Abstract
The strain screened from sludge can selectively hydrolyze (S)-ethyl indoline-2-carboxylate to produce (S)-indoline-2-carboxylic acid. It was identified as the Bacillus aryabhattai strain based on its morphology, metabolism, and 16S rDNA sequence analysis. Glucose and yeast powder were used as the best carbon and nitrogen sources to cultured cells with an initial pH of seven. Subsequently, we optimized the key parameters for selective hydrolysis. Finally, when the substrate concentration had reached 3%, with a 35 °C water bath, a pH of seven, and a speed of 600 rpm, the e.e.p value attained 96% with a 33% yield. Thus, we had developed a new method for producing (S)-indoline-2-carboxylic acid that used whole microbial cells as the biocatalyst.
1. Introduction
(S)-indoline-2-carboxylic acid is a key intermediate for the synthesis of perindopril, which not only exhibits a general antihypertensive effect, but also acts as a liver X-receptor modulator with anticancer activity [1,2]. Perindopril is a third-generation angiotensin-converting enzyme inhibitor lacking mercapto groups. It can expand the arteries and reduce blood volume, while exhibiting long action duration, limited side effects, and good tolerance; it is widely used in the treatment of various types of hypertension and congestive heart failure [3,4]. Therefore, understanding the means for producing (S)-indoline-2-carboxylic acid with high optical purity is essential.
The industrial process for the preparation of (S)-indoline-2-carboxylic acid uses racemic indoline-2-carboxylic acid as its basis, along with (R)-α-methyl benzyl amine in an ethanol solvent as a resolving agent to obtain (S)-indoline-2-carboxylic acid [5]. The asymmetric synthesis method has also been reported as being used to obtain (S)-indoline-2-carboxylic acid through a free-radical cyclization reaction with bromobenzyl bromide with a glycine tert-butyl ester derivative as a reactant; it is catalyzed by a cinchona base derivative. The target product, (S)-indoline-2-carboxylic acid, is obtained by hydrolysis [6].
Biocatalysts have been gradually introduced to the field of medicine. With the advantage of their mildly reaction conditions, fewer by-products, and high product purity, they have gradually replaced traditional chemical methods in some synthetic fields. Asada et al. obtained chiral indoline-2-carboxylic acid by hydrolyzing the (R, S)-indoline-2-carboxylic acid methyl ester with an immobilized esterase in a buffer solution [7]. De Vries et al.’s patent reported condensing O-halo (chloro or bromo) benzaldehyde condensed with acetic anhydride. The cinnamic acid derivative obtained was aminolyzed with the phenylalanine aminolysis enzyme to product optically pure O-halo-L-phenylalanine, and then subjected to cyclization to obtain optically pure (S)-indoline-2-carboxylic acid [8].
Some of these biocatalytic methods display satisfactory activity and enantioselectivity. However, reports on the production of (S)-indoline-2-carboxylic acid by enzymatic methods are still rare. Furthermore, because of the high price of the enzyme, the cost of large-scale production can be very expensive. Compared to purified enzymes, the use of whole cells of microorganisms as biocatalysts is not only inexpensive, but also yields more cycles. Therefore, it is desirable to develop a biological process based on whole-cell biocatalysts to prepare (S)-indoline-2-carboxylic acid.
Thus, the aim is to prepare (S)-indoline-2-carboxylic acid in a single step using microbial whole cells. We isolated 216 candidates through substrate-targeted screening from sludge, and the microorganism Bacillus aryabhattai had the best enantioselectivity of these. The strain can selectively hydrolyze (R, S)-ethyl indoline-2-carboxylate in the aqueous phase to produce an optically pure (S)-indoline-2-carboxylic acid (the reaction formula is shown in Figure 1).
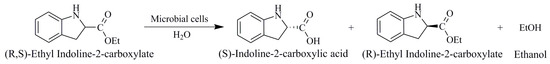
Figure 1.
The whole cell catalysis reaction route.
2. Results
2.1. The Identification of Microorganisms Hydrolizing (R, S)-Ethyl Indoline-2-Carboxylate
Among the 216 strains of microorganisms isolated from the sludge, four strains displayed a higher substrate enantioselectivity (e.e.s > 75%). The strain numbered C26-1 demonstrated the best substrate enantioselectivity(Table 1). The high performance liquid phase results are shown in Figure 2.

Table 1.
A comparison of the enantioselective hydrolysis properties of (R, S)-ethyl indoline-2-carboxylate in sludge strains. Conditions: six mL of resting cells, 1.5 mL of phosphate buffer (200 mM, pH 7), 0.015 g of (R, S)-ethyl indoline-2-carboxylate, at 180 rpm, and 30 °C for 24 h.
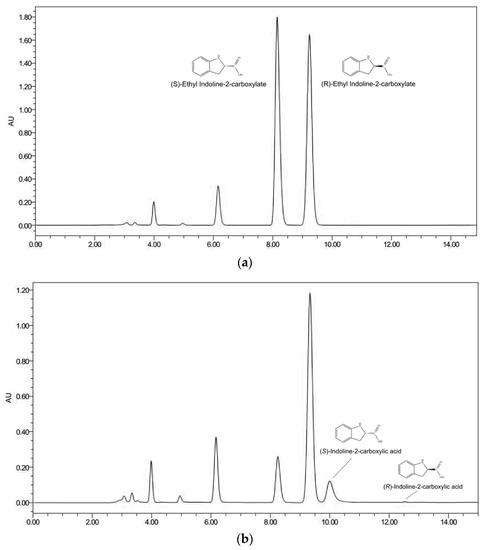
Figure 2.
(a) HPLC chromatogram of (R, S)-ethyl indoline-2-carboxylate; (b) The HPLC chromatogram of the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate via strain C26-1. Conditions: AD-H, 1 mL/min, UV at 220 nm, running time 15 min. (S)-ethyl indoline-2-carboxylate and (R)-ethyl indoline-2-carboxylate peaked at 8.1 min and 9.3 min, respectively. (S)-indoline-2-carboxylic acid and (R)-indoline-2-carboxylic acid peaked at 10.1 min and 12.1 min, respectively.
We also compared the enantiomeric resolution of (R, S)-ethyl indoline-2-carboxylate using some commercially available enzymes and laboratory-preserved enzyme preparations. The results revealed(show in Table 2) that these enzymes possess low enantioselective hydrolysis ability.

Table 2.
The ability of some commercially available enzymes and laboratory-preserved enzyme preparations for the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate. Conditions: 0.02 g of enzyme, 1.5 mL of phosphate buffer (200 mM, pH 7), 0.015 g of (R, S)-ethyl indoline-2-carboxylate, at 180 rpm, and 30 °C for 12 h.
Single colonies were obtained by coating and inoculating them onto agar plates. The colonies were large, white, and nearly round, with dry, opaque surfaces, and clear boundaries (Figure S1).
A single colony was selected by Gram staining and observed with an optical microscope. Strain C26-1 was a purple, rod-shaped cell, and a plurality of these cells could be connected end to end to form a long chain (Figure S2). Preliminary labeling identified the group as Gram-positive bacteria.
The strain was tested for 94 phenotypes, including 71 carbon source utilization assays and 23 chemical sensitivities, using the Biolog (GENIII) automated microbial identification system. Metabolic fingerprints were analyzed by a Biolog reader, and the results are displayed in Table S1.
The Biolog system’s 24-hour identification results reveal the strain as having the highest degree of similarity with Bacillus aryabhattai. 16S rDNA was extracted (Figure S3) and sequenced by Sangon Biotech (Shanghai) Co., Ltd. The total length of the strain’s 16S rDNA sequence was 1470 bp (Figure S4).
The sequence was submitted to NCBI(National Center for Biotechnology Information) for alignment; it displayed a 100% similarity to Bacillus aryabhattai. Subsequently, the strain was deposited in the China Centre for Type Culture Collection (CCTCC), and its accession number was M 2017751.
2.2. Bacillus Aryabhattai’s Growth Curve
The optimal culture conditions are as follows: the best carbon and nitrogen sources are glucose and yeast powder, respectively; the inoculation amount was 1%, 180 rpm, and 30 °C for 24 h. Biomass (OD600) and enzyme activity were measured every two hours. The growth curve was as Figure 3:
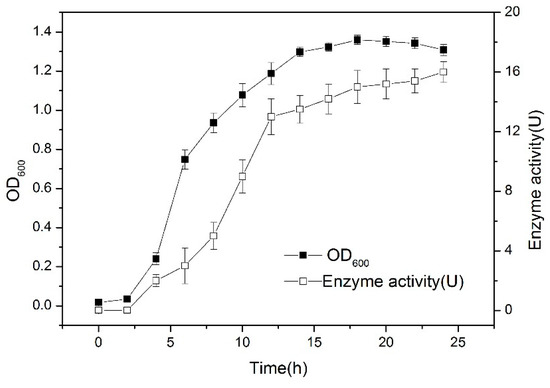
Figure 3.
Growth curve for strain Bacillus aryabhattai. The cells were cultured at 180 rpm, 30 °C for 24 h; then, they were sampled every two hours. The sterile medium was used as a control group. All of the data were in triplicate.
The data indicates that the cells were in a lag phase between zero and two hours, and in the logarithmic growth phase between three and 10 h. The cells were in a stable phase after 20 h. The lipase activity of the cells increased rapidly between three and 12 h, and then gradually remained stable after 20 h.
2.3. The Effects of pH, Temperature, Substrate Concentration, Co-Solvent, and Co-Solvent Addition on the Resolution of (R, S)-Ethyl Indoline-2-Carboxylate
As a typical ester hydrolysis reaction, we hope the equilibrium moves toward the positive direction. The methods generally employed include increasing the amount of substrate (increase substrate concentration and substrate solubility), improving the temperature, removing the product in time (neutralize the acid produced), and the like. However, this reaction is an enzymatic reaction, and it is necessary to satisfy the above conditions as much as possible while ensuring the activity and selectivity of the enzyme. Therefore, we studied the optimal conditions of the enzymatic reaction from the pH, temperature, substrate concentration, co-solvent, and co-solvent addition.
The pH can change the charge density, surface conformation, and the dissociation of the active center groups of the enzyme [9], thus affecting the enzyme’s activity in aqueous solutions. The pH can also affect the equilibrium of the reaction. Figure 4 shows the changes in enzyme activity and e.e.p within a 20-minute reaction at pH values of between 5.0–9.5 at 30 °C.
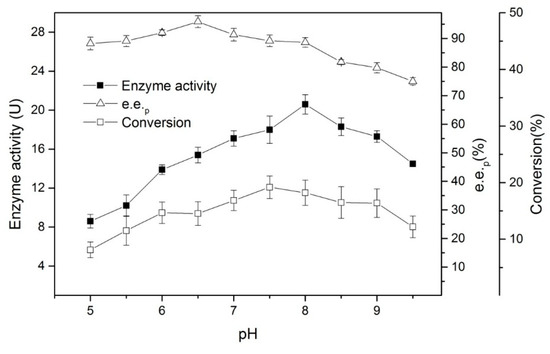
Figure 4.
The effects of pH on the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate. Conditions: 0.05 g of lyophilized cells, 1.5 mL of buffer (A series of pH from 5.0 to 9.5), 0.015 g of (R, S)-ethyl indoline-2-carboxylate, at 180 rpm, and 30 °C for 20 min.
The results reveal that hydrolysis at a neutral to weakly alkaline pH can increase enzyme activity; this makes it easy to obtain improved e.e.p and conversion rates. Conversely, too high a pH will cause the e.e.p to drop. Based on the results of the HPLC, it is speculated that this may be due to the spontaneous hydrolysis of the ester and the irreversible inactivation of the enzyme under alkaline conditions. Therefore, it is necessary to maintain a reaction pH of around seven.
Temperature plays an important role in the thermodynamic equilibrium of reactions, and diverse reactions have different temperature requirements in industrial production. Therefore, an enzyme’s thermal stability is also an essential reference indicator. We studied the catalytic ability of Bacillus aryabhattai at varied temperatures (Figure 5). As the temperatures increased, the enzyme activity gradually increased, reaching a maximum at 35 °C. When the temperature exceeded 40 °C, the enzyme activity decreased rapidly. The e.e.p and conversion rates had the same trend as the enzyme activity. This indicates that a high temperature can seriously damage enzyme activity and selectivity.
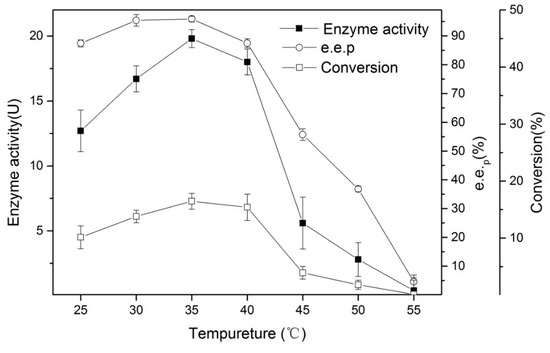
Figure 5.
The effects of temperature on the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate. Conditions: 0.05 g of lyophilized cells, 1.5 mL of phosphate buffer (200 mM, pH 7), 0.015 g of (R, S)-ethyl indoline-2-carboxylate, at 180 rpm, and every 5 °C interval from 25 °C to 50 °C for 20 min.
Substrate concentration is also one of the key factors in the enzymatic process. Excessive substrate concentration may cause substrate inhibition and affect enzyme activity and selectivity. We established a series of substrate concentration gradients (by mass fraction) and ran the hydrolysis reaction for 20 min (in Figure 6). The results showed that when the substrate concentration exceeded 1.5%, the enzymatic activity revealed a downward trend. The causes of its substrate inhibition were multifaceted. For example, the catalysts that were used in the reaction were from a lyophilized cell, and if the expression level of the cell’s target enzyme was low, it was possible for it to be suppressed at a lower substrate concentration. However, from the e.e.p trend, maintaining a certain substrate inhibition level assisted in increasing the e.e.p value of the final product. This necessitated reevaluating the amount of substrate that was added to the final scale production to obtain an improved e.e.p and yield.
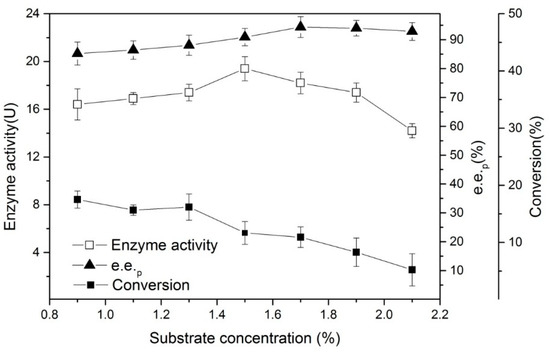
Figure 6.
The effects of the substrate concentration on the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate. Conditions: 0.05 g of lyophilized cells, 1.5 mL of phosphate buffer (200 mM, pH 7), different mass fractions of (R, S)-ethyl indoline-2-carboxylate, at 180 rpm, and 35 °C for 20 min.
Lipase is a type of interfacial enzyme that catalyzes the cleavage of ester bonds at the “water–oil” interface, producing corresponding alcohols and acids [10]. The (R, S)-ethyl indoline-2-carboxylate in this reaction is a water-insoluble solid that may undergo external mass transfer limitations. Therefore, the addition of the appropriate type and volume of organic solvent can effectively increase the activity and enantioselectivity of the enzyme [11]. We evaluated changes in the activity and enantioselectivity following the addition of different organic reagents, as shown in the Table 3.

Table 3.
The effects of the different organic solvents on the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate. Conditions: 0.05 g of lyophilized cells, 1.5 mL of phosphate buffer (200 mM, pH 7), 0.025 g of (R, S)-ethyl indoline-2-carboxylate, 180 µL of different kinds of organic solvents, at 180 rpm, and 35 °C for 20 min.
The results showed that the experimental groups to which dichloromethane and acetonitrile were added no longer exhibited enzyme activity and selectivity. This indicates that these two organic reagents may cause the rapid inactivation or competitive inhibition of the enzyme. The group that was added to n-hexane slightly increased the enzyme’s activity while retaining e.e.p’s stability. Interesting, the addition of tetrahydrofuran reversed the enantioselectivity of the enzyme. Finally, we chose n-hexane as a co-solvent. In general, an appropriate amount of organic solvent helped increase the activity of the lipase, while excessive organic solvent can inactivate the enzyme [12], resulting in a decrease in enzyme activity.
Therefore, we also studied the effects of the amount of n-hexane added (its volume ratio with the reaction system) on the enzyme activity. As shown in Figure 7, when the ratio of water: n-hexane is 5:1, a good balance can be obtained between enzyme activity and enantioselectivity.
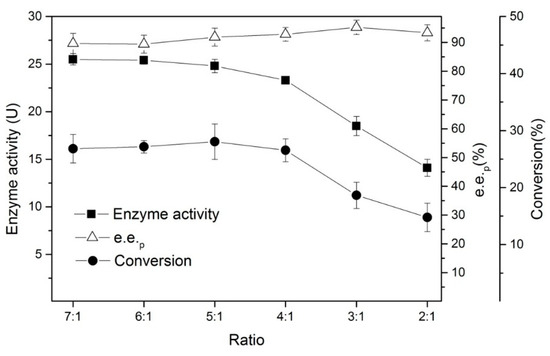
Figure 7.
The effects of the amount of n-hexane added to the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate. Conditions: 0.05 g of lyophilized cells, 1.5 mL of phosphate buffer (200 mM, pH 7), 0.025 g of (R, S)-ethyl indoline-2-carboxylate, different ratio of aqueous:n-hexane, at 180 rpm, and 35 °C for 20 min.
2.4. The Time Course of the Hydrolysis Reaction
The time course of the enantioselective hydrolysis of (R, S)-ethyl indoline-2-carboxylate using the lyophilized cells of Bacillus aryabhattai C26-1 is shown in Figure 8, while the reaction conditions are described as follow: 50 mL of water, one gram of lyophilized cells of Bacillus aryabhattai, and one gram of (R, S)-ethyl indoline-2-carboxylate were added to a 100-mL three-necked flask. The pH of the reaction system was monitored in real time using a pH meter, and 50 mM of NaOH was removed in the reaction system to maintain the pH at seven (Julabo ACE® Reaction Controller), while eight mL of n-hexane was added as a co-solvent. The reaction was stirred using a magnetic stirring device at 600 rpm, run in a 35 °C water bath, and sampled every hour. All the data were in triplicate.
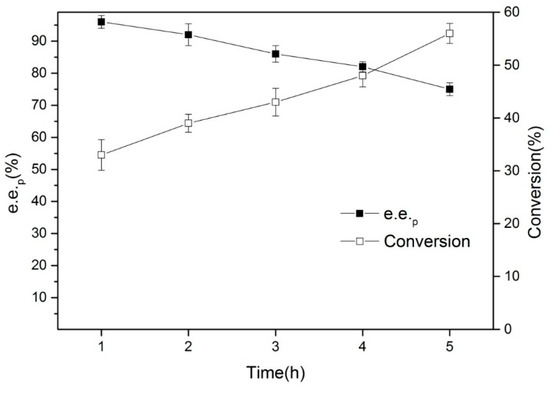
Figure 8.
The time course of the whole cell catalyzed resolution of (R, S)-ethyl indoline-2-carboxylate of Bacillus aryabhattai.
After one hour, the reaction solution was acidified by adding four M of HCl to halt the reaction until a pH of two was reached; it was then extracted with an equal volume of n-hexane to remove the unreacted substrate. The product was extracted with an equal volume of ethyl acetate and evaporated at 40 °C under reduced pressure. Finally, 0.12 g of solid was obtained with a yield of 36%. The solid was dissolved into DMSO, while the 1H NMR nuclear magnetic spectrum was recorded (Figure 9).
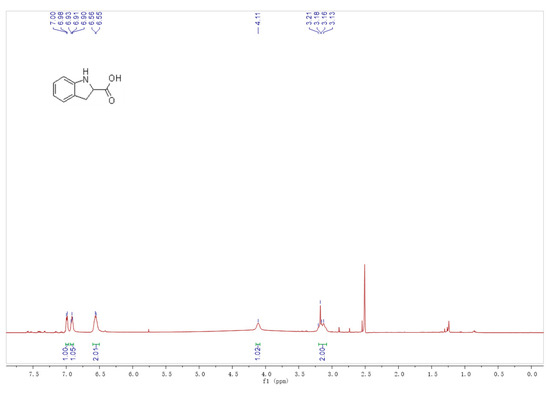
Figure 9.
The 1H NMR spectrum of the product.
3. Discussion
(S)-indoline-2-carboxylic acid is a widely used pharmaceutical intermediate with a large market demand. The strains that we selected have high activity toward (S)-ethyl indoline-2-carboxylate. At optimized conditions, the reaction can reach a 33% yield and 96% e.e.p, with the E value being 78. However, the experimental results show that the reaction will for an extended period cause the hydrolysis of ethyl (R)-indoline-2-carboxylate to produce (R)-indoline-2-carboxylic acid, which will eventually reduce the e.e.p. At the same time, a lower substrate concentration tolerance was observed for the whole cell hydrolysis. Therefore, we employed a batch feed method to add the substrate every hour to bring the final substrate concentration to 30 g/L. The results revealed similar conversion rates and e.e.p values.
The chemical route to obtaining (S)-indoline-2-carboxylic acid usually initially produces (R, S)-indoline-2-carboxylic acid. A reduction method is mainly used; for instance, Hudson et al. used phosphine iodide and concentrated hydroiodic acid as reducing agents to reduce indole-2-carboxylic acid to indoline-2-carboxylic acid with a yield of 86% [13]. Youn et al. successfully reduced ethyl indole-2-carboxylate to ethyl indoline-2-carboxylate, with a yield of 96%, using magnesium powder and methanol as the reducing agents [14]. One-step synthesis methods have also been reported. Katritzky et al. reported that indoline directly reacted with carbon dioxide to obtain indoline-2-carboxylic acid [15], after which (S)-indoline-2-carboxylic acid was obtained using a resolution reagent. In addition to (R)-α-phenethylamine, Wang et al. resolved (S)-indoline-2-carboxylic acid with camphorsulfonic acid [16]. Researchers have also explored techniques that did not involve resolving agents. Liu et al. nitrated L-phenylalanine to give 6-nitro-(S)-indoline-2-carboxylic acid, which was then reduced to give (S)-indoline-2-carboxylic acid [17]. These methods not only required high pressure or corrosion-resistant equipment, but were also highly polluting. The use of resolving reagents is also problematic in that it is expensive and difficult to recycle.
On the contrary, the biocatalytic method has attracted greater attention due to its mild reaction conditions, fewer by-products, and reduced heavy metal ions and organic reagents pollution. Cho’s patent used Savinase to hydrolyze (R, S)-methyl indoline-2-carboxylate [18]; the substrate concentration for this was 10%. They first obtained (S)-methyl indoline-2-carboxylate with e.e.s values at up to 99% and with a yield of 47% after five hours. Further hydrolysis in aqueous alkaline solution yielded (S)-indoline-2-carboxylic acid. Compared with the existing reports, the innovation of this research was that the whole cells of microorganisms were used as catalysts, reducing production costs by facilitating separation. Moreover, the reaction directly hydrolyzed the ethanol ester of (S)-indoline-2-carboxylic acid to obtain the products, reducing the number of reaction steps.
In summary, we screened strains with the capability of enantioselectively hydrolyzing (R, S)-ethyl indoline-2-carboxylate from sludge. Following morphological observation, 16S rDNA sequence analysis and physiological and biochemical identification, the strain with the highest enantioselectivity was identified as Bacillus aryabhattai. At the same time, a practical biochemical pathway was developed for the preparation of (S)-indoline-2-carboxylic acid. The reaction was carried out in water under mild conditions, and its subsequent processing was simple. The biocatalyst was easily mass produced by fermentation, effectively reducing the production cost of (S)-indoline-2-carboxylic acid. This is consequently advantageous for large-scale production, and further research into this approach is predicted.
4. Materials and Methods
4.1. Sources of Chemicals and Enzymes
(R, S)-ethyl indoline-2-carboxylate was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan), while (S)-indoline-2-carboxylic acid and (R)-indoline-2-carboxylic acid were supplied by Zhejiang Huahai Pharmaceutical Co., Ltd (Zhejiang, China). The enzymes Novozym 435 and TLIM (Thermomyces lanuginosus) were purchased from Novo Nordisk company (Beijing, China). The rest were purified and immobilized from wild strains and preserved in our laboratory, and the other reagents were of analytical grade. The soil samples were derived from pharmaceutical factory drainage port sludge in Zhejiang Province, China.
4.2. The Detection of (R, S)-Ethyl Indoline-2-Carboxylate and Its Products
Normal phase HPLC (Waters, 1525, Waters Ltd., Milford, MA, USA) was used to detect (R, S)-ethyl indoline-2-carboxylate and its products. Column DAICEL Chiral Pak® AD-H (5 μm, 4.6×250 mm, Daicel Chiral Technologies Ltd., Tokyo, Japan), mobile phase n-hexane:isopropanol = 5:1 (v/v), flow rate one mL/min, UV detection at 220 nm, column temperature 25 ° C, injection volume 10 μL. The NMR spectrum (500 MHz for one H of NMR) was recorded in DMSO using a Bruker Avance spectrometer (Bruker Ltd., Zurich, Switzerland); the chemical shift was reported in ppm, and the coupling constant was expressed in Hz.
An enzyme activity unit (U) is defined as the amount of enzyme required to produce one n mol of (S)-indoline-2-carboxylic acid per minute under certain conditions. The enantiomeric excess of the product (e.e.p) was calculated in Equation (1) with peak areas of (S)-indoline-2-carboxylic acid and (R)-indoline-2-carboxylic acid, and the enantiomeric ratio (E) was calculated according to Equation (2) [19]:
4.3. Activity Strains’ Enantioselective Hydrolysis Enrichment and Separation
The strains with enantioselective hydrolysis activity on (R, S)-ethyl indoline-2-carboxylate were isolated from sludge. An enrichment culture was carried out prior to the screening. Fresh sludge samples were suspended in a 50-mL medium containing 0.5 g of (R, S)-ethyl indoline-2-carboxylate, 2.0 g of NaNO3, 1.0 g of K2HPO4, 0.5 g of MgSO4∙7H2O, and 0.5 g of KCl, and incubated for five days at 30 °C and 180 rpm. The culture solution was then inoculated onto a 2% 50-mL fresh medium of the same composition, and incubated for a further five days under the same conditions. Then, 100 µL of culture solution was spread on a pH indicator agar plate containing the same components and bromothymol blue, and cultured at 30 °C until a yellow region appeared. The yellow colonies were selected and inoculated onto a 50-mL medium consisting of 10 g/L peptone, 5 g/L of yeast extract, and 10 g/L of NaCl, at an initial pH of seven. After 24 h of culturing, the cells were collected by centrifugation (10,000 rpm, two minutes) in a six-mL culture medium, and washed twice with phosphate buffer (200 mM, pH 7). The cells were resuspended in 1.5 mL of phosphate buffer (200 mM, pH 7), to which 0.015 g of (R, S)-ethyl indoline-2-carboxylate was added, and the culture was then stirred at 180 rpm, 30 °C for 24 h. The sample was extracted with ethyl acetate and monitored by HPLC.
4.4. Isolation and Identification of the Strains
The screened strains were streaked onto agar plates and cultured at 30 °C for one to three days. The shape, size, and color of the single colonies were observed. Following this, the single colonies were selected for Gram staining and observed using an optical microscope (Nikon Ys2-H). The whole genomes of the cells were extracted with a SK8255 column bacterial genomic DNA extraction kit, and PCR amplification of 16S rDNA was performed using the universal primer 27F/1492R primer (forward 5′-AGTTTGATCMTGGCTCAG-3′; reverse 5′-GGTTACCTTGTTACGACTT-3′). The conditions were as follows: 94 °C for four minutes, 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 90 s, repeating for 30 cycles; followed by 72 °C for 10 min. The amplified DNA was purified using a SK8131 DNA column gel recovery kit, and the nucleotide sequence of 16S rDNA was determined by Sangon Biotech Co., Ltd (Shanghai, China). The sequence alignments were BLAST (Basic Local Alignment Search Tool) on the NCBI [20].
4.5. The Production of Lipase in Bacillus Aryabhattai
Bacillus aryabhattai was cultured in a 250-mL shake flask to produce esterase. One liter of medium consisted of 20 g of yeast extract, 20 g of glucose, five g of (NH4)2SO4, one g of MgSO4∙7H2O, and 0.5 g of NaCl. The initial pH was seven, and the inoculums was 50 mL. The culture was carried out at 180 rpm, 30 °C for 24 h, and the biomass (OD600) and enzyme activity of Bacillus aryabhattai were measured periodically. The culture solution was centrifuged to obtain cells, and washed twice with phosphate buffer (200 mM, pH seven) to obtain resting cells. The cells were lyophilized using a freeze dryer and stored at ‒20 °C until use.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/2/206/s1, Figure S1: Colony morphology of Strain C26-1. The colonies were large, white, and nearly round, with dry, opaque surfaces and clear boundaries, Figure S2: The Gram-staining pattern of the strain. The cells observed with an optical microscope are the group of Gram-positive bacteria, Figure S3: 16S rDNA sequence agarose gel electrophoresis. The strip size is approximately 1500 bp, Figure S4: 16S rDNA of strain Bacillus aryabhattai was sequenced and the total length was 1470 bp, Table S1: Strain C26-1 on Biolog GEN III plates: Its utilization of 71 carbon sources and sensitivity to 23 chemicals.
Author Contributions
Conceptualization, Y.Z. and S.W.; methodology, Y.Z. and S.W.; software, J.C.; validation, C.C.; investigation, J.C.; resources, Y.Z.; data curation, C.C.; writing—original draft preparation, J.C.; writing—review and editing, J.C.; visualization, C.C.; project administration, Y.Z.
Funding
This study was funded by the Zhejiang Provincial Natural Science Foundation (LY18B020021).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; nor in the collection, analyses, or interpretation of data, the writing of the manuscript, or, the decision to publish the results.
References
- Lebreton, L.; Dumas, C.; Massardier, C. Novel compounds of indoline. F.R. Patent 2,886,293, 12 January 2006. [Google Scholar]
- Kujala, T.; Loponen, J.; Pihlaja, K. Betalains and phenolics in red beetroot (beta vulgaris) peel extracts: Extraction and characterisation. Z. Naturforsch. Sect. C J. Biosci. 2001, 56, 343–348. [Google Scholar] [CrossRef]
- Thuillez, C.; Richard, C.; Loueslati, H.; Auzepy, P.; Giudicelli, J.F. Systemic and regional hemodynamic effects of perindopril in congestive heart failure. J. Cardiovasc. Pharmacol. 1990, 15, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F. Angiotensin-converting enzyme inhibitor and spironolactone combination therapy: New objectives in congestive heart failure treatment. Am. J. Cardiol. 1993, 71, A34–A39. [Google Scholar] [CrossRef]
- Vincent, M.; Baliarda, J.; Marchand, B.; Remond, G. Alpha-methyl Benzyl Amine Salt of Indoline-2-Carboxylic Acid. U.S. Patent 4,954,640, 4 September 1990. [Google Scholar]
- Viswanathan, R.; Prabhakaran, E.N.; Plotkin, M.A.; Johnston, J.N. Free radical-mediated aryl amination and its use in a convergent [3 + 2] strategy for enantioselective indoline alpha-amino acid synthesis. J. Am. Chem. Soc. 2003, 125, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Masanori, A.; Shigaki, H.; Hidetoshi, K.; Yoshio, N.; Hideyuki, T.; Kenji, T. Process for Preparing Optically Active Indoline-2-Carboxylic Acid. U.S. Patent 4,898,822, 6 February 1990. [Google Scholar]
- De Lange, B.; Hyett, D.J.; Maas, P.J.D.; Mink, D.; Van Assema, F.B.J.; Sereinig, N. Asymmetric synthesis of (s)-2-indolinecarboxylic acid by combining biocatalysis and homogeneous catalysis. Chemcatchem 2011, 3, 289–292. [Google Scholar] [CrossRef]
- Huang, J.; Yan, R.; Shi, H.; Wang, P.; He, J. The characteristic and kinetic studies on the enantioselective hydrolysis of (r, s)-α-ethyl-2-oxo-1-pyrrolidine acetic acid ethyl ester catalyzed by tsukamurella tyrosinosolvens, e105-derived hydrolase. Asia-Pac. J. Chem. Eng. 2015, 9, 941–949. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. (Int. Ed.) 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A. Enzymatic catalysis in organic media at 100 degrees C. Science 1984, 224, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yun, Z.; Sun, A.; Hu, Y. Enantio-selective preparation of (S)-1-phenylethanol by a novel marine GDSL lipase MT6 with reverse stereo-selectivity. Chin. J. Catal. 2016, 37, 1966–1974. [Google Scholar] [CrossRef]
- Hudson, C.; Robertson, A. The synthesis and chemistry of DL-indoline-2-carboxylic acid. Aust. J. Chem. 1967, 20, 1935. [Google Scholar] [CrossRef]
- Youn, I.K.; Yon, G.H.; Pak, C.S. Magnesium-methanol as a simple convenient reducing agent for α,β-unsaturated esters. Cheminform 1986, 27, 2409–2410. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Sengupta, S. α-metallation of tetrahydroquinoline and indoline via their lithium carbamates: A versatile one-pot procedure. J. Chem. Soc. Perkin Trans. 1989, 1, 17–19. [Google Scholar] [CrossRef]
- Wang, Z.X.; Raheem, M.A.; Weeratunga, G. New Processes for the Preparation of Optically Pure Indoline-2-Carboxylic Acid and n-Acetyl-Indoline-2-Carboxylic Acid. Patent WO/2006/053440, 26 May 2006. [Google Scholar]
- Liu, J.Q.; Chen, X.Z.; Ji, B.; Zhao, B.T. Transformation of l-phenylalanine to (s)-indoline-2-carboxylic acid without group-protection. Res. Chem. Intermed. 2013, 39, 1143–1152. [Google Scholar] [CrossRef]
- Cho, N.R.; Lim, J.H.; Kim, J.K. Method for Preparing (s)-Indoline-2-Carboxylic Acid and (s)-Indoline-2-Carboxylic Acid Methyl Ester Using Hydrolytic Enzyme. U.S. Patent US20,070,077,632A1, 5 April 2007. [Google Scholar]
- Hsiao, H.T.; Lin, S.Y.; Tsai, S.W. Quantitative insights and improvements of enzyme activity and stereoselectivity for calb-catalyzed alcoholysis in two-step desymmetrization. J. Mol. Catal. B Enzym. 2016, 127, 82–88. [Google Scholar] [CrossRef]
- Wilson, K.H.; Blitchington, R.B. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 1996, 62, 2273–2278. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).