Abstract
In this paper, the Ti2AlNb alloy was bonded to TC4 alloy using the vacuum diffusion bonding method with a Ti interlayer. The interfacial microstructure of the Ti2AlNb/Ti/TC4 joint was characterized. The relationship between the bonding parameters and the microstructure and mechanical property of the joints was explored. Results indicated that the interdiffusion of Nb and Al elements between the interlayer and substrates promoted the formation of the lamellar α + β dual-phase structure in the joint. The bonding parameters determined the diffusion distance of Nb and Al elements, thus controlling the characteristics of the lamellar α + β dual-phase structure. When the Ti2AlNb alloy and TC4 alloy were bonded at 950 °C for 30 min under a pressure of 10 MPa, the elemental diffusion in the bonding couple was sufficient and the joint possessed the maximum shear strength of 549 MPa.
1. Introduction
In 1960, researchers found that adding an appropriate amount of Nb in a Ti3Al-based alloy could develop a new kind of alloy, i.e., the Ti2AlNb alloy, which possesses high specific strength, excellent creep resistance, and oxidation resistance [1,2,3,4,5,6]. TC4 alloy (Ti-6Al-4V) is a typical structural material, since it has the advantages of low density, good workability, and outstanding fracture toughness [7,8]. Due to the outstanding performance of the Ti2AlNb alloy and TC4 alloy, they have been regarded as promising structural materials in the aerospace industry [9,10]. Ti2AlNb alloy can serve at a higher temperature (600~750 °C) than the TC4 alloy (<600 °C) [11]. However, the Ti2AlNb alloy has a comparatively higher density and cost than the TC4 alloy. When manufacturing the airspace engine, it is often necessary to join the Ti2AlNb alloy to the TC4 alloy to produce composite components [12,13]. If a part is working at a temperature below 600 °C, the TC4 alloy can be used. For a part working at high temperatures (600~750 °C), the Ti2AlNb alloy can be used. Thus, these two materials can develop their full potential. A balance can be achieved between structure–property and manufacturing cost. Meanwhile, the coefficient of thermal expansion (CTE) mismatch between Ti2AlNb alloy and TC4 alloy is small. The CTE of Ti2AlNb alloy is 8.8 × 10−6 °C−1 [5], while that of the TC4 alloy is 9–9.5 × 10−6 °C−1 [14]. The effect of the CTE mismatch is small when fabricating the large-scale Ti2AlNb/TC4 component. In some ways, the joining of the Ti2AlNb alloy and TC4 alloy is inevitable and in high demand. From the above viewpoint, developing a technique for reliably joining these two materials will further broaden their applications and is of great meaning.
Up to now, many welding techniques have been employed for joining Ti2AlNb alloys and TC4 alloys [15,16,17,18,19]. However, each technique has both strengths and weaknesses. When the Ti2AlNb alloy and TC4 alloy are welded by fusion welding methods, it is very difficult to precisely control the interfacial microstructure in the joining pair [20], such as the phase composition and distribution of interfacial products, and the thickness of reaction layers. Defects like voids and hot cracks often appear in the joint. To eliminate the adverse effects, post-weld heat treatment is required. Brazing and transient liquid phase (TLP) bonding are also used to join Ti2AlNb alloy [21] and TC4 alloy [22,23]. Researchers have obtained satisfactory results using the Ti-Zr-Ni-Cu [24], Ti-Ni-Cu [25], and Ti-Ni-Nb [26] filler metals or interlayers. However, during the joining, brittle intermetallic compounds (such as Ti2Ni, Ti2Cu, Zr2Cu, et al.) are inevitably formed in the joint.
As a solid-state bonding method, diffusion bonding has some inherent advantages in comparison with fusion welding, brazing, and TLP, etc. During diffusion bonding, the joint microstructure can be well controlled to avoid the formation of voids, cracks, and brittle intermetallic compounds. A sound diffusion bonded joint relies on the occurrence of self-diffusion [27]. When the Ti2AlNb alloy and TC4 alloy are direct diffusion bonded, a high bonding temperature and a long holding time are often needed to enhance the atomic diffusion at the bonding interfaces, which may be harmful to the base metals. This is because that when the bonding temperature is high and the holding time is long, the fine acicular orthorhombic (O) phase in Ti2AlNb alloy will largely transform to the B2 phase. The precipitation hardening effect of the O phase will be attenuated, leading to the decrease of the strength. Meanwhile, the α phases in TC4 alloys will largely transform to β phases, forming the coarse β phases and significantly decreasing the ductility of the TC4 alloy. Thus, the strength of the direct diffusion bonded Ti2AlNb/TC4 joint is relatively low. It is difficult to reach the full potential of the Ti2AlNb alloy and TC4 alloy. Adding a suitable interlayer in the diffusion couple can improve the reliability of the contact at the bonding interface. Therefore, a sound joint can be produced at a relatively low bonding temperature and short holding time. Diffusion bonding with Ti/Al [28], Ni [29], Ni/Al [30], and Ti/Cu [31] interlayers have been applied to the joining of Ti-based alloys, and sound joints have been obtained without damaging the base metals. However, the interfacial reactions between the interlayers and parent alloys are too strong, leading to the formation of brittle intermetallic compounds. To achieve a reliable joint of Ti2AlNb alloy and TC4 alloy, a suitable interlayer should be chosen. It should have an appropriate interdiffusion degree and not form intermetallic compounds with the base metals. Considering the chemical and physical properties of the Ti2AlNb alloy and TC4 alloy, using pure Ti foil as the bonding interlayer may be a feasible solution. First, Ti2AlNb alloys and TC4 alloys have good chemical compatibility with the Ti interlayer. No brittle intermetallic compounds will be formed during the bonding. Second, Ti is softer than the Ti2AlNb alloy and TC4 alloy. It can improve the reliability of the contact at the interface and lower the bonding pressure. Unfortunately, to the best of our knowledge, the diffusion bonding of Ti2AlNb alloys and TC4 alloys with a Ti interlayer has not been reported.
In this study, the Ti2AlNb alloy and TC4 alloy were diffusion bonded using the pure Ti interlayer. The typical joint microstructure was characterized. The relationship between the microstructural evolution and mechanical performance of the Ti2AlNb/Ti/TC4 joint is discussed systematically.
2. Experimental Procedures
Figure 1 shows the microstructures of the Ti2AlNb alloy and TC4 alloy. The Ti2AlNb alloy is in the as-cast status and has a chemical composition of Ti-22Al-23Nb-2V (at.%). It includes several different phase types: orthorhombic phase (O) (Cmcm system based on Ti2AlNb), hexagonal close-packed (hcp) α2 phase (D019 structure base on Ti3Al), and bcc B2 phase (ordered structure). The TC4 alloy has a chemical composition of Ti-6Al-4V (at.%) and consists of the α-Ti matrix phase and β-Ti phase.
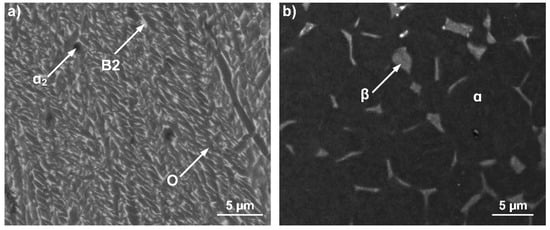
Figure 1.
Microstructures of the (a) Ti2AlNb alloy and (b) TC4 alloy.
Before diffusion bonding, the Ti2AlNb and TC4 substrates were cut into small pieces by the wire-cut electrical discharge machine. The sizes of the Ti2AlNb and TC4 substrates were 5 mm × 5 mm × 3 mm and 10 mm × 8 mm × 2 mm, respectively. Then the joining surfaces were polished using sandpapers. Finally, all the samples were placed in acetone and cleaned for 10 min under ultrasound treatment. A thin Ti sheet (30 μm thick) was placed between the substrates as the bonding interlayer. The bonding triples (Ti2AlNb/Ti/TC4) were placed in a graphite fixture under a bonding pressure of 10 MPa. The diffusion bonding experiment was conducted in a vacuum furnace (Centorr 6-1650-15T). The vacuum level was about 4 × 10−4 Pa. The heating rate was 30 °C/min. The bonding temperature ranged from 850~980 °C. The holding time ranged from 10~90 min. When the holding time was over, the bonding couple was firstly cooled down to 400 °C at a cool rate of 15 °C/min. Then, the heat power was turned off and the bonding couple was cooled down to room temperature in the furnace.
After the diffusion bonding, the cross-section and the fracture surface of the joints were characterized using scanning electron microscopy (SEM, Hitachi S-4700) equipped with the energy-dispersive spectrometer (EDS) and X-ray diffraction (XRD, D8 ADVANCE). The joint room-temperature shear strength was tested using the universal test machine (Instron-1186). The shear rate was set as 0.5 mm·min−1. The schematic diagram is shown in Figure 2.
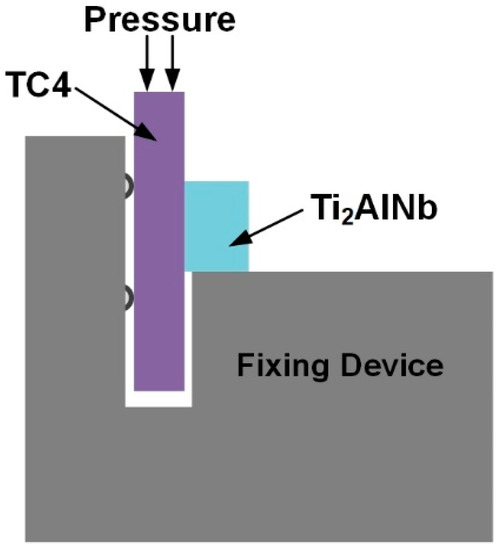
Figure 2.
Schematic diagram of the joint shear test.
3. Results and Discussion
3.1. Interfacial Microstructure of the Ti2AlNb/Ti/TC4 Joint
The diffusion bonding joint of the Ti2AlNb alloy and TC4 alloy was obtained using the Ti interlayer at 950 °C for 30 min under pressure of 10 MPa. Figure 3 shows the typical joint microstructure. References [32,33] indicated that the yield strengths of Ti2AlNb and TC4 alloys at 950 °C were much lower than those at room temperature. Under the bonding pressure, plastic deformations occurred in the Ti2AlNb and TC4 alloys. The indentation effect of the Ti2AlNb sample into the TC4 plate was observed in the joint. The deformation of the Ti2AlNb and TC4 alloys led to better contact between the bonding surfaces and the interlayer and was beneficial to the diffusion bonding. Figure 3 indicates that the bonding between the parent alloys and Ti interlayer was sound without any voids or cracks. No residual Ti interlayer was observed. Due to the atomic diffusion during the bonding process, a ~30 μm thick reaction zone was formed. There were two phases in the reaction zone, which were the lamellar phase (phase B) and the matrix phase (phase A). To confirm the phase composition of the reaction zone, the EDS analysis was conducted. Table 1 represents the EDS results of each phase in Figure 3. It indicates that the matrix phase mainly consisted of Ti. As for the lamellar phase, it not only had a majority of Ti element but also had a rather high content of Al and Nb elements. According to the EDS results and Ti-Al-Nb phase diagram [34], the matrix phase should be the α-Ti phase and the lamellar phase was supposed to be the β-Ti phase. To further confirm the phase composition, an XRD analysis was also carried out. Before the XRD test, the TC4 alloy was removed by grinding until the reaction zone was reached. Then, the XRD test was carried out to identify the phase composition of the reaction zone. The data in Figure 4 indicate that the α-Ti phase and β-Ti phase were detected.
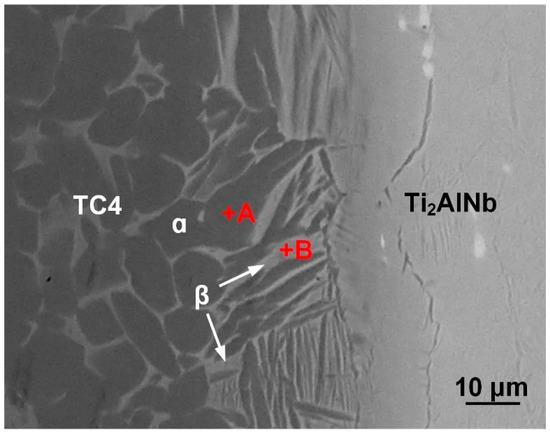
Figure 3.
Interfacial microstructure of the Ti2AlNb/Ti/TC4 joint. (950 °C, 30 min, 10 MPa).

Table 1.
EDS analysis results.
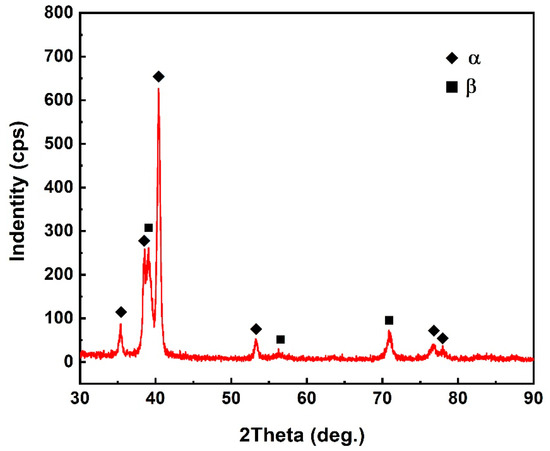
Figure 4.
XRD analysis results.
Figure 5 shows the distribution of Nb, Al, and Ti elements along the red line across the final joint. Before the diffusion bonding, there were differences in the content of Nb, Al, and Ti elements between the pure Ti interlayer and the parent alloys. When the diffusion bonding process began, Ti diffused from the pure Ti interlayer to the adjacent parent alloys under the effect of concentration gradient at the bonding interface. At the same time, Nb and Al atoms migrated from parent alloys to the pure Ti interlayer. Thus, an obvious concentration change of Ti, Al, and Nb elements was observed in the diffusion bonding joint. From Figure 5, it can be seen that in the final joint, there was more Ti in the TC4 alloy and in the diffusion zone (formed in place of the pure Ti foil) and, naturally, less in the Ti2AlNb alloy. It should be noticed that the concentration change of the Ti and Nb elements was much more significant than the Al element at the bonding interface. This phenomenon is mainly due to the different atomic radii of Ti, Nb, and Al atoms. Compared with Nb and Ti, Al had a smaller atomic radius. During the diffusion bonding process, the Al atom migrated to the Ti interlayer more easily than the Ti and Nb atoms, resulting in the uniform distribution at the bonding interface. Researchers suggest that Nb is the β-stabilizing element [35]. Therefore, the volume fraction of the lamellar β phase gradually decreased as the distance from the Ti2AlNb alloy increased, which was in line with the Nb concentration.
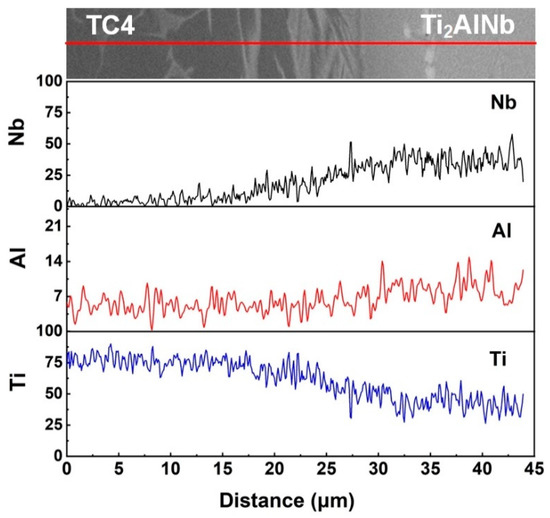
Figure 5.
Elemental distribution at the bonding interface.
3.2. Influence of Bonding Parameter on Microstructure of the Ti2AlNb/Ti/TC4 Joint
In this section, the joints were bonded with various bonding parameters to investigate their effects on the joint microstructure. Figure 6 illustrates the images of Ti2AlNb/Ti/TC4 joints bonded at different conditions. Figure 6a–c shows the microstructure of joints obtained at different bonding temperatures. The figures demonstrate that the interfacial morphology of the joint changed significantly at various bonding temperatures. The change of the joint microstructure was mainly due to the atomic diffusion at various bonding conditions. Higher bonding temperatures and longer holding times led to the increase of the atomic diffusion distance. When the bonding temperature was 850 °C, the interdiffusion in the bonding couple was insufficient [36,37]. The interfaces on the two sides were clear and straight. Some voids were observed at the bonding interfaces. The high temperature increased the elemental diffusion coefficient and atomic mobility of Al, Nb, and V elements. Therefore, when the bonding temperature increased to 900 °C and 950 °C, the diffusion of Al, Nb, and V elements from the parent alloys, as well as that of the Ti from the Ti interlayer, was greatly promoted. The voids on two sides disappeared. On the TC4 side, the TC4/Ti interface became indistinct. On the Ti2AlNb side, a lamellar α + β dual-phase structure was produced at the Ti2AlNb/Ti interface. The appearance of the β phase in the Ti interlayer was mainly due to the diffusion of the Nb element from the Ti2AlNb alloy to the Ti interlayer. High bonding temperature enhanced the diffusion of the Nb element. Therefore, when the bonding temperature was high (950 °C), the lamellar α + β dual-phase area was much larger than that at 900 °C.
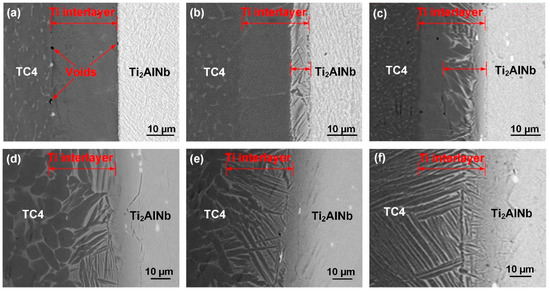
Figure 6.
Microstructure of the joints bonded at various bonding parameters (10 MPa): (a) 850 °C, 10 min; (b) 900 °C, 10 min; (c) 950 °C, 10 min; (d) 950 °C, 30 min; (e) 950 °C, 60 min; (f) 950 °C, 90 min.
Figure 6c–f shows the microstructure of the Ti2AlNb/Ti/TC4 joint bonded with different holding times. Since the bonding temperature had reached a relatively high temperature (950 °C), the parent alloys formed a reliable bonding with the Ti interlayer. As the holding time increased, the morphology of the Ti interlayer had an obvious change. The lamellar α + β dual-phase structure gradually extended to the Ti interlayer from the Ti2AlNb substrate and joined together with the TC4 substrate. The diffusion of the Nb element increased with the extending of holding time. When the holding time was 10 min, the diffusion distance of the Nb in the Ti interlayer was ~20 μm. Thus, the lamellar α + β dual-phase structure only formed in the region near the Ti2AlNb alloy, as shown in Figure 6c. A long holding time increased the diffusion distance of Nb and promoted the growth of the lamellar α + β dual-phase structure. When the holding time increased to 30 min, the entire interlayer transformed to the lamellar α + β dual-phase structure. Further increasing the holding time decreased the interlamellar spacing of the lamellar α + β dual-phase structure. When the holding time increased to 90 min, the diffusion distance of the Nb increased significantly. The Nb element diffused across the Ti interlayer and entered the TC4 alloy. Since Nb is the beta-stabilizing element, the increase of Nb content led to the α→β transformation and the formation of lamellar α + β dual-phase structure. Thus, the TC4 alloy adjacent to the bonding interface transformed to the lamellar α + β dual-phase structure. Ankem et al. [38] reported that the α + β dual-phase structure had a better mechanical performance than the Ti interlayer. In consequence, to obtain a sound Ti2AlNb/Ti/TC4 joint with better mechanical property, an appropriately high bonding temperature and an appropriately long holding time is recommended.
3.3. Shear Strength and Fracture Analysis of the Ti2AlNb/Ti/TC4 Joint
The room-temperature shear test was conducted to assess the mechanical property of the joints. Figure 7 shows the test results of the joints achieved under various bonding conditions. Figure 7a demonstrates that as the bonding temperature increased, the joint shear strength increased first from 240 MPa to 494 MPa, then decreased slightly to 489 MPa. The variation of joint shear strength depended on the microstructure evolution of the joints. As shown in Figure 6a–c, the bonding temperature determined the atomic diffusion, thus influencing the bonding quality. At a low bonding temperature (850 °C), the interdiffusion in the bonding couple was insufficient due to the low atomic mobility. The interfacial bonding was weak. Voids were formed at the bonding interfaces, leading to the low shear strength (240 MPa). The elevation of bonding temperature enhanced the atomic diffusion and improved the interfacial bonding quality. The Nb element diffused from the Ti2AlNb substrate to the interlayer, forming the lamellar α + β dual-phase structure near the Ti2AlNb substrate. A previous study [39] indicated that the lamellar α + β dual-phase structure has excellent combination properties compared to the pure Ti interlayer (α phase). Thus, the formation of a lamellar α + β dual-phase structure in the Ti interlayer is beneficial to improve the joint shear strength. A higher bonding temperature led to a larger dual-phase region. As a result, when the bonding temperature increased, the joint shear strength increased rapidly from 240 MPa to 494 MPa. When the bonding temperature further increased to 980 °C, the yield strength of the TC4 alloy decreased to <20 MPa. The TC4 alloy became very soft at 980 °C, leading to excessive deformation under the bonding pressure. Thus, it was not desirable for the diffusion bonding of the TC4 alloy and Ti2AlNb alloy at a higher temperature.
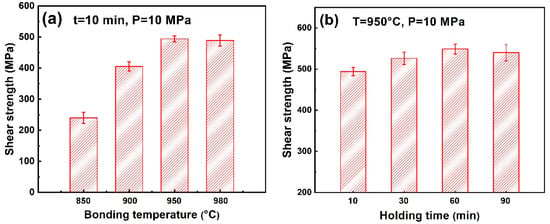
Figure 7.
Effect of (a) bonding temperature and (b) holding time on the shear strength of the Ti2AlNb/Ti/TC4 joints.
Figure 7b shows the shear strength of the joints bonded at various holding times. Unlike the obvious variation in Figure 7a, the joints bonded at various holding times all had a relatively high shear strength (higher than 500 MPa). This was mainly because of the fact that when the bonding temperature was 950 °C, the atomic activity was already very high. Even the holding time was as short as 10 min and the interdiffusion at the bonding interface still ensured a high bonding quality and the joint shear strength reached a relatively high value of 494 MPa. The further increase of bonding time only improved the bonding quality and the joint shear strength to a limited extent. In consequence, the influence of holding time was small on the joint shear strength. As shown in Figure 6, when the holding time further increased, the Ti interlayer gradually transformed to the lamellar α + β dual-phase structure. The formation of lamellar α + β dual-phase structure in the Ti interlayer is beneficial to improve the joint shear strength. Thus, as the holding time extended, the joint shear strength gradually rose to 549 MPa. However, when the holding time further increased to 90 min, the high-hardness β phase was excessive in the joint. It affected the ductility of the joint, leading to a slight decrease in the joint shear strength. From the above analysis, it could be seen that for the diffusion bonding of the Ti2AlNb alloy and TC4 alloy with a Ti interlayer, a high shear strength of the joint could be achieved over a large range of bonding parameters. The maximum joint shear strength achieved in this study was much higher than the previously reported studies (brazing (359 MPa [26]) and TLP bonding (428 MPa) [37]). It proved the feasibility of diffusion bonding of the Ti2AlNb alloy and TC4 alloy using a Ti interlayer.
Figure 8 shows the fracture morphology of the Ti2AlNb/Ti/TC4 joint achieved at 950 °C for 60 min under a pressure of 10 MPa. To demonstrate more details on the fracture surface, two figures with different magnifications are given. They indicate that the fracture surface was characterized by a combination of ductile and brittle features. As shown in Figure 8a, the fracture surface consisted of two parts, which were the central part and the rest part, respectively. The enlargement in Figure 8b shows that the central part was covered with a large number of dimples and its fracture was a ductile rupture. In the rest part of the fracture surface, cleavage-like structures could be observed. The XRD analysis was conducted on the fracture surface. The results in Figure 9 suggest that the phase composition of the fracture surface was a mixture of α + β phases. Since both the TC4 alloy and the lamellar α + β dual-phase region consisted of α phase and β phase, it was supposed that the fracture occurred within the TC4 substrate or the lamellar α + β dual-phase region rather than the Ti2AlNb substrate.
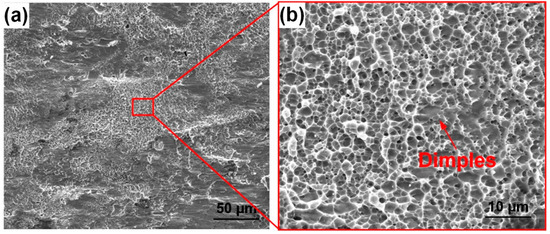
Figure 8.
Joint fracture surface (950 °C, 60 min, 10 MPa): (a) overall morphology; (b) enlarged morphology.
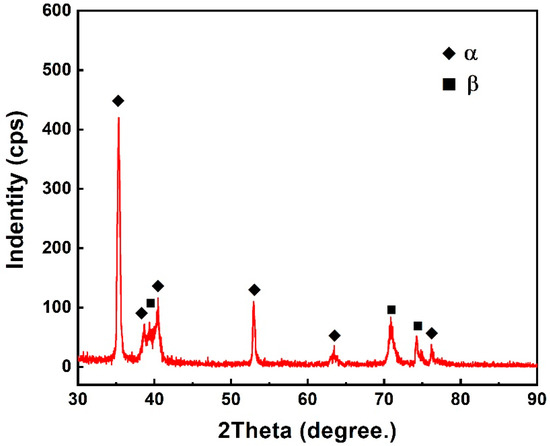
Figure 9.
XRD analysis results of the joint fracture surface.
4. Conclusions
(1) The diffusion of Nb from the Ti2AlNb substrate to the Ti interlayer led to the formation of a β phase in the Ti interlayer. The typical interfacial microstructure of the Ti2AlNb/Ti/TC4 joint was characterized by the lamellar α + β dual-phase zone.
(2) The bonding parameters determined the diffusion distance of Nb and Al elements, thus controlling the growth of the lamellar α + β dual-phase structure. With the increase of bonding temperature and holding time, the lamellar α + β dual-phase structure first appeared at the Ti2AlNb/Ti interface and then gradually extended to the entire Ti interlayer.
(3) When the Ti2AlNb alloy and TC4 alloy were bonded at 950 °C for 60 min under a pressure of 10 MPa, the elemental diffusion in the bonding couple was sufficient and the joint shear strength reached the maximum of 549 MPa.
Author Contributions
Conceptualization, G.F. and Y.W. (Yifeng Wang); methodology, G.F.; software, Y.W. (Yan Wei); validation, B.H. and Y.W. (Yan Wei); formal analysis, G.F. and Y.W. (Yan Wei); investigation, G.F. and Y.W. (Yifeng Wang); resources, B.H.; data curation, Y.W. (Yan Wei); writing—original draft preparation, G.F.; writing—review and editing, G.F. and Y.W. (Yan Wei); visualization, D.D.; supervision, Y.W. (Yifeng Wang) and D.D.; project administration, G.F.; funding acquisition, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 51905055), the Natural Science Foundation of Chongqing (No. cstc2020jcyj-msxmX0115), and the Fundamental Research Funds for the Central Universities Project (No. 2020CDJ-LHZZ-086).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest and non-financial interest requiring disclosure in this article.
References
- Dadé, M.; Esin, V.A.; Nazé, L.; Sallot, P. Short- and long-term oxidation behaviour of an advanced Ti2AlNb alloy. Corros. Sci. 2019, 148, 379–387. [Google Scholar] [CrossRef]
- Esin, V.A.; Mallick, R.; Dadé, M.; Denand, B.; Delfosse, J.; Sallot, P. Combined synchrotron X-ray diffraction, dilatometry and electrical resistivity in situ study of phase transformations in a Ti2AlNb alloy. Mater. Charact. 2020, 169, 110654. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, W.; Zhang, P.; Ma, H.; Li, D.; Xu, J.; Ma, X. Anti-correlation between solution strengthening and precipitation strengthening in lamellar O microstructures of a Ti2AlNb based alloy. J. Alloys Compd. 2020, 847, 156470. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yu, L.; Liang, H.; Huang, Y.; Ma, Z. Microstructures and tensile properties of Ti2AlNb and Mo-modified Ti2AlNb alloys fabricated by hot isostatic pressing. Mat. Sci. Eng. A 2020, 776, 139043. [Google Scholar] [CrossRef]
- Kumpfert, J. Intermetallic alloys based on orthorhombic titanium aluminide. Adv. Eng. Mater. 2001, 3, 851–864. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, N.; Liang, H.; Liu, Y. Phase transformation and microstructure control of Ti2AlNb-based alloys: A review. J. Mater. Sci. Technol. 2021, 80, 203–216. [Google Scholar] [CrossRef]
- Guo, X.; Ren, Z.; Ma, X.; Li, N.; Bian, L.; Wang, T. Effect of temperature and reduction ratio on the interface bonding properties of TC4/304 plates manufactured by EA rolling. J. Manuf. Process. 2021, 64, 664–673. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J.; Hu, S.; Wang, T.; Bu, X. Investigation of welding crack in laser welding-brazing welded TC4/6061 and TC4/2024 dissimilar butt joints. J. Manuf. Process. 2020, 60, 54–60. [Google Scholar] [CrossRef]
- Fu, Y.; Lv, M.; Zhao, Q.; Zhang, H.; Cui, Z. Investigation on the size and distribution effects of O phase on fracture properties of Ti2AlNb superalloy by using image-based crystal plasticity modeling. Mat. Sci. Eng. A 2021, 805, 140787. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Q.; Ma, Z.; Li, C.; Yu, L.; Liu, Y. Solution treatment for enhanced hardness in Mo-modified Ti2AlNb-based alloys. J. Alloys Compd. 2019, 805, 1184–1190. [Google Scholar] [CrossRef]
- Zhang, T.B.; Huang, G.; Hu, R.; Li, J.S. Microstructural stability of long term aging treated Ti–22Al–26Nb–1Zr orthorhombic titanium aluminide. Trans. Nonferrous Met. Soc. China 2015, 25, 2549–2555. [Google Scholar] [CrossRef]
- Du, Y.J.; Xiong, J.T.; Jin, F.; Li, S.W.; Yuan, L.; Feng, D.; Shi, J.M.; Li, J.L. Microstructure evolution and mechanical properties of diffusion bonding Al5(TiZrHfNb)95 refractory high entropy alloy to Ti2AlNb alloy. Mat. Sci. Eng. A 2021, 802, 140610. [Google Scholar] [CrossRef]
- Yuan, L.; Xiong, J.T.; Du, Y.J.; Wang, Y.; Shi, J.M.; Li, J.L. Effects of pure Ti or Zr powder on microstructure and mechanical properties of Ti6Al4V and Ti2AlNb joints brazed with TiZrCuNi. Mat. Sci. Eng. A 2020, 788. [Google Scholar] [CrossRef]
- Shi, J.M.; Zhang, L.X.; Chang, Q.; Sun, Z.; Feng, J.C. Strengthening the ZrC-SiC ceramic and TC4 alloy brazed joint using laser additive manufactured functionally graded material layers. Ceram. Int. 2018, 44, 11060–11069. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, M.; Yang, Z.; Wang, D.; Liu, Y. Vacuum brazing of Ti2AlNb and TC4 alloys using Ti–Zr–Cu–Ni and Ti–Zr–Cu–Ni+Mo filler metals: Microstructural evolution and mechanical properties. Arch. Civ. Mech. Eng. 2018, 18, 546–556. [Google Scholar] [CrossRef]
- Yang, R.; Lin, F.; Zhu, Y.; Yao, Y.; Lin, P. Microstructure and mechanical properties of diffusion bonded joints of TC4/Ti2AlNb dissimilar alloys. Hot Work. Technol. 2020, 49, 20–24. [Google Scholar] [CrossRef]
- Lei, Z.L.; Dong, Z.J.; Chen, Y.B.; Huang, L.; Zhu, R.C. Microstructure and mechanical properties of laser welded Ti–22Al–27Nb/TC4 dissimilar alloys. Mat. Sci. Eng. A 2013, 559, 909–916. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, M.; Zhang, K.; Chen, Y. Effect of laser beam displacement on characteristics of laser welded Ti2AlNb/TC4 dissimilar alloy. Chin. J. Lasers 2015, 42, 1203008. [Google Scholar] [CrossRef]
- Galeyev, R.M.; Valiakhmetov, O.R.; Safiullin, R.V.; Imayev, V.M.; Imayev, R.M.; Fecht, H.J. Development of laminated composite materials based on orthorhombic aluminide/titanium alloy and their tensile mechanical properties. Mater. Sci. Tech. Lond. 2009, 25, 1485–1488. [Google Scholar] [CrossRef]
- Tan, L.; Yao, Z.; Qin, C.; Guo, H.; Li, S. Linear friction and electron beam welded joints of Ti2AlNb/TC11. Adv. Mat. Res. 2010, 97, 3895–3898. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, Y.; Zhang, B.; Zhang, L. Controlling interfacial reactions of Ti3SiC2/Ti2AlNb brazed joints by transferring graphene layers. Mat. Sci. Eng. A 2020, 771, 138624. [Google Scholar] [CrossRef]
- Xiong, J.T.; Sun, J.R.; Wang, J.C.; Zhang, H.; Shi, J.M.; Li, J.L. Evaluation of the bonded ratio of TC4 diffusion bonded joints based on ultrasonic C-scan. J. Manuf. Process. 2019, 47, 238–243. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Q.; Tian, X.; Xiong, J.; Li, J.; Zhang, L.; Feng, J. Brazing of Gr/2024Al composite and TC4 using AgCuTi filler with the assist of Ti-Al-C-Cu interlayer. J. Manuf. Process. 2019, 47, 211–218. [Google Scholar] [CrossRef]
- Ren, H.S.; Xiong, H.P.; Chen, B.; Pang, S.J.; Chen, B.Q.; Ye, L. Vacuum brazing of Ti3Al-based alloy to TiAl using TiZrCuNi(Co) fillers. J. Manuf. Process. 2015, 224, 26–32. [Google Scholar] [CrossRef]
- He, Y.; Lu, C.; Ni, C.; Chen, Q.; Zheng, W.; Wang, D.; Wei, L.; Wang, L.; Sun, Y.; Zou, H.; et al. Tailoring microstructure and mechanical performance of the TC4 titanium alloy brazed joint through doping rare-earth element Dy into Ti-Cu-Ni filler alloy. J. Manuf. Process. 2020, 50, 255–265. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.Q.; Yang, Z.W.; Wang, D.P.; Liu, X.G.; Liu, Y.C. Effects of Nb content in Ti-Ni-Nb brazing alloys on the microstructure and mechanical properties of Ti-22Al-25Nb alloy brazed joints. J. Mater. Sci. Technol. 2017, 33, 682–689. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.Q.; Yang, Z.W.; Wang, D.P.; Liu, X.G.; Liu, Y.C. Diffusion bonding of Ti2AlNb alloy using pure Ti foil as an interlayer. J. Alloys Compd. 2018, 756, 163–174. [Google Scholar] [CrossRef]
- Duarte, L.I.; Ramos, A.S.; Vieira, M.F.; Viana, F.; Vieira, M.T.; Kocak, M. Solid-state diffusion bonding of gamma-TiAl alloys using Ti/Al thin films as interlayers. Intermetallics 2006, 14, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Zhang, J.H.; Zhou, R.L.; Li, X.Q. Diffusion bonding technology of a titanium alloy to a stainless steel web with an Ni interlayer. Mater. Charact. 1999, 43, 287–292. [Google Scholar] [CrossRef]
- Simoes, S.; Viana, F.; Ventzke, V.; Kocak, M.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Diffusion bonding of TiAl using Ni/Al multilayers. J. Mater. Sci. 2010, 45, 4351–4357. [Google Scholar] [CrossRef]
- Ren, H.S.; Ren, X.Y.; Xiong, H.P.; Li, W.W.; Pang, S.J.; Ustinov, A.I. Nano-diffusion bonding of Ti2AlNb to Ni-based superalloy. Mater. Charact. 2019, 155. [Google Scholar] [CrossRef]
- Pederson, R.; Gaddam, R.; Antti, M.L. Microstructure and mechanical behavior of cast Ti-6Al-4V with addition of boron. Cent. Eur. J. Eng. 2012, 2, 347–357. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Chen, M.H.; Wang, H.; Wang, N.; Ouyang, J.D.; Li, X.X. Thermal deformation behavior and mechanism of intermetallic alloy Ti2AlNb. Trans. Nonferrous Met. Soc. China 2016, 26, 722–728. [Google Scholar] [CrossRef]
- Xu, S.; Liang, Y.; Lin, J.; Zhang, H.; Yang, G.; Guo, X.; Fashu, S.; He, J. The partial liquidus projection and solidification sequences in Ti-(40-60) Al-(5-30) Nb alloys. J. Alloys Compd. 2021, 858. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Xin, S.W.; Zeng, W.D. Effect of major alloying elements on microstructure and mechanical properties of a highly beta stabilized titanium alloy. J. Alloys Compd. 2009, 481, 190–194. [Google Scholar] [CrossRef]
- Lin, P.; Xi, X.Z.; Zhao, W.K.; Yang, R.H.; Lin, F.; Cui, X.L.; Liu, G. Microstructure and mechanical properties of Ti-6Al-4V/Ti-22Al-25Nb joint formed by diffusion bonding. Trans. Nonferrous Met. Soc. China 2021, 31, 1339–1349. [Google Scholar] [CrossRef]
- Cai, X.Q.; Wang, Y.; Yang, Z.W.; Wang, D.P.; Liu, Y.C. Transient liquid phase (TLP) bonding of Ti2AlNb alloy using Ti/Ni interlayer: Microstructure characterization and mechanical properties. J. Alloys Compd. 2016, 679, 9–17. [Google Scholar] [CrossRef]
- Ankem, S.; Margolin, H.; Greene, C.A.; Neuberger, B.W.; Oberson, P.G. Mechanical properties of alloys consisting of two ductile phases. Prog. Mater. Sci. 2006, 51, 632–709. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J.C. Perspectives on titanium science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).