Stabilization of Superionic-Conducting High-Temperature Phase of Li(CB9H10) via Solid Solution Formation with Li2(B12H12)
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
2.3. Battery Test
3. Results and Discussion
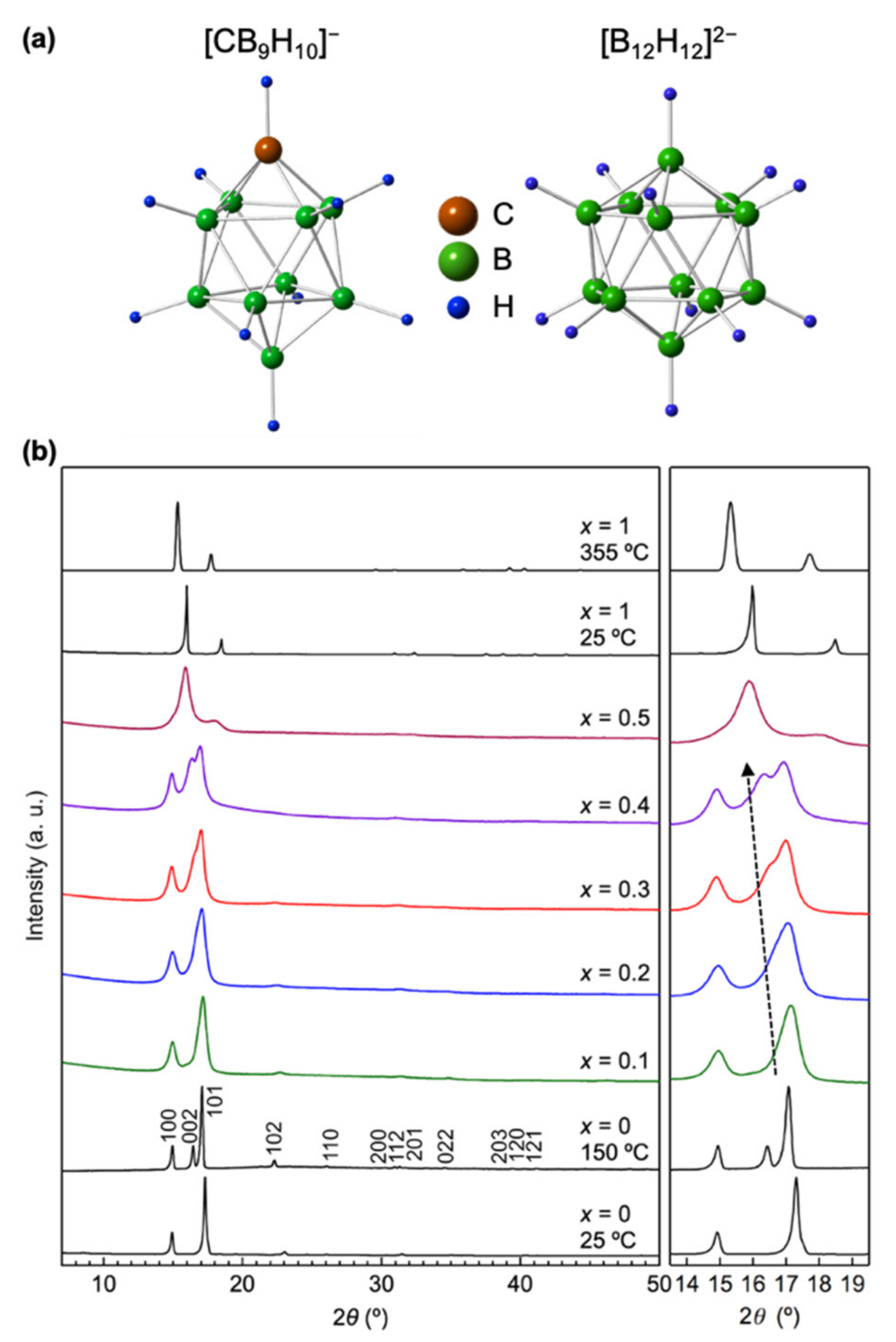
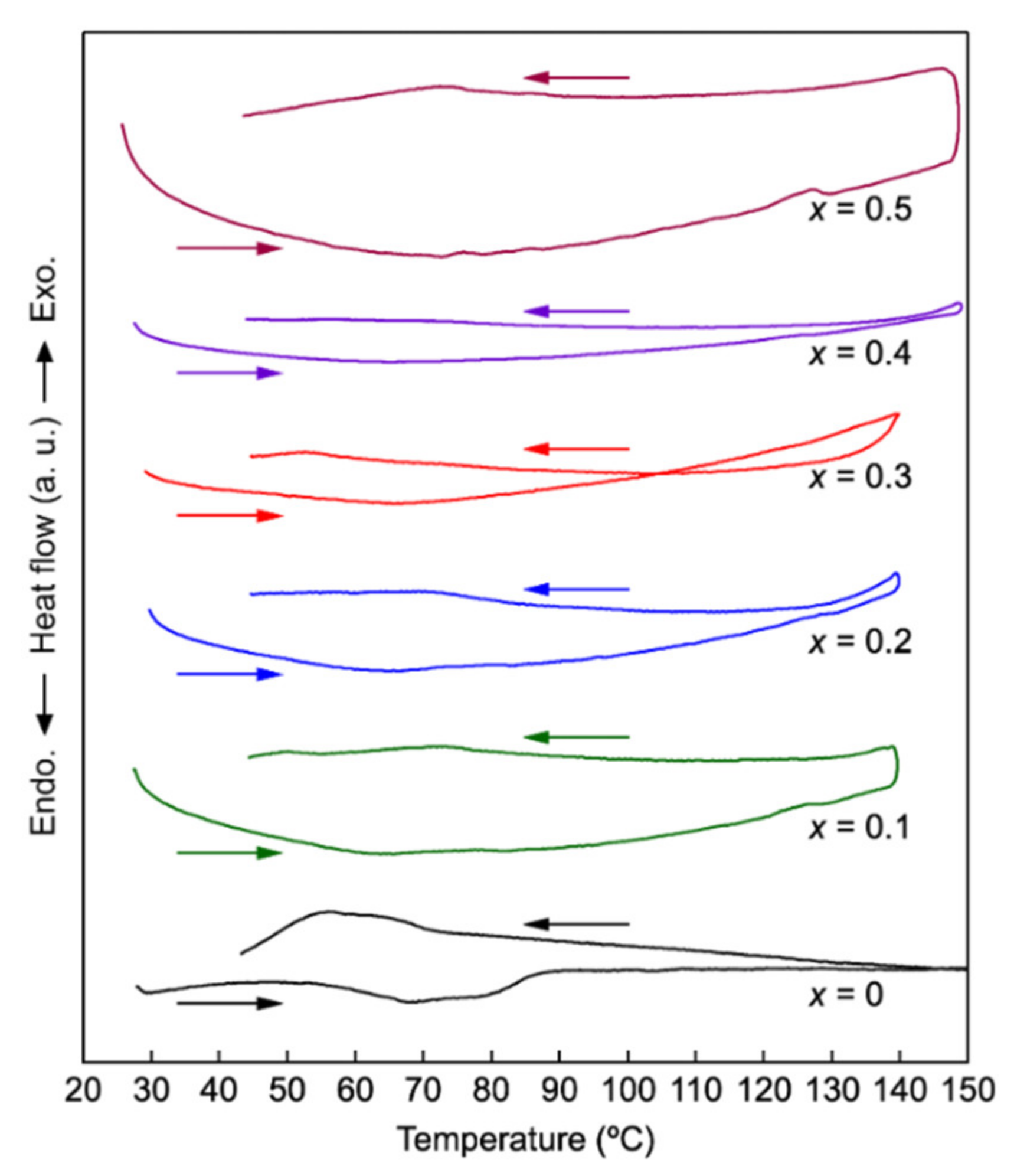
3.1. Synthesis and Characterization
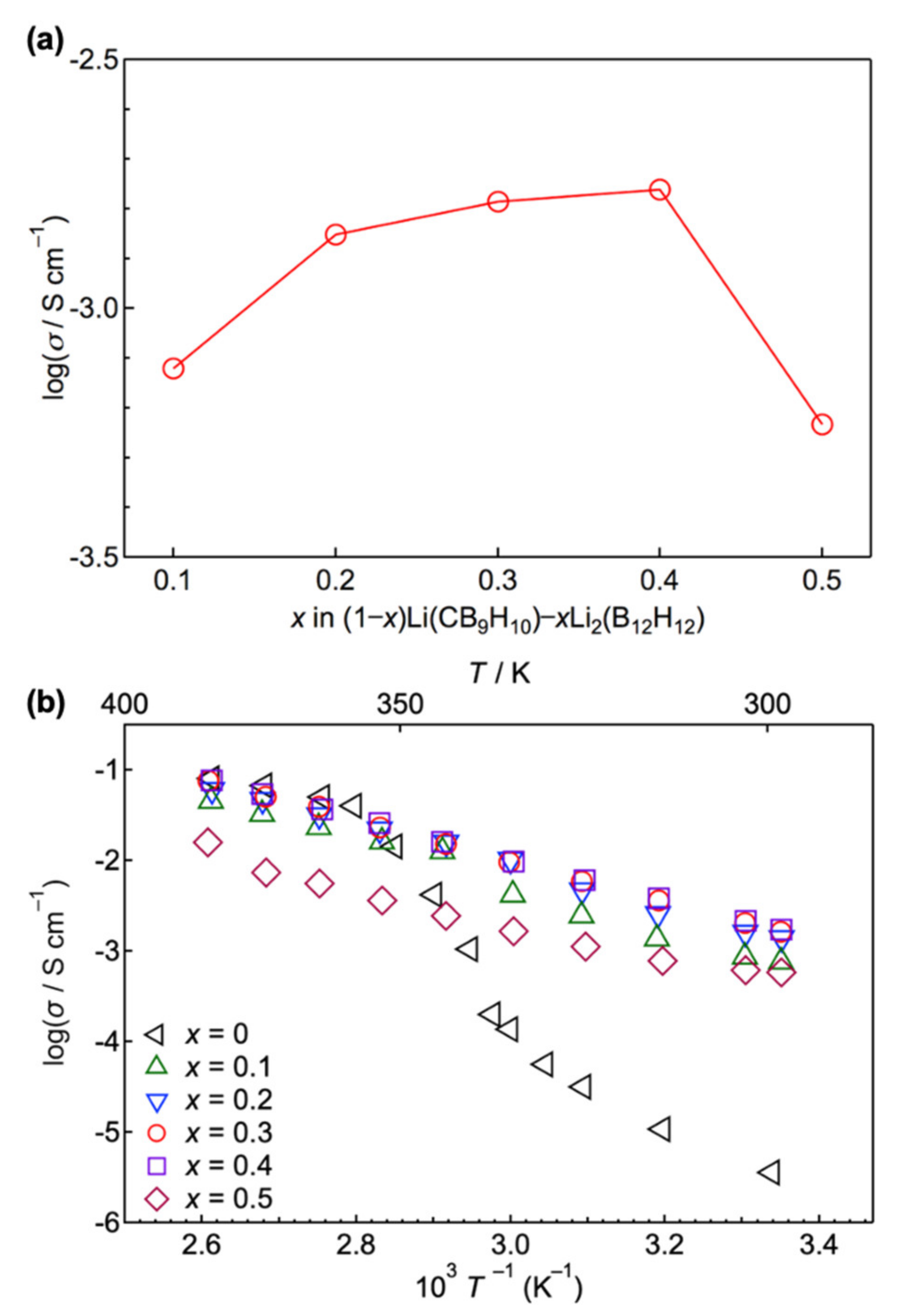
3.2. Ionic Conductivity
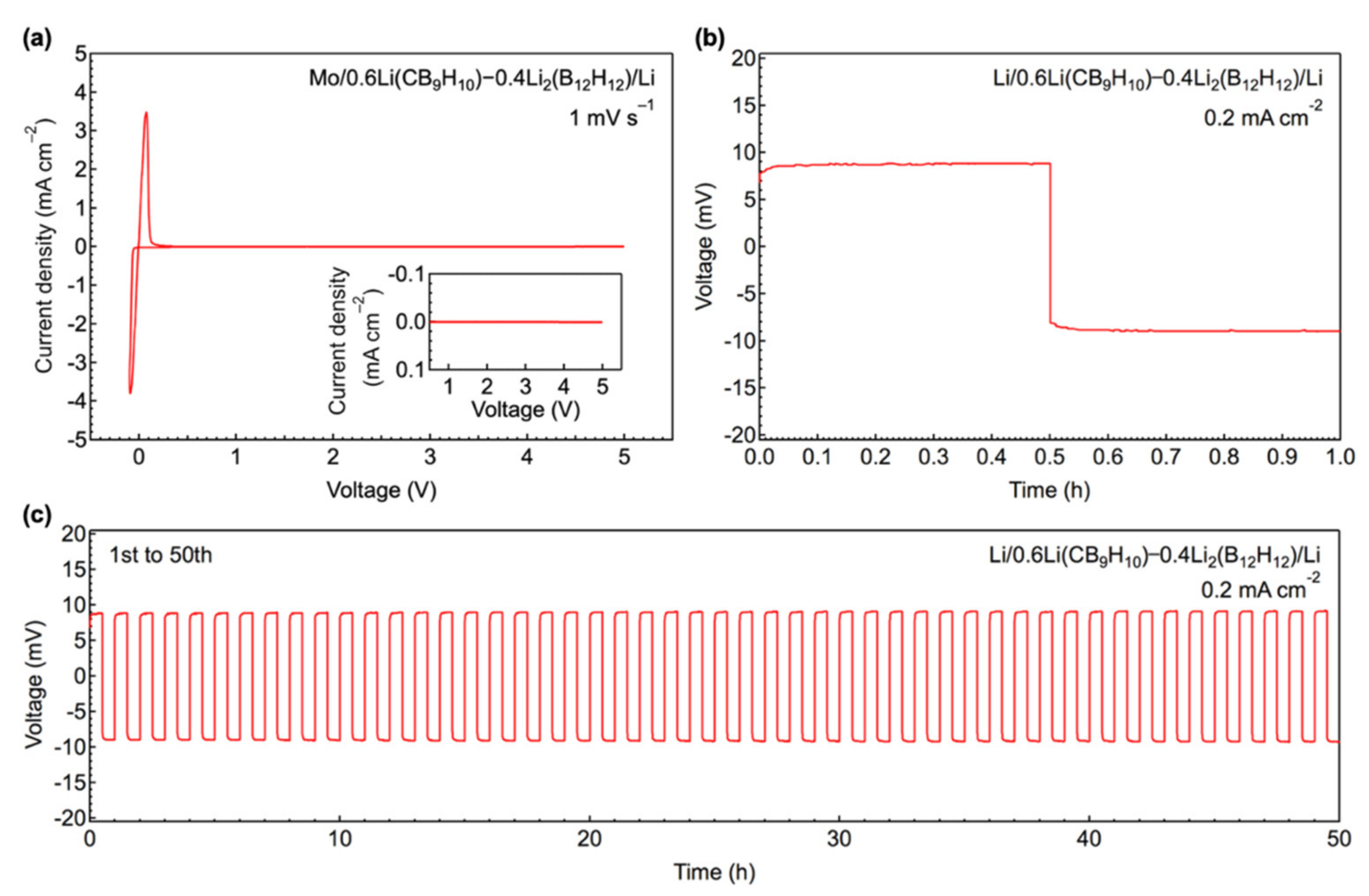
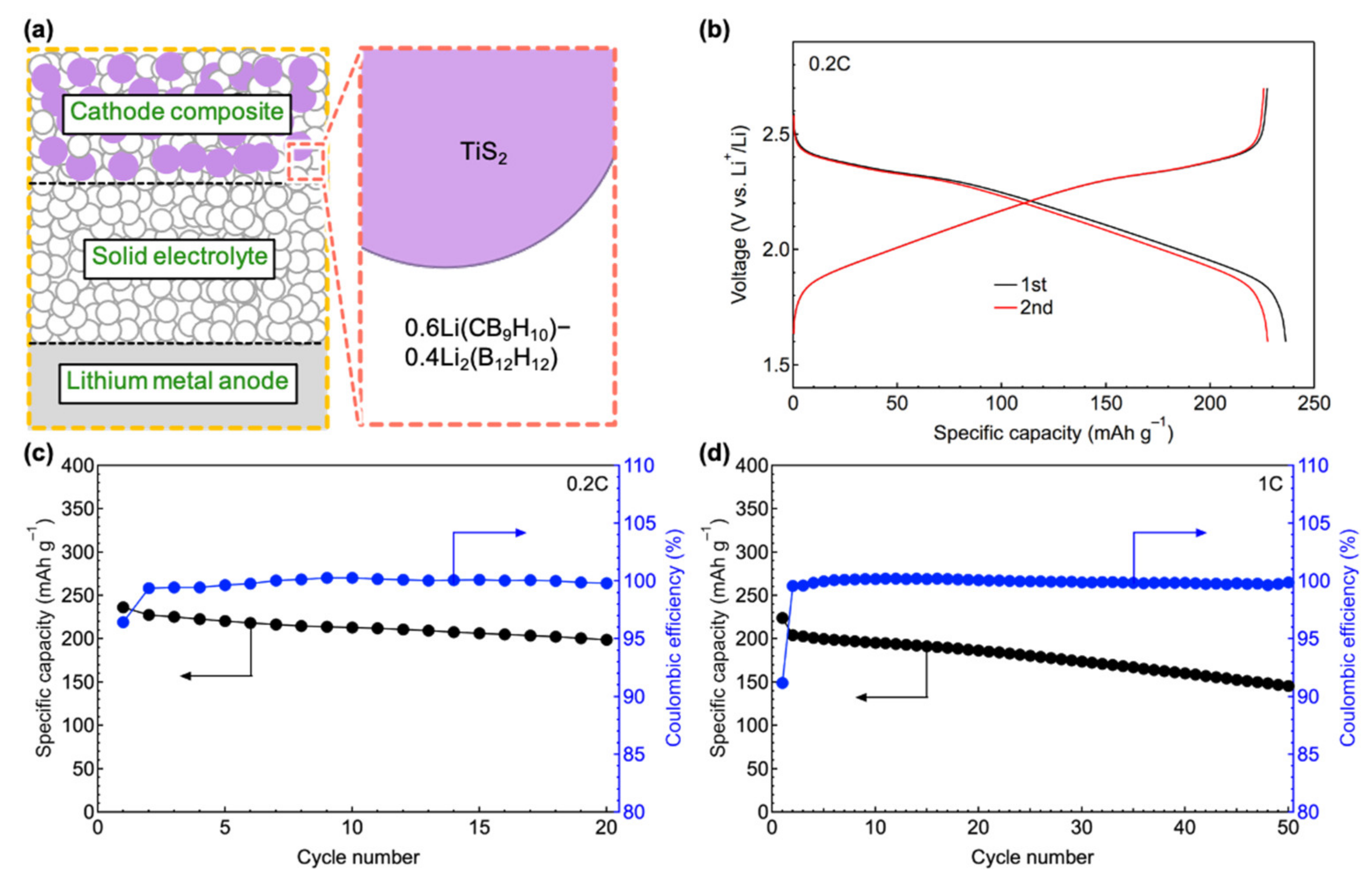
3.3. All-Solid-State Battery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Kim, S.; Oguchi, H.; Toyama, N.; Sato, T.; Takagi, S.; Otomo, T.; Arunkumar, D.; Kuwata, N.; Kawamura, J.; Orimo, S.-I. A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Deiseroth, H.-J.; Kong, S.-T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiss, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids with an Unusually High Li+ Mobility. Angew. Chem. Int. Ed. 2008, 47, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, K.; Heubner, C.; Kusnezoff, M. Reduced Sintering Temperatures of Li+ Conductive Li1.3Al0.3Ti1.7(PO4)3 Ceramics. Crystals 2020, 10, 408. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Kim, S.; Hirayama, M.; Taminato, S.; Kanno, R. Epitaxial growth and lithium ion conductivity of lithium-oxide garnet for an all solid-state battery electrolyte. Dalton Trans. 2013, 42, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hirayama, M.; Cho, W.; Kim, K.; Kobayashi, T.; Kaneko, R.; Suzuki, K.; Kanno, R. Low temperature synthesis and ionic conductivity of the epitaxial Li 0.17 La 0.61 TiO 3 film electrolyte. CrystEngComm. 2014, 16, 1044–1049. [Google Scholar] [CrossRef]
- Shi, J.; Hong, Y.; Zhu, C. Effect of Chromium on Electrochemical and Mechanical Properties of Beta-Al2O3 Solid Electrolyte Synthesized Via a Citrate-nitrate Combustion Method. Crystals 2020, 10, 987. [Google Scholar] [CrossRef]
- Matsuo, M.; Nakamori, Y.; Orimo, S.-I.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Kim, S.; Harada, K.; Toyama, N.; Oguchi, H.; Kisu, K.; Orimo, S.-I. Room temperature operation of all-solid-state battery using a closo-type complex hydride solid electrolyte and a LiCoO2 cathode by interfacial modification. J. Energy Chem. 2020, 43, 47–51. [Google Scholar] [CrossRef]
- Asakura, R.; Duchêne, L.; Kühnel, R.-S.; Remhof, A.; Hagemann, H.; Battaglia, C. Electrochemical Oxidative Stability of Hydroborate-Based Solid-State Electrolytes. ACS Appl. Energy Mater. 2019, 2, 6924–6930. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Hu, Y.; Sørby, M.; Mæhlen, J.; Vullum, P.; Fjellvåg, H.; Hauback, B. Reversibility of metal-hydride anodes in all-solid-state lithium secondary battery operating at room temperature. Solid State Ion. 2018, 317, 263–267. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage-past, recent progress and future outlook. J. Alloy. Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S.-I. Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef]
- Blanchard, D.; Nale, A.; Sveinbjörnsson, D.; Eggenhuisen, T.M.; Verkuijlen, M.H.W.; Vegge, T.; Kentgens, A.P.M.; De Jongh, P.E. Nanoconfined LiBH4as a Fast Lithium Ion Conductor. Adv. Funct. Mater. 2015, 25, 184–192. [Google Scholar] [CrossRef]
- Oguchi, H.; Kim, S.; Maruyama, S.; Horisawa, Y.; Takagi, S.; Sato, T.; Shimizu, R.; Matsumoto, Y.; Hitosugi, T.; Orimo, S.-I. Epitaxial Film Growth of LiBH4 via Molecular Unit Evaporation. ACS Appl. Electron. Mater. 2019, 1, 1792–1796. [Google Scholar] [CrossRef]
- Matsuo, M.; Remhof, A.; Martelli, P.; Caputo, R.; Ernst, M.; Miura, Y.; Sato, T.; Oguchi, H.; Maekawa, H.; Takamura, H.; et al. Complex Hydrides with (BH4)−and (NH2)−Anions as New Lithium Fast-Ion Conductors. J. Am. Chem. Soc. 2009, 131, 16389–16391. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Song, T.; Miyaoka, H.; Shinzato, K.; Miyaoka, H.; Ichikawa, T.; Shi, S.; Zhang, X.; Isobe, S.; et al. Ammonia, a Switch for Controlling High Ionic Conductivity in Lithium Borohydride Ammoniates. Joule 2018, 2, 1522–1533. [Google Scholar] [CrossRef]
- Tang, W.S.; Unemoto, A.; Zhou, W.; Stavila, V.; Matsuo, M.; Wu, H.; Orimo, S.-I.; Udovic, T.J. Unparalleled lithium and sodium superionic conduction in solid electrolytes with large monovalent cage-like anions. Energy Environ. Sci. 2015, 8, 3637–3645. [Google Scholar] [CrossRef]
- Tang, W.S.; Matsuo, M.; Wu, H.; Stavila, V.; Zhou, W.; Talin, A.A.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; et al. Liquid-Like Ionic Conduction in Solid Lithium and Sodium Monocarba- closo -Decaborates Near or at Room Temperature. Adv. Energy Mater. 2016, 6, 1502237. [Google Scholar] [CrossRef]
- Kim, S.; Toyama, N.; Oguchi, H.; Sato, T.; Takagi, S.; Ikeshoji, T.; Orimo, S.-I. Fast Lithium-Ion Conduction in Atom-Deficient closo-Type Complex Hydride Solid Electrolytes. Chem. Mater. 2018, 30, 386–391. [Google Scholar] [CrossRef]
- Toyama, N.; Kim, S.; Oguchi, H.; Sato, T.; Takagi, S.; Tazawa, M.; Nogami, G.; Orimo, S.-I. Lithium ion conductivity of complex hydrides incorporating multiple closo-type complex anions. J. Energy Chem. 2019, 38, 84–87. [Google Scholar] [CrossRef]
- Kim, S.; Kisu, K.; Takagi, S.; Oguchi, H.; Orimo, S.-I. Complex Hydride Solid Electrolytes of the Li(CB9H10)–Li(CB11H12) Quasi-Binary System: Relationship between the Solid Solution and Phase Transition, and the Electrochemical Properties. ACS Appl. Energy Mater. 2020, 3, 4831–4839. [Google Scholar] [CrossRef]
- Sun, Y.; Suzuki, K.; Hori, S.; Hirayama, M.; Kanno, R. Superionic Conductors: Li10+δ[SnySi1–y]1+δP2−δS12 with a Li10GeP2S12-type Structure in the Li3PS4–Li4SnS4–Li4SiS4 Quasi-ternary System. Chem. Mater. 2017, 29, 5858–5864. [Google Scholar] [CrossRef]
- Tang, W.S.; Yoshida, K.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; Dimitrievska, M.; Stavila, V.; Orimo, S.-I.; Udovic, T.J. Stabilizing Superionic-Conducting Structures via Mixed-Anion Solid Solutions of Monocarba-closo-borate Salts. ACS Energy Lett. 2016, 1, 659–664. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693. [Google Scholar] [CrossRef]
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg rechargeable batteries: An on-going challenge. Energy Environ. Sci. 2013, 6, 2265–2279. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kisu, K.; Orimo, S.-i. Stabilization of Superionic-Conducting High-Temperature Phase of Li(CB9H10) via Solid Solution Formation with Li2(B12H12). Crystals 2021, 11, 330. https://doi.org/10.3390/cryst11040330
Kim S, Kisu K, Orimo S-i. Stabilization of Superionic-Conducting High-Temperature Phase of Li(CB9H10) via Solid Solution Formation with Li2(B12H12). Crystals. 2021; 11(4):330. https://doi.org/10.3390/cryst11040330
Chicago/Turabian StyleKim, Sangryun, Kazuaki Kisu, and Shin-ichi Orimo. 2021. "Stabilization of Superionic-Conducting High-Temperature Phase of Li(CB9H10) via Solid Solution Formation with Li2(B12H12)" Crystals 11, no. 4: 330. https://doi.org/10.3390/cryst11040330
APA StyleKim, S., Kisu, K., & Orimo, S.-i. (2021). Stabilization of Superionic-Conducting High-Temperature Phase of Li(CB9H10) via Solid Solution Formation with Li2(B12H12). Crystals, 11(4), 330. https://doi.org/10.3390/cryst11040330




