Abstract
Redox reactions catalyzed by highly selective nicotinamide-dependent oxidoreductases are rising to prominence in industry. The cost of nicotinamide adenine dinucleotide coenzymes has led to the use of well-established elaborate regeneration systems and more recently alternative synthetic biomimetic cofactors. These biomimetics are highly attractive to use with ketoreductases for asymmetric catalysis. In this work, we show that the commonly studied cofactor analogue 1-benzyl-1,4-dihydronicotinamide (BNAH) can be used with alcohol dehydrogenases (ADHs) under certain conditions. First, we carried out the rhodium-catalyzed recycling of BNAH with horse liver ADH (HLADH), observing enantioenriched product only with unpurified enzyme. Then, a series of cell-free extracts and purified ketoreductases were screened with BNAH. The use of unpurified enzyme led to product formation, whereas upon dialysis or further purification no product was observed. Several other biomimetics were screened with various ADHs and showed no or very low activity, but also no inhibition. BNAH as a hydride source was shown to directly reduce nicotinamide adenine dinucleotide (NAD) to NADH. A formate dehydrogenase could also mediate the reduction of NAD from BNAH. BNAH was established to show no or very low activity with ADHs and could be used as a hydride donor to recycle NADH.
1. Introduction
The field of biocatalysis has flourished in the past few decades, with enzymes increasingly being used in industrial processes for fine chemical commodities. Alcohol dehydrogenases ADHs (EC 1.1.1.X, also ketoreductases KREDs) are one example, reversibly catalyzing the reduction of ketones or aldehydes to the corresponding (enantioenriched) alcohols through the use of one equivalent of nicotinamide adenine dinucleotide cofactor NAD(P). ADHs are known to be specific to either the phosphorylated NADP or non-phosphorylated NAD, although a few have been found to accept both [1,2,3]. These cofactors act as hydride acceptor and donor intermediates between the substrate and product.
Enzyme recognition of NAD(P), usually by a cofactor (binding) motif such as the Rossmann fold, is important for cellular processes, however when using in vitro systems, the adenine dinucleotide moiety of the cofactor becomes obsolete and disadvantageous, being prone to hydrolysis [4]. Synthetic nicotinamide coenzyme biomimetics (NCBs) have been shown to replace NAD(P)H in flavin-dependent enzymes such as nitroreductase and NAD(P)H quinone oxidoreductase [5,6,7], a cytochrome P450 BM3 variant [8], ene-reductases [9,10,11,12,13,14,15,16], and styrene monooxygenase [17]. In all cases described, a flavin was involved as an electron mediator [18,19,20,21,22].
Jones pioneered the use of synthetic cofactor analogues with horse liver ADH (HLADH) [23,24,25,26], followed by Fish [27] and others [28,29]. A dehydrogenase from the aldo-reductase superfamily from Pyrococcus furiosus (AdhD) was engineered to increase catalytic efficiency towards nicotinamide mononucleotide (NMN, Figure 1A) [30,31]. Acyclic analogues of NAD (Figure 1B) were shown not only to be used with HLADH, but also to alter the substrate specificity of the enzyme [32]. More recently, the group of Sieber showed the use of synthetic NCBs with an NADH oxidase [33,34], and a glucose dehydrogenase (GDH), producing variants displaying activity, albeit low, with 1-benzylnicotinamide (BNA, Figure 1C) [35].
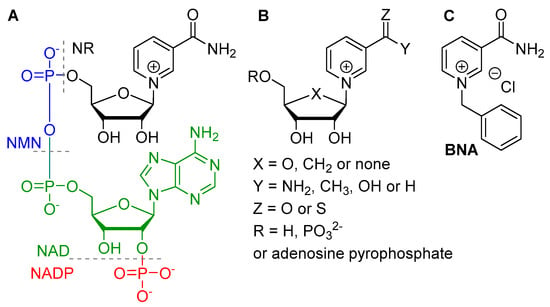
Figure 1.
(A) Schematic representation of the natural NAD(P); (B) Naturally-based analogues; (C) synthetic analogue.
These examples are important steps towards the use of NCBs with dehydrogenases, and more specifically ADHs, which would be advantageous for large scale applications, although NCB recycling and stability is still in its early stages [36]. In a previous study, cofactors NR, NMN, and carbocyclic analogues showed no observable activity in the HLADH-catalyzed oxidation of ethanol [37,38], corroborated by computational studies on the mechanism for hydride transfer in HLADH [39].
Here the research was carried out to establish whether the commonly used and now commercially available BNAH analogue could be used with ADHs and other ketoreductases for asymmetric catalysis. First, we explored the rhodium-catalyzed recycling of BNAH with HLADH [27], which seemed to show that BNAH could replace NADH, contrary to recent attempts [16,35]. This work was followed by screening series of ADHs and KREDs to assess biomimetic acceptance. BNAH was further used as a hydride source in a preliminary recycling system with a formate dehydrogenase (FDH).
2. Results and Discussion
2.1. Rh-Catalyzed Cofactor Recycling
The group of Fish reported activity with HLADH and cofactor analogues BNA and NMN using a Rh-catalyzed recycling system [27]. For this study we kept 4-phenyl-2-butanone as a substrate, as the enantiomeric excess (ee) of the reduced product would probe the enantioselectivity of the enzymatic reaction (Figure 2A). The same HLADH enzyme was purchased, although the provider and purity are different, as the enzyme is now being produced by recombinant expression (see Supplementary Information (SI) Section 1).
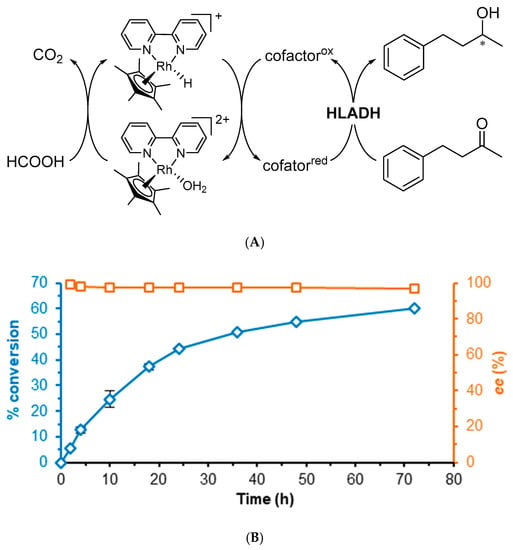
Figure 2.
(A) Representative scheme of the HLADH-catalyzed reduction of 4-phenyl-2-butanone to enantioenriched 4-phenyl-2-butanol; (B) Time course of the reaction (blue diamonds, ee orange squares); NADH regeneration system with [Cp*Rh(bpy)(H2O)]2+ using sodium formate as a hydride source. Reaction conditions: 100 mM KPi pH 7.0, HLADH (2 U), [NAD] = 2.2 mM, [Cp*Rh(bpy)(H2O)]2+ = 0.52 mM, [HCO2Na] = 52 mM, [4-phenyl-2-butanone] = 16.7 mM, shaken at 30 °C, 800 rpm. Conversion and ee determined by chiral GC.
We could produce the expected results with the Rh-catalyzed regeneration of NADH under the same conditions (Figure 2B, 60% conversion 97% ee after 72 h) [27]. Next, we compared reaction results between the commercially available ‘crude’ (unpurified cell-free extract) HLADH and the dialyzed enzyme, with either NAD, nicotinamide mononucleotide (NMN) or BNA (Figure 3 and SI Table S3). When using either crude or dialyzed HLADH with NAD, after 18 h, conversions were similar (38–42%) with a good enantiomeric excess (>90% ee). However, the same reactions carried out in the presence of NMN, BNA, or in the absence of a cofactor showed 4–11% conversion and 4–37% ee compared to 18–78% conversion and <1% ee with the dialyzed enzyme (Figure 3).
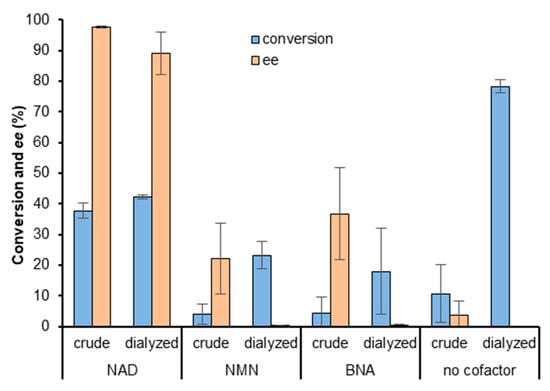
Figure 3.
HLADH-catalyzed reduction of 4-phenyl-2-butanone with the Rh complex recycling system using either NAD, NMN, BNA, or no cofactor. HLADH was used as lyophilized cell-free extracts (crude) or dialyzed (conversion, blue; ee, orange). Reaction conditions: 100 mM KPi pH 7.0, HLADH (2 U), [cofactor] = 2.2 mM (where applicable), [Cp*Rh(bpy)(H2O)]2+ = 0.52 mM, [HCO2Na] = 52 mM, [4-phenyl-2-butanone] = 16.7 mM, shaken at 30 °C, 800 rpm, 18 h.
A rationale for the higher conversions achieved with dialyzed HLADH with respect to the crude HLADH, with NMN, BNA or without cofactor, could be found by looking closer at the ee obtained with crude HLADH (4–37% ee) and with dialyzed HLADH (<1% ee), suggesting direct reduction of the ketone by the Rh complex. With the crude enzyme preparation, trace amounts of NAD could be reduced by the Rh complex, hence showing some enzymatic reduction of the ketone. In this case, Rh-catalyzed reduction of the ketone could also be inhibited by the presence of other low molecular weight molecules in the crude enzyme preparation [40]. Removal of these low molecular weight species (NAD or other) by dialysis (cut-off at 12 kDa) would favor the non-enzymatic Rh-catalyzed ketone reduction and account for the higher conversions and lack of enantioselectivity of this transformation.
The redox potentials of NAD and NMN are identical [41], therefore chemical reactivity cannot explain the difference in the enzyme-catalyzed reaction. Extensive investigation by Plapp and others through analyses of high resolution X-ray structures of HLADH reveals that the binding of NAD to the coenzyme-binding domain induces a conformational modification [42,43,44] necessary for catalytic activity of the enzyme [45]. With the truncated nicotinamide cofactors NR, NMN and the synthetic BNA, the conformational change most likely does not occur, indicating the complete coenzyme is required [46]. The importance of specific interactions between coenzyme and enzyme (SI Figure S2) is supported by molecular dynamics simulations [47].
Therefore, we established that trace amounts of NAD present in the commercial HLADH preparation are responsible for the low to modest conversions and ee observed with NMN and BNA, and once removed only racemic product was observed due to non-enzymatic reduction. HLADH showed no observable or very low activity with NMN and BNA.
2.2. Screening of ADHs and Ketoreductases
Libraries of ADHs and KREDs were screened for activity with BNAH. A panel of KREDs from a Codexis kit was used for the reduction of cyclohexanone with BNAH as the sole cofactor (Figure 4, grey bars). Control reactions were run without cofactor (white bars). Product was observed in all cases in varying percent conversions, in the control reactions (no BNAH added) as well. Further screening of various available ADHs and KREDs in the form of cell-free extracts (see SI Sections 3.3 and 3.4) showed similar activity.
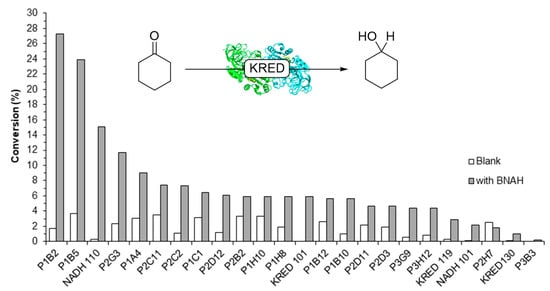
Figure 4.
Screening of the ketoreductases (KREDs) Codexis kit. X-axis: KREDs. Y-axis: conversion to cyclohexanol after 24 h, measured by GC analysis, with BNAH (grey) and without BNAH (white). Reactions conditions: 50 mM KPi pH 7.0, [cyclohexanone] = 10 mM, [KRED] = 10 mg/mL, [BNAH] = 10 mM (where applicable).
We hypothesized that the conversion obtained was due to the presence of NAD(P)/NAD(P)H from the lyophilized crude cell extracts. The additional product formation observed with BNAH can be attributed to its ability to reduce trace amounts of NAD(P), through a intermolecular hydride transfer, which was well established and demonstrated by Jones [23].
Using the heat purified TADH (Thermus sp. ATN1), no product was observed with BNAH as the sole cofactor. Further testing of other purified ADHs, HvADH2 (Haloferax volcanii ADH2) [48], HvADH2-F108G, HwADH (Haloquadratum walsbyi ADH), and HLADH-EE [49] with the natural cofactors and a series of synthetic NCBs (Figure S1) showed the latter afforded no observable activity, as well as no significant inhibitory effects (SI Section 3.3).
Thus, the conclusion from screening cell free extracts and purified ADHs and KREDs was that BNAH gave no detectable activity but could be used as a hydride source.
2.3. BNAH as a Hydride Donor
2.3.1. Direct Hydride Transfer to NAD
Due to the lack of activity with purified enzyme, we investigated the use of BNAH as a hydride source to recycle NADH for HLADH-catalyzed reactions (Figure 5 top scheme) [23,24,26]. Using the dialyzed HLADH for the reduction of 4-phenyl-2-butanone, we could reach 1.4 mM of the enantioenriched alcohol (98% ee) product after 18 h (Figure 5 bottom graph), affording a turnover frequency (TOF) of 7 h−1 with stoichiometric BNAH and 1 mM NAD, compared to a TOF of 23 h−1 with 1 equivalent of NADH, and 21 h−1 with the Rh recycling system. We ascribe the decrease in reaction kinetics after 3 h to the instability of BNAH over time, especially in the phosphate buffer as previously described [34].
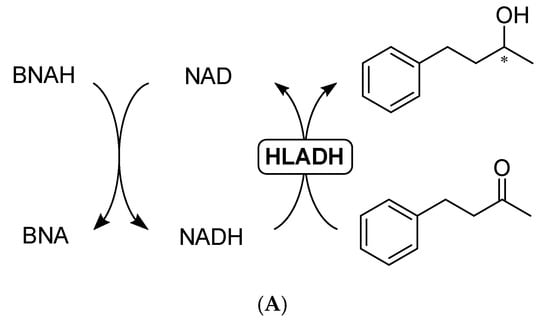
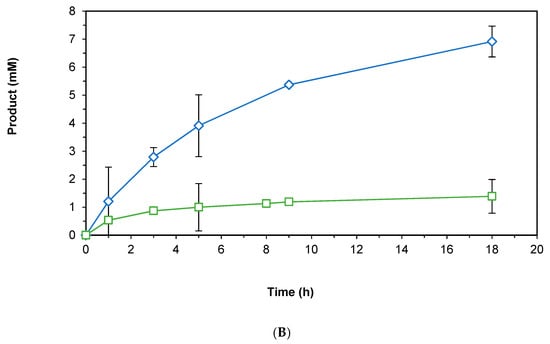
Figure 5.
(A) Representative scheme of the HLADH-catalyzed reduction of 4-phenyl-2-butanone to enantioenriched 4-phenyl-2-butanol; (B) Time course of the reaction with NADH (blue diamonds) or BNAH + NAD (green squares). Reaction conditions: 100 mM KPi pH 7.0, 1 mL final volume, HLADH (2 U), [NADH] = 16.7 mM (blue diamonds), [BNAH] = 16.7 mM + [NAD] = 1 mM (green squares), [4-phenyl-2-butanone] = 16.7 mM, shaken at 30 °C and 800 rpm over time.
2.3.2. Formate Dehydrogenase with BNAH
To improve the use of BNAH as a hydride donor to reduce NAD, a formate dehydrogenase (FDH)-catalyzed recycling system was established. The system shown in Figure 6A uses the NAD-dependent FDH1 β-subunit from Methylobacterium extorquens AM1 (MeFDH) as a relay between BNAH and NAD. The iron-sulfur cluster contained in MeFDH can be reduced by BNAH, subsequently reducing NAD. This new recycling system was coupled to a purified ADH (ADH-A from Rhodococcus ruber). The use of MeFDH shows catalytic behavior and results in 1.7 mM of product after 24 h (Figure 6B). The control reaction without MeFDH shows almost 5 times lower conversion with 0.3 mM of product. Catalytic turnover numbers of 17 (NAD), 338 (MeFDH) and 11 698 (ADH-A) were observed. The significant decrease of the reaction kinetics after 3 h applying the MeFDH could indicate stability problems regarding the enzyme. Immobilization of the enzymes could be envisaged to improve the system. Further optimizations to improve the robustness of the system could lead to even higher catalytic turnover.
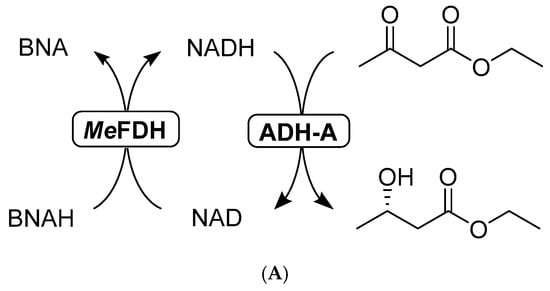
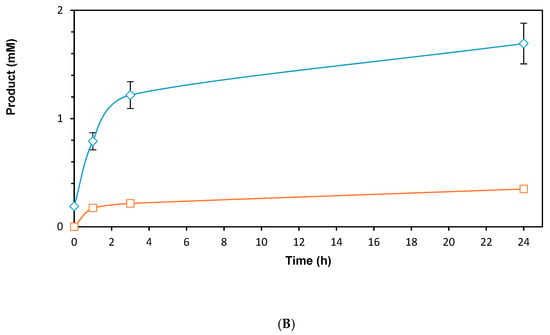
Figure 6.
(A) Representative scheme of the ADH-A-catalyzed reduction of ethyl acetoacetate with MeFDH; (B) Time course of the reaction (blue diamonds) or without MeFDH (orange squares). Reaction conditions: 50 mM NaPi pH 7.0, ADH-A (0.6 U), [MeFDH] = 5 µM (blue diamonds) or 0 µM (orange squares), [NAD] = 0.1 mM, [BNAH] = 10 mM (solid), [ethyl acetoacetate] = 10 mM, shaken at 30 °C, 800 rpm, anaerobic.
3. Materials and Methods
3.1. Chemicals and Enzymes
All commercially available reagents and solvents were purchased with the highest purity available and used as received. Enzymes were either commercially available or recombinantly expressed. Further details are provided in the Supplemental Information (SI).
The HLADH was obtained from Evocatal GmbH as ADH 210 HLADH (≥0.5 U/mg, protein concentration 0.22 mg/mg) and from Sigma-Aldrich (St. Louis, MO, USA) as alcohol dehydrogenase equine (recombinant, expressed in E. coli, ≥0.5 U/mg). When referring to the ‘crude’ preparation of HLADH, the enzyme was used as received. When referring to the ‘dialyzed’ HLADH, the enzyme was further purified through dialysis. The activity of both the crude and dialyzed HLADH was measured by UV spectroscopy before use. 1-Benzyl-1,4-dihydronicotinamide BNAH, other analogues and rhodium complexes were synthesized as previously described [7,9,40].
3.2. Instrumentation
UV spectroscopy measurements were made using an Agilent Cary 60 UV-Vis spectrophotometer (Santa Clara, CA, USA) at the designated wavelength.
Gas chromatography (GC) analyses were carried out on a Shimadzu GC-2010 (Kyoto, Japan) gas chromatograph equipped with a flame ionization detector (FID).
Nuclear magnetic resonance (NMR) spectra were recorded on an Agilent Technologies spectrometer (Santa Clara, CA, USA) at 400 MHz (1H).
3.3. Experimental Setup
Screenings and biocatalytic reactions were run in a microcentrifuge plastic tube (1.5 mL in volume). The ADH enzyme (10 mg of lyophilized ADH per 1 mL reaction), potassium phosphate buffer (50 mM, pH 7.0; final volume of 1 mL), cofactor BNAH (10 mM, added in acetonitrile, 2% final volume: 20 µL) and cyclohexanone (10 mM) were added. The reaction mixture was placed in a thermomixer at 25 °C and 800 rpm for 24 h. The reaction was stopped through extraction (500 μL of ethyl acetate containing 5 mM dodecane as an internal standard). After centrifugation (13,000 rpm, 1 min) and separation of the two phases, the ethyl acetate layer was dried with anhydrous magnesium sulfate, centrifuged (12,000 rpm, 1 min), transferred to gas chromatography (GC) vials and analyzed by GC. Blank reactions were carried out in the absence of BNAH or any type of cofactor.
For the biocatalytic Rh recycling experiments, stock solutions of NAD, NMN and BNA (10 mM), [Cp*Rh(bpy)(H2O)]Cl2, [Cp*Rh(bpy)(H2O)]OTf2 (5 mM), sodium formate (1 M) and HLADH (3 mg, 2 U/mg) in deoxygenated phosphate buffer (100 mM, pH 7.0) were freshly prepared before the experiments. In a microcentrifuge plastic tube (2 mL in volume), potassium phosphate buffer (100 mM, pH 7.0; final volume of 1 mL), cofactor (2.2 mM or none), [Cp*Rh(bpy)(H2O)]2+ (104 μL, 0.52 mM), sodium formate (52 μL, 52 mM), HLADH (200 μL, 2 U), and substrate 4-phenyl-2-butanone (2.5 μL, 16.7 mM) were added under nitrogen flow. The reaction mixture was placed in an Eppendorf thermomixer at 30 °C and 600 rpm for 18 h, or other indicated times. The reaction was stopped at the indicated time intervals through extraction (500 μL of ethyl acetate containing 5 mM dodecane as an internal standard). After centrifugation (13,000 rpm, 1 min) and separation of the two phases, the ethyl acetate layer was dried with anhydrous magnesium sulfate, centrifuged (12,000 rpm, 1 min), transferred to GC vials and analyzed on a chiral GC column.
4. Conclusions
From the Rh-catalyzed recycling of BNAH we could establish that unpurified ADH led to product formation via reduction of trace amounts of NAD. The screening of purified ADHs from various sources with biomimetic cofactors showed no detectable or very low activity. Additionally, no significant inhibition by these analogues was observed. BNAH can be used as a hydride donor to recycle NADH due to an intermolecular hydride transfer. For the first time, we show a regeneration system using formate dehydrogenase MeFDH and BNAH as a hydride donor, albeit with low catalytic turnover.
Future prospects towards both coenzyme and protein engineering would be very promising for ADHs, based on encouraging results with other dehydrogenases AdhD and SsGDH from the enzyme perspective, and knowledge on the structure-activity relationship from the coenzyme point of view. With these prospects in progress, industrial applications of synthetic biomimetics would be of interest once their instability is overcome.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/3/207/s1: the experiment details on enzymatic activity assays, syntheses of the rhodium complex and NCBs, biocatalytic reactions, analytical data and product characterization.
Author Contributions
Conceptualization, F.H. and C.E.P.; validation, S.G., D.J.O., J.-H.X., F.P., F.H. and C.E.P.; investigation, L.J.-C., A.S.K.L., A.R., G.T.H., S.G., Y.Y.L., J.C. and C.E.P.; resources, J.-H.X., F.P., F.H. and C.E.P.; writing—original draft preparation, F.H. and C.E.P.; writing—review and editing, all authors; supervision, F.H. and C.E.P.; project administration, C.E.P.; funding acquisition, F.H. and C.E.P.
Funding
This research was funded by the Netherlands Organization for Scientific Research (NWO), VENI grant number 722.015.011 (for C.E.P.); by the European Union through the Seventh Framework People Programme, grant number 316955 (for L.J.-C., A.L. and A.R.); and by the European Research Council ERC Consolidator, grant number 648026 (for G.T.H. and F.H.).
Acknowledgments
We kindly thank Yong Hwan Kim at UNIST for the MeFDH.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Faber, K. Biotransformations in Organic Chemistry; Springer: Berlin, Germany, 2011; ISBN 978-3-642-17393-6. [Google Scholar]
- Gröger, H.; Hummel, W.; Borchert, S.; Kraußer, M. Reduction of ketones and aldehydes to alcohols. In Enzyme Catalysis in Organic Synthesis; Drauz, K., Gröger, H., May, O., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 1035–1110. [Google Scholar]
- Hollmann, F.; Bühler, K.; Bühler, B. Oxidation of alcohols, aldehydes, and acids. In Enzyme Catalysis in Organic Synthesis; Drauz, K., Gröger, H., May, O., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 1325–1437. [Google Scholar]
- Oppenheimer, N.J. NAD hydrolysis: Chemical and enzymatic mechanisms. Mol. Cell. Biochem. 1994, 138, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.J.; Friedlos, F.; Jarman, M.; Davies, L.C.; Goddard, P.; Anlezark, G.M.; Melton, R.G.; Sherwood, R.F. Virtual cofactors for an Escherichia coli nitroreductase enzyme—Relevance to reductively activated prodrugs in antibody directed enzyme prodrug therapy (ADEPT). Biochem. Pharmacol. 1995, 49, 1641–1647. [Google Scholar] [CrossRef]
- Friedlos, F.; Jarman, M.; Davies, L.C.; Boland, M.P.; Knox, R.J. Identification of novel reduced pyridinium derivatives as synthetic cofactors for the enzyme DT diaphorase (NAD(P)H dehydrogenase (quinone), EC 1.6.99.2). Biochem. Pharmacol. 1992, 44, 25–31. [Google Scholar] [CrossRef]
- Knox, R.J.; Jenkins, T.C.; Hobbs, S.M.; Chen, S.A.; Melton, R.G.; Burke, P.J. Bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by human NAD(P)H quinone oxidoreductase 2: A novel co-substrate-mediated antitumor prodrug therapy. Cancer Res. 2000, 60, 4179–4186. [Google Scholar] [PubMed]
- Ryan, J.D.; Fish, R.H.; Clark, D.S. Engineering cytochrome P450 enzymes for improved activity towards biomimetic 1,4-NADH cofactors. ChemBioChem 2008, 9, 2579–2582. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.E.; Gargiulo, S.; Opperman, D.J.; Lavandera, I.; Gotor-Fernández, V.; Gotor, V.; Taglieber, A.; Arends, I.W.C.E.; Hollmann, F. Mimicking nature: Synthetic nicotinamide cofactors for C=C bioreduction using enoate reductases. Org. Lett. 2013, 15, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Knaus, T.; Paul, C.E.; Levy, C.W.; de Vries, S.; Mutti, F.G.; Hollmann, F.; Scrutton, N.S. Better than nature: Nicotinamide biomimetics that outperform natural coenzymes. J. Am. Chem. Soc. 2016, 138, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Geddes, A.; Paul, C.E.; Hay, S.; Hollmann, F.; Scrutton, N.S. Donor-acceptor distance sampling enhances the performance of “better than Nature” nicotinamide coenzyme biomimetics. J. Am. Chem. Soc. 2016, 138, 11089–11092. [Google Scholar] [CrossRef] [PubMed]
- Löw, S.A.; Löw, I.M.; Weissenborn, M.J.; Hauer, B. Enhanced ene-reductase activity through alteration of artificial nicotinamide cofactor substituents. ChemCatChem 2016, 8, 911–915. [Google Scholar] [CrossRef]
- Scholtissek, A.; Tischler, D.; Westphal, A.H.; van Berkel, W.J.H.; Paul, C.E. Old yellow enzyme-catalysed asymmetric hydrogenation: Linking family roots with improved catalysis. Catalysts 2017, 7, 130. [Google Scholar] [CrossRef]
- Scholtissek, A.; Gadke, E.; Paul, C.E.; Westphal, A.H.; van Berkel, W.J.H.; Tischler, D. Catalytic performance of a class III old yellow enzyme and its cysteine variants. Front. Microbiol. 2018, 9, 2410. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, A.; Ullrich, S.R.; Mühling, M.; Schlömann, M.; Paul, C.E.; Tischler, D. A thermophilic-like ene-reductase originating from an acidophilic iron oxidizer. Appl. Microbiol. Biotechnol. 2017, 101, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Falcone, N.; She, Z.; Syed, J.; Lough, A.; Kraatz, H.-B. Synthesis and biochemical evaluation of nicotinamide derivatives as NADH analogues in ene reductase. ChemBioChem 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.E.; Tischler, D.; Riedel, A.; Heine, T.; Itoh, N.; Hollmann, F. Nonenzymatic regeneration of styrene monooxygenase for catalysis. ACS Catal. 2015, 5, 2961–2965. [Google Scholar] [CrossRef]
- Paul, C.E.; Hollmann, F. A survey of synthetic nicotinamide cofactors in enzymatic processes. Appl. Microbiol. Biotechnol. 2016, 100, 4773–4778. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.E.; Arends, I.W.C.E.; Hollmann, F. Is simpler better? Synthetic nicotinamide cofactor analogues for redox chemistry. ACS Catal. 2014, 4, 788–797. [Google Scholar] [CrossRef]
- Hollmann, F.; Paul, C.E. Synthetische nikotinamide in der biokatalyse. BIOspektrum 2015, 21, 376–378. [Google Scholar] [CrossRef]
- Qi, J.X.; Paul, C.E.; Hollmann, F.; Tischler, D. Changing the electron donor improves azoreductase dye degrading activity at neutral pH. Enzyme Microb. Technol. 2017, 100, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Hollmann, F.; Ho, T.V.; Schnyder, A.; Fish, R.H.; Schmid, A. Bioorganometallic chemistry: Biocatalytic oxidation reactions with biomimetic NAD+/NADH co-factors and [Cp*Rh (bpy) H]+ for selective organic synthesis. J. Organomet. Chem. 2004, 689, 4783–4790. [Google Scholar] [CrossRef]
- Taylor, K.E.; Jones, J.B. Nicotinamide coenzyme regeneration by dihydropyridine and pyridinium compounds. J. Am. Chem. Soc. 1976, 98, 5689–5694. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B.; Taylor, K.E. Nicotinamide coenzyme regeneration-rates of some 1,4-dihydropyridine, pyridinium salt, and flavin mononucleotide hydrogen-transfer reactions. Can. J. Chem. 1976, 54, 2974–2980. [Google Scholar] [CrossRef]
- Jones, J.B.; Taylor, K.E. Nicotinamide coenzyme regeneration-flavin mononucleotide (riboflavin phosphate) as an efficient, economical, and enzyme-compatible recycling agent. Can. J. Chem. 1976, 54, 2969–2973. [Google Scholar] [CrossRef]
- Jones, J.B.; Taylor, K.E. Use of pyridinium and flavin derivatives for recycling of catalytic amounts of NAD+ during preparative-scale horse liver alcohol dehydrogenase-catalyzed oxidations of alcohols. J. Chem. Soc. Chem. Commun. 1973, 6, 205–206. [Google Scholar] [CrossRef]
- Lo, H.C.; Fish, R.H. Biomimetic NAD+ models for tandem cofactor regeneration, horse liver alcohol dehydrogenase recognition of 1,4-NADH derivatives, and chiral synthesis. Angew. Chem. Int. Ed. 2002, 41, 478–481. [Google Scholar] [CrossRef]
- Ansell, R.J.; Lowe, C.R. Artificial redox coenzymes: Biomimetic analogues of NAD+. Appl. Microbiol. Biotechnol. 1999, 51, 703–710. [Google Scholar] [CrossRef]
- Sunderland, J.R.; Tao, X.; Butrick, E.E.; Keilich, L.C.; Villa, C.E.; Miecznikowski, J.R.; Jain, S.S. Investigation of liver alcohol dehydrogenase catalysis using an NADH biomimetic and comparison with a synthetic zinc model complex. Polyhedron 2016, 114, 145–151. [Google Scholar] [CrossRef]
- Campbell, E.; Meredith, M.; Minteer, S.D.; Banta, S. Enzymatic biofuel cells utilizing a biomimetic cofactor. Chem. Commun. 2012, 48, 1898–1900. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Wheeldon, I.R.; Banta, S. Broadening the cofactor specificity of a thermostable alcohol dehydrogenase using rational protein design introduces novel kinetic transient behavior. Biotechnol. Bioeng. 2010, 107, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Malver, O.; Sebastian, M.J.; Oppenheimer, N.J. Alteration in substrate specificity of horse liver alcohol dehydrogenase by an acyclic nicotinamide analog of NAD. DNA Repair 2014, 23, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Nowak, C.; Beer, B.; Pick, A.; Roth, T.; Lommes, P.; Sieber, V. A water-forming NADH oxidase from Lactobacillus pentosus suitable for the regeneration of synthetic biomimetic cofactors. Front. Microbiol. 2015, 6, 957. [Google Scholar] [CrossRef] [PubMed]
- Nowak, C.; Pick, A.; Csepei, L.I.; Sieber, V. Characterization of biomimetic cofactors according to stability, redox potentials, and enzymatic conversion by NADH oxidase from Lactobacillus pentosus. ChemBioChem 2017, 18, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Nowak, C.; Pick, A.; Lommes, P.; Sieber, V. Enzymatic reduction of nicotinamide biomimetic cofactors using an engineered glucose dehydrogenase: Providing a regeneration system for artificial cofactors. ACS Catal. 2017, 7, 5202–5208. [Google Scholar] [CrossRef]
- Zachos, I.; Nowak, C.; Sieber, V. Biomimetic cofactors and methods for their recycling. Curr. Opin. Chem. Biol. 2018, 49, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sicsic, S.; Durand, P.; Langrene, S.; Legoffic, F. A new approach for using cofactor dependent enzymes—Example of alcohol-dehydrogenase. FEBS Lett. 1984, 176, 321–324. [Google Scholar] [CrossRef]
- Sicsic, S.; Durand, P.; Langrene, S.; Legoffic, F. Activity of NMN+, nicotinamide ribose and analogs in alcohol oxidation promoted by horse-liver alcohol-dehydrogenase—Improvement of this activity and structural requirements of the pyridine-nucleotide part of the NAD+ coenzyme. Eur. J. Biochem. 1986, 155, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Webb, S.P.; Hammes-Schiffer, S. Computational studies of the mechanism for proton and hydride transfer in liver alcohol dehydrogenase. J. Am. Chem. Soc. 2000, 122, 4803–4812. [Google Scholar] [CrossRef]
- Poizat, M.; Arends, I.W.C.E.; Hollmann, F. On the nature of mutual inactivation between [Cp*Rh (bpy) (H2O)]2+ and enzymes-analysis and potential remedies. J. Mol. Catal. B Enzym. 2010, 63, 149–156. [Google Scholar] [CrossRef]
- Srinivasan, R.; Fisher, H.F. Configurational, conformational, and solvent effects on the reduction of a Schiff-base by reduced pyridine-nucleotide analogs. Arch. Biochem. Biophys. 1983, 223, 453–457. [Google Scholar] [CrossRef]
- Eklund, H.; Nordström, B.; Zeppezauer, E.; Söderlund, G.; Ohlsson, I.; Boiwe, T.; Söderberg, B.O.; Tapia, O.; Brändén, C.-I.; Akeson, A. 3-Dimensional structure of horse liver alcohol-dehydrogenase at 2.4 Å resolution. J. Mol. Biol. 1976, 102, 27–59. [Google Scholar] [CrossRef]
- Eklund, H.; Samama, J.-P.; Wallen, L.; Brändén, C.-I.; Akeson, A.; Jones, T.A. Structure of a triclinic ternary complex of horse liver alcohol-dehydrogenase at 2.9 Å resolution. J. Mol. Biol. 1981, 146, 561–587. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Biellmann, J.-F.; Nördstrom, B.; Brändén, C.-I. Conformation of adenosine diphosphoribose and 8-bromoadenosine diphosphoribose when bound to liver alcohol-dehydrogenase. Eur. J. Biochem. 1975, 50, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Samama, J.-P.; Zeppezauer, E.; Biellmann, J.-F.; Brändén, C.-I. Crystal-structure of complexes between horse liver alcohol-dehydrogenase and coenzyme analogs 3-iodopyridine-adenine dinucleotide and pyridine-adenine dinucleotide. Eur. J. Biochem. 1977, 81, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Plapp, B.V. Conformational changes and catalysis by alcohol dehydrogenase. Arch. Biochem. Biophys. 2010, 493, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.; Kitao, A. Molecular dynamics simulations of NAD+-induced domain closure in horse liver alcohol dehydrogenase. Biophys. J. 2006, 91, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Timpson, L.M.; Liliensiek, A.K.; Alsafadi, D.; Cassidy, J.; Sharkey, M.A.; Liddell, S.; Allers, T.; Paradisi, F. A comparison of two novel alcohol dehydrogenase enzymes (ADH1 and ADH2) from the extreme halophile Haloferax volcanii. Appl. Microbiol. Biotechnol. 2013, 97, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, D.; Irwin, J.A.; Paradisi, F. Horse liver alcohol dehydrogenase: New perspectives for an old enzyme. Mol. Biotechnol. 2012, 52, 244–250. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).