Abstract
Enzyme catalyzed reactions are rapidly becoming an invaluable tool for the synthesis of many active pharmaceutical ingredients. These reactions are commonly performed in batch, but continuous biocatalysis is gaining interest in industry because it would allow seamless integration of chemical and enzymatic reaction steps. However, because this is an emerging field, little attention has been paid towards the suitability of different reactor types for continuous biocatalytic reactions. Two types of continuous flow reactor are possible: continuous stirred tank and continuous plug-flow. These reactor types differ in a number of ways, but in this contribution, we focus on residence time distribution and how enzyme kinetics are affected by the unique mass balance of each reactor. For the first time, we present a tool to facilitate reactor selection for continuous biocatalytic production of pharmaceuticals. From this analysis, it was found that plug-flow reactors should generally be the system of choice. However, there are particular cases where they may need to be coupled with a continuous stirred tank reactor or replaced entirely by a series of continuous stirred tank reactors, which can approximate plug-flow behavior. This systematic approach should accelerate the implementation of biocatalysis for continuous pharmaceutical production.
1. Introduction
As the pharmaceutical industry moves towards flow chemistry [1], the real advantage of continuous manufacturing is that common (and even standardized) technologies will start to be used for development and ultimately production. For example, production can use scaled-out versions of the identical tubular reactors which were run in the laboratory. The use of such common technologies, will enable enormous savings to be made in development time, meaning that processes can be implemented more quickly, and products launched into the market earlier. These are major drivers in virtually all pharmaceutical companies and so the field has grown considerably in recent years. The complementary field of biocatalysis has also grown tremendously in the last decade. This has been fueled firstly by protein engineering developments [2,3,4,5],which allow the individual tuning of catalytic properties of enzymes, and secondly by the ability to operate cascades of enzymes [6,7,8] under similar conditions. Additionally, biocatalysis offers the possibility of cutting the number of process steps [9]. Many in the biocatalysis field therefore believe it timely to investigate flow biocatalysis [10,11], not least because this enables the smooth integration of chemical and biocatalytic methods together in continuous processes [12,13]. Indeed, this special issue is testament to the interest in this field. There are already some superb examples of flow biocatalysis both in the area of enzyme characterization and property measurements [14,15], as well as development and production [16,17]. Nevertheless, an often-cited challenge is that biocatalytic reactions in general are rather slow (compared to their chemical counterparts) and thereby the use of flow technology is hard to justify. In reality, the arguments for flow technology are perhaps a little different for enzymes and, in this brief review, we will discuss the issue of reactor selection, in order to capitalize upon the benefits of both flow technology and biocatalysis. Downstream unit operations are not included in this discussion.
2. Reactor Types
Figure 1 shows schematic diagrams of the three ideal reactor types, namely the batch stirred tank reactor (BSTR), the continuous stirred tank reactor (CSTR) and the continuous plug-flow reactor (CPFR). The characteristics of these reactors have been described in extensive detail elsewhere [18] and will therefore be only briefly summarized here.
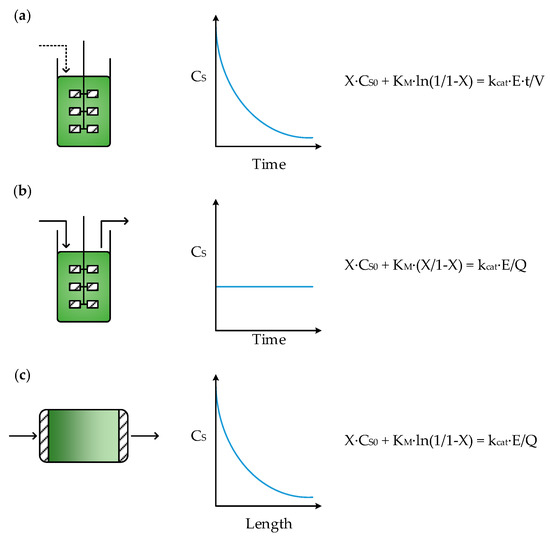
Figure 1.
Reactor schematics, substrate concentration (CS) profiles and design equations for: (a) batch stirred tank reactor (BSTR), (b) continuous stirred tank reactor (CSTR) and (c) continuous plug-flow reactor (CPFR).
The design equations for each of the reactors in Figure 1 can be used to determine the concentration of enzyme (E) required to achieve a desired fractional conversion of substrate (X) for a given initial or feed substrate concentration (CS0). In a BSTR, the time of reaction (t) and reaction volume (V) are also required, whilst for CSTRs and CPFRs the volumetric flowrate through the reactor (Q) is needed. KM is the affinity constant of the enzyme towards the substrate and kcat is its turnover number.
In a BSTR, the mechanically stirred vessel is first filled with substrate and enzyme, to initiate the reaction, after which no material is removed until the reaction is stopped. BSTRs are well-mixed reactors, meaning that concentrations are the same regardless of location within the reactor. Typical enzyme kinetics follow Michaelis–Menten behavior, where rate is independent of substrate (zero order) at high concentrations but becomes proportional to the amount of substrate (first order) at lower concentrations, with a transition in between. This means that in a BSTR the substrate is initially consumed quickly, whilst later in the reaction, as it enters the first order regime, the reaction rate slows, as illustrated by the substrate concentration profile in Figure 1a. However, given sufficient time in the reactor, complete conversion can be achieved, provided the equilibrium is favorable. BSTRs are commonly used for biocatalytic reactions [19,20] due to their simplicity and flexibility. For instance, substrate concentrations can be kept below toxic or inhibitory levels by adopting a fed-batch approach [21,22] where substrate is fed to the reactor, resulting in a reaction volume that increases with time. Additionally, pH changes caused by the biocatalytic reaction can be neutralized through the addition of an acid or base to maintain the optimal pH of the enzyme.
The design of a CSTR is similar to that of a BSTR, except that material is continuously added to, and removed from, the reactor such that the working volume remains constant. In this case, the biocatalyst must either be fed continuously to the reactor (to make up for loss of catalyst in the effluent) or it must be retained within the reactor by immobilization and/or partially permeable membranes. Like BSTRs, CSTRs are well-mixed and so the reactor contents and effluent are homogenous. However, since there must be enough substrate in the reactor to achieve an adequate reaction rate, the effluent will always contain some substrate and so full substrate conversion is not possible [23]. This trade-off between reaction rate and conversion is an important characteristic of CSTRs. Furthermore, since the reactor contents are homogenous, the substrate concentration, and subsequently the reaction rate, throughout the reactor remain constant with respect to time, as shown in the profile in Figure 1b.
In a CPFR, reactants are pumped into a long tubular reactor where, unlike stirred tanks, material flowing through does not mix with any material flowing ahead of it, or behind it. This results in concentration gradients over the length of the reactor, identical to the concentration gradients over time in a BSTR. Therefore, if the reactor is sufficiently long, the substrate can be fully converted. For this reason, in Figure 1c the concentration profile is given with respect to length, since the time material spends in a CPFR is simply a function of the reactor length and volumetric flowrate. Although it is possible to operate a CPFR with a soluble catalyst, biocatalysts are typically immobilized onto the reactor wall or on particles of a carrier material, which are then packed into a tube to form a continuous packed-bed reactor (CPBR) [24] that exhibits plug-flow behavior. However, this can potentially introduce mass transfer limitations and large pressure drops over the reactor [25].
3. Batch vs. Continuous
There are many considerations which need to be evaluated when selecting the type of reactor to use. However, even for simple single product reactions in a single liquid phase, two considerations which are always of relevance are the residence time distribution (RTD) and the kinetics of the enzyme catalyzed reaction. BSTRs and CPFRs have identical kinetic behavior and both afford good control over RTDs. Consequently, it would appear that there is little motivation to invest in the shift from batch operation to continuous since both give the same result. However, much research has highlighted the benefits of continuous biocatalysis over batch processing, for both production as well as research and development, and these are summarized in Figure 2 [26,27,28]. Nonetheless, it has also been shown that, for single-phase homogenous reactions, the differences in performance between batch and continuous reactors at the laboratory scale are negligible [29]. Therefore, in such instances, shifting from batch to continuous would appear to be a time-consuming and resource-intensive process that yields few improvements. But, at production scales, where mixing and heat transfer in large batch reactors are less efficient, shifting to continuous operation could be beneficial. Additionally, steady-state operation of a continuous reactor affords simpler control and greater consistency than a dynamic batch process at large scales.
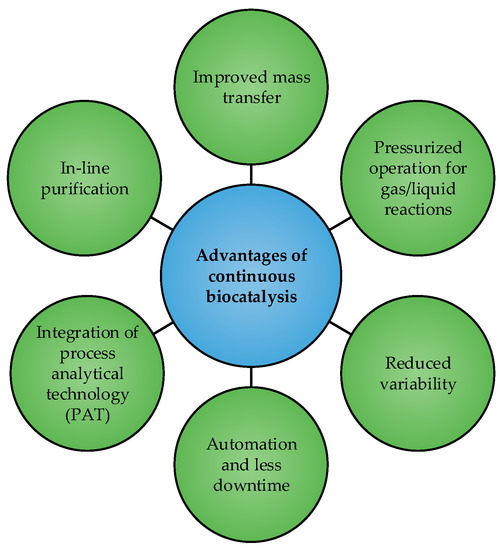
Figure 2.
Benefits of shifting towards flow biocatalysis.
Moreover, active pharmaceutical ingredients (APIs) are often the products of numerous reaction steps. Some of these reaction steps, especially those involving optically active compounds, may be biocatalytic, but many reactions are still more efficient using chemical catalysts. Chemocatalytic reactions are frequently operated continuously to benefit from rapid mixing and heat transfer [30], particularly in the case of exothermic reactions, or to avoid the storage of unstable or toxic intermediates. Therefore, when selecting a reactor for a biocatalytic reaction that has to be integrated into a combined chemo/biocatalytic reaction sequence [31,32,33], opting for a continuous approach may be more practical, potentially allowing for end-to-end manufacturing that could even include downstream processing and formulation [34]. For this reason, it may also make more sense to use continuous reactors for research and development in the pharmaceutical industry, as it simplifies the transition to production scale through the process of scale-out, reducing time-to-market.
Finally, continuous operation is generally more efficient than batch operation, which is plagued with lengthy start-up and shutdown times, before and after each reaction, and mandatory downtimes as the reactors need to be routinely cleaned. Of course, there are still some cases where BSTRs may be the most appropriate reactor to use. For instance, if the reaction rates of an enzyme are very low, then continuous operation in a CPFR would require very low flowrates or an impractically long reactor. In such a case, it would be beneficial to simply run the reaction in a BSTR for a long period of time until the reaction reaches completion. However, even in such cases where BSTRs are in use it is still possible to gain some of the benefits of flow chemistry, by simulating continuous behavior. For example, this could be done by operating three BSTRs in parallel, but with each at a different stage in the process (i.e., start-up, operation, shutdown), termed pseudo-continuous operation.
4. Residence Time Distribution
Residence time defines the length of time material is in a reactor. For an ideal BSTR it is simply the time from the addition of the final reagent (usually biocatalyst) to the quenching time of the reaction. In other words, all material is in the reactor for the same length of time and can be said to have a defined residence time. If this time is sufficiently long, then all the reactant will have been converted to product. Therefore, in the production of APIs, batch reactors have frequently been used simply because complete conversion was achievable. Moreover, if some substrate remains at the end of the reaction, more catalyst can be added to complete the conversion. This flexibility is very attractive. Even in cases where the equilibrium prevents complete conversion of substrate to product, all material has an identical residence time and thereby the reaction mixture has a defined conversion. For more complex reactions (with multiple reactants, products or phases) this is critical, because otherwise what might leave the reactor is a variable mixture of compounds that complicates downstream processing. Small-molecule APIs are complex, and their production is strictly regulated (e.g., by the Federal Drug Administration or European Medicines Agency). For this reason, there is a demand for precision chemistry in the pharmaceutical industry to achieve high product quality in a reproducible manner, ensuring the safety of the patient. It is for this reason that enzymes are particularly attractive due to their high selectivity [35], compared to most conventional catalysts, which minimizes by-product formation. Additionally, they operate at mild conditions [36] which also greatly reduces the occurrence of spontaneous degradation of reactants, intermediates or products. This allows for the precise production of APIs with simpler downstream processing steps. However, to truly capitalize upon this, the reactor should also give a precise residence time. In other words, a precision catalyst used in a precision reactor. In this way the benefit of flow biocatalysis becomes clear.
Residence time is an important characteristic of any reactor. It is desirable to have a well-defined residence time for accurate control of reactions. In an ideal CPFR, where no back-mixing occurs, material exits the reactor in the same order as it enters with a single residence time (τ) which can easily be calculated from the volume of the reactor and the volumetric flowrate, as follows:
The ideal BSTR and CPFR are the only two cases where a reactor has a single residence time. For all other reactor types and configurations, multiple residence times exist and so residence times are typically expressed as a function of time, known as the residence time distribution. For instance, the RTD of the ideal BSTR (or ideal CPFR) is represented mathematically by the following Dirac delta function, shown graphically in Figure 3:
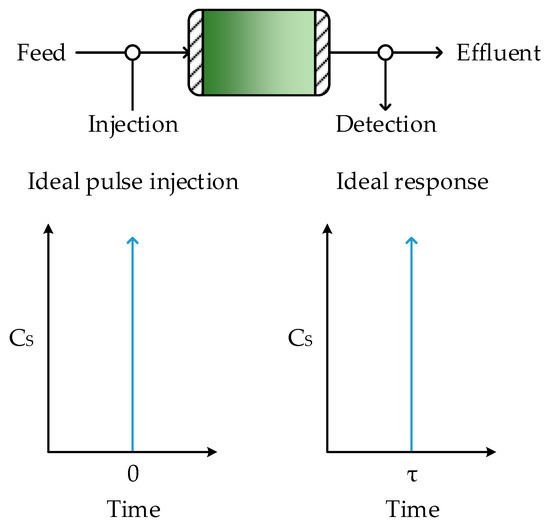
Figure 3.
Response of a CPFR to a pulse injection under ideal conditions.
In an ideal CSTR it is assumed that mixing is complete and instantaneous, such that the composition of the entire reactor volume and the reactor outlet are homogenous. As a result, some of the feed molecules exit the reactor immediately, since fluid is constantly being removed at the outlet, whilst others remain in the reactor almost indefinitely. Therefore, the RTD can be represented by an exponential decay function (Equation (3)), illustrated in Figure 4, although the mean residence time can still be calculated using Equation (1).
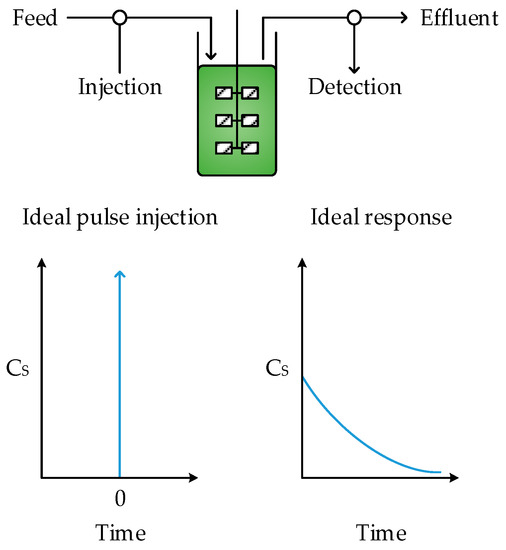
Figure 4.
Response of a CSTR to a pulse injection under ideal conditions.
5. Enzyme Kinetics
Enzyme kinetics are often modeled using the basic Michaelis–Menten equation, shown below. Modified versions of this equation also exist to describe more complex systems, such as those with substrate or product inhibition, or multiple substrates or products.
Equation (4) relates the reaction rate (v) to the substrate concentration. The maximum rate of the enzyme reaction (Vmax) is dependent on the turnover number of the enzyme and its concentration. The affinity constant of the enzyme towards a specific substrate, KM, corresponds to the substrate concentration at which the initial rate will be half of the maximum rate. Therefore, to achieve the maximum rate of an enzymatic reaction, the substrate concentration must be sufficiently high (relative to its KM) that the rate equation approximates the following zero order form:
If an enzyme has a very high affinity towards a particular substrate, characterized by a very low KM, the maximum rate of the enzymatic reaction can still be achieved at low substrate concentrations. This is a highly desirable scenario because it means that the enzyme can likely be used effectively regardless of reactor type. This can be illustrated by dividing the design equation of a CSTR (Figure 1b) with that of a CPFR (Figure 1c) to determine the ratio of enzyme concentrations (ECSTR/ECPFR) required to reach a desired fractional conversion, plotted in Figure 5. It can be seen that, to reach high fractional conversions, much higher enzyme concentrations are required in a CSTR than a CPFR unless the substrate concentration in the feed is a few orders of magnitude higher than the affinity constant of the enzyme. For this reason, protein engineering efforts should be focused on increasing the affinity of enzymes towards industrially attractive molecules [37,38]. In the meantime, however, enzymes generally have low affinities towards pharmaceutical intermediates due to their complex, non-native structures. Therefore, for pharmaceutical production in particular, the choice of reactor can greatly affect the reaction rates that can be achieved in a biocatalytic reaction.
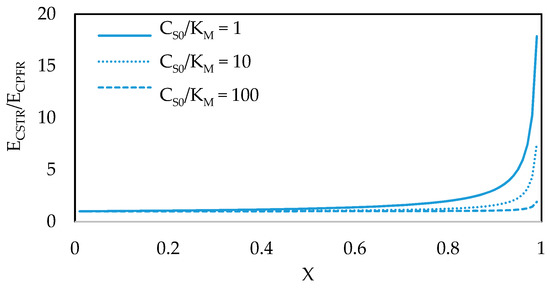
Figure 5.
Enzyme concentrations required in a CSTR vs. a CPFR to achieve a desired fractional conversion of substrate.
For instance, in a CSTR, substrate is fed into a much larger, well-mixed volume. This means the reaction takes place at a single, constant substrate concentration far more dilute than that of the feed, resulting in reduced rates throughout the reactor. In contrast, the substrate concentration in a CPFR is equal to that of the feed at the inlet, where the enzyme can operate closer to its maximum rate, and progressively decreases across the length of the reactor, as does the reaction rate. This is typically a more effective way of using the catalyst. As expressed earlier, this kinetic behavior is identical to that of a biocatalytic reaction in a BSTR [18].
Enzyme inhibition by substrate or product is also very important to consider when selecting a reactor for biocatalysis. On the one hand, if the enzyme is inhibited by high concentrations of the substrate, the substrate dilution that occurs in a CSTR may be desirable, whereas the high initial substrate concentration in a CPFR could have a negative impact. On the other hand, if the enzyme is inhibited by the product, operating in a CSTR is undesirable as the homogeneity guarantees inhibition throughout the reactor unless a much larger, more dilute reaction volume is used, which would increase capital costs as well as the cost of downstream processing. Conversely, in a BSTR product inhibition would only become problematic towards the end of the reaction. Likewise, in a CPFR the rate would only be severely inhibited towards the end of the reactor, whilst maintaining a smaller, more concentrated and cost-effective volume. The concentrations of substrate and product throughout the reactor also influence the thermodynamic equilibrium of the reaction, but this has been discussed in detail elsewhere [39].
The difference in capital costs between a CSTR and CPFR is best demonstrated with an example. One of the most successful instances where a biocatalytic reaction has been implemented in the pharmaceutical industry is the use of a highly engineered transaminase in the production of sitagliptin [40]. The kinetic parameters of this enzyme have not been published. However, another transaminase from Halomonas elongata (HEWT), which has been characterized, was recently used for the continuous production of amines at laboratory scale [41]. The turnover number of this enzyme was found to be 0.094 s−1, and its KM values were 2.57 mM and 0.56 mM towards (S)-1-phenylethylamine (amino donor) and pyruvate (amino acceptor), respectively, for the production of acetophenone at 25 °C [42]. Due to the high affinity of the enzyme towards pyruvate, it will be assumed that pyruvate is supplied in sufficient excess such that the reaction rate is only dependent on the concentration of (S)-1-phenylethylamine, according to Equation (4). Equation (7) shows how the Michaelis–Menten expression can be transformed into a function of the fractional conversion, where FS0 is the inlet molar flowrate of substrate.
A typical industrial enzyme concentration of 1 g/L has been assumed as well as a desired annual production target of 100 kg, with a final product concentration of 10 g/L. Figure 6 shows the Levenspiel plot for this reaction, which can be used to determine the reactor volume required to achieve a desired conversion in a continuous reactor with these conditions [43]. For a CSTR, the required volume is equal to the area of a rectangle with a height of FS0/v and a width of X, whereas for a CPFR, the required volume is the area under the curve. It is clear from Figure 6 that a similar volume would be required for both reactors to achieve most fractional conversions. In fact, only 2.5 L more volume would be required in a CSTR than a CPFR to achieve 80% substrate conversion. However, in most biocatalytic reactions the only difference between the substrate and product is a single functional group, which can make separating them extremely challenging. Therefore, to avoid adding more complexity to the downstream process it is best to aim for complete conversion of the substrate and this is where the gap between the CSTR and the CPFR becomes apparent. From Figure 6, it can be calculated that, to achieve 99% substrate conversion, a CPFR with a volume of 38 L would be sufficient, but a CSTR would require a volume of 134 L, nearly 4 times larger. This problem becomes significantly worse if a conversion of 99.9% is desired, a perfectly common target in the pharmaceutical industry, in which case a CSTR would require 23 times more volume than a CPFR, greatly increasing capital and downstream processing costs.
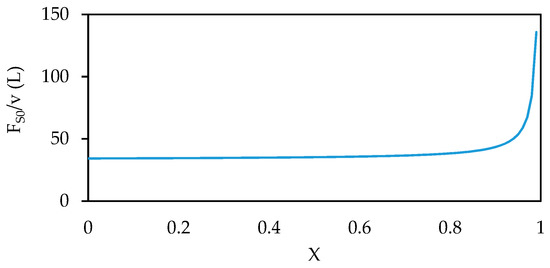
Figure 6.
Levenspiel plot for the production of acetophenone from (S)-1-phenylethylamine and pyruvate by an amine transaminase from Halomonas elongata (HEWT).
6. pH Control and Multiphase Systems
Much of the previous discussion has shown that CPFRs are generally better suited for continuous biocatalytic production of pharmaceuticals than CSTRs. Nevertheless, CPFRs and CPBRs do have some limitations that may preclude their use with certain systems. For instance, although residence time and temperature are easily controlled in these reactors, control of pH across the length of the reactor is often more challenging due to the concentration gradients that arise from the lack of mixing [44]. To overcome this problem, engineered enzymes that can tolerate the range of pH expected to occur across the reactor would be required. Alternatively, the effluent from the CPFR can be recycled through a CSTR where acid or base can be added to adjust the pH back to the optimal value, as illustrated in Figure 7. Here, the well-mixed behavior of a CSTR is extremely beneficial to quickly counteract pH changes. Another possibility is to operate multiple shorter CPFRs in series since the performance will be the same as a single long CPFR. This allows additional pH adjustments to be made in between CPFRs, as shown in Figure 8. This configuration could also allow intermediate substrate feeding to avoid substrate inhibition.
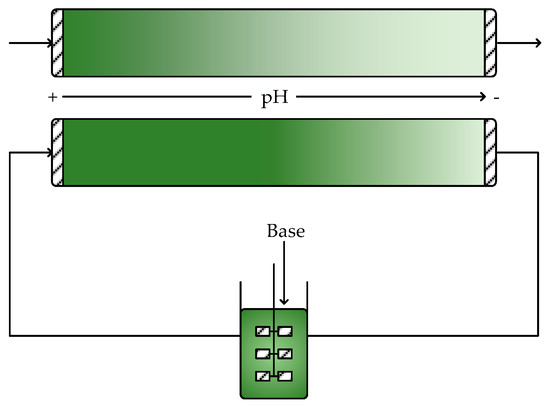
Figure 7.
Comparison of pH profiles in a CPFR when pH is controlled via the feed stream and when coupled to a CSTR with base addition through a recycle loop.
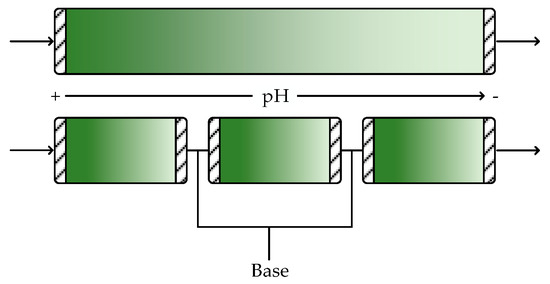
Figure 8.
Operating multiple CPFRs in series allows intermediate pH adjustment without increasing the total reactor volume required to reach the desired conversion.
Another instance where CPFRs are generally ill-suited for use is with multiphase systems, commonly encountered in the field of biocatalysis, as channeling often occurs due to the lack of mixing [45]. In fact, multiphase biocatalytic systems are often limited by mass transfer between phases, such as the supply of molecular oxygen to biocatalytic oxidation reactions or when using a water-immiscible organic solvent as a means of in situ substrate supply (ISSS) or in situ product removal (ISPR) to combat inhibition [46,47]. In such systems, it is imperative to maintain large interfacial areas between the immiscible phases to facilitate mass transfer. For small scale applications, microfluidic reactors are being used increasingly because their high surface-to-volume ratios afford excellent mass transfer rates between immiscible phases [48]. However, for large scale applications, surface-to-volume ratios tend to be much lower and so mechanical mixing is required to speed up mass transfer. As such, CSTRs would appear to be much better suited than CPFRs for handling multiphase systems. Nonetheless, as previously discussed, they also have many disadvantages. One way of overcoming these limitations would be to operate a series of CSTRs, shown in Figure 9, which approximates plug-flow behavior. This approach may also be generally attractive to the pharmaceutical industry as very few modifications would have to be made to existing batch infrastructure to switch to continuous operation.
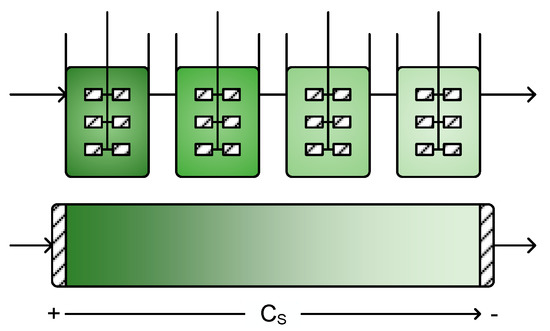
Figure 9.
A series of CSTRs approximates the plug flow behavior of a CPFR, but also allows intermediate addition of substrates, pH adjustment and good mass transfer in multiphase systems.
7. Reactor Selection
The analyses of reactor configurations in Section 4, Section 5 and Section 6 are established, even if not widely discussed in the scientific literature. However, we have also taken inspiration from this to develop a reactor selection tool, so that for the first time, decisions about continuous biocatalytic reactors can be made on a rational basis. To effectively use this tool, a number of prerequisites should be satisfied, namely that the enzyme of interest has been characterized, the operating conditions (temperature, pH, co-solvents etc.) have been set and the enzyme has been shown to be stable at these conditions for a sufficient duration, which is case dependent. Additionally, it is recommended that the main limitations of the enzyme [49] are identified and its performance is compared with relevant economic targets [50], but this is outside the scope of this article.
Figure 10 shows a proposed workflow for selecting a continuous reactor configuration. Initially, it is important to assess whether the enzyme or downstream purification is likely to be the dominant operating cost of the process, since the objectives in each case are different. For example, if an expensive enzyme is required then it is crucial to select a reactor configuration that will make the most effective use of the enzyme kinetics. However, if the enzyme is inexpensive, relative to product isolation and purification, the kinetics become less important since higher enzyme concentrations can always be used to increase reaction rates. In such a case, it would instead be desirable to simplify downstream processing by ensuring that effluent concentrations do not vary significantly to maintain the necessary driving force for separation. This can be achieved by having a well-defined residence time. Additionally, if possible, the substrate should be fully converted to avoid difficult separations.
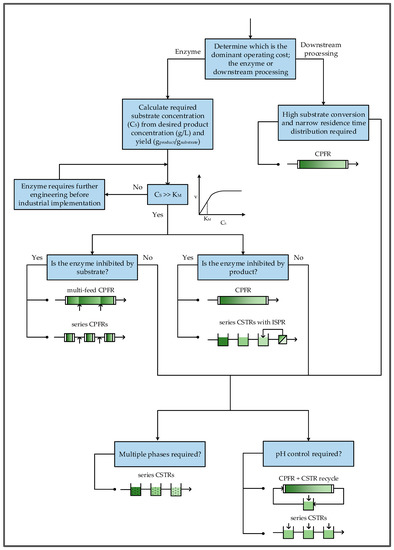
Figure 10.
Workflow for selection of continuous biocatalytic reactor configurations.
If the enzyme is determined to be the dominant operating cost, the next step is to calculate the required substrate concentration from the desired product concentration and reaction yield. For high value products like pharmaceuticals a product concentration of at least 60 g/L is generally economically feasible [50]. Once the substrate concentration is determined it should be compared to the affinity constant of the enzyme to determine whether it is high enough for the enzyme to operate close to its maximum rate. Since enzymatic reactions typically take place in aqueous environments, the water solubility of the substrate may be a limiting factor, especially for large, complex, organic molecules like pharmaceutical intermediates. Therefore, organic solvents may be required to increase substrate solubility. Protein engineering has recently been applied to allow enzymes to operate in the presence of organic solvents [51]. However, the ideal scenario would be to have enzymes operating in neat substrate, completely solvent-free [52]. If the substrate concentration is not above the affinity constant, it means that the enzyme will not even operate at half of its maximum rate, which is far too ineffective for many industrial processes. Consequently, it would be better to continue engineering the enzyme to reduce its KM until it is below the required substrate concentration.
If the substrate concentration is sufficient for good rates, the next step is to determine whether the enzyme is inhibited by the substrate or product. If the enzyme is inhibited at substrate concentrations close to the desired feed concentration, a CPFR can be used if it is fed with a lower substrate concentration at multiple points along the reactor. However, the lack of mixing could make it difficult to radially disperse the substrate in reactors with larger diameters, especially if the substrate is fed from one side of the reactor. Therefore, a better alternative may be to operate a series of shorter CPFRs, using the same total reaction volume, with a substrate feed between each reactor. If the product inhibits the enzyme at the desired product concentration, a CPFR can be used to ensure that product inhibition is only severe towards the end of the reactor. Alternatively, plug-flow behavior can be approximated with a series of CSTRs, each with a higher product concentration than the previous. In this way, only the last reactors should be severely inhibited. Nevertheless, the last reactor in the series should be equipped with a means of selectively removing the product to prevent complete inhibition in the reactor and avoid substrate in the effluent.
If downstream processing is found to have a higher overall cost contribution than the enzyme, plug-flow behavior is critical for approaching full conversion; so too having good control over the residence time, and consequently the concentration profiles at the outlet. Therefore, a CPFR would be the most appropriate reactor configuration.
Finally, the need for pH control and/or multiple phases should be considered. In both of these cases, good mixing is required to neutralize pH changes or generate high interfacial areas between phases. For multiphase reactions, a series of CSTRs should be used to approximate plug-flow behavior while still allowing sufficient dispersion of the phases. This configuration would also be beneficial for pH control because it provides multiple acid/base feed points. Alternatively, a CPFR can be coupled with a recycle loop through a CSTR where pH can be controlled, although the presence of a recycle complicates the process.
8. Immobilization
Due to the high cost of enzymes, it is desirable to recycle them for continuous operation, provided they are stable enough for repeated use. Isolation of the enzyme downstream of the reactor, using selectively permeable membranes, is one possibility, but introduces an additional unit operation and further complexity to the process. A better alternative is to retain the enzyme within the reactor. This can be done by placing membranes at the reactor outlet, however, fouling of the membrane and concentration polarization are likely to become problematic [53]. For this reason, the most common method of retaining enzymes within a reactor is by binding them to larger carrier particles that are easier to separate from the reactor effluent than the soluble enzyme. Recently, the use of paramagnetic nanoparticles as supports has received much attention due to their high specific surface areas and simple retention within the reactor by a magnetic field [54]. Numerous methods and support materials have been described for the immobilization of a wide variety of enzymes [55]. Depending on the support material used, immobilization can improve the stability of an enzyme towards harsher operating conditions, such as elevated temperatures or the presence of organic co-solvents [56,57,58], since bonds are formed between the enzyme and the support. However, in some cases, these bonds, particularly strong covalent bonds, may prevent the enzyme from adopting a more stable conformation or one that is required to catalyze the desired reaction and so, immobilization of an enzyme can also negatively affect its stability or activity [59,60]. Physical adsorption is an alternative for immobilizing enzymes without forming such strong bonds [61] but this makes it easier for the enzyme to leach off the support during reactor operation. Affinity immobilization, whereby enzymes are engineered to contain specific tags [62] that bind very selectively to ligands on specialized support materials, minimizes leaching and maintains the flexibility of the enzyme so that activity loss is reduced. This method of immobilization also ensures that only the desired enzyme binds to the support instead of other proteins or impurities that may be present in crude cell extracts.
In stirred tank reactors, whether they are operated in batch or continuously, the use of immobilized biocatalysts is often limited because the catalyst loading in the reactor is restricted to about 10% (v,v), compared to 60% (v,v) in a CPBR [18]. This is because the shear forces from stirring [63] may break apart the carrier material, making the biocatalyst difficult to separate from the reaction media and potentially contaminating the effluent. Loss of biocatalyst in the effluent may also reduce the productivity of a CSTR. Cross-linked enzyme aggregates (CLEAs) [64] are a more suitable form of immobilization for use in stirred tank reactors [65,66] due to their smaller size compared to typical carrier-bound biocatalysts.
Although immobilization has proven to be advantageous in some cases, it is important to recognize that the support and immobilization process add additional cost to the biocatalyst and this should always be taken into consideration when assessing the feasibility of a process. Furthermore, although most immobilization supports allow for high protein loadings due to their large specific surface areas, internal diffusion limitations frequently make such high loadings ineffective [67,68]. As a result, immobilization often limits the amount of enzyme that can be loaded into a reactor, compared to soluble enzymes. Nevertheless, as protein expression and engineering continue to improve, the costs of enzymes may decrease until eventually it becomes feasible to utilize soluble enzymes in continuous processes [69,70,71]. This would be especially attractive for the pharmaceutical industry, where the high value of the products can help offset the cost of the biocatalyst, to simplify production and downstream processing. Additionally, the enzyme would only need to be stable at the desired operating conditions for the length of the residence time in the reactor.
9. Outlook
In this review, some important criteria have been investigated to assist reactor selection for continuous biocatalytic pharmaceutical production and a novel selection tool is presented. We have shown that many reactor configurations, using only the ideal CSTR and CPFR, are possible to ensure effective enzyme use, overcome inhibitory effects, simplify downstream processing, operate with multiple phases or control operating conditions like pH, depending on the major limitations of a given system. Nevertheless, there are other important factors that have yet to be considered, such as:
- Regeneration and retention of expensive cofactors in a continuous reactor;
- Cost-effective and benign methods for retaining enzymes to reduce their overall cost contributions;
- The need for metrics to evaluate and compare different continuous biocatalytic systems;
- Effective downstream unit operations for continuous product isolation and purification.
Future efforts will need to be directed towards these aspects and eventually be used to update the selection tool presented here.
Author Contributions
The methodology presented here was conceptualized by R.M.L. and J.M.W.; the original draft was prepared by R.M.L., reviewed and edited by J.M.W.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| Symbol | Definition | Unit |
| CS | Substrate concentration | mol L−1 |
| CS0 | Initial or inlet substrate concentration | mol L−1 |
| X | Fractional substrate conversion | - |
| t | Reaction time | s |
| V | Reactor volume | L |
| Q | Volumetric flowrate | L s−1 |
| KM | Substrate affinity constant | mol L−1 |
| kcat | Enzyme turnover number | s−1 |
| τ | Residence time | s |
| FS0 | Inlet molar flowrate of substrate | mol s−1 |
References
- Porta, R.; Benaglia, M.; Puglisi, A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Proc. Res. Dev. 2015, 20, 2–25. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, G.A.; Pichler, H.; May, O.; Gruber-Khadjawi, M. Application of designed enzymes in organic synthesis. Chem. Rev. 2011, 111, 4141–4164. [Google Scholar] [CrossRef] [PubMed]
- Ghislieri, D.; Green, A.P.; Pontini, M.; Willies, S.C.; Rowles, I.; Frank, A.; Grogan, G.; Turner, N.J. Engineering an enantioselective amine oxidase for the synthesis of pharmaceutical building blocks and alkaloid natural products. J. Am. Chem. Soc. 2013, 135, 10863–10869. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Chuaboon, L.; Wongate, T.; Punthong, P.; Kiattisewee, C.; Lawan, N.; Hsu, C.; Lin, C.; Bornscheuer, U.T.; Chaiyen, P. One-Pot Bioconversion of L-Arabinose to L-Ribulose in an Enzymatic Cascade. Angew. Chem. 2019, 10. [Google Scholar] [CrossRef]
- Aumala, V.; Mollerup, F.; Jurak, E.; Blume, F.; Karppi, J.; Koistinen, A.; Schuiten, E.; Voss, M.; Bornscheuer, U.; Deska, J.; et al. Biocatalytic production of amino-carbohydrates through oxidoreductase and transaminase cascades. ChemSusChem 2018. [Google Scholar] [CrossRef]
- Ma, S.K.; Gruber, J.; Davis, C.; Newman, L.; Gray, D.; Wang, A.; Grate, J.; Huisman, G.W.; Sheldon, R.A. A green-by-design biocatalytic process for atorvastatin intermediate. Green Chem. 2010, 12, 81–86. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; McClean, K.; Housden, S.; Gasparini, G.; Archer, I. Biocatalytic oxidase: Batch to continuous. Chem. Eng. Res. Des. 2012, 90, 726–731. [Google Scholar] [CrossRef]
- Rudroff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- Dawood, A.W.H.; Bassut, J.; de Souza, R.; Bornscheuer, U.T. Combination of the Suzuki-Miyaura Cross-Coupling Reaction with Engineered Transaminases. Chem. Eur. J. 2018, 24, 16009–16013. [Google Scholar] [CrossRef] [PubMed]
- Ringborg, R.H.; Pedersen, A.T.; Woodley, J.M. Automated Determination of Oxygen-Dependent Enzyme Kinetics in a Tube-in-Tube Flow Reactor. ChemCatChem 2017, 9, 3285–3288. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Eisl, I.; Nidetzky, B. Advanced characterization of immobilized enzymes as heterogeneous biocatalysts. Catal. Today 2015, 259, 66–80. [Google Scholar] [CrossRef]
- Andrade, L.H.; Kroutil, W.; Jamison, T.F. Continuous flow synthesis of chiral amines in organic solvents: Immobilization of E. coli cells containing both omega-transaminase and PLP. Org. Lett. 2014, 16, 6092–6095. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Archer, I.; Jones, E.; Ashe, R. Scaling Up Biocatalysis Reactions in Flow Reactors. Org. Process Res. Dev. 2012, 16, 1013–1016. [Google Scholar] [CrossRef]
- Woodley, J.M. Scale-Up and Development of Enzyme-Based Processes for Large-Scale Synthesis Applications. In Science of Synthesis: Biocatalysis in Organic Synthesis; Faber, K., Fessner, W.D., Turner, N.J., Eds.; Thieme: Stuttgart, Germany, 2015; Volume 3, pp. 515–546. [Google Scholar]
- Zhou, X.; Lü, S.; Xu, Y.; Mo, Y.; Yu, S. Improving the performance of cell biocatalysis and the productivity of xylonic acid using a compressed oxygen supply. Biochem. Eng. J. 2015, 93, 196–199. [Google Scholar] [CrossRef]
- Dennewald, D.; Hortsch, R.; Weuster-Botz, D. Evaluation of parallel milliliter-scale stirred-tank bioreactors for the study of biphasic whole-cell biocatalysis with ionic liquids. J. Biotechnol. 2012, 157, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, X.; Zhu, X.-L.; Li, Y.; Xu, H.-P.; Zhao, B.-F.; Chen, L.; Zhang, X.-D. Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioenergy 2011, 35, 1906–1911. [Google Scholar] [CrossRef]
- Gomes, N.; Teixeira, J.A.; Belo, I. Fed-batch versus batch cultures of Yarrowia lipolytica for gamma-decalactone production from methyl ricinoleate. Biotechnol. Lett. 2012, 34, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Lima-Ramos, J.; Neto, W.; Woodley, J.M. Engineering of Biocatalysts and Biocatalytic Processes. Top. Catal. 2014, 57, 301–320. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Continuous biodiesel conversion via enzymatic transesterification catalyzed by immobilized Burkholderia lipase in a packed-bed bioreactor. Appl. Energ. 2016, 168, 340–350. [Google Scholar] [CrossRef]
- Xu, Y.; Nordblad, M.; Woodley, J.M. A two-stage enzymatic ethanol-based biodiesel production in a packed bed reactor. J. Biotechnol. 2012, 162, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Fisher, K.; Scrutton, N.S.; Goddard, N.J.; Fielden, P.R. Continuous two-phase flow miniaturised bioreactor for monitoring anaerobic biocatalysis by pentaerythritol tetranitrate reductase. Lab A Chip 2010, 10, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Raston, C.L.; Weiss, G.A. Rapid protein immobilization for thin film continuous flow biocatalysis. Chem. Commun. 2016, 52, 10159–10162. [Google Scholar] [CrossRef]
- Pedersen, A.T.; de Carvalho, T.M.; Sutherland, E.; Rehn, G.; Ashe, R.; Woodley, J.M. Characterization of a Continuous Agitated Cell Reactor for Oxygen Dependent Biocatalysis. Biotechnol. Bioeng. 2017, 114, 1222–1230. [Google Scholar] [CrossRef]
- Valera, F.E.; Quaranta, M.; Moran, A.; Blacker, J.; Armstrong, A.; Cabral, J.T.; Blackmond, D.G. The flow’s the thing... or is it? Assessing the merits of homogeneous reactions in flask and flow. Angew. Chem. 2010, 49, 2478–2485. [Google Scholar] [CrossRef]
- Wegner, J.; Ceylan, S.; Kirschning, A. Ten key issues in modern flow chemistry. Chem. Commun. 2011, 47, 4583–4592. [Google Scholar] [CrossRef]
- Yuryev, R.; Strompen, S.; Liese, A. Coupled chemo(enzymatic) reactions in continuous flow. Beilstein J. Org. Chem. 2011, 7, 1449–1467. [Google Scholar] [CrossRef]
- Morales, M.; Dapsens, P.Y.; Giovinazzo, I.; Witte, J.; Mondelli, C.; Papadokonstantakis, S.; Hungerbühler, K.; Pérez-Ramírez, J. Environmental and economic assessment of lactic acid production from glycerol using cascade bio- and chemocatalysis. Energy Environ. Sci. 2015, 8, 558–567. [Google Scholar] [CrossRef]
- Groger, H.; Hummel, W. Combining the ‘two worlds’ of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr. Opin. Chem. Biol. 2014, 19, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Biocatalysis-key to sustainable industrial chemistry. Curr. Opin. Biotechnol. 2010, 21, 713–724. [Google Scholar] [CrossRef]
- Bordeaux, M.; Galarneau, A.; Fajula, F.; Drone, J. A regioselective biocatalyst for alkane activation under mild conditions. Angew. Chem. 2011, 123, 2123–2127. [Google Scholar] [CrossRef]
- Woodley, J.M. Protein engineering of enzymes for process applications. Curr. Opin. Chem. Biol. 2013, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.M. Integrating protein engineering with process design for biocatalysis. Phil. Trans. R. Soc. A 2017, 376. [Google Scholar] [CrossRef] [PubMed]
- Abu, R.; Woodley, J.M. Application of Enzyme Coupling Reactions to Shift Thermodynamically Limited Biocatalytic Reactions. ChemCatChem 2015, 7, 3094–3105. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef]
- Planchestainer, M.; Contente, M.L.; Cassidy, J.; Molinari, F.; Tamborini, L.; Paradisi, F. Continuous flow biocatalysis: Production and in-line purification of amines by immobilised transaminase from Halomonas elongata. Green Chem. 2017, 19, 372–375. [Google Scholar] [CrossRef]
- Cerioli, L.; Planchestainer, M.; Cassidy, J.; Tessaro, D.; Paradisi, F. Characterization of a novel amine transaminase from Halomonas elongata. J. Molec. Catal. B Enzym. 2015, 120, 141–150. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B. Continuous production of β-cyclodextrin by cyclodextrin glycosyltransferase immobilized in mixed gel beads: Comparative study in continuous stirred tank reactor and packed bed reactor. Biochem. Eng. J. 2016, 105, 107–113. [Google Scholar] [CrossRef]
- Tufvesson, P.; Fu, W.; Jensen, J.S.; Woodley, J.M. Process considerations for the scale-up and implementation of biocatalysis. Food Bioprod. Process. 2010, 88, 3–11. [Google Scholar] [CrossRef]
- Xue, R.; Woodley, J.M. Process technology for multi-enzymatic reaction systems. Bioresour. Technol. 2012, 115, 183–195. [Google Scholar] [CrossRef]
- Schmolzer, K.; Madje, K.; Nidetzky, B.; Kratzer, R. Bioprocess design guided by in situ substrate supply and product removal: Process intensification for synthesis of (S)-1-(2-chlorophenyl)ethanol. Bioresour. Technol. 2012, 108, 216–223. [Google Scholar] [CrossRef]
- Gruber, P.; Marques, M.P.C.; O’Sullivan, B.; Baganz, F.; Wohlgemuth, R.; Szita, N. Conscious coupling: The challenges and opportunities of cascading enzymatic microreactors. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Karande, R.; Schmid, A.; Buehler, K. Miniaturizing Biocatalysis: Enzyme-Catalyzed Reactions in an Aqueous/Organic Segmented Flow Capillary Microreactor. Adv. Synth. Catal. 2011, 353, 2511–2521. [Google Scholar] [CrossRef]
- Nordblad, M.; Gomes, M.D.; Meissner, M.P.; Ramesh, H.; Woodley, J.M. Scoping Biocatalyst Performance Using Reaction Trajectory Analysis. Org. Proc. Res. Dev. 2018, 22, 1101–1114. [Google Scholar] [CrossRef]
- Lima-Ramos, J.; Tufvesson, P.; Woodley, J.M. Application of environmental and economic metrics to guide the development of biocatalytic processes. Green Process Synth 2014, 3. [Google Scholar] [CrossRef]
- Doukyu, N.; Ogino, H. Organic solvent-tolerant enzymes. Biochem. Eng. J. 2010, 48, 270–282. [Google Scholar] [CrossRef]
- Lee, A.; Chaibakhsh, N.; Rahman, M.B.A.; Basri, M.; Tejo, B.A. Optimized enzymatic synthesis of levulinate ester in solvent-free system. Ind. Crop. Prod. 2010, 32, 246–251. [Google Scholar] [CrossRef]
- Luo, J.; Morthensen, S.T.; Meyer, A.S.; Pinelo, M. Filtration behavior of casein glycomacropeptide (CGMP) in an enzymatic membrane reactor: Fouling control by membrane selection and threshold flux operation. J. Membr. Sci. 2014, 469, 127–139. [Google Scholar] [CrossRef]
- Bezerra, R.M.; Neto, D.M.A.; Galvão, W.S.; Rios, N.S.; Carvalho, A.C.L.d.M.; Correa, M.A.; Bohn, F.; Fernandez-Lafuente, R.; Fechine, P.B.A.; de Mattos, M.C.; et al. Design of a lipase-nano particle biocatalysts and its use in the kinetic resolution of medicament precursors. Biochem. Eng. J. 2017, 125, 104–115. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Qing Max Lu, G. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Munoz, G.; Fernandez-Lorente, G.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Rios, N.S.; Pinheiro, M.P.; dos Santos, J.C.; Fonseca, T.D.; Lima, L.D.; de Mattos, M.C.; Freire, D.M.; da Silva Júnior, I.J.; Rodríguez-Aguado, E.; Goncalves, L.R. Strategies of covalent immobilization of a recombinant Candida antarctica lipase B on pore-expanded SBA-15 and its application in the kinetic resolution of (R,S)-Phenylethyl acetate. J. Mol. Catal. B Enzym. 2016, 133, 246–258. [Google Scholar] [CrossRef]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef]
- Manoel, E.A.; Dos Santos, J.C.; Freire, D.M.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Matosevic, S.; Lye, G.J.; Baganz, F. Immobilised enzyme microreactor for screening of multi-step bioconversions: Characterisation of a de novo transketolase-omega-transaminase pathway to synthesise chiral amino alcohols. J. Biotechnol. 2011, 155, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Rosa, M.E.; Fernandes, P.C.; Ribeiro, M.H. Operational stability of naringinase PVA lens-shaped microparticles in batch stirred reactors and mini packed bed reactors-one step closer to industry. Bioresour. Technol. 2014, 164, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Cross-Linked Enzyme Aggregates as Industrial Biocatalysts. Org. Proc. Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Xu, D.Y.; Yang, Z. Cross-linked tyrosinase aggregates for elimination of phenolic compounds from wastewater. Chemosphere 2013, 92, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-Y.; Chen, J.-Y.; Yang, Z. Use of cross-linked tyrosinase aggregates as catalyst for synthesis of l-DOPA. Biochem. Eng. J. 2012, 63, 88–94. [Google Scholar] [CrossRef]
- Lage, F.A.; Bassi, J.J.; Corradini, M.C.; Todero, L.M.; Luiz, J.H.; Mendes, A.A. Preparation of a biocatalyst via physical adsorption of lipase from Thermomyces lanuginosus on hydrophobic support to catalyze biolubricant synthesis by esterification reaction in a solvent-free system. Enzym. Microb. Technol. 2016, 84, 56–67. [Google Scholar] [CrossRef]
- Manoel, E.A.; Ribeiro, M.F.P.; dos Santos, J.C.S.; Coelho, M.A.Z.; Simas, A.B.C.; Fernandez-Lafuente, R.; Freire, D.M.G. Accurel MP 1000 as a support for the immobilization of lipase from Burkholderia cepacia: Application to the kinetic resolution of myo -inositol derivatives. Process Biochem. 2015, 50, 1557–1564. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Yang, S.; Zhu, M.; Wang, X. Technology prospecting on enzymes: Application, marketing and engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209017. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.; Dinu, C. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).