Abstract
Ultrathin magnetic materials with room-temperature ferromagnetism/ferrimagnetism hold great potential in spintronic applications. In this work, we report the successful controllable growth of Fe3O4 thin films using a facile chemical vapor deposition method. Room-temperature ferrimagnetism was maintained in the as-grown Fe3O4 thin films down to 4 nm. Raman spectroscopy, X-ray diffraction and X-ray photoelectron spectroscopy analysis were conducted to reveal the structure and quality of the Fe3O4 film. Magnetization measurement showed ferrimagnetic hysteresis loops in all Fe3O4 thin films. A saturation magnetization of 752 emu/cm3 was observed for the 4 nm Fe3O4 film, which was higher than that of bulk Fe3O4 materials (480 emu/cm3). Additionally, the Verwey transition at ~120 K was visible for the Fe3O4 thin films. This work provides an alternative method of synthesizing ferrimagnetic ultrathin films for electronic, spintronic, and memory device applications.
1. Introduction
Room-temperature ferromagnetic/ferrimagnetic thin films have greatly promoted the development of electronic devices and spintronic devices [1,2,3]. The recent emergence of two-dimensional magnetic materials provides possibilities for new physics and new devices [4,5]. Several atomically thin 2D magnetic materials, e.g., CrI3 [6,7], Cr2Ge2Te6 [8], Fe3GeTe2 [9,10] and Cr2S3 [11], have been discovered. However, the Curie temperature of most 2D magnetic materials is still far below room temperature. Another alternative approach to obtain room-temperature ferromagnetic/ferrimagnetic films is to grow non-layered ultrathin materials with maintained magnetic properties.
Magnetite (Fe3O4) has been widely studied due to its half metallicity, high Curie temperature (TC = 860 K) and metal–insulator transitions (Verwey transition, TV ≈ 120 K) [12,13,14]. These properties make Fe3O4 a superior candidate for spintronics devices [15,16,17,18,19]. Above TV, Fe3O4 possesses the cubic-inverse-spinel structure with lattice constants a = 8.3967 Å and belongs to the space group Fd-3m [20]. In the crystal structure of Fe3O4, oxygen ions are closely packed in a cubic arrangement, and Fe ions fill in the interstices. There are two types of interstices: a tetrahedral interstice (the Fe ion is surrounded by four oxygen ions) and an octahedral interstice (the Fe ion is surrounded by six oxygen ions). In a unit cell of Fe3O4, there are 64 tetrahedral interstices and 32 octahedral interstices. One eighth of the tetrahedral interstices are occupied by half of the Fe3+ ions. Half of the octahedral interstices are occupied by the other half of the Fe3+ ions and all of the Fe2+ ions.
The magnetic property of Fe3O4 thin films varies with the thickness. Fe3O4 thin films (with thicknesses less than 20 nm) epitaxially deposited on MgO substrate are still ferrimagnetic with high magnetic moments. The saturation magnetization (Ms) for a 5 nm Fe3O4 film is found to be 922 emu/cm3, which is much greater than that of bulk Fe3O4 [21]. Epitaxial Fe3O4 films on SrTiO3 substrate showed an increase in Ms for thickness below 15 nm. The Ms of 3 nm Fe3O4 film reached 1017 emu/cm3. As for films with thicknesses above 15 nm, Ms is close to the bulk Fe3O4 value [22]. According to previous reports, film thickness has a great influence on the magnetic properties of Fe3O4.
Fe3O4 thin films are usually synthesized with pulsed laser deposition (PLD) [23,24], molecular beam epitaxy (MBE) [25], magnetron sputtering [26], sol-gel and spin-coater [27] as well as chemical vapor deposition (CVD). PLD, MBE and magnetron sputtering can produce high-quality films but with high costs. Sol-gel and spin-coater techniques are low-cost and convenient methods, but the film quality is limited. CVD is an intermediate method with low costs and good film quality. There are several works on CVD growth of Fe3O4 films. In Mantovan’s work [28], the magnetoresistance of polycrystalline Fe3O4 thin films by using the cyclohexadiene iron tricarbonyl liquid precursor was mainly studied. In another report [29], the Ms of Fe3O4 film prepared under ambient conditions by using a mist CVD method was 500 emu/cm3. A space-confined CVD method developed by Xiong’s group [30] obtained ultrathin Fe3O4 nanosheets, with the size of about 2–4 µm, whose ultrabroadband photoresponse was demonstrated. Here, we choose the CVD method using Fe, PtCl2 and KMnO4 as precursors to prepare Fe3O4 thin films and thin triangles and investigate their electrical and magnetic properties.
Here, we report CVD growth of Fe3O4 thin films with thicknesses down to a few nanometers on (0001) α-Al2O3 substrate. Raman spectroscopic characterization and XRD measurement showed that Fe3O4 films were compressive strained due to the lattice mismatch between Fe3O4 and Al2O3. A strain of −6.50‰ was observed for the 4 nm Fe3O4 thin film. Magnetic measurement revealed robust ferrimagnetism in the 4 nm Fe3O4 thin film. The Ms of thinner Fe3O4 film (4 nm thickness) was significantly larger than that of thicker film.
2. Materials and Methods
2.1. Materials
Fe (99.99%) foil with 0.03 mm thickness was purchased from Taizhou Senuo Material Technology Co., Ltd., Beijing, China. KMnO4 (CAS:7722-64-7, 99.8%) was purchased from Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China. PtCl4 (CAS:13454-96-1, Pt 58.0%) was purchased from Beijing Yanuo Xincheng Technology Co., Ltd., Beijing, China. All the materials were analytical grade and were used without further purification.
2.2. CVD Growth of Fe3O4
An alumina boat containing KMnO4 (~0.40 g) was put outside of the furnace and heated to 180 ℃. Another alumina boat containing PtCl4 powder (~0.02 g) was placed in the upstream of the furnace, and sapphire substrates were placed in the center of the furnace. Iron foil was placed in the downstream of the furnace. The temperature of the furnace was raised to 400 °C in 20 min and kept at 400 °C for 30 min. Then, the furnace was naturally cooled down to room temperature. The carrier gas was 30 sccm Ar. The growth was carried out at atmospheric pressure.
2.3. Characterization
The crystal structure and composition of the as-grown films were checked using X-ray photoelectron spectroscopy (ESCALAB250Xi, Thermo Fisher Scientific Co., Ltd., Loughborough, England) and X-ray diffraction (XRD). XRD patterns were collected using an X-ray diffractometer (D/MAX-TTRIII, Rigaku Co., Tokyo, Japan) with a scan rate of 6°/min. Atomic force microscopic (AFM) images were captured on a multimode scanning probe microscope (Bruker Multimode-8, Bruker Co., Billerica, MA, USA). Energy dispersive spectroscopy (EDS) elemental mapping were taken on a Hitachi S4800 equipped with an energy dispersive X-ray spectroscopy detector.
Raman spectroscopic measurements were performed on a home-made vacuum, variable temperature, low-wavenumber Raman system with 532 nm excitation. An NA = 0.82 low temperature objective (LT-APO/VIS/0.82, attocube systems AG, Haar, Germany) was used for laser focusing and signal collection.
Resistance measurements were performed in a physical property measurement system (PPMS-9, Quantum Design Inc., San Diego, CA, USA) under He-purged conditions. The copper transmission electron microscopy grid was used as a shadow mask. For electrical measurement, 10 nm Ti and 60 nm Au was electron-beam evaporated on Fe3O4 film. The magnetic properties were measured using the vibrational sample magnetometer at different temperatures.
3. Results
Fe3O4 thin film was prepared via the oxidation of iron species in a CVD system, in which Cl2 decomposed from PtCl4 was used to produce volatile iron species from the iron foil (Figure 1a). The distance between the substrate and the iron foil was changed to tune the thickness of Fe3O4 films. Figure 1b,c show optical microscopic images and AFM images of Fe3O4 films grown on sapphire. The EDS elemental mapping of Fe, O and Al (Figure S1 in the Supplementary Materials) illustrate that the Fe and O atoms were homogeneously distributed on the substrate. The synthesis of ultrathin magnetic films via CVD is simple and convenient, and the synthesized materials can be transferred and stacked to prepare heterojunctions and functional devices.
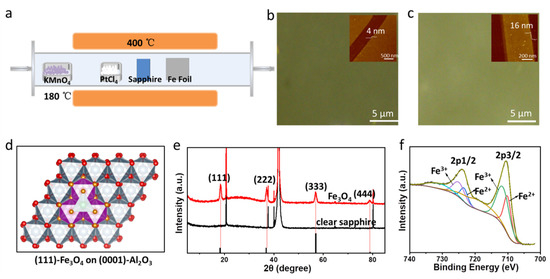
Figure 1.
(a) Illustration of tube furnace for the growth of Fe3O4. Optical microscopic images of (b) 4 nm and (c) 16 nm Fe3O4 films grown on sapphire; insets of (b,c) are the AFM images; (d) schematic diagram of (111) faced Fe3O4 crystal structure on (0001) plane of Al2O3; (e) XRD patterns of the Fe3O4 film; (f) XPS spectra of Fe3O4 film.
Figure 1d presents a schematic diagram of (111) faced Fe3O4 film on (0001) plane of Al2O3. The distance between adjacent oxygen atoms is 2.96 Å in Fe3O4, while it is 2.89 Å in Al2O3, yielding a compressive lattice mismatch of about 2.3% for Fe3O4. Sapphire is composed of identical layers of face-sharing AlO6 octahedron. For the Fe3O4 film grown on sapphire, the diffraction peaks at 18.518°, 37.308°, 57.208° and 79.213° are clearly observed (Figure 1e) and assigned to (111), (222), (333) and (444), respectively. Other peaks were from the α-Al2O3 substrate. The above results suggest that the Fe3O4 films are epitaxially grown on (0001) α-Al2O3 substrates, and the preferred orientation of the obtained epitaxial Fe3O4 films is the [111] crystal orientation.
X-ray photoelectron spectroscopy (XPS) is used to determine the valence states of Fe and the proportion of different valence states in Fe3O4 films. Figure 1f shows the Fe2p core level XPS of Fe3O4 thin film. The Fe2p splits into Fe2p3/2 and Fe2p1/2 due to spin–orbit coupling [31]. The binding energies positioned at 711 eV and 724 eV could be denoted as Fe2p3/2 and Fe2p1/2 of Fe2+ and Fe3+, respectively, which is consistent with a literature report [32]. The binding energy of 2p3/2 and 2p1/2 of Fe2+ are about 710.06 eV and 723.35 eV, while those of Fe3+ are about 711.47 eV and 724.97 eV, respectively. The ratio of Fe2+/Fe3+ calculated from the peak area is 0.468 (1:2.1), which is lower than 1/2 of stoichiometric Fe3O4, which indicates that the film is fully oxidized. Only the binding energies at 711 eV and 724 eV, corresponding to the Fe2p3/2 and Fe 2p1/2 of Fe3O4, are observed, with no satellite peaks of FeO (at 715.5 eV) or γ-Fe2O3 (at 719.1 eV), which finally confirms that all samples are Fe3O4 rather than γ-Fe2O3.
The strain of the films can be measured using XRD. Figure 2a,b show XRD patterns of the Fe3O4 thin films with various thicknesses grown on (0001) α-Al2O3 substrates. The positions of diffraction peak and the calculated lattice parameters are shown in Table S1 in the Supplementary Materials. The lattice parameter decreases from 8.3796 Å to 8.3414 Å when the thickness decreases from 16 nm to 4 nm (Figure 2c). The diffraction peak of the film with 4 nm is narrower, indicating a narrower distribution of the lattice parameters. All the lattice parameters of Fe3O4 thin films are smaller than that of the bulk Fe3O4 (8.3967 Å) [20], which confirms a compressive strain in the thin films [33]. The compressive strain of the 4 nm film and 16 nm film is 6.50‰ and 1.95‰, respectively (Figure 2c).
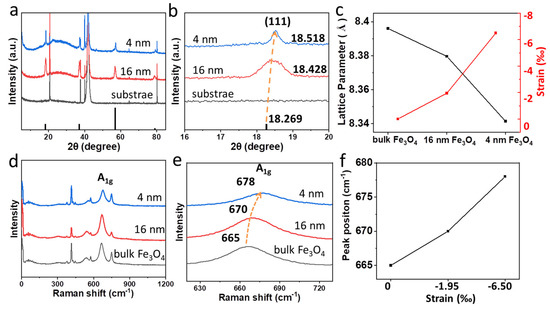
Figure 2.
(a,b) XRD patterns of Fe3O4 films with different thicknesses; (c) the lattice parameter and strain of 4 nm film, 16 nm film and bulk Fe3O4. (d,e) Raman spectra of the 4 nm film, 16 nm film and bulk Fe3O4; (f) Strain−dependence of Raman peak position.
In addition, the Raman spectra can reflect the change in the strain in the sample [34]. Figure 2d,e display the Raman spectra of the Fe3O4 grown on sapphire. The Raman peaks located at 665 cm−1, 670 cm−1 and 678 cm−1 are observed, corresponding to the A1g of bulk Fe3O4, 16 nm and 4 nm Fe3O4 film, respectively. The relationship between Raman peak position and strain are shown in Figure 2f. The Raman peak shifts to a higher frequency at a stronger compressive strain.
In addition to the Fe3O4 films, triangular Fe3O4 nanosheets down to 5 nm thickness were grown on sapphire via CVD. Figure 3a displays the Raman spectra of the triangle Fe3O4 nanosheets with different thicknesses grown on sapphire at room temperature. The optical microscopic images of triangle Fe3O4 nanosheet grown on sapphire are shown in Figure 3b. A strong peak at about 665 cm−1 is observed in triangle Fe3O4 nanosheets, which is assigned to the A1g mode [30]. Other Raman peaks located at 194, 300, and 536 cm−1 correspond to the T2g(1), Eg, and T2g(2) modes, respectively. The A1g mode is the symmetric stretch of oxygen atoms along Fe-O bonds; the T2g(2) mode is the asymmetric stretch of Fe and O; the Eg mode is the symmetric bend of oxygen with respect to Fe; T2g(1) is the translatory movement of the whole FeO4. The thickness of triangle Fe3O4 nanosheets with different thickness is presented in Figure 3c–f. No significant Raman peak shift is observed as the thickness changes. The absence of strain in the Fe3O4 nanosheets is very possibly due to a different interface structure.
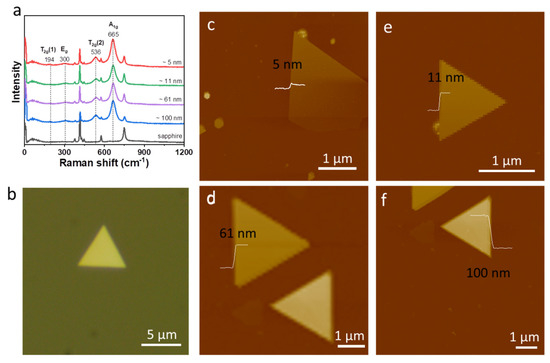
Figure 3.
(a) Raman spectra of triangle Fe3O4 nanosheets with various thicknesses; (b) optical microscopic images of triangle Fe3O4 nanosheet; (c–f) the AFM images of triangle Fe3O4 nanosheets.
To determine the magnetic properties of Fe3O4 ultrathin films, we measured the temperature-dependent magnetization curves of Fe3O4 films. The microscopic origin of the change in the magnetic properties of Fe3O4 can been studied in detail via XAS and XMCD advanced characterization techniques [27,35]. Currently, we study the macroscopic magnetic properties of Fe3O4 films using the conventional vibrational sample magnetometer technique due to experimental conditions. The magnetic hysteresis (M-H) loops are shown in Figure 4. At 300 K, the Ms of 16 nm Fe3O4 film is about 445 emu/cm3, which is close to the value obtained in bulk Fe3O4 (480 emu/cm3) [36]. Surprisingly, the Ms of 4 nm Fe3O4 film is dramatically increased to 752 emu/cm3. Compared with Fe3O4 films synthesized using different methods (Table 1), ultra films with high Ms were obtained in our work. As shown in Table 1, Ms for a 5 nm Fe3O4 film grown on MgO substrate by MBE is 922 emu/cm3 [21]; epitaxial 3 nm Fe3O4 film on SrTiO3 substrate shows a Ms of 1017 emu/cm3 [22]. The Ms of Fe3O4 film on SrTiO3 via CVD is about 500 emu/cm3 [29]. The Ms obtained in the above experiments are all larger than the Ms of the bulk Fe3O4. In our work, 4 nm Fe3O4 film was obtained, and the Ms is 752 emu/cm3. At 2 K, the Ms of Fe3O4 films exhibit a similar thickness dependence. Representative in-plane and out-of-plane M-H loops of the 4 nm film are shown in Figure 4c,d. The magnetic coercivity of the film increased with the cooling down process, from 238 Oe (or 912 Oe) at room temperature to 772 Oe (or 2702 Oe) at 2 K in the in-plane (or out-of-plane) magnetic field. This is normal since the reverse of magnetic moment requires more energy at low temperature due to the suppression of thermal disturbance. The in-plane M-H loops of the 4 nm Fe3O4 film show better rectangularity than that of out-of-plane M-H loops at 300 K and 2 K. At 300 K, in-plane and out-of-plane M-H curves of 4 nm Fe3O4 are saturated at 0.1359 T and 0.2679 T, respectively. This indicates that the in-plane M-H curve is easy to saturate under a small applied magnetic field, while the out-of-plane M-H curve is saturated at a larger magnetic field. This suggests that the easy axis of Fe3O4 film lies in the film plane with a small magnetic anisotropy.
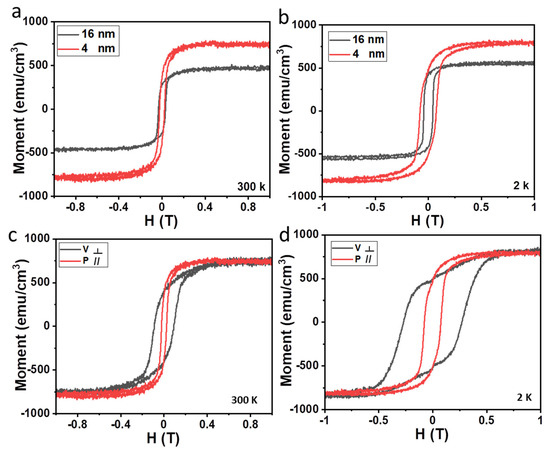
Figure 4.
In-plane magnetic hysteresis loops of Fe3O4 films with the thicknesses of 4 nm and 16 nm measured at 300 K (a) and 2 K (b). In-plane and out-of-plane magnetic hysteresis loops of 4 nm Fe3O4 films measured at 300 K (c) and 2 K (d).

Table 1.
Saturation magnetization and thickness of Fe3O4 films synthesized by different methods.
As shown in Figure 5a, the resistance of a 4 nm Fe3O4 film first increases gradually as the temperature decreases and then rapidly rises at TV. The conductivity of an Fe3O4 film is mainly contributed to the electron hopping between Fe2+ and Fe3+ at octahedral positions. Above TV, the electrons are easily exchanged between the Fe2+ and Fe3+ on the octahedral sites, which accounts for the relatively high electrical conductivity of cubic Fe3O4 at high temperatures. Low temperatures can freeze the electron hopping and thus result in much higher resistance.
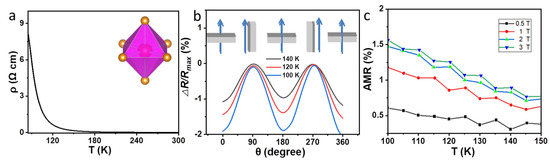
Figure 5.
(a) Temperature−dependent resistance of a 4 nm Fe3O4 thin film; (b) angular−dependent resistance for the 4 nm Fe3O4 film measured at 3 T; (c) temperature−dependent AMR at different fields for the 4 nm Fe3O4 film.
Figure 5b shows the angular−dependent resistance for the 4 nm Fe3O4 film with a fixed magnetic field of 3 T. The △R/Rmax (%) is defined as △R/Rmax (%) = (Rθ − Rmax)/Rmax × 100), where Rmax is the maximum of resistivity for each scan. θ is the angle between the applied magnetic field and film normal direction. θ = 0° corresponds to the out-of-plane field, and θ = 90° is the in-plane magnetic field. The curves maintain two-fold symmetry at different temperatures near TV (140 K, 120 K, 100 K), following the typical sin2θ dependence with valleys at 0°/180° and peaks at 90°/270°, respectively. The details of the origin resistance of 4 nm Fe3O4 film are seen in Figure S2. Sin2θ symmetry indicates that the thin films have a uniaxial magnetic anisotropy.
In addition, we measured magnetoresistance (MR) at various applied fields. The MR is defined as MR = (RH − R0)/R0 × 100%. A representative MR of the 4 nm Fe3O4 film as a function of magnetic field measured at different temperatures (300 K, 180 K and 120 K) are shown in Figure S3. As shown in Figure S3a, in-plane MR at 120 K shows abnormal behavior compared with 180 K and 300 K, which is due to the occurrence of phase transfer at about 120 K. However, the abnormal behavior at 120 K is absent when the applied magnetic field is perpendicular to the film (Figure S3b). The possible reason is that the out-of-plane direction is the hard magnetization direction.
The effect of temperature and magnetic field on the anisotropic magnetoresistance (AMR, AMR (%) = (Rθ=90° − Rθ=0°)/Raverage × 100), where Rθ=90° and Rθ=0° are the resistivities for magnetic field applied parallel and perpendicular to the current direction, respectively, and Raverage = Rθ=90°/3 + 2 × Rθ=0°/3, should be further explored. The in-plane and out-of-plane resistance as a function of temperature at different fields were investigated (Figure S4). As shown in Figure 5c, the AMR is enhanced at higher magnetic fields and is suppressed at lower temperatures.
4. Conclusions
In summary, we report the growth of ultrathin Fe3O4 films and triangle nanosheets using a low-cost and highly efficient CVD method. The 4 nm Fe3O4 ultrathin film exhibits room-temperature ferrimagnetism and a higher magnetic moment. This work contributes greatly to the synthesis of ultrathin magnetic films from non-layered magnetic materials for next-generation electronic and spintronic devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12040485/s1, Table S1: Positions of diffraction peaks and the calculated lattice parameters for Fe3O4; Figure S1: EDS elemental mapping of Fe (a), O (b) and Al (c) of Fe3O4 film; Figure S2: Angular−dependent resistance for the 4 nm Fe3O4 film measured at 140 K (a), 120 K (b) and 100 K (c); Figure S3: In-plane MR (a) and out-of-plane MR (b) of the 4 nm Fe3O4 film as a function of magnetic field at 300 K, 180 K and 120 K; Figure S4. Temperature−dependent in-plane resistance (a) and out-of-plane resistance (b) at different magnetic fields.
Author Contributions
Conceptualization: L.X.; data curation: F.L. and R.Z.; formal analysis: F.L. and Y.C.; methodology: F.L. and Z.Q.; resources: R.Z. and Z.Q.; validation: F.L.; writing—original draft: F.L.; writing—review and editing: R.Z., Z.Q., Y.C. and L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Strategic Priority Research Program of CAS (XDB30000000).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deng, Y.; Yu, Y.; Song, Y.; Zhang, J.; Wang, N.Z.; Sun, Z.; Yi, Y.; Wu, Y.Z.; Wu, S.; Zhu, J.; et al. Gate-tunable room-temperature ferromagnetism in two-dimensional Fe3GeTe2. Nature 2018, 563, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Kang, K.; Shayan, K.; Yoshimura, A.; Dadras, S.; Wang, X.; Zhang, L.; Chen, S.; Liu, N.; Jindal, A.; et al. Enabling room temperature ferromagnetism in monolayer MoS2 via in situ iron-doping. Nat. Commun. 2020, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
- Ostwal, V.; Shen, T.; Appenzeller, J. Efficient Spin-orbit torque switching of the semiconducting van der Waals ferromagnet Cr2Ge2Te6. Adv. Mater. 2020, 32, 1906021. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chen, C.; Chen, J.; He, T.; Li, H.; Yang, Z.; Xie, L.; Wang, Z.; Zhang, K. High-performance junction field-effect transistor based on black phosphorus/β-Ga2O3 heterostructure. J. Semicond. 2020, 41, 082002. [Google Scholar] [CrossRef]
- Huang, B.; Clark, G.; Navarro-Moratalla, E.; Klein, D.R.; Cheng, R.; Seyler, K.L.; Zhong, D.; Schmidgall, E.; McGuire, M.A.; Cobden, D.H.; et al. Layer-dependent ferromagnetism in a van der Waals crystal down to the monolayer limit. Nature 2017, 546, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Clark, G.; Klein, D.R.; MacNeill, D.; Navarro-Moratalla, E.; Seyler, K.L.; Wilson, N.; McGuire, M.A.; Cobden, D.H.; Xiao, D.; et al. Electrical control of 2D magnetism in bilayer CrI3. Nat. Nanotechnology 2018, 13, 544–548. [Google Scholar]
- Gong, C.; Kim, E.M.; Wang, Y.; Lee, G.; Zhang, X. Multiferroicity in atomic van der Waals heterostructures. Nat. Commun. 2019, 10, 2657. [Google Scholar] [CrossRef]
- Fei, Z.; Huang, B.; Malinowski, P.; Wang, W.; Song, T.; Sanchez, J.; Yao, W.; Xiao, D.; Zhu, X.; May, A.F.; et al. Two-dimensional itinerant ferromagnetism in atomically thin Fe3GeTe2. Nat. Mater. 2018, 17, 778–782. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, T.; Ding, M.; Dong, B.; Li, Y.; Chen, M.; Li, X.; Huang, J.; Wang, H.; Zhao, X.; et al. Electric-field control of magnetism in a few-layered van der Waals ferromagnetic semiconductor. Nat. Nanotechnol. 2018, 13, 554–559. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, X.; Xu, J.; Tang, B.; Shang, Q.; Shi, J.; Huan, Y.; Liao, J.; Chen, Q.; Hou, Y. Controlled growth and thickness-fependent vonduction- type Transition of 2D Ferrimagnetic Cr2S3 semiconductors. Adv. Mater. 2020, 32, 1905896. [Google Scholar] [CrossRef]
- Senn, M.S.; Wright, J.P.; Attfield, J.P. Charge order and three-site distortions in the Verwey structure of magnetite. Nature 2011, 481, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Piekarz, P.; Parlinski, K.; Oles, A.M. Mechanism of the Verwey transition in magnetite. Phys. Rev. Lett. 2006, 97, 156402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verwey, E.J.W. Electron conduction of magnetite (Fe3O4) and its transition point at low temperatures. Nature 1939, 144, 327–328. [Google Scholar] [CrossRef]
- Dijken, S.V.; Fain, X.; Watts, S.M.; Coey, J. Negative magnetoresistance in Fe3O4/Au/Fe spin valves. Phys. Rev. B 2004, 70, 052409. [Google Scholar] [CrossRef]
- Iwata-Harms, J.M.; Chopdekar, R.V.; Wong, F.J.; Nelson-Cheeseman, B.B.; Jenkins, C.A.; Arenholz, E.; Suzuki, Y. Magnetotransport in La0.7Sr0.3MnO3/CuCr2O4/Fe3O4 magnetic junctions. Appl. Phys. Lett. 2015, 106, 012405. [Google Scholar] [CrossRef]
- Yoon, K.S.; Koo, J.H.; Do, Y.H.; Kim, K.W.; Kim, C.O.; Jin, P.H. Performance of Fe3O4/AlOx/CoFe magnetic tunnel junctions based on half-metallic Fe3O4 electrodes. J. Magn. Magn. Mater. 2005, 285, 125–129. [Google Scholar] [CrossRef]
- Wang, W.G.; Li, M.; Hageman, S.; Chien, C.L. Electric-field-assisted switching in magnetic tunnel junctions. Nat. Mater. 2011, 11, 64–68. [Google Scholar] [CrossRef]
- Zhao, L.B.; Mi, W.B.; Jiang, E.Y.; Bai, H.L. Spin-polarized transport of electrons from polycrystalline Fe3O4 to amorphous Si. Appl. Phys. Lett. 2007, 91, 052113. [Google Scholar] [CrossRef]
- Ong, H.C.; Zhu, A.X.E.; Du, G.T. Dependence of the excitonic transition energies and mosaicity on residual strain in ZnO thin films. Appl. Phys. Lett. 2002, 80, 941–943. [Google Scholar] [CrossRef]
- Arora, S.K.; Wu, H.-C.; Choudhary, R.J.; Shvets, I.V.; Mryasov, O.N.; Yao, H.; Ching, W.Y. Giant magnetic moment in epitaxial Fe3O4 thin films on MgO(100). Phys. Rev. B 2008, 77, 134443. [Google Scholar] [CrossRef]
- Guan, X.; Zhou, G.; Xue, W.; Quan, Z.; Xu, X. The investigation of giant magnetic moment in ultrathin Fe3O4 films. APL Mater. 2016, 4, 036104. [Google Scholar] [CrossRef] [Green Version]
- Kado, T. Structural and magnetic properties of magnetite-containing epitaxial iron oxide films grown on MgO(001) substrates. J. Appl. Phys. 2008, 103, 043902. [Google Scholar] [CrossRef]
- Ziese, M.; Blythe, H.J. Magnetoresistance of magnetite. J. Phys. Condens. Matter 2000, 12, 13. [Google Scholar] [CrossRef]
- Sterbinsky, G.E.; Cheng, J.; Chiu, P.T.; Wessels, B.W.; Keavney, D.J. Investigation of heteroepitaxial growth of magnetite thin films. J. Vac. Sci. Technol. B 2007, 25, 1389–1392. [Google Scholar] [CrossRef]
- Lu, Z.L.; Xu, M.X.; Zou, W.Q.; Wang, S.; Liu, X.C.; Lin, Y.B.; Xu, J.P.; Lu, Z.H.; Wang, J.F.; Lv, L.Y.; et al. Large low field magnetoresistance in ultrathin nanocrystalline magnetite Fe3O4 films at room temperature. Appl. Phys. Lett. 2007, 91, 102508. [Google Scholar] [CrossRef]
- Dawn, R.; Zzaman, M.; Bharadwaj, R.R.; Kiran, C.; Shahid, R.; Verma, V.K.; Sahoo, S.K.; Amemiya, K.; Singh, V.R. Direct evidence to control the magnetization in Fe3O4 thin films by N2 ion implantation: A soft X-ray magnetic circular dichroism study. J. Sol-Gel Sci. Technol. 2021, 99, 461–468. [Google Scholar] [CrossRef]
- Mantovan, R.; Vangelista, S.; Cocco, S. Chemical vapor deposition of polycrystalline Fe3O4 thin films by using the cyclohexadiene iron tricarbonyl liquid precursor. J. Appl. Phys. 2012, 111, 312. [Google Scholar] [CrossRef]
- Kan, D.; Sugano, S.; Kosugi, Y.; Kobayashi, K.; Uebayashi, N.; Koganezawa, T.; Shimakawa, Y. Selective growth of α-Fe2O3, γ- Fe2O3 and Fe3O4 at low temperatures and under ambient pressure. Jpn. J. Appl. Phys. 2019, 58, 095504. [Google Scholar] [CrossRef]
- Yin, C.; Gong, C.; Chu, J.; Wang, X.; Yan, C.; Qian, S. Ultrabroadband photodetectors up to 10.6 μm based on 2D Fe3O4 nanosheets. Adv. Mater. 2020, 32, 2002237. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Cao, L.; Guo, Q.; Liang, J.; Kou, Z.; Zhou, X.; Huang, Z.; Zhai, Y.; Du, J.; You, B.; Zhao, H.; et al. Preparation of sputtered Fe3O4 thin film. J. Mater. Sci. Mater. Electron. 2021, 32, 23645–23653. [Google Scholar] [CrossRef]
- Zhou, H.; Yuan, C.; Yang, Y.; Yu, T.; Luo, X. The role of strain on the magnetic properties of confined Fe3O4 nanocrystals in Al2O3 matrix. Mater. Lett. 2019, 239, 52–55. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, C.; Fang, X.; Zhang, D.; Zhu, M.; Zhao, D.; Zhen, C.; Ma, L.; Hou, D. Modulation on the magnetic and electrical properties of Fe3O4 thin films through strain relaxation. J. Magn. Magn. Mater. 2021, 536, 168128. [Google Scholar] [CrossRef]
- Nongjai, R.; Samad, R.; Singh, V.R.; Verma, V.K.; Kandasami, A. Magnetic and electronic structures of N implanted iron oxide thin films. J. Magn. Magn. Mater. 2021, 527, 167703. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Sun, J.R.; Han, Y.N.; Xie, X.Y.; Shen, J.; Rong, C.B.; He, S.L.; Shen, B.G. Microstructure and magnetic properties of strained Fe3O4 films. J. Appl. Phys. 2008, 103, 07D703. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).