Abstract
Aldol condensation of furfural and acetone (an important initial step to obtain diesel from biomass) was studied over MgAl mixed oxides. The influence of the utilization of microwaves and/or a surfactant (Pluronic 123) during the synthesis as well as the use of water (either pre-hydrating the solids before catalytic studies or in water/toluene mixtures as the reaction medium) is discussed. The combined use of Pluronic 123 and microwaves led to solids with bigger pore sizes, exhibiting lower basicity and higher acidity than the conventional synthetic method, thus resulting in an increase in the yield of the desired product of condensation, comprising two molecules of furfural and one of acetone (F2Ac). As for the influence of water, re-hydration of the mixed oxides was detrimental to activity, probably as a result of the partial blocking (solvation) of active sites. On the contrary, the increase in water percentage in the reaction medium resulted in higher conversions, though selectivity to F2Ac decreased. The weakening of the C=O bond of furfural in the presence of water as well as the higher solubility of the first condensation product (FAc) in toluene, as compared to water, could account for that. A 44.5% yield of F2Ac (66% conversion) after 16 h was obtained with the most active solid, which maintained the activity for three consecutive reactions.
1. Introduction
Fossil fuel depletion and environmental concern have boosted the search for renewable energies, one of the possible sources being biomass [1,2]. Furfural is a so-called platform molecule from biomass obtained through xylose dehydration [3,4] and can be transformed into a wide range of chemicals via hydrogenation, oxidation, decarbonylation, nitration, or condensation processes, just to cite some of them [5]. For instance, aldol condensation and subsequent hydrogenation and hydrodeoxygenation can lead to liquid hydrocarbons for use as diesel [6,7,8].
Aldol condensation is a well-known C–C bond formation process which can occur in acidic or basic sites, the latter being more frequently reported in the literature [6,7,8,9,10,11,12,13,14,15]. It requires the existence of a reactive hydrogen in alpha position, with respect to a carbonyl compound able to form an enol, which reacts with another carbonyl compound, and after dehydration, yields a conjugated enone. Focusing on aldol condensation between furfural and acetone (Figure 1), it can initially lead to 4-(2-furanyl)-3-buten-2-one (FAc), a subsequent aldol condensation with another furfural molecule, forming 1,5-bis-(2-furanyl)-1,4-pentadien-3-one (F2Ac) (Figure 1a) [16,17]. Some side reactions include acetone self-condensation to form diacetone-alcohol and mesityl oxide (Figure 1b), condensation between FAc and acetone (Figure 1c), and multiple aldol condensations between different carbonyl compounds, thus forming polymers [18,19] (Figure 1d).
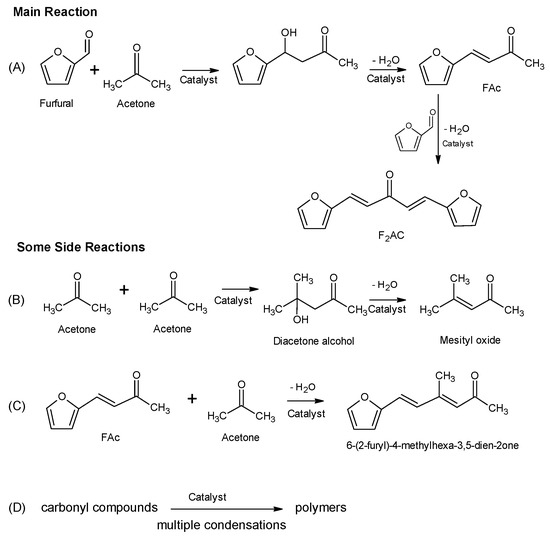
Figure 1.
Reaction scheme for aldol condensation of furfural and acetone. Some side reactions have also been included. (A) aldol condensation between furfural and acetone, (B) acetone self-condensation, (C) condensation between FAc and acetone and (D) multiple aldol condensations between different carbonyl compounds.
Aldol condensations have been traditionally performed in organic media, using base catalysts such as sodium or calcium hydroxides. Nevertheless, the existence of corrosion problems and the difficult reutilization have led to the use of some other base heterogeneous catalysts, such as hydrotalcites and hydrotalcite-derived mixed oxides [20,21], amorphous aluminophosphate [15], and diamine-functionalized MCM-41 [22], just to cite some examples.
In the present work, different AlMg mixed oxides were obtained through calcination of layered double hydroxides (LDHs) and tested for aldol condensation with acetone to form F2Ac. The influence on the catalytic results of two synthetic variables (conventional or microwave heating with the presence or absence of Pluronic 123 as the surfactant) was explored. Furthermore, the effect of water (either pre-hydrating the solids before catalytic studies or in water/toluene mixtures as the reaction medium) is discussed.
2. Results and Discussion
2.1. Textural, Structural and Acid–Base Characterization of the Solids
X-ray diffractograms of uncalcined and calcined hydrotalcites are shown in Figure 2. As can be seen, uncalcined solids exhibit a typical hydrotalcite crystallinity profile (JCPDS 22-700), with symmetric reflections at 2θ = 11°, 22°, 36°, 37°, 45°, 60°, and 62°. Therefore, sharper peaks corresponded to (003), (006), (010), and (013) reflections, whereas broader signals were obtained for (009), (015), and (016) reflections, all of them representative of layered materials. As for calcined solids (Figure 2b), diffraction patterns are very similar to each other, exhibiting (111), (200), and (220) reflections ascribed to periclase. In a previous paper, Aramendia et al. [23], using 27Al NMR-MAS, demonstrated that the coordination of Al3+ changed from octahedral to tetrahedral upon calcination of hydrotalcites, Al3+ ions thus isomorphically substituting Mg2+ ions, forming MgAlOx periclase.
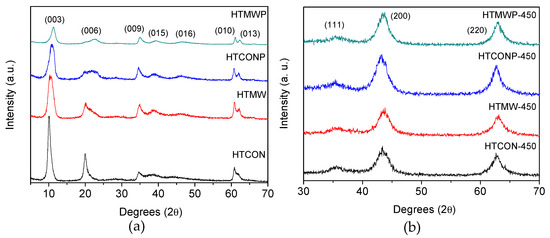
Figure 2.
X-ray diffractograms of the different solids synthesized in the present work. (a) Uncalcined solids. (b) Solids calcined at 450 °C.
Thermal stability of hydrotalcites was determined by TG-DTA (Figure 3). In all cases, weight loss percentage is in the 42–45% range (Figure 3a). HTCON and HTMW thermogravimetric profiles are consistent with those reported in the literature for hydrotalcites [24,25]. Therefore, two main weight losses are observed. The first one at 100–200 °C is ascribed to the loss of intercalated water molecules, whereas nitrate coming from both the precursor and hydroxyl groups can account for the second loss at higher temperatures (250–500 °C). For the solids synthesized using the surfactant (HTCONP and HTMWP), the second weight loss seems to be produced quicker (i.e., at lower temperatures), thus suggesting that for those systems, re-structuration to form periclase is somehow favored by Pluronic 123. Heat flow profiles (Figure 3b) seem to confirm this hypothesis, the exothermal peak centered at 450 °C in HTCON and HTMW being shifted to lower temperatures (300–350 °C) for HTCONP and HTMWP.
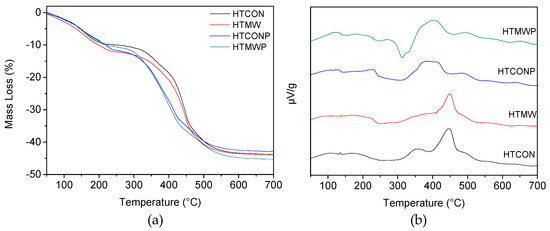
Figure 3.
TG analyses (a) and heat flow (b) of hydrotalcites.
Raman spectra of uncalcined solids (i.e., hydrotalcites) are represented in Figure 4. The band appearing at 557 cm−1 can be assigned to vibrations of brucite-like octahedral layers, Al-O-Mg, which are present in all Mg-Al hydrotalcites [26]. Moreover, the spectra also exhibit bands at 710 and 1055 cm−1, corresponding to nitrate vibrations [27] and bands at ca. 3500 cm−1, due to surface hydroxyl groups. In the case of HTCONP and HTMWP solids, there are also some intense bands of C-H stretching Pluronic 123 at 2986, 2941, and 2933 cm−1 [28].
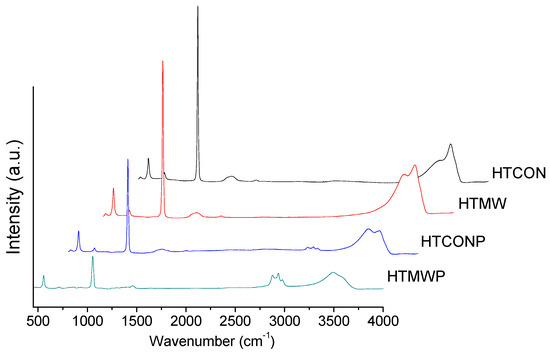
Figure 4.
Raman spectra of the uncalcined solids (hydrotalcites).
N2 adsorption–desorption isotherms of calcined solids are shown in Figure 5. In all cases, type IV isotherms corresponding to mesoporous materials were obtained. BET surface areas, pore volume and average pore diameter values are given in Table 1. With regards to the BET areas, they are in the 160–210 m2·g−1 range, the highest value corresponding to HTCONP-450. Modification of conventional synthesis by using microwave irradiation and/or in the presence of the surfactant (Pluronic 123) led in all cases to an increase in BET area. Solids that aged under microwave irradiation exhibit smaller pore diameters than their conventionally-heated counterparts (compare HTMW-450 vs. HTCON-450 or HTMWP-450 vs. HTCONP-450). Systems synthesized in the presence of the surfactant present bigger pores (compare HTCONP-450 vs. HTCON-450 and HTMWP-450 vs. HTMW-450). Therefore, the effect of microwaves and the presence of a surfactant on pore volume is the opposite. However, if both variables are changed simultaneously, the influence of the surfactant is more important, thus resulting in the pore diameter increasing (compare HTCON-450 and HTMWP-450 with pore diameters of 6.8 and 8.4 nm, respectively).
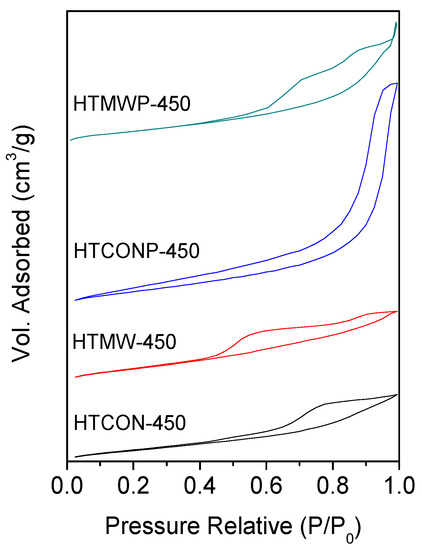
Figure 5.
Nitrogen adsorption–desorption isotherms corresponding to the mixed oxides.

Table 1.
Summary of the main features of the mixed oxides synthesized in this work.
X-Ray fluorescence results (Table 1) evidenced a good incorporation of Mg and Al to the solids, with Mg/Al ratios very similar to the nominal value (Mg/Al = 2).
Base characterization of the solids was performed using thermal programmed desorption of pre-adsorbed CO2 (CO2-TPD) and the results are given in Table 1 and Table 2, and in Figure 6. In all cases, signals were deconvoluted in peaks, which, depending on the desorption temperature, were ascribed to weak (80–200 °C), medium (200–300 °C), or strong (>300 °C) basic sites, respectively. Taking HTCON-450 as the reference, the use of microwave irradiation and/or the presence of the surfactant in the synthesis results in a drop in total basicity. Interestingly, as far as the base site distribution is concerned, the effect of microwave irradiation, the presence of the surfactant, or both variables simultaneously considered is different. Therefore, in the absence of Pluronic 123, microwave irradiation does not vary base site distribution. On the contrary, the presence of Pluronic 123 results in an increase in the strong base sites’ percentage, to the detriment of weak ones. Finally, simultaneous use of microwaves and Pluronic 123 leads to an increase in the percentage of weak base sites.

Table 2.
Base site distribution of the solids expressed as µmol CO2/g. Values in brackets represent the percentage of the total basicity.
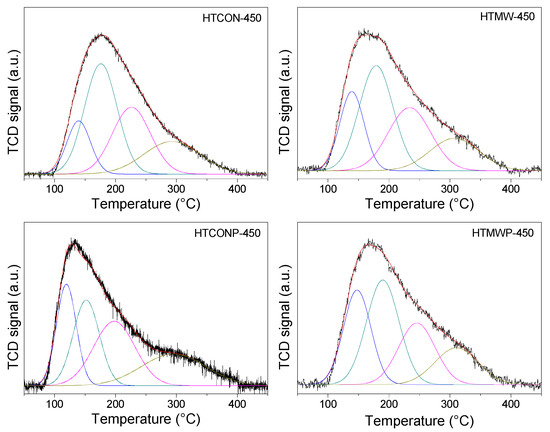
Figure 6.
Temperature-programmed desorption profiles of CO2 for the mixed oxides synthesized in this work.
Acid characterization of the solids was performed by thermal programmed desorption of pre-adsorbed pyridine (Py-TPD), results being given in Table 1 and Table 3, and in Figure 7. Taking HTCON-450 as the reference, contrary to basicity, total acidity increases when the solids are synthesized utilizing a microwave and/or in the presence of Pluronic 123. Furthermore, with regards to acid site distribution, only microwave irradiation has some effect (acid strength decreases), whereas the presence of Pluronic 123, either under conventional heating or microwave irradiation, does not vary acid site distribution.

Table 3.
Acid site distribution of the solids expressed as µmol Py/g. Values in brackets represent the percentage of the total basicity.
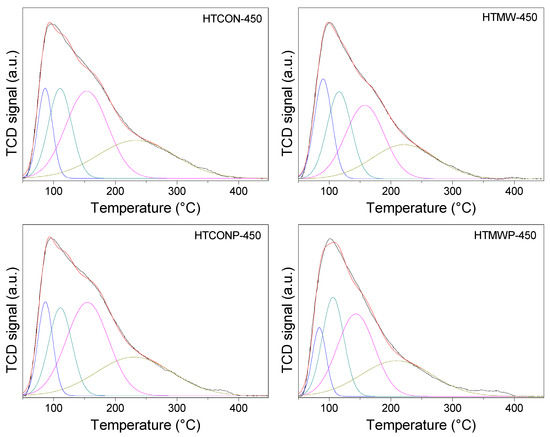
Figure 7.
Temperature-programmed desorption profiles of pyridine for the solids synthesized in this work.
All in all, microwave irradiation and/or the use of Pluronic 123 as the surfactant result in an increase in total acidity and a decrease in total basicity together with an increase in BET areas.
2.2. Catalytic Activity
The solid synthesized under conventional heating and in the absence of the surfactant (HTCON-450) was used as the reference material in order to study the influence of reaction temperature and the presence of water in the reaction medium on catalytic activity.
2.2.1. Influence of Reaction Temperature
Table 4 summarizes catalytic results obtained for t = 3 h at different temperatures. From that table, it is evident that the increase in temperature results in the increase in conversion, whereas selectivity to F2Ac, the desired product, hardly changes. Therefore, from then on, 100 °C was selected as the reaction temperature.

Table 4.
Aldol condensation of furfural and acetone on HTCON-450: Influence of temperature on catalytic activity. Reaction conditions: Reactor pressurized to 5 bar with N2; 10 mmol of furfural, 20 mmol acetone, 20 mL toluene, and 400 mg catalyst, t = 3 h.
2.2.2. Influence of Water
Two different approaches were made to study the influence of water on catalytic activity of HTCON-450. On the one hand, the solid was re-hydrated using a N2 flow saturated in water. On the other hand, reactions were performed in water/toluene mixtures (0%, 5%, 10%, 50%). Regarding the former approach (Table 5), the pretreatment of HTCON-450 with a flow of nitrogen saturated in water results in a drop in conversion (35.0% and 23.7% for HTCON-450 and HTCON-450-rehydrated, respectively, at t = 3 h), whereas selectivity values to F2Ac are quite similar. This suggests that rehydration results in the elimination of active sites to a certain extent, which could be ascribed to solvation. Results obtained for the uncalcined HTCON solid (which exhibits very low catalytic activity) are also given for the sake of comparison.

Table 5.
Aldol condensation of furfural and acetone on HTCON-450: Influence of calcination and rehydration of the solid. Reaction conditions: Reactor pressurized to 5 bar with N2; 10 mmol of furfural, 20 mmol acetone, 20 mL toluene, and 400 mg catalyst, t = 3 h, 100 °C.
Table 6 summarizes the results obtained for the study conducted using different water/toluene ratios as the reaction medium. As can be seen, the higher the percentage of water, the higher the conversion, but in general, the lower the selectivity to the desired product, F2Ac. In a previous paper [29] on chemoselective hydrogenation of alpha,beta-unsaturated carbonyl compounds, our research group found evidence by Raman spectroscopy that water interacted with the carbonyl group and made the double bond weaker and thus more reactive (carbonyl band shifted to lower wavelength values). The same could occur in the C=O group in furfural and account for its higher conversion in the presence of water. With regards to the change in selectivity, one should consider that we are working in a biphasic media and thus the catalyst hydrophilic character, as well as the relative solubility of reactants and products both in toluene and water, is important. Active sites in the catalyst will probably interact better with water than with toluene. Furfural is partially soluble in water (50–100 mg·mL−1). Its condensation with one molecule of acetone will produce FAc, whose solubility in water is much lower (1–10 mg·mL−1). Therefore, once formed, FAc will pass to the organic phase (toluene) and will not be able to undergo subsequent condensation with another acetone molecule to produce F2Ac. This results in the increase in selectivity to FAc and probably in conversion, since FAc is retired of the aqueous phase as the reaction proceeds. All in all, the highest yield to F2Ac, the desired product, is achieved with pure toluene. Therefore, this reaction medium was selected for subsequent studies on other catalysts.

Table 6.
Aldol condensation of furfural and acetone on HTCON-450: Influence of the presence of water in water/toluene mixtures. Reaction conditions: Reactor pressurized to 5 bar with N2; 10 mmol of furfural, 20 mmol acetone, 20 mL (toluene + water) and 400 mg catalyst, t = 3 h, 100 °C.
2.3. Catalytic Activity of the Other Mixed Oxides
Once pure toluene and 100 °C had been selected as the reaction conditions for aldol condensation of furfural and acetone in order to obtain F2Ac, the study was extended to the other mixed oxides. Reactions were conducted at 3 h and 16 h, the main catalytic results being summarized in Table 7.

Table 7.
Results obtained for aldol condensation of furfural and acetone on the different solids. Reaction conditions: Reactor pressurized to 5 bar with N2; 10 mmol of furfural, 20 mmol acetone, 20 mL toluene and 400 mg catalyst, 100 °C.
A first conclusion from that table is that the lowest conversion values correspond to HTMW-450. It is important to note that this solid was the one exhibiting the lowest pore diameter (4.2 nm, Table 1), which could account for that. Focusing on the other solids, the highest conversion value at 3 h is achieved for HTCON-450, whereas as the reaction proceeds, the rate is higher for the systems synthesized using Pluronic 123, which together with their higher selectivity to F2Ac results in F2Ac yields of 24.6%, 28.3%, and 44.5% for HTCON-450, HTCONP-450, and HTMWP-450, respectively, at t = 16 h. As evidenced by thermal-programmed desorption of pyridine and CO2, the use of microwave irradiation and/or the presence of Pluronic 123 in the reaction medium during the synthesis resulted in an increase in total acidity and a decrease in total basicity. In a previous paper, Climent et al. [15] described the cooperative effect of weak acid and base sites of an amorphous aluminophosphate in aldol condensation, thus resulting in higher selectivities than those presented by stronger acid or base catalysts. This effect together with the increase in pore size could explain the higher yields obtained for the solids synthesized using the surfactant.
2.4. Reutilization of HTCON-450 and HTMWP-450
Finally, some reutilization studies were conducted on HTCON-450 and HTMWP-450 solids, results being summarized in Table 8. In all cases, the Mg/Al ratio of the solids was quite similar to the nominal value (Mg/Al = 2). Moreover, after the reactions, the reaction medium was analyzed by inductively coupled plasma mass spectrometry (ICP-MS). No Mg or Al was detected which is evidence of the stability of the solids, which do not undergo leaching.

Table 8.
Results obtained for reutilization studies. Reaction conditions: Reactor pressurized to 5 bar with N2; 10 mmol of furfural, 20 mmol acetone, 20 mL toluene and 400 mg catalyst, 100 °C.
As far as the catalytic activity is concerned, neither HTCON-450 nor HTMWP-450 exhibited any remarkable deactivation keeping F2Ac yield in the ca. 20% order after three hours. In the case of the most active solid at long reaction times (HTMWP-450), its activity and selectivity only decreased slightly (from 67.1 to 58.5%) after three consecutive uses.
3. Materials and Methods
Hydrotalcites were synthesized by a co-precipitation method, starting from two solutions containing 0.2 mol Mg(NO3)2·6H2O and 0.1 mol Al(NO3)3·9H2O in 25 mL deionized water, respectively (Mg/Al = 2). The mixture was slowly added to a pH 10 aqueous solution under continuous stirring and an inert atmosphere (N2), with temperature maintained at 60 °C. During precipitation, the pH value was maintained, adding NaOH 1M. The suspension was divided into four portions for further treatment. One part was kept under conventional heating at 80 °C for 24 h, followed by filtration and washing with deionized water, thus obtaining the solid called HTCON. A second portion was aged under microwave heating at 80 °C for 1 h, thus leading, after filtration and washing, to the solid termed as HTMW. A flexiWave platform for microwave synthesis (22 V, 50 Hz) with an IR temperature sensor (p/n IRT0500) was used. The other two portions were submitted to the same conventional or microwave heating while performing the synthesis in the presence of surfactant Pluronic 123 (2% by weight), thus leading to the solids named HTCONP and HTMWP, respectively. Finally, all four solids were calcined at 450 °C in the air for 8 h (1 °C·min−1 ramp). Nomenclature of these solids include the suffix 450, referring to calcination temperature (HTCON-450, HTMW-450, HTCONP-450, and HTMWP-450). Subsequent treatment of HTCON-450 for 2 h at 450 °C in the presence of a N2 flow (50 mL·min−1) saturated in water at 20 °C led to a solid called HTCON-450-rehydrated.
A Setaram SetSys 12 instrument (SETARAM Instrumentation, Caluire, France) was used for thermogravimetric analyses (TGA). Experiments were performed on 20 mg samples placed in an alumina crucible and heated in the 30–600 °C range (10 °C·min−1, 50 mL·min−1 air stream).
Textural properties (BET surface area, cumulative pore volume, and average pore diameter) were measured in a Micromeritics ASAP-2010 instrument (Micromeritics, Norcross, GA, USA.). Samples were heated at 120 °C and degassed to 0.1 Pa before measurement.
The measure of magnesium or aluminium leaching (presence in filtered reaction medium) was performed by inductively coupled plasma mass spectrometry (ICP-MS) on a Perkin–Elmer ELAN DRC-e instrument.
The Mg/Al ratio of solids was measured by X-ray fluorescence (XRF) spectroscopy (Rigaku ZSK PrimusIV wavelength X-ray spectrometer (Rigaku, The Woodlands, TX, USA). Further details are given elsewhere [30].
Raman spectra were recorded on a Renishaw spectrometer (InVia Raman Microscope, Renishaw, Gloucestershire, UK), equipped with a Leica microscope with various lenses, monochromators, filters, and a CCD detector. Spectra were recorded over the 150–4000 cm−1 range, using green laser light excitation (532 nm) and gathering 32 scans.
X-ray diffraction (XRD) analysis was performed on a Siemens D-5000 diffractometer (Bruker Corporation, Billerica, MA, USA) using CuKα radiation over the range 5–80°.
Surface acidity of samples was measured by thermal programmed desorption of pre-adsorbed pyridine (Py-TPD) using TC detection. Samples (30 mg) were cleaned by heating to 450 °C (10 °C·min−1 ramp) under He flow (75 mL·min−1) and then cooled down to 50 °C. The catalysts were subsequently saturated with pyridine for 30 min, cleaned for 60 min with He and TPD monitored from 50 to 450 °C (10 °C·min−1), the final temperature being held for 45 min.
Surface basicity of the catalysts was determined on a Micromeritics Autochem II instrument by thermal programmed desorption of pre-absorbed CO2 (CO2-TPD) with TCD detection. Samples (100 mg) were cleaned in an Air stream (20 mL·min−1 Ar, heating at 450 °C at a rate of 10 °C·min−1 for 60 min and then cooled down to 40 °C). Then, solids were saturated with CO2 (5% CO2/Ar flow at 20 mL·min−1 for 60 min), physisorbed CO2 removed with Ar flow (20 mL·min−1 for 30min) and TPD monitored from 50 to 450 °C (5 °C·min−1), the final temperature being held for 60 min.
The solids were tested for aldol condensation of furfural using a Berghof HR-100 stainless steel high-pressure autoclave equipped with a 75 mL PTFE insert vessel. Under standard conditions, 10 mmol of furfural, 20 mmol acetone, 20 mL toluene, and a 400 mg catalyst were introduced in the vessel. Reactor was purged with nitrogen and pressurized to 5 bar of N2. The reaction temperature was set to 100 °C and started by switching on the stirring at 750 rpm. To stop the reaction, the vessel was submerged in an ice bath. The choice of toluene as the organic medium was motivated by a previous paper [3] on xylose dehydration to furfural where toluene was found to give the highest yield to furfural. The final strategy would be to make the one-pot transformation of xylose to furfural and then F2Ac.
Experiments to evaluate the influence of the presence of water in the reaction medium were conducted varying the water/toluene ratio (0%, 5%, 10%, and 50% volume) while keeping the total solvent volume constant (20 mL).
Once the reactions were finished, the products were analyzed by gas chromatography (Agilent 7890) with a flame ionization detector (GC-FID), using a Supelco NukolTM capillary column. In the case of using biphasic media (toluene/water mixtures), products were extracted from the aqueous phase with dichloromethane before GC-FID analysis. Quantification of furfural and condensation products was performed using the appropriate calibration curves. In all cases, mass balance considering unreacted furfural, FAc, and F2Ac was over 95%.
For reutilization experiments, after the reaction, the solids were filtered, washed with ethanol, and dried at 100 °C, followed by calcination at 450 °C under the same conditions as described in the synthesis. Nomenclature of reused catalysts include the suffix R (one reuse) or R2 (two reuses).
Furfural conversion and FAc and F2Ac selectivity were defined by Equations (1)–(3):
4. Conclusions
The synthesis of hydrotalcites in the presence of Pluronic 123 led, after calcination, to MgAl mixed oxides with bigger pore sizes than untreated solids. On the other hand, microwave irradiation led to smaller pore sizes as compared to conventional thermal treatment. As far as acid–base characteristics are concerned, the use of both microwave irradiation and Pluronic 123 during the synthesis resulted in a decrease of total basicity and an increase in total acidity.
Rehydration of mixed oxides by treating them with a nitrogen flow saturated with water led to solids exhibiting lower catalytic activity in aldol condensation of furfural, probably as a result of the partial blocking (solvation) of active sites. By contrast, the increase in the percentage of water in water/toluene biphasic media resulted in an increase in conversion values, though selectivity to FAc also increased at the expense of the desired product F2Ac. A plausible explanation is that water weakens the C=O bond in furfural, thus favoring its transformation. Moreover, once FAc is produced, its higher solubility in toluene, as compared to water, favors its transfer to the organic medium, thus avoiding its subsequent reaction with another furfural molecule to yield F2Ac. The fact that the produced FAc is retired to the organic phase could also account for the observed increase in conversion.
A comparison of catalytic activity of the reference material (HTCON-450) with that of the other solids allows us to conclude that the use of Pluronic 123 during synthesis (especially in combination with microwave irradiation) resulted in solids exhibiting higher F2Ac yields at long reaction times. This could be the result of the combination of two factors: The above-mentioned larger pore size achieved with the surfactant and the increase in total acidity which could favor aldol condensation.
HTMWP-450 exhibited a good stability without any significant loss of activity after three uses.
Author Contributions
Conceptualization, C.J.-S. and F.J.U.; methodology, A.M. and J.R.R.; validation, F.J.U., J.R.R. and J.H.-C.; formal analysis, A.M. and J.R.R.; investigation, A.P. and D.C.; data curation, A.M., J.H.-C. and A.P.; writing—original draft preparation, A.P. and D.C.; writing—review and editing, J.H.-C and A.M.; supervision, C.J.-S. and F.J.U.
Funding
This research was funded by Ramón Areces Foundation.
Acknowledgments
The scientific support from the Central Service for Research Support (SCAI) at the University of Cordoba is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Parejas, A.; Montes, V.; Hidalgo-Carrillo, J.; Sanchez-Lopez, E.; Marinas, A.; Urbano, F.J. Microemulsion and Sol-Gel Synthesized ZrO2-MgO Catalysts for the Liquid-Phase Dehydration of Xylose to Furfural. Molecules 2017, 22, 2257. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- O’Neill, R.E.; Vanoye, L.; De Bellefon, C.; Aiouache, F. Aldol-condensation of furfural by activated dolomite catalyst. Appl. Catal. B Environ. 2014, 144, 46–56. [Google Scholar] [CrossRef]
- Smolakova, L.; Frolich, K.; Kocik, J.; Kikhtyanin, O.; Capek, L. Surface Properties of Hydrotalcite-Based Zn(Mg)Al Oxides and Their Catalytic Activity in Aldol Condensation of Furfural with Acetone. Ind. Eng. Chem. Res. 2017, 56, 4638–4648. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Aqueous-phase furfural-acetone aldol condensation over mixed oxides. Appl. Catal. B Environ. 2012, 113–114, 201–211. [Google Scholar] [CrossRef]
- Chheda, J.N.; Dumesic, J.A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today 2007, 123, 59–70. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Shen, W.Q.; Tompsett, G.A.; Hammond, K.D.; Xing, R.; Dogan, F.; Grey, C.P.; Conner, W.C.; Auerbach, S.M.; Huber, G.W. Liquid phase aldol condensation reactions with MgO-ZrO2 and shape-selective nitrogen-substituted NaY. Appl. Catal. A Gener. 2011, 392, 57–68. [Google Scholar] [CrossRef]
- Cota, I.; Ramirez, E.; Medina, F.; Sueiras, J.E.; Layrac, G.; Tichit, D. New synthesis route of hydrocalumite-type materials and their application as basic catalysts for aldol condensation. Appl. Clay Sci. 2010, 50, 498–502. [Google Scholar] [CrossRef]
- West, R.M.; Liu, Z.Y.; Peter, M.; Gaertner, C.A.; Dumesic, J.A. Carbon-carbon bond formation for biomass-derived furfurals and ketones by aldol condensation in a biphasic system. J. Mol. Catal. A Chem. 2008, 296, 18–27. [Google Scholar] [CrossRef]
- Daniel, E.; Resasco, S.S.; Faria, J.; Prasomsri, T.; Ruiz, A.M.P. Furfurals as chemical platform forbiofuels production. In Heterogeneous Catalysis in Biomass to Chemicals and Fuels; D.K.A.I., Ed.; Stanford Court: Irvine, CA, USA, 2011. [Google Scholar]
- Bao, Q.; Qi, H.; Zhang, C.; Ning, C.; Zhang, Y.; Wu, Y.; Gui, W.; Wang, Z. Highly Catalytic Activity of Ba/γ-Ti–Al2O3 Catalyst for Aldol Condensation of Methyl Acetate with Formaldehyde. Catal. Lett. 2018, 148, 3402–3412. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Fornés, V.; Guil-Lopez, R.; Iborra, S. Aldol condensations on solid catalysts: A cooperative effect between weak acid and base catalysts. Adv. Synth. Catal. 2002, 344, 1090–1096. [Google Scholar] [CrossRef]
- Fakhfakh, N.; Cognet, P.; Cabassud, M.; Lucchese, Y.; Rios, M.D.D.L. Stoichio-kinetic modeling and optimization of chemical synthesis: Application to the aldolic condensation of furfural on acetone. Chem. Eng. Process. 2008, 47, 349–362. [Google Scholar] [CrossRef]
- Xing, R.; Subrahmanyam, A.V.; Olcay, H.; Qi, W.; van Walsum, G.P.; Pendse, H.; Huber, G.W. Production of jet and diesel fuel range alkanes from waste hemicellulose-derived aqueous solutions. Green Chem. 2010, 12, 1933–1946. [Google Scholar] [CrossRef]
- Patel, A.A.; Patel, S.R. Synthesis and characterization of furfural-acetone polymers. Eur. Polym. J. 1983, 19, 231–234. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. Furans in polymer chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379. [Google Scholar] [CrossRef]
- Hora, L.; Kelbichova, V.; Kikhtyanin, O.; Bortnovskiy, O.; Kubicka, D. Aldol condensation of furfural and acetone over Mg-Al layered double hydroxides and mixed oxides. Catal. Today 2014, 223, 138–147. [Google Scholar] [CrossRef]
- Ordonez, S.; Diaz, E.; Leon, M.; Faba, L. Hydrotalcite-derived mixed oxides as catalysts for different C-C bond formation reactions from bioorganic materials. Catal. Today 2011, 167, 71–76. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Sreekanth, P.; Bandopadhyay, T.; Figueras, F.; Tuel, A. Knoevenagel and aldol condensations catalysed by a new diamino-functionalised mesoporous material. J. Mol. Cataly. A Chem. 1999, 142, 361–365. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Aviles, Y.; Borau, V.; Luque, J.M.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Thermal decomposition of Mg Al and Mg Ga layered-double hydroxides: A spectroscopic study. J. Mater. Chem. 1999, 9, 1603–1607. [Google Scholar] [CrossRef]
- Morato, A.; Alonso, C.; Medina, F.; Cesteros, Y.; Salagre, P.; Sueiras, J.E.; Tichit, D.; Coq, B. Palladium hydrotalcites as precursors for the catalytic hydroconversion of CCl2F2 (CFC-12) and CHClF2 (HCFC-22). Appl. Catal. B Environ. 2001, 32, 167–179. [Google Scholar] [CrossRef]
- Xu, C.; Gao, Y.; Liu, X.; Xin, R.; Wang, Z. Hydrotalcite reconstructed by in situ rehydration as a highly active solid base catalyst and its application in aldol condensations. RSC Adv. 2013, 3, 793–801. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J.; Theiss, F. Synthesis and Raman spectroscopic characterisation of hydrotalcites based on the formula Ca6Al2(CO3)(OH)16·4H2O. J. Raman Spectrosc. 2010, 42, 1163–1167. [Google Scholar] [CrossRef]
- Frost, R.L.; Erickson, K.L. Vibrational spectroscopic study of the nitrate containing hydrotalcite Mbobomkulite, Spectrochim. Acta Part A 2005, 61, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, P.; Seyed-Rasulzade, S.K.; Nikzad-Kojanag, B. Effect of Preparation Methods and Pluronic Template on the Catalytic Activity of Ca/SBA-15. Iran. J. Chem. Chem. Eng. 2018, 37, 53–60. [Google Scholar]
- Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Delgado, J.J.; Raya-Miranda, R.; Urbano, F.J. Water as solvent in the liquid-phase selective hydrogenation of crotonaldehyde to crotyl alcohol over Pt/ZnO: A factorial design approach. Appl. Catal. B Environ. 2014, 154, 369–378. [Google Scholar] [CrossRef]
- Cosano, D.; Esquivel, D.; Mateos, L.D.; Quesada, E.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Spectroscopic analysis of corrosion products in a bronze cauldron from the Late Iberian Iron Age. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 489–496. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).