat. Technol. 2010, 205, 258–265. [Google Scholar] [CrossRef]
- Bhatt, R.T.; Choi, S.R.; Cosgriff, L.M.; Fox, D.S.; Lee, K.N. Impact Resistance of Environmental Barrier Coated SiC/SiC Composites. Mater. Sci. Eng. A 2008, 476, 8–19. [Google Scholar] [CrossRef]
- Eaton, H.E.; Linsey, G.D. Accelerated Oxidation of SiC CMC’s by Water Vapor and Protection via Environmental Barrier Coating Approach. J. Eur. Ceram. Soc. 2002, 22, 2741–2747. [Google Scholar] [CrossRef]
- Ueno, S.; Ohji, T.; Lin, H.T. Corrosion and Recession of Mullite in Water Vapor Environment. J. Eur. Ceram. Soc. 2008, 28, 431–435. [Google Scholar] [CrossRef]
- Stolzenburg, F.; Kenesei, P.; Almer, J.; Lee, K.N.; Johnson, M.T.; Faber, K.T. The Influence of Calcium- Magnesium-Aluminosilicate Deposits on Internal Stresses in Yb2Si2O7 Multilayer Environmental Barrier Coatings. Acta Mater. 2016, 105, 189–198. [Google Scholar] [CrossRef]
- Bakan, E.; Marcano, D.; Zhou, D.; Sohn, Y.J.; Mauer, G.; Vaßen, R. Yb2Si2O7 Environmental Barrier Coatings Deposited by Various Thermal Spray Techniques: A Preliminary Comparative Study. J. Therm. Spray Technol. 2017, 26, 1011–1024. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Liu, Y.-C.; Song, P.; Li, W.-X.; Yang, G.; Liu, B. Recent progress in thermal/environmental barrier coatings and their corrosion resistance. Rare Met. 2020, 5, 498–512. [Google Scholar] [CrossRef]
- Laura, R.T.; Nitin, P.P. Towards multifunctional thermal environmental barrier coatings (TEBCs) based on rare-earth pyrosilicate solid-solution ceramics. Scripta Mater. 2018, 154, 111–117. [Google Scholar]
- Botero, C.A.; Jimenez-Pique, E.; Martin, R.; Kulkarni, T.; Sarin, V.K.; Llanes, L. Influence of Temperature and Hot Corrosion on the Micro-Nanomechanical Behavior of Protective Mullite EBCs. Int. J. Refract. Met. Hard Mater. 2015, 49, 383–391. [Google Scholar] [CrossRef]
- Price, J.R.; van Roode, M.; Stala, C. Ceramic Oxide-Coated Silicon Carbide for High Temperature Corrosive Environments. Key Eng. Mater. 1992, 72, 71–84. [Google Scholar] [CrossRef]
- Lee, K.N.; Miller, R.A.; Jacobson, N.S. New Generation of Plasma-Sprayed Mullite Coatings on Silicon Carbide. J. Am. Ceram. Soc. 1995, 78, 705–710. [Google Scholar] [CrossRef]
- Garcia, E.; Mesquita-Guimaraes, J.; Miranzo, P.; Osendi, M.I.; Wang, Y.; Lima, R.S.; Moreau, C. Mullite and Mullite/ZrO2-7wt.%Y2O3 Powders for Thermal Spraying of Environmental Barrier Coatings. J. Therm. Spray Technol. 2010, 19, 286–293. [Google Scholar] [CrossRef][Green Version]
- Cojocaru, C.V.; Levesque, D.; Moreau, C.; Lima, R.S. Performance of Thermally Sprayed Si/Mullite/BSAS Environmental Barrier Coatings Exposed to Thermal Cycling in Water Vapor Environment. Surf. Coat. Technol. 2013, 216, 215–223. [Google Scholar] [CrossRef]
- Garcia, E.; Mesquita-Guimaraes, J.; Miranzo, P.; Osendi, M.I. Crystallization Studies in Mullite and Mullite-YSZ Beads. J. Eur. Ceram. Soc. 2010, 30, 2003–2008. [Google Scholar] [CrossRef]
- Mah, T.I.; Mazdiyasni, K.S. Mechanical Properties of Mullite. J. Am. Ceram. Soc. 2010, 66, 699–703. [Google Scholar] [CrossRef]
- Hamidouche, M.; Bouaouadja, N.; Olagnon, C.; Fantozzi, G. Thermal shock behaviour of mullite ceramic. Ceram. Int. 2003, 29, 599–609. [Google Scholar] [CrossRef]
- Lee, K.N.; Miller, R.A. Development and Environmental Durability of Mullite and Mullite/YSZ Dual Layer Coatings for SiC and Si3N4 Ceramics. Surf. Coat. Technol. 1996, 86, 142–148. [Google Scholar] [CrossRef]
- Latzel, S.; VaBen, R.; Stover, D. New Environmental Barrier Coating System on Carbon-Fiber Reinforced Silicon Carbide Composites. J. Therm. Spray Technol. 2005, 14, 268–272. [Google Scholar] [CrossRef]
- Mesquita-Guimaraes, J.; García, E.; Miranzo, P.; Osendi, M.I.; Cojocaru, C.V.; Lima, S.R. Mullite-YSZ Multilayered Environmental Barrier Coatings Tested in Cycling Conditions under Water Vapor Atmosphere. Surf. Coat. Technol. 2012, 209, 103–109. [Google Scholar] [CrossRef]
- Nasiri, N.A.; Patra, N.; Horlait, D.; Jayaseelan, D.D.; Lee, W.E. Thermal Properties of Rare-Earth Monosilicates for EBC on Si-Based Ceramic Composites. J. Am. Ceram. Soc. 2016, 99, 589–596. [Google Scholar] [CrossRef]
- Harder, B.J.; Zhu, D.; Schmitt, M.P.; Wolfe, D.E. Microstructural Effects and Properties of Non-line-of-Slight Coating Processing via Plasma Spray-Physical Vapor Depositon. J. Therm. Spray Technol. 2017, 26, 1052–1061. [Google Scholar] [CrossRef]
- Li, J.F.; Liao, H.; Wang, X.Y.; Coddet, C.; Chen, H.; Ding, C.X. Plasma spraying of nanostructured partially yttria stabilized zirconia powders. Thin Solid Film. 2004, 460, 101–115. [Google Scholar] [CrossRef]
- Cojocaru, C.V.; Wang, Y.; Moreau, C.; Lima, R.S.; Mesquita-Guimarães, J.; Garcia, E.; Miranzo, P.; Osendi, M.I. Mechanical Behavior of Air Plasma-Sprayed YSZ Functionally Graded Mullite Coatings Investigated via Instrumented Indentation. J. Therm. Spray Technol. 2011, 20, 100–107. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.; Li, J.; Guo, H.; Xu, H. Microstructure and High-Temperature Oxidation Behavior of Plasma-Sprayed Si/Yb2SiO5 Environmental Barrier Coatings. Chin. J. Aeronaut. 2019, 32, 1994–1999. [Google Scholar] [CrossRef]
- Lima, R.S.; Kucuk, A.; Berndt, C.C. Evaluation of Microhardness and Elastic Modulus of Thermally Sprayed Nanostructured Zirconia Coatings. Surf. Coat. Technol. 2001, 135, 166–172. [Google Scholar] [CrossRef]
- Ahmed, I.; Bergman, T.L. Thermal Modeling of Plasma Spray Deposition of Nanostructured Ceramics. J. Therm. Spray Technol. 1999, 8, 315–322. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, M.; Huang, J.; Ding, C. Vacuum-Plasma Sprayed Nanostructured Titanium Oxide Films. J. Therm. Spray Technol. 1999, 8, 219–222. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Wang, M.; Wang, S.; Xiao, T.D.; Strutt, P.R. Abrasive Wear Characteristics of Plasma Sprayed Nanostructured Alumina/Titania Coatings. Wear 2000, 237, 176–185. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Sun, X.G.; He, J.Q.; Pan, Z.Y.; Yu, L.L. Preparation and characterization of nanostructured La2Zr2O7 feedstock used for plasma spraying. Powder Technol. 2011, 212, 267–277. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Yang, Y. Preparation and characterization of re modified nanocrystalline Al2O3/13wt%TiO2 feedstock for plasma spraying. J. Nanosci. Nanotechnol. 2009, 9, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Imose, M.; Takano, Y.; Yoshinaka, M.; Hirota, K.; Yamaguchi, O. Novel Synthesis of Mullite Powder with High Surface Area. J. Am. Ceram. Soc. 1998, 81, 1537–1540. [Google Scholar] [CrossRef]
- Gören, R.; Özgür, C. Rapid synthesis of mullite powders. J. Ceram. Process. Res. 2012, 13, 262–266. [Google Scholar]
- Klug, F.J.; Prochazka, S. Alumina-Silica Phase Diagram in the Mullite Region. J. Am. Ceram. Soc. 1987, 70, 750–759. [Google Scholar] [CrossRef]
- Vargas, F.; Restrepo, E.; Rodriguez, J.E.; Vargas, F.; Arbelaez, L.; Caballero, P.; Arias, J.; Lopez, E.; Latorre, G.; Duarte, G. Solid-state synthesis of mullite from spent catalysts for manufacturing refractory brick coatings. Ceram. Int. 2018, 44, 3556–3562. [Google Scholar] [CrossRef]
- He, K.; Chen, N.; Wang, C.; Wei, L.; Chen, J. Method for Determining Crystal Grain Size by X-ray Diffraction. Cryst. Res. Technol. 2018, 53, 1700157. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological Characterization of X-ray Diffraction Methods at Different Acquisition Geometries for Determination of Crystallite Size in Nano-Scale Materials. Mater. Charact. 2013, 85, 111–123. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y.; Liu, M.; Deng, C.; Li, Y.; Wang, Y.; Zhang, X. Bonding strength and thermal conductivity of novel nanostructured La2(Zr0.75Ce0.25)2O7/8YSZ coatings. Appl. Surf. Sci. 2019, 481, 460–465. [Google Scholar] [CrossRef]
- Guo, H.B.; Murakami, H.; Kuroda, S. Microstructures and properties of plasma sprayed segmented thermal barrier coatings. J. Am. Ceram. Soc. 2006, 89, 1432–1439. [Google Scholar] [CrossRef]
- Harder, B.J.; Almer, J.D.; Weyant, C.M.; Lee, K.N.; Faber, K.T. Residual Stress Analysis of Mullite Environmental Barrier Coatings. J. Am. Ceram. Soc. 2009, 92, 452–459. [Google Scholar] [CrossRef]


| Spray Current/A | Spray Voltage/V | Flow Rate of Primary Gas/SCFH * | Flow Rate of Carrier Gas/SCFH | Powder Giving Rate/g/min | Spray Distance/cm |
|---|---|---|---|---|---|
| 550 | 55 | 120 | 12 | 20 | 40 |
| Spray Current/A | Spray Voltage/V | Flow Rate of Primary Gas/SCFH | Flow Rate of Carrier Gas/SCFH | Powder Giving Rate/g/min | Spray Distance/cm | |
|---|---|---|---|---|---|---|
| Si | 450 | 45 | 80 | 12 | 35 | 12 |
| mullite | 550 | 55 | 120 | 12 | 35 | 12 |
| T (°C) | Bulk Density (g/cm3) | Tap Density (g/cm3) | The Angle of Repose (°) | Flowability (s/50 g) |
|---|---|---|---|---|
| 1200 | 0.81 | 1.03 | 32 | 222.2 |
| 1300 | 0.87 | 1.11 | 32 | 192.3 |
| 1400 | 0.96 | 1.15 | 30 | 159.6 |
| 1500 | 1.07 | 1.26 | 30 | 123.5 |
| T (°C) | 1200 | 1300 | 1400 | 1500 |
|---|---|---|---|---|
| Grain size (nm) | 18 | 19 | 43 | 52 |
| Grain Size (nm) | Bulk Density (g/cm3) | Tap Density (g/cm3) | The Angle of Repose (°) | Flowability (s/50 g) |
|---|---|---|---|---|
| 48 | 1.21 | 1.39 | 28 | 88.7 |
| Contribution | Layer Thickness (µm) | Porosity (%) | Crack | References |
|---|---|---|---|---|
| Nanostructured mullite | 60 | 6.5 | No cracks | This work |
| Fused or sintered mullite purchased from commercial vendors | 200–400 | Obvious porosity | Obvious cracks | K.N. Lee et al. [13]. |
| #1020 mullite, d50 30 µm (Saint-Gobain, Worchester, MA, USA) | 50 | 11 | No cracks in mullite coating, 15 vertical cracks/cm in BSAS layer (BSAS/mullite/Si) | V. Cojocaru et al. [15]. |
| 1–49 mullite powder (Praxair Wiggensbach, Germany) | 800/150 | Not mention | Through-thickness cracking 1.8–2.9 cracks/mm | S. Latzel et al. [20]. |
| Spray-dried and freeze-granulated mullite (both are porous-type structure) | 50–100 | Spray-dried 9 and freeze-granulated 30 | Periodically spaced vertical cracks | C.V. Cojocaru et al. [25]. |
| Mullite powder, HC Stark, Karlsruhe, Germany | 200 | 11 | Through-thickness cracking between BSAS and mullite coating was evident (BSAS/mullite/Si) | B.J. Harder et al. [41]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Gong, X.; Liu, S.; Jia, J.; Zhang, X.; Wang, L.; Yan, M.; Wang, Y. A Novel Nanostructured Mullite Feedstock for Environmental Barrier Coatings via Atmosphere Plasma Spraying. Crystals 2022, 12, 726. https://doi.org/10.3390/cryst12050726
Xiao F, Gong X, Liu S, Jia J, Zhang X, Wang L, Yan M, Wang Y. A Novel Nanostructured Mullite Feedstock for Environmental Barrier Coatings via Atmosphere Plasma Spraying. Crystals. 2022; 12(5):726. https://doi.org/10.3390/cryst12050726
Chicago/Turabian StyleXiao, Fei, Xue Gong, Saiyue Liu, Jin Jia, Xiaodong Zhang, Lan Wang, Mufu Yan, and You Wang. 2022. "A Novel Nanostructured Mullite Feedstock for Environmental Barrier Coatings via Atmosphere Plasma Spraying" Crystals 12, no. 5: 726. https://doi.org/10.3390/cryst12050726
APA StyleXiao, F., Gong, X., Liu, S., Jia, J., Zhang, X., Wang, L., Yan, M., & Wang, Y. (2022). A Novel Nanostructured Mullite Feedstock for Environmental Barrier Coatings via Atmosphere Plasma Spraying. Crystals, 12(5), 726. https://doi.org/10.3390/cryst12050726






