Abstract
The alleviation of iron corrosion in 3.5% NaCl sodium chloride solution using N,N′-bis[2-methoxynaphthylidene]amino]oxamide (MAO) as a corrosion inhibitor has been reported. The work was achieved using various investigation techniques. Potentiodynamic cyclic polarization (PCP) displayed a powerful inhibition for the corrosion via reducing the iron’s cathodic and anodic reactions. This was reflected in reduced corrosion currents and increased polarization resistances in the presence and upon the increase of MAO concentration. The electrochemical impedance spectroscopy results indicated that MAO molecules provoke the corrosion resistance via increasing polarization resistance. The power of MAO on decreasing pitting attack was also investigated through measuring the change of current with time at −0.475 V(Ag/AgCl). Scanning electron microscopy images were taken of the surface after the current–time measurements were performed in the absence and presence of MAO. The current-time experiments indicated that MAO highly mitigates the corrosion of iron. The energy dispersive X-ray analyzer reported the products found on the tested surfaces. The effect of extending the exposure time from 1 h to 48 h was also tested and was found to alleviate the corrosion of iron, whether MAO molecules are absent or present.
1. Introduction
Iron is known to be the base metal for most of the current employed pipelines that have been used in water and petroleum oil transportation and many other applications. Iron is a main component that is essential in making hundreds of alloys in addition to the manufacture of ships, rails, bridges, pipes, boilers, reinforcement steel, etc. The issue of corrosion as a general and important phenomenon is well known to disturb the majority of iron’s applications. Interestingly, iron corrosion causes a loss of a quarter of the world’s production in a year, and this is an economic catastrophe. Some industrial practices using iron in a chloride medium can cause potential corrosion, including the corrosion of the food cans, pipes buried in the soil, ships, bridges, and oil pipelines due to the high salinity of the water, and there are other examples of the corrosion of many metal parts exposed to industrial media, such as acids, bases, salt water, etc. Moreover, the concentration of 3.5% NaCl represents the percent of sodium chloride in the natural seawater solution. Most of the pipelines are crossing or passing nearby seawater and marine environments. The dissolution of iron due to the presence of the chloride ions is considered as one of the main reasons for the occurrence of corrosion of these materials. From the point of economical view, the development of methods and materials to minimize corrosion should be applied [1,2,3,4,5]. Corrosion inhibitors are generally believed to effectively mitigate the destructive impacts of aggressive environments and prevent the dissolution of metals and alloys. The effective action of those corrosion inhibitors is attributed to their molecules interacting with the metals via the functional groups of these inhibitors [6,7,8,9,10,11,12,13,14].
It has been reported [9,10,11,12] that organic compounds that have the ability to be adsorbed onto the surface of metals and alloys can act as corrosion inhibitors. These inhibitors control corrosion via delaying the anodic dissolution or restricting the cathodic reduction or inhibiting both anodic and cathodic reactions at the same time. Corrosion inhibitors can also be incorporated within coatings and the resultant coating can be applied onto the surface of metals before being in contact with the aggressive media [11,13].
Several investigators have reported [15,16,17,18,19,20,21,22] that organic compounds can act as powerful corrosion inhibitors for numerous metals and alloys in various corrosive media. Particularly, those compounds with polar groups that include N, O, and S and double bonds are excellent corrosion inhibitors. This is attributed to their tendency to interact with surfaces of the metals via their adsorption [23]. Moreover, this adsorption is affected by the number of points such as the charge of the surface of metal, its nature, the mode of adsorption, the structure of the inhibitor, and finally the corrosiveness of the medium [18]. Several compounds with these characteristics have been reported to be efficient corrosion inhibitors for iron in corrosive media; for example, 3-amino-5-mercapto-1,2,4-triazole [1], benzotriazole [2], 5-(3-aminophenyl)-tetrazole [3], 1,1’-thiocarbonyldiimidazole [5], amino acids [7], monoalkyl phosphate esters [19], methionine [21], and cetylpyridinium chloride [22], etc.
The objective of this work was to control the corrosion of iron in 3.5% NaCl solution using N,N′-bis[2-methoxynaphthylidene]amino]oxamide (MAO). Lower concentrations of MAO; namely, 5 × 10−5 M, 1 × 10−4 M, and 5 × 10−4 M, were added to 3.5% NaCl solution and the corrosion behavior was evaluated in these solutions after two periods of time; i.e., 1 h and 48 h. To the best of our knowledge, MAO has not been used before as a corrosion inhibitor for any metal or alloy. Various electrochemical methods were employed; specifically, the potentiodynamic cyclic polarization, the change of the current with time at an active potential, and the traditional electrochemical impedance spectroscopy. The surface after corrosion was inspected using a scanning electron microscopy (SEM) along with an energy dispersive X-ray unit that was attached to the SEM.
2. Experimental Materials and Methods
An iron (spec pure, 99.999% purity) electrode was purchased from Goodfellow (Ermine Business Park, Huntingdon, England) and was used in this study. The electrochemical experiments were collected using a three-electrode electrochemical cell that accommodates 0.250 L NaCl solution. The iron served as the working electrode and was prepared via welding one face with a copper wire, then impeding it in nonconductive epoxy resin. The surface of the iron electrode was finished by grinding it with different emery papers starting from 200 grit to 800 grit before abrasion with alumina slurry of 0.5 µ and 0.3 µ, respectively. A Pt sheet and silver–silver chloride were the auxiliary and reference electrodes, respectively. The organic compound MAO powder was dissolved in absolute ethanol to prepare a stock solution of 10−2 M; other MAO concentrations were prepared by dilution. At the same time, a 1000 mL of 7 wt.% NaCl stock solution was prepared by weighing 70 gm NaCl powder, which was dissolved in a bidistilled water and the 3.5% NaCl solutions were prepared also by dilution.
For all electrochemical techniques, a computer-controlled Auto-lab Potentiostat-Galvanostat model PGSTAT302N was purchased from Metrohm (Amsterdam, The Netherlands), employed using a version 4.9 general purpose electrochemical software (GPES). The GPES software was used to collect the experimental curves and spectra. Potentiostatic cyclic polarization (PCP) forward and backward curves were obtained between a potential ranging from −1.800 V to −0.500 V (Ag/AgCl) with a scan rate of 1.67 mV/s. The potentiostatic current versus time curves were obtained after maintaining the potential at a fixed amount of −0.600. The electrochemical impedance spectroscopy (EIS) data were imported from the corrosion potential (OCP) values at a range of frequency between 105 Hz and 10−1 Hz. The EIS data were fitted using the ZSimpWin v3.1 program to obtain an equivalent circuit model and the impedance parameters. A complete procedure for EIS data has been reported in the previous studies [1,3,5]. To ensure the reproducibility, all experiments were running in triplicate employing a fresh sample surface and a new solution portion for each experiment. The UV–visible absorption was scanned on a Shimadzu (UV-1900) spectrophotometer within 200–1100 nm. A scanning electron microscope (SEM, JEOL, Tokyo, Japan) was used to acquire the surface examination for iron. A unit of an energy dispersive X-ray (EDX) analyzer was attached to the SEM instrument and used to obtain the elemental analysis for the investigated surfaces via the EDX profiles. The SEM machine and the EDX unit were operated at 15 kV.
3. Results and Discussion
3.1. Chemistry and Characterization of the Inhibitor
N,N′-bis[2-methoxynaphthylidene]amino]oxamide (MAO) derived from the interaction between ethanedihydrazide and o-anisaldehyde was isolated and structurally identified in previous works (see also Supplementary Materials file, Figures S1 and S2) [24,25,26,27,28]. The UV–visible spectra of the inhibitor were performed in Dimethylformamide (DMF, Figure 1). The electronic data ascertained the MAO structure and exhibited five absorption bands at λmax (nm) equal to 376 (n-π*, C=N), 374 (n-π*, C=O), 372 (π-π*, C=N), 369 (π-π*, C=O) and 366 (π-π*, aromatic ring). The template synthesis of the inhibitor (aromatic oxamide, MAO) derived from dihydrazide and aldehyde is represented in Scheme 1.
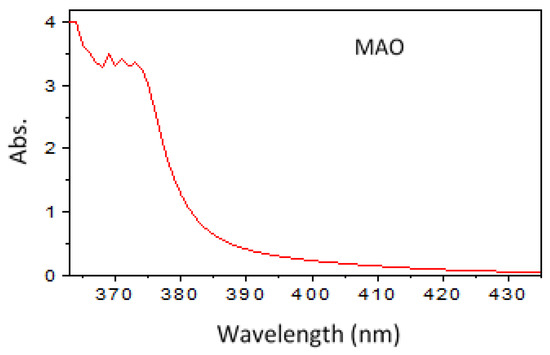
Figure 1.
UV–visible spectrum of the utilized inhibitor, N,N′-bis[2-methoxynaphthylidene]amino]oxamide (MAO), in dimethylformamide (DMF).
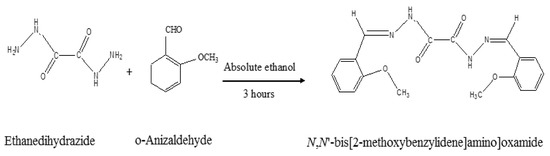
Scheme 1.
Synthesis and proposed structure of N,N′-bis[2-methoxynaphthylidene]amino]oxamide (MAO).
3.2. Potentiodynamic Cyclic Polarization (PCP)
The measured PCP curves for iron after 1 h in (a) 3.5% NaCl solutions in the absence and presence of (b) 1 × 10−4 M MAO, (c) 5 × 10−4 M MAO, and (d) 1 × 10−3 M MAO are shown respectively in Figure 2. Similar curves were acquired for iron at the same condition after 48 h in the different solutions and the curves are exhibited in Figure 3. The polarization data estimated from the PCP curves are listed in Table 1. Herein, ECorr = the corrosion potential, jCorr = corrosion current density, βc = cathodic Tafel slope, βa = anodic Tafel slope, RP = polarization resistance, and RCorr = corrosion rate. Here, the values of jCorr and ECorr were drawn as in the previous works [1,3,5,29,30,31,32,33,34]. The values of RP and RCorr were calculated from these relations [29,30,31,32,33,34]:
where, the values for iron for k = 3272, EW = 27.92, d = 7.87 g/cm3, and A = 1 cm2; the definition of these variables is reported elsewhere [1,3]. For the CPP curve obtained for iron in NaCl solution without MAO present (Figure 2a), the current is seized in the cathodic branch by sweeping the potential in the positive direction. This resulted from the reduction of oxygen on iron [1,3,5]:
2H2O + O2 + 4e− → 4OH−
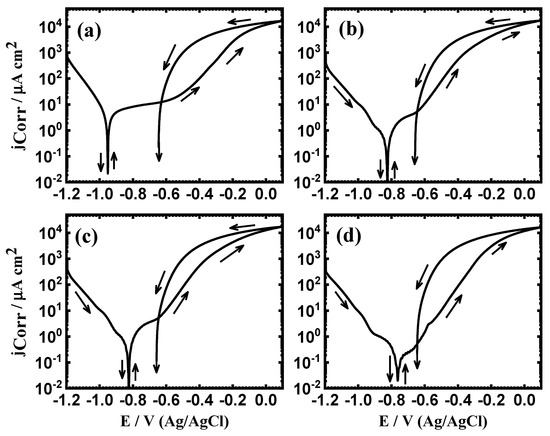
Figure 2.
Potentiodynamic cyclic polarization (PCP) curves for iron rod after 1 h immersion in (a) 3.5% NaCl without and with (b) 5 × 10−5 M MAO, (c) 1 × 10−4 M MAO, and (d) 5 × 10−5 M MAO present, respectively.
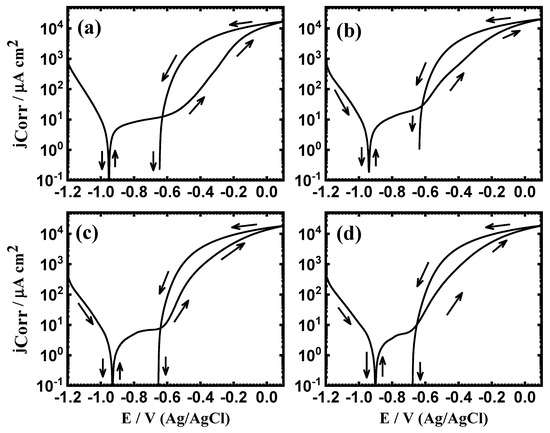
Figure 3.
PCP curves of iron after 48 h in (a) 3.5% NaCl without and with (b) 5 × 10−5 M MAO, (c) 1 × 10−4 M MAO, and (d) 5 × 10−5 M MAO present, respectively.

Table 1.
PCP data for iron in the different test solutions.
Upon proceeding with the potential in the positive side, the current increases in the anodic side due to the oxidation of iron via the anodic dissolution as follows [1,3,5]:
Fe0 = Fe2+ + 2e−
The increase of current then gets stabilized due to the formation of a passive region of an oxide film on the surface as follows [1]:
Fe0 + ½ O2 + H2O → Fe(OH)2
The formed hydroxide is unstable and thus transfers to a stable oxide as per this reaction:
½ O2 + 3Fe(OH)2 → Fe3O4 + 3H2O
After this passive region, the current rapidly and suddenly increases due to the breakdown of Fe3O4 surface film. This promotes the occurrence of pitting attack, as was confirmed by the swept potential in the opposite direction. Here, the currents in the backward side were greater than the ones in the forward. The difference between the values in the forward and backward directions led to a huge hysteresis loop; the bigger the size of this loop, the higher the intensity of the pitting corrosion [1,3,5,35,36].
The addition of MAO at 5 × 10−5 M seized the cathodic and anodic reactions and also minimized the current of the passive region. This was further confirmed by the values recorded in Table 1, where jCorr and RCorr were lower compared to those for the iron in 3.5% NaCl only. In addition, the value of polarization resistance, RP, was augmented. Increasing the concentration of MAO to 1 × 10−4 M showed more decrements in the corrosion of iron. The lowest corrosion currents and corrosion rate and the highest polarization resistance were recorded when MAO addition was raised to 5 × 10−4 M.
To give a quantification on the ability of MAO on the inhibition of iron corrosion, the inhibition efficiency of MAO at the different concentrations was given via this relation [1,3,5]:
Herein, is the jCorr for iron in NaCl solution alone, while is the jCorr for iron in MAO + NaCl solutions. The values of IE% are also listed in Table 1. The increase of MAO concentration highly increases the values of IE%, which reflects a high protection of iron versus corrosion in NaCl solution. It is worth mentioning that increasing the time to 48 h (Figure 3) provides more inhibition. This was again confirmed via the data listed in Table 1. The polarization measurements thus indicated that the presence of 5 × 10−5 M and the increase of this concentration to 1 × 10−4 M and even more to 5 × 10−4 M highly decreased the corrosion of iron. Extending the time to 48 h is also effective in inhibiting the iron corrosion.
In order to confirm the ability of MAO on the inhibition of iron corrosion in the chloride (3.5% NaCl) solution, we compared its efficiency with the inhibition efficiencies of other compounds. Here, the values of IE% obtained from the polarization measurements using the concentration of 5 × 10−4 M of 3-amino-5-mercapto-1,2,4-triazole (AMTA) [1], 5-(3-aminophenyl)-tetrazole (APT) [3], ethanedihydrazide (EH) [30], and N,N′-bis[2-hydroxynaphthylidene]amino]oxamide (HAO) [31], when added to 3.5% NaCl solution, are listed in Table 2. It is indicated from Table 2 that MAO gives the highest inhibition efficiency amongst all reported compounds. The polarization data and the comparison with other investigated inhibitors reveals that MAO is a good corrosion inhibitor that works on both cathodic and anodic reactions decreasing its intensity at shorter and longer immersion periods of time.

Table 2.
Percentage of the inhibition efficiency (IE%) values obtained on the surface of iron after its immersion for 1 h in 3.5% NaCl + 5 × 10−4 M of different organic compounds at the same conditions.
3.3. Potentiostatic Current versus Time (PCT) Measurements
PCT is a method that has been successfully performed to report the behavior of metallic materials in corrosive media, particularly the pitting attack [1,3,5,25,26,27,28,29]. Herein, the PCT experiments were carried out to investigate the influence of adding MAO molecules on the corrosion of iron in NaCl solution. Figure 4 displays the PCT curves acquired for iron in 3.5% NaCl solutions containing (1) 0.0 MAO, (2) 5 × 10−5 M MAO, (3) 1 × 10−4 M MAO, and (4) 5 × 10−5 M MAO after 1 h exposure at −475 mV (Ag/AgCl). The same data were obtained after 48 h and are displayed in Figure 5. It is seen that the current starts from very low values and continuously increases with time. The low current values at the initial readings are due to the formation an oxide layer during the exposure of the electrode. On the other side, the continued increase of current with time is due to the dissolution of iron and the occurrence of pitting corrosion. This dissolution may undergo as per the following equations [1,3,5,30,31]:
Fe (s) + 2Cl− (aq) = FeCl2 (s) + 2e−
FeCl2 (s) = FeCl2 (interface) → FeCl2 (aq)
FeCl2 (s) + Cl− (aq) = FeCl3 (s) + e−
FeCl3 (s) = FeC13 (interface) = FeCl3 (aq)
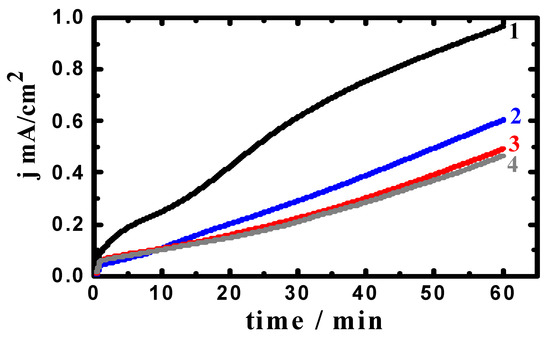
Figure 4.
Current–time curves collected at −475 mV (Ag/AgCl) for an iron rod in 3.5% NaCl solutions containing (1) 0.0 MAO, (2) 5 × 10−5 M MAO, (3) 1 × 10−4 M MAO, and (4) 5 × 10−5 M MAO after 1 h immersion.
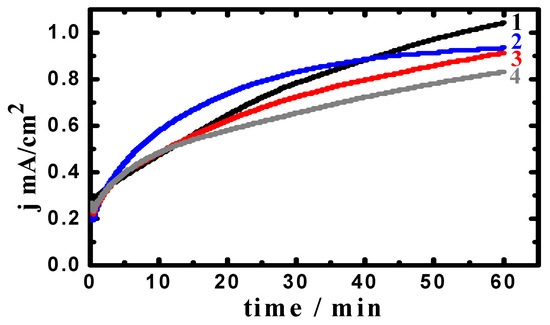
Figure 5.
Current–time curves collected at −475 mV (Ag/AgCl) for an iron rod in 3.5% NaCl solutions containing (1) 0.0 MAO, (2) 5 × 10−5 M MAO, (3) 1 × 10−4 M MAO, and (4) 5 × 10−5 M MAO after 48 h immersion.
The corrosion of iron can be explained as follows; at first, the iron surface forms ferrous chloride (Equation (8)) due to its reaction with the chloride ions from the solution. This ferrous chloride dissolves from the surface into the solution via diffusion (Equation (9)). The rest of the ferrous chloride left on the surface would react with more chloride ions to form a ferric chloride layer on the surface (Equation (10)), which in turn goes to the solution via Equation (11). The later reaction leads to the occurrence of pitting corrosion surrounded by the rest of the corrosion products [30,31].
Addition of MAO (Figure 4) highly decreases the absolute currents, although the current–time behavior is similar. The increase of MAO concentration from 5 × 10−5 M to 5 × 10−4 M gives the same behavior but with decreases in the absolute current. This means that MAO molecules decrease the uniform corrosion of iron and its impact gets greater with the increase of MAO concentration. Moreover, MAO decreases the intensity of pitting by lowering the current as compared to the current acquired for iron in NaCl without MAO present.
Prolonging the time of exposure to 48 h (Figure 5), the PCT behavior showed higher initial currents, whether MAO is there or not. Its values then increase with time, displaying the highest currents for Fe in NaCl solutions without MAO present. The absolute currents were lower in the presence of MAO, where the lowest absolute currents were for the iron in 3.5% NaCl + 5 × 10−4 M MAO. The PCT curves obtained after a long immersion period of time thus confirm that MAO molecules decrease the general corrosion, and, to some extent, the pitting attack. The PCT experiments of whether iron was present for 1 h or 48 h indicated that MAO molecules remarkably inhibit the iron corrosion, and this effect gets enhanced with increasing MAO concentration.
3.4. Surface Examination
The SEM and EDX investigations were performed to examine the layer that may be formed on the surface of iron. For that, the iron was immersed in 3.5% NaCl solution alone and also in 3.5% NaCl + 5 × 10−4 M for 48 h, before applying −0.475 V for 1 h. Figure 6 depicts (a) the SEM image and (b) EDX analysis for the surface of iron after 48 h immersion in NaCl before setting the iron’s potential at −0.475 V for 1 h. The same experiment was done in the same conditions to discover the surface morphology and to detect the elements on the iron surface in 3.5% NaCl + 5 × 10−4 M; these are shown in Figure 7. The SEM micrograph that was taken for the surface of iron in NaCl alone (Figure 6a shows a deep wide pit indicating that the iron suffers pitting corrosion. The presence of pits on the surface of iron confirms the PCP results that iron corrodes in NaCl solution via general and pitting corrosion. The weight percentages (wt. %) for the elements given by the EDX profile for iron in NaCl alone were 86.96 % Fe, 4.39% O, 7.17 % C, 0.84% Cl, and 0.64% Na, respectively. The wt. % of Fe is by far the highest, while O, Cl, and Na were very low, implying that the surface of iron is almost bare or has a very thin iron oxide layer. The SEM and EDX investigations thus confirm that applying a high anodic potential for 1 h on iron in NaCl solution allows the dissolution of iron via both general and localized corrosion.
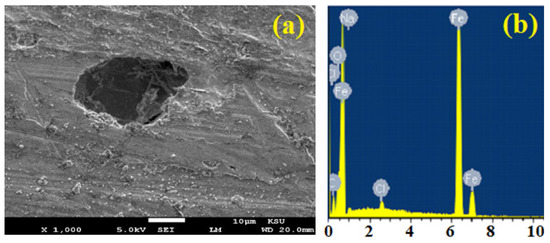
Figure 6.
(a) SEM image and (b) EDX profile spectrum after 1 h holding the potential at −475 mV (Ag/AgCl) for the surface of the iron rod that was immersed for 48 h in 3.5% NaCl solution.
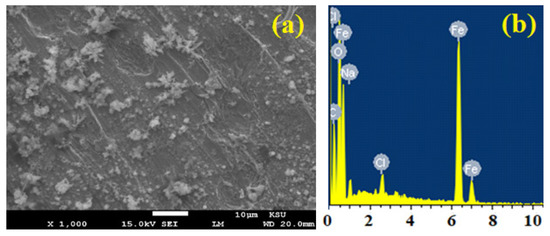
Figure 7.
(a) SEM image and (b) EDX profile spectrum after 1 h holding the potential at −475 mV (Ag/AgCl) for the surface of the iron rod that was immersed for 48 h in 3.5% NaCl + 5 × 10−4 M MAO solution.
Addition of 5 × 10−4 M MAO led to a complete changing of the iron surface when compared to its surface in 3.5% NaCl alone, and this is depicted in Figure 7a. Herein, the SEM image of Figure 7a exhibits that the surface is covered with an oxide and/or a corrosion product. Moreover, the surface of iron appeared to not show any pits. The wt.% collected for the elements given by EDX spectra for iron in NaCl + 5 × 10−4 M MAO were 55.61% Fe, 21.60% O, 19.33% C, 1.58% Cl, and 1.89% Na, respectively. The wt.% of Fe and O are high exhibiting that a layer of iron oxide (Fe2O3 and/or Fe3O4) is formed on the surface of iron. The formation of this kind of layer inhibits the corrosion of iron from the severe attack of the chloride ions. Furthermore, the presence of low percentages of Cl and Na at almost similar quantities indicate that a little NaCl salt was deposited on the surface of iron as the experiment was running. The examination of the surface of iron in NaCl and NaCl + MAO via SEM and EDX analyses supported the results obtained by PCP and PCT techniques.
3.5. EIS Measurements
Figure 8 exhibits the Nyquist spectra for iron after 1 h immersion in (1) 3.5% NaCl solutions in the absence and presence of (2) 5 × 10−5 M MAO, (3) 1 × 10−4 M MAO, and (4) 5 × 10−5 M MAO, respectively. The Nyquist spectra were also plotted for iron after its exposure to the same solutions for 48 h, which are seen in Figure 9. EIS experiments have successfully reported the corrosion and passivation processes [37,38,39,40,41,42,43,44]. The data estimated from EIS data were fitted to an equivalent circuit and the best fit is presented in Figure 10. Table 3 lists the values of the elements of this circuit. Moreover, the values of the inhibition efficiency (IE%) have been also calculated as reported before and are depicted also in Table 3. The impedance data herein can be defined as follows: RS, RP1 and RP2 are the solution resistance, the first polarization resistance, and the second polarization resistance, respectively; Q1 and Q2 are the first and the second constant phase elements, (CPEs), respectively. RP1 also represents the resistance of the interface between the iron surface and a layer may be formed on its surface, while RP2 can represent the resistance of the interface between the formed layer on the iron surface and the solution.
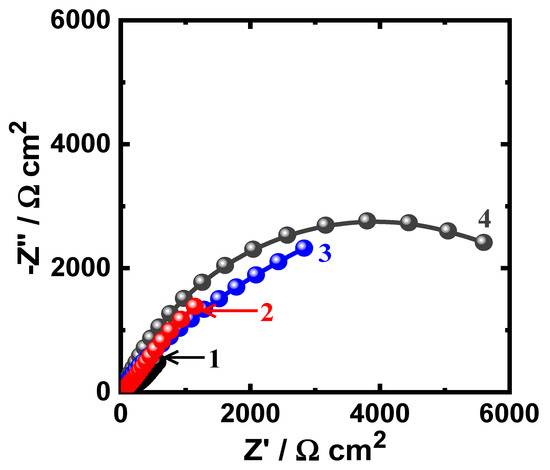
Figure 8.
Nyquist spectra for iron after its exposure for 1 h to (1) 3.5% NaCl solutions in the absence and presence of (2) 5 × 10−5 M MAO, (3) 1 × 10−4 M MAO, and (4) 5 × 10−5 M MAO, respectively.
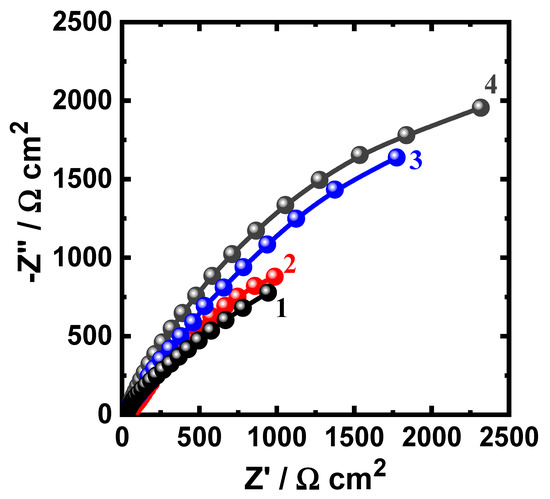
Figure 9.
Nyquist spectra for iron after its exposure for 48 h to (1) 3.5% NaCl solutions in the absence and presence of (2) 5 × 10−5 M MAO, (3) 1 × 10−4 M MAO, and (4) 5 × 10−5 M MAO, respectively.
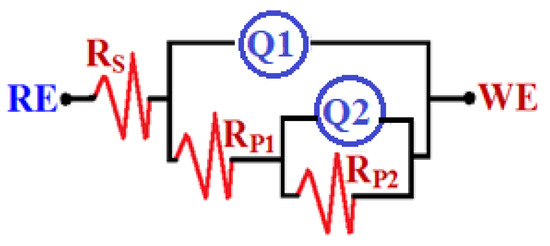
Figure 10.
Equivalent circuit model.
The Nyquist spectra of Figure 8 exhibiting only a semicircle indicate that its diameter increases when the concentration of MAO increased. The iron in NaCl solution alone showed the lowest diameter, indicating that it has the lowest resistance. Adding MAO molecules boosts the diameter of the semicircle, indicating the increase of the corrosion resistance of the surface and that impact is enhanced with adding higher concentrations of MAO.
Prolonging the time of exposure to 48 h (Figure 9) displays similar behavior with lower diameters for the plotted semicircles. The values of the elements of Table 3 confirmed the spectra of Figure 8 and Figure 9, where the values of RS, RP1, and RP2 were the lowest for iron in NaCl solution alone. In addition, the presence of MAO and the increase of its concentrations increases the values of all these resistances. Furthermore, the increase of MAO contents decreased the values of YQ1 and YQ2, where the highest values of YQ1 and YQ2 were recorded for iron in the chloride solutions alone. Here, the values of n that accompany the first constant phase elements (YQ1) are circa 1.0, giving a strong indication that Q1 is a double layer capacitor with some porosities. Additionally, the presence of Q2 with its n exponent is a bit higher than 0.5, giving the indication that the CPEs behaves like a Warburg, which reflects on the passivation of the surface, particularly in the presence of MAO molecules. From these EIS results, MAO molecules have the ability to protect the surface of iron from the corrosiveness action of NaCl solution and this impact is magnified when MAO concentration is elevated. EIS experiments thus confirm and are in good agreement with the PCP and PCT results. All these measurements indicated that the presence of iron in contact with 3.5% NaCl solution leads to the corrosion of iron via both uniform and pitting corrosion. The inhibition of iron corrosion at this condition is achieved via the adsorption of the MAO molecules onto the surface of iron. The adsorption of MAO blocks the active sites that present on the surface of iron and leads to the protection it from being attacked by the Cl ions. The adsorption process of the MAO molecules does not only inhibit the uniform corrosion of iron, but also minimizes the pitting attack through preventing the localized attack that occurs due to the presence of the corrosive Cl−.
4. Conclusions
The corrosion of pure iron in 3.5% NaCl solution after two different exposure periods of time was reported. Alleviation of the corrosion of iron after 1 h and 48 h in the chloride solution by N,N′-bis[2-methoxynaphthylidene]amino]oxamide as a corrosion inhibitor (MAO) was also investigated. The work was carried out employing PCP, PCT, EIS, SEM, and EDX techniques. It was found that iron corrodes in 3.5% NaCl via uniform and pitting corrosion forms. Addendum of 5 × 10−5 M MAO to 3.5% NaCl solution reduces the corrosion of iron as a result of detracting the cathodic and anodic reactions, which reflects on boosting its corrosion resistance. Adding a higher concertation of MAO, 1 × 10−4 M highly inhibits the corrosion of iron via presenting further detraction in its corrosion current and corrosion rate and boosting its corrosion resistance. The highest inhibition efficiency was acquired when the concentration of MAO was augmented to 5 × 10−4 M, where MAO molecules cover the surface of iron and strongly prevent it from being in a contact with the solution. SEM and EDX inspections confirmed that iron suffers both uniform and pitting corrosion, while the presence of MAO reduces both corrosion forms to the minimum limit. Protracting the immersion time of iron in the solutions to 48 h also allows the MAO molecules to restrain the corrosion of iron as confirmed by PCP, PCT, and EIS data.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11121516/s1, Figure S1: IR spectra of N,N′-bis[2-methoxynaphthylidene]amino]oxamide (MAO), Figure S2: Mass spectra of MAO.
Author Contributions
Conceptualization, E.-S.M.S. and A.H.A.; methodology, E.-S.M.S. and A.H.A.; formal analysis, E.-S.M.S. and A.H.A.; funding, E.-S.M.S.; writing—original draft preparation, E.-S.M.S.; writing—review and editing, E.-S.M.S. and A.H.A.; supervision, E.-S.M.S. and A.H.A.; project administration, E.-S.M.S. and A.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded through the Researchers Supporting Project Number (RSP-2021/33) at King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the manuscript.
Acknowledgments
Researchers Supporting Project Number (RSP-2021/33), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar] [CrossRef]
- Yao, J.L.; Ren, B.; Huang, Z.F.; Cao, P.G.; Gub, R.A.; Tian, Z.-Q. Extending surface Raman spectroscopy to transition metals for practical applications IV. A study on corrosion inhibition of benzotriazole on bare Fe electrodes. Electrochim. Acta 2003, 48, 1263–1271. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Effects of 5-(3-aminophenyl)-tetrazole on the inhibition of unalloyed iron corrosion in aerated 3.5% sodium chloride solutions as a corrosion inhibitor. Mater. Chem. Phys. 2011, 129, 961–967. [Google Scholar] [CrossRef]
- Melendres, C.A.; Camillone, N., III; Tipton, T. Laser Raman spectroelectrochemical studies of anodic corrosion and film formation on iron in phosphate solutions. Electrochim. Acta 1989, 34, 281–286. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Corrosion and corrosion inhibition of pure iron in neutral chloride solutions by 1,1’-thiocarbonyldiimidazole. Int. J. Electrochem. Sci. 2011, 6, 3077–3092. [Google Scholar]
- Khaled, K.F.; Abdel-Rehim, S.S.; Sakr, G.B. On the corrosion inhibition of iron in hydrochloric acid solutions, Part I: Electrochemical DC and AC studies. Arab. J. Chem. 2012, 5, 213–218. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 5, 1684–1695. [Google Scholar] [CrossRef]
- Shainy, K.M.; Rugmini Ammal, P.; Unni, K.N.; Sailas, B.; Abraham, J. Surface Interaction and Corrosion Inhibition of Mild Steel in Hydrochloric Acid Using Pyoverdine, an Eco-Friendly Biomolecule. J. Bio. Tribo. Corros. 2016, 2, 20. [Google Scholar] [CrossRef]
- Lakshminarayanan, V.; Kannan, R.; Rajagopalan, S.R. Cyclic voltammetric behavior of certain copper-azole systems using carbon paste electrodes. J. Electroanal. Chem. 1994, 364, 79–86. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions. J. Appl. Surf. Sci. 2006, 252, 8615–8622. [Google Scholar] [CrossRef]
- Andreeva, D.; Shchukin, D.; Mohwald, H. Corrosion Inhibiting Coating for Active Corrosion Protection of Metal Surfaces, Comprising a Sandwich-like Inhibitor Complex. WO Patent 046915, 16 April 2009. [Google Scholar]
- May, M.; Khalifa, K.; Ali, B. Corrosion Inhibition of Mild Steel by Using Carbimazole/Zn+ System in NaCl Medium. Amer. J. Mech. Mater. Eng. 2019, 3, 70–77. [Google Scholar] [CrossRef]
- Asatyas, S.; Wahyuningrum, D.; Bundjali, B. Adsorption Mechanism of 4-(4,5-diphenyl-1H-imidazole)-N,N-dimethylbenzenamine as a Corrosion Inhibitor Towards Carbon Steel in 1% NaCl Solution. Inter. J. Sci. Basic Appl. Res. 2020, 53, 175–182. [Google Scholar]
- Fontana, M.G. Corrosion Engineering, 3rd ed.; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Zhang, S.; Tao, Z.; Li, W.; Hou, B. The effect of some triazole derivatives as inhibitors for the corrosion of mild steel in 1M hydrochloric acid. Appl. Surf. Sci. 2009, 255, 6757–6763. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.-M. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim. Acta 2006, 51, 1313–1321. [Google Scholar] [CrossRef]
- Sliem, M.H.; Radwan, A.B.; Mohamed, F.S.; Alnuaimi, N.A.; Abdullah, A.M. An efficient green ionic liquid for the corrosion inhibition of reinforcement steel in neutral and alkaline highly saline simulated concrete pore solutions. Sci. Rep. 2020, 10, 14565. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Sliem, M.H.; Shakoor, R.A.; Radwan, A.B.; Kahraman, R.; Umer, M.A.; Manzoor, U.; Abdullah, A.M. Electrochemical and thermodynamic study on the corrosion performance of API X120 steel in 3.5% NaCl solution. Sci. Rep. 2020, 10, 4314. [Google Scholar] [CrossRef]
- Gao, X.; Liu, S.; Lu, H.; Gao, F.; Ma, H. Corrosion inhibition of iron in acidic solutions by monoalkyl phosphate esters with different chain lengths. Ind. Eng. Chem. Res. 2015, 54, 1941–1952. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Liu, Y.; Wu, S.; Li, W.; Sun, W.; Guod, D.; Jiang, J. Molecule adsorption and corrosion mechanism of steel under protection of inhibitor in a simulated concrete solution with 3.5% NaCl. RSC Adv. 2018, 8, 20648. [Google Scholar] [CrossRef]
- Lai, Y.; Gao, Y.; Jin, Y.; Wen, L. Study of methionine as green corrosion inhibitor for TWIP steel in neutral chloride solution. Mater. Res. Exp. 2021, 8, 046533. [Google Scholar] [CrossRef]
- ArockiaSelvi, J.; Kamaraj, P.; Arthanareeswari, M.; PushpaMalini, T.; Mohanapriya, S.; Subasree, N. Effect of cetylpyridinium chloride on corrosion inhibition of mild steel in chloride environment. Mater. Today 2019, 14, 264–270. [Google Scholar] [CrossRef]
- Fontana, M.G.; Staehle, K.W. Advances in Corrosion Science and Technology; Plenum: New York, NY, USA, 1970; Volume 1. [Google Scholar]
- Ahmed, A.H. N,N′-bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells. Open Chem. 2020, 18, 426–437. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M. Nickel(II)-oxaloyldihydrazone complexes: Characterization, indirect band gap energy and antimicrobial evaluation. Cogent Chem. 2016, 2, 1142820. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M.; Omran, A.A. Copper(II)-oxaloyldihydrazone complexes: Physico-chemical studies, energy band gap and inhibition evaluation of free oxaloyldihydrazones toward the corrosion of copper metal in acidic medium. Arab. J. Chem. 2019, 12, 4287–4302. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M. Physicochemical studies on some selected oxaloyldihydrazones and their novel palladium(II) complexes along with using oxaloyldihydrazones as corrosion resistants. Inorg. Nano-Met. Chem. 2017, 47, 1652–1663. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M. Mn2+-complexes of N,O-dihydrazone: Structural studies, indirect band gap energy and corrosion inhibition on aluminum in acidic medium. J. Chil. Chem. Soc. 2018, 63, 4159–4168. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Ahmed, A.H. Synthesizing new hydrazone derivatives and studying their effects on the inhibition of copper corrosion in sodium chloride solutions. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2010, 40, 365–372. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Sherif, E.-S.M.; Abdo, H.S.; Gad, E.S. Ethanedihydrazide as a Corrosion Inhibitor for Iron in 3.5% NaCl Solutions. ACS Omega 2021, 6, 14525–14532. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.-S.M.; Ahmed, A.H.; Abdo, H.S.; DefAllah, M.N. Impediment of Iron Corrosion by N,N0-bis[2-hydroxynaphthylidene]amino]oxamide in 3.5% NaCl solution. Crystals 2021, 11, 1263. [Google Scholar] [CrossRef]
- Khalil, K.A.; Sherif, E.-S.M.; Almajid, A.A. Corrosion passivation in Simulated Body Fluid of Magnesium/Hydroxyapatite Nanocomposites Sintered by High Frequency Induction Heating. Int. J. Electrochem. Sci. 2011, 6, 6184–6199. [Google Scholar]
- Sherif, E.-S.M.; Latief, F.H.; Abdo, H.S.; Alharthi, N.H. Electrochemical and Spectroscopic Study on the Corrosion of Ti–5Al and Ti–5Al–5Cu in Chloride Solutions. Met. Mater. Inter. 2019, 25, 1511–1520. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Potgieter, J.H.; Comins, J.D.; Cornish, L.; Olubambi, P.A.; Machio, C.N. The beneficial effect of ruthenium additions on the passivation of duplex stainless steel corrosion in sodium chloride solutions. Corros. Sci. 2009, 51, 1364–1371. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Li, W.; Nobe, K.; Pearlstein, A.J. Potential/current oscillations and anodic film characteristics of iron in concentrated chloride solutions. Corros. Sci. 1990, 31, 615–620. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.E.; Bayoumi, R.S. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid part I. weight loss, polarization, EIS, PZC, EDX and ESM studies. Electrochim. Acta 2007, 52, 3588–3599. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Kuriakose, N.; Kakkassery, J.T.; Raphael, V.P.; Shanmughan, S.K. Electrochemical Impedance Spectroscopy and Potentiodynamic Polarization Analysis on Anticorrosive Activity of Thiophene-2-Carbaldehyde Derivative in Acid Medium. Ind. J. Mater. Sci. 2014, 2014, 124065. [Google Scholar] [CrossRef][Green Version]
- Sherif, E.M.; Park, S.-M. Inhibition of copper corrosion in 3.0% NaCl by N-phenyl-1,4-phenylenediamine. J. Electrochem. Soc. 2005, 152, 428–433. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Mehmeti, V.; Haldhar, R.; Berdimurodov, E.; Hamed, O.; Jodeh, S.; Lgaz, H.; Sherif, E.S.; Ebenso, E.E. Epoxy coating as effective anti-corrosive polymeric material for aluminum alloys: Formulation, electrochemical and computational approaches. J. Mol. Liq. 2021, 117886. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.-M. Inhibition of copper corrosion in acidic pickling solutions by N-phenyl-1,4-phenylenediamine. Electrochim. Acta 2006, 51, 4665–4673. [Google Scholar] [CrossRef]
- Mashuga, M.E.; Olasunkanmi, L.O.; Lgaz, H.; Sherif, E.S.; Ebenso, E.E. Aminomethylpyridazine isomers as corrosion inhibitors for mild steel in 1 M HCl: Electrochemical, DFT and Monte Carlo simulation studies. J. Mol. Liq. 2021, 117882. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.-M. Effects of 2-amino-5-ethylthio-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim. Acta 2006, 51, 6556–6562. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).