Galvanic Exchange as a Novel Method for Carbon Nitride Supported CoAg Catalyst Synthesis for Oxygen Reduction and Carbon Dioxide Conversion
Abstract
1. Introduction
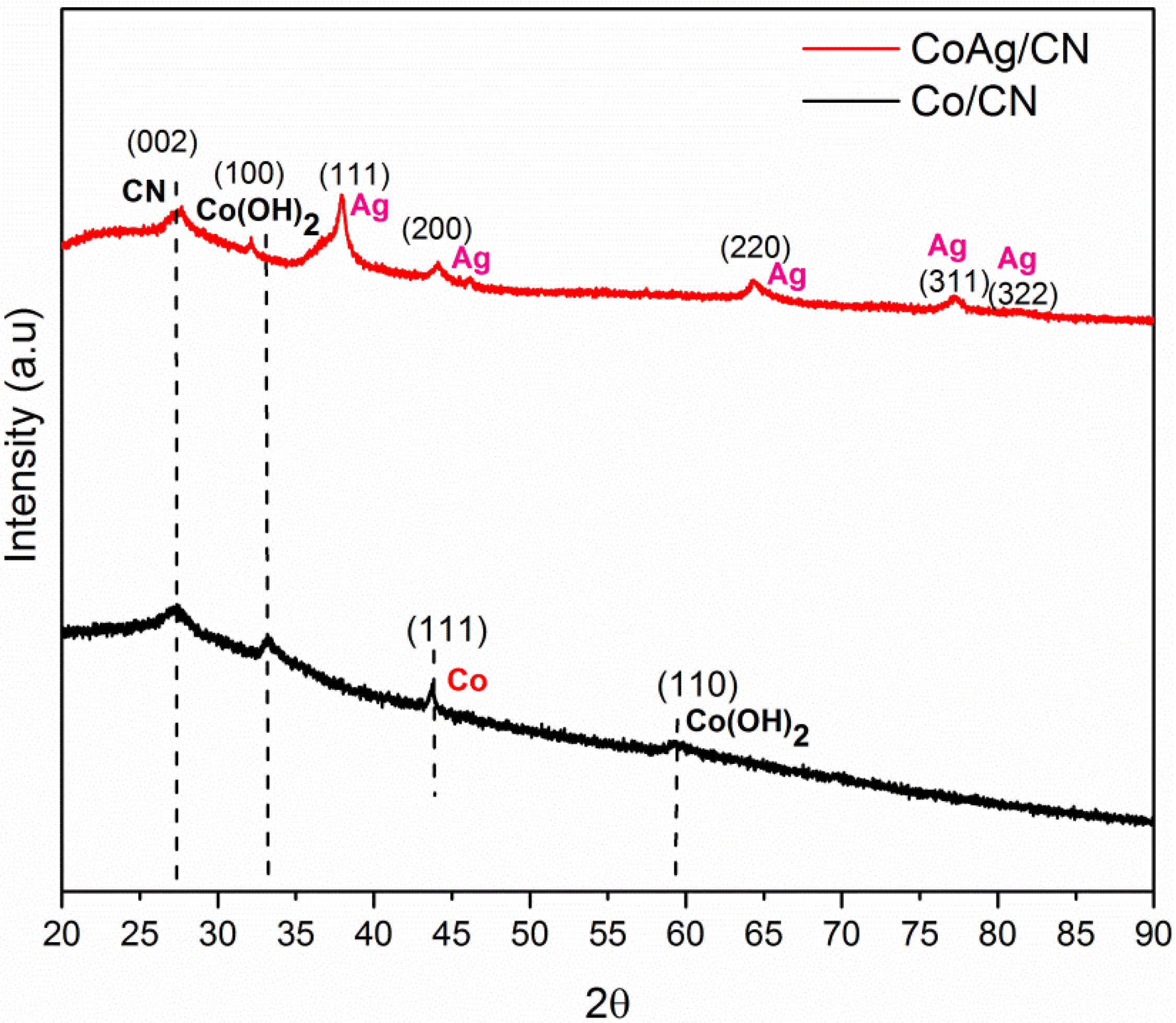
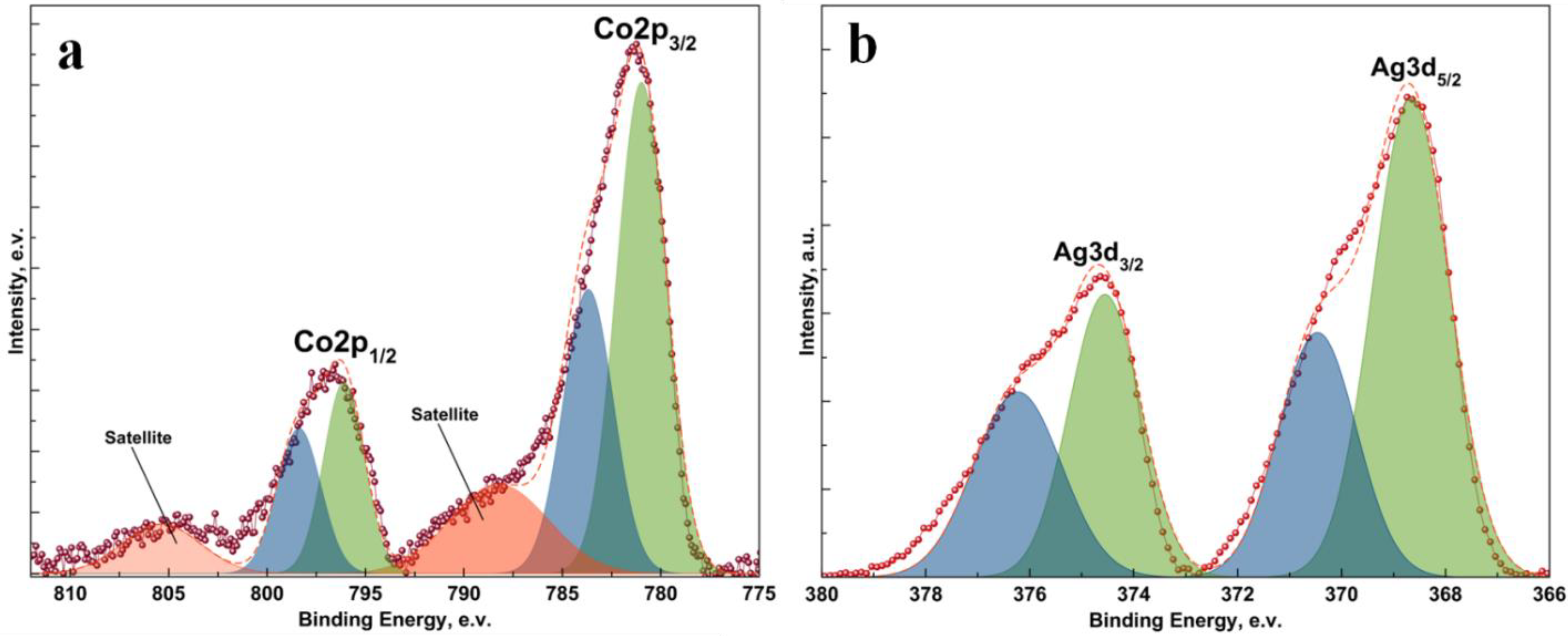
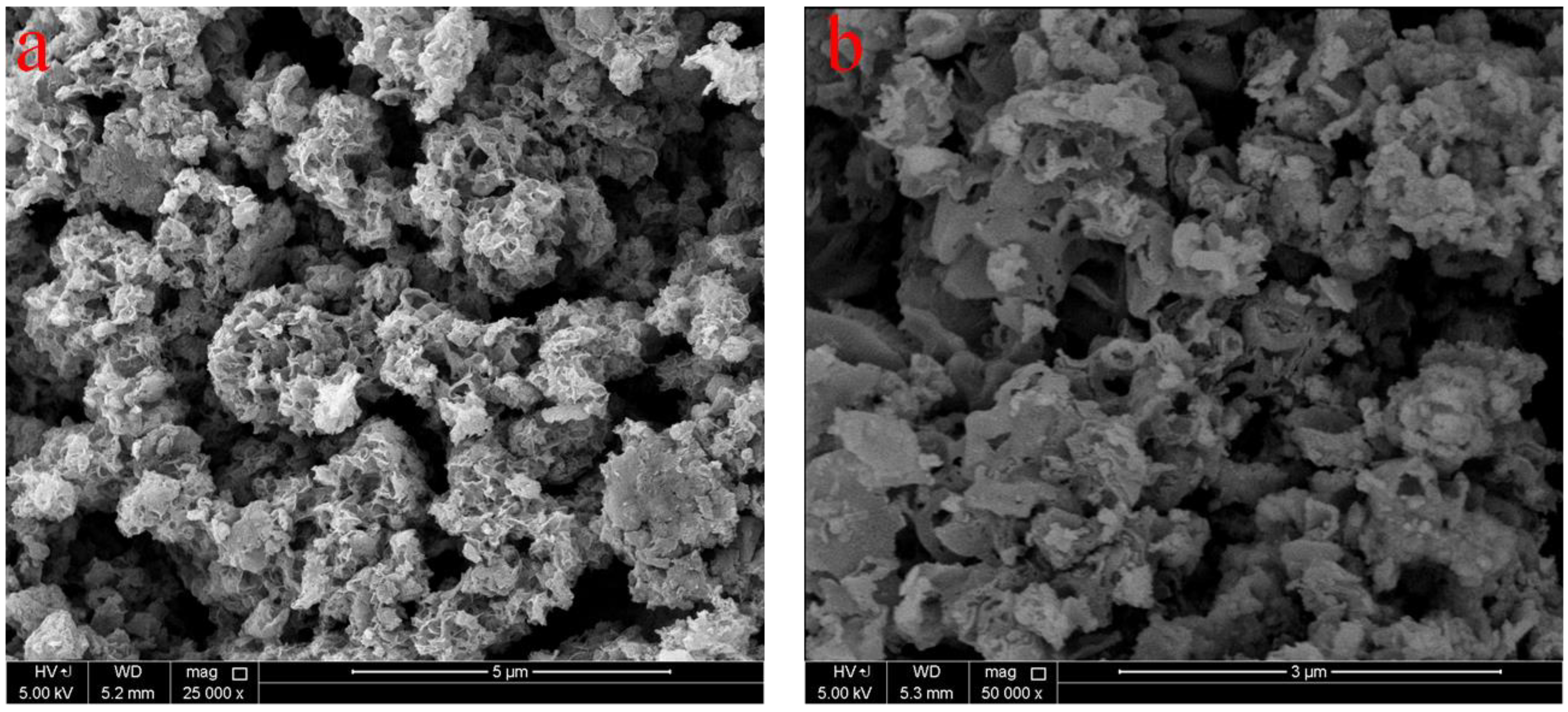
2. Results and Discussion
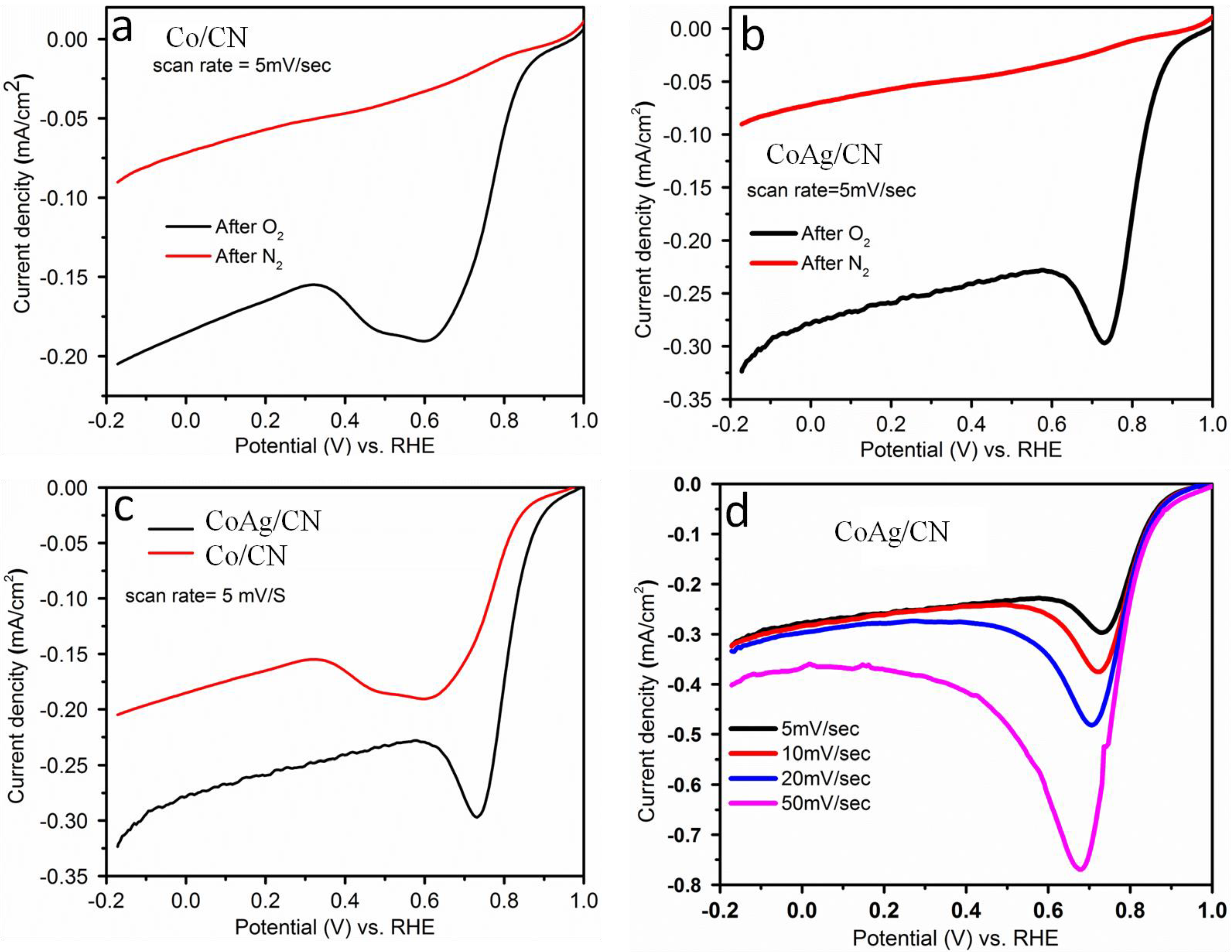
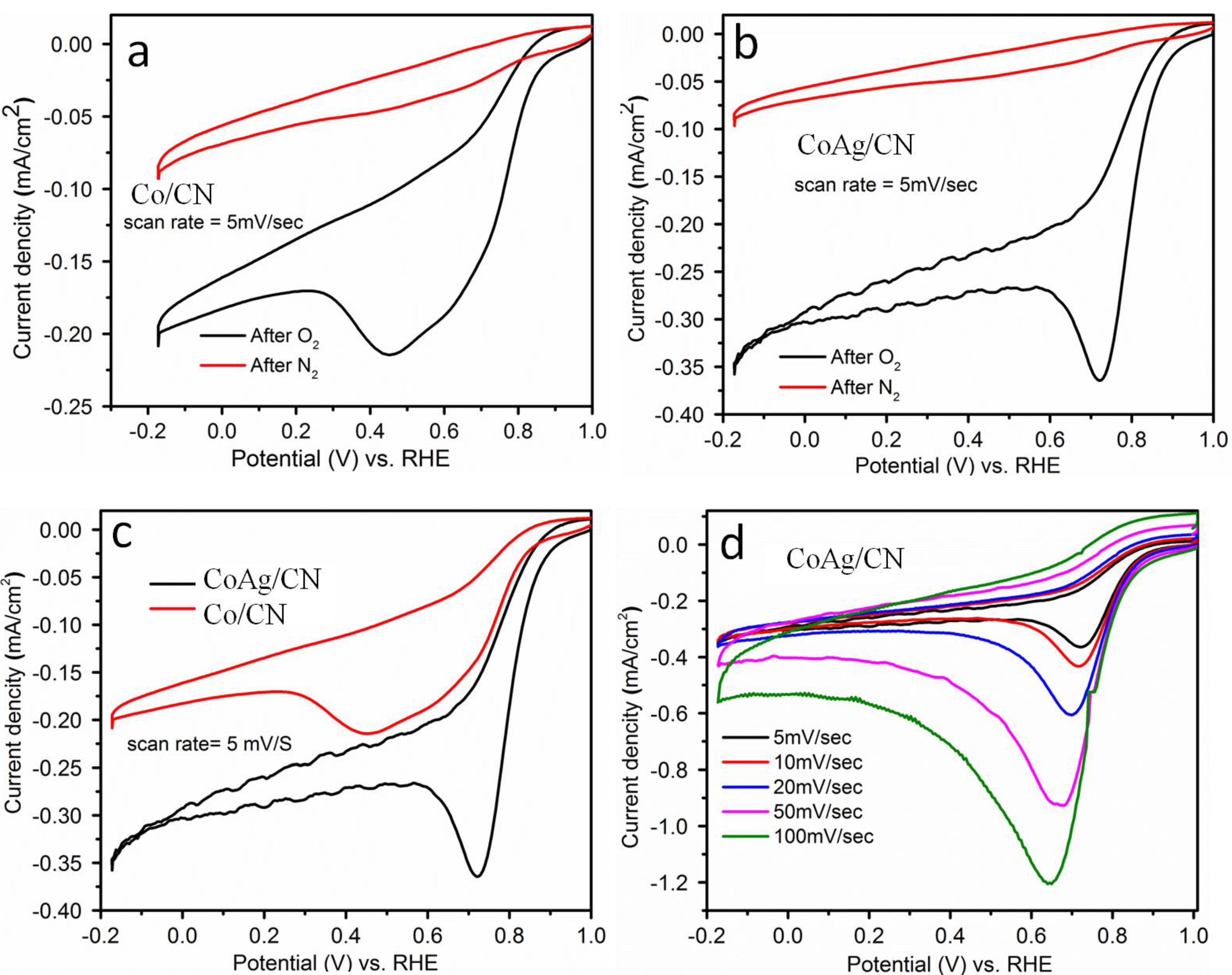
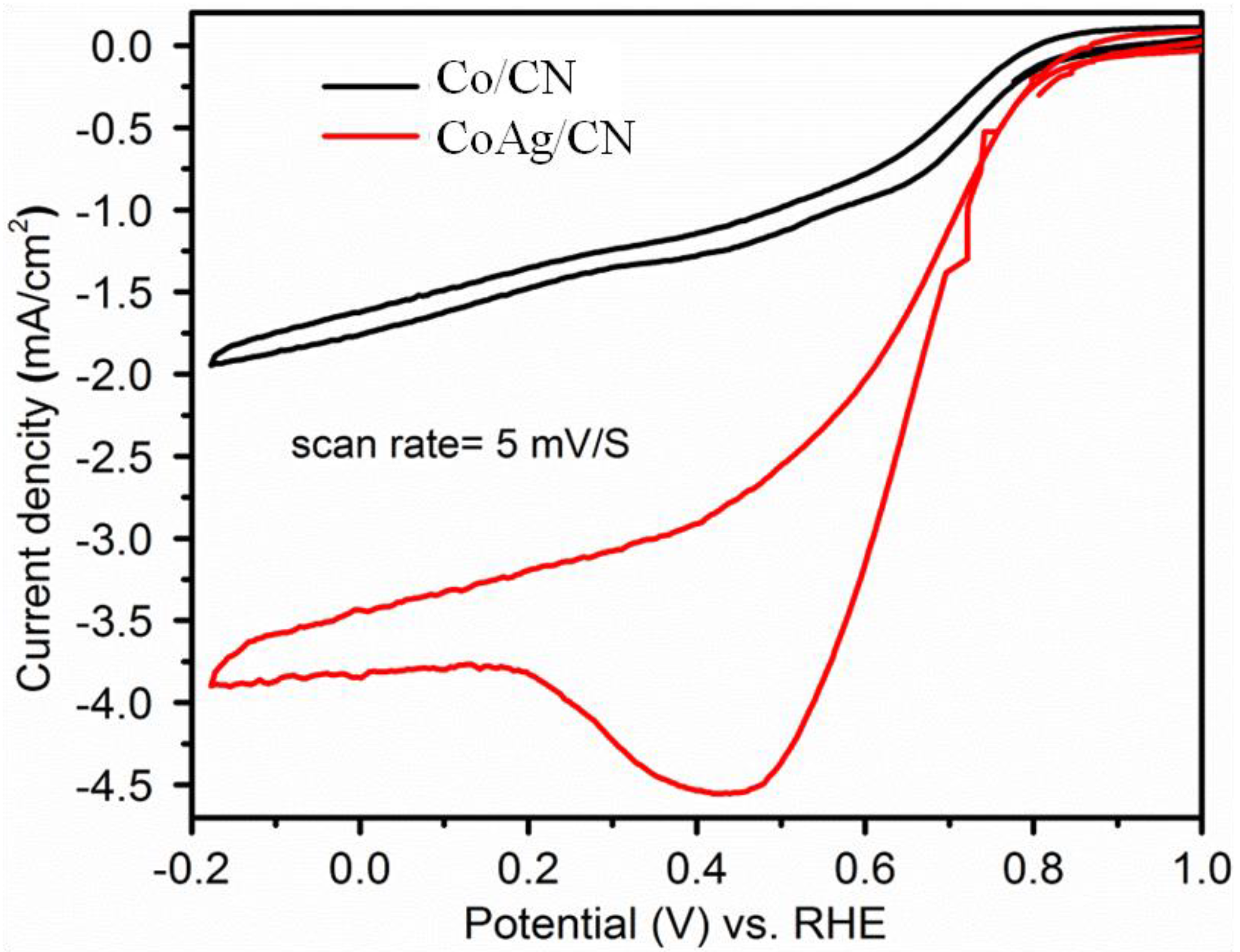
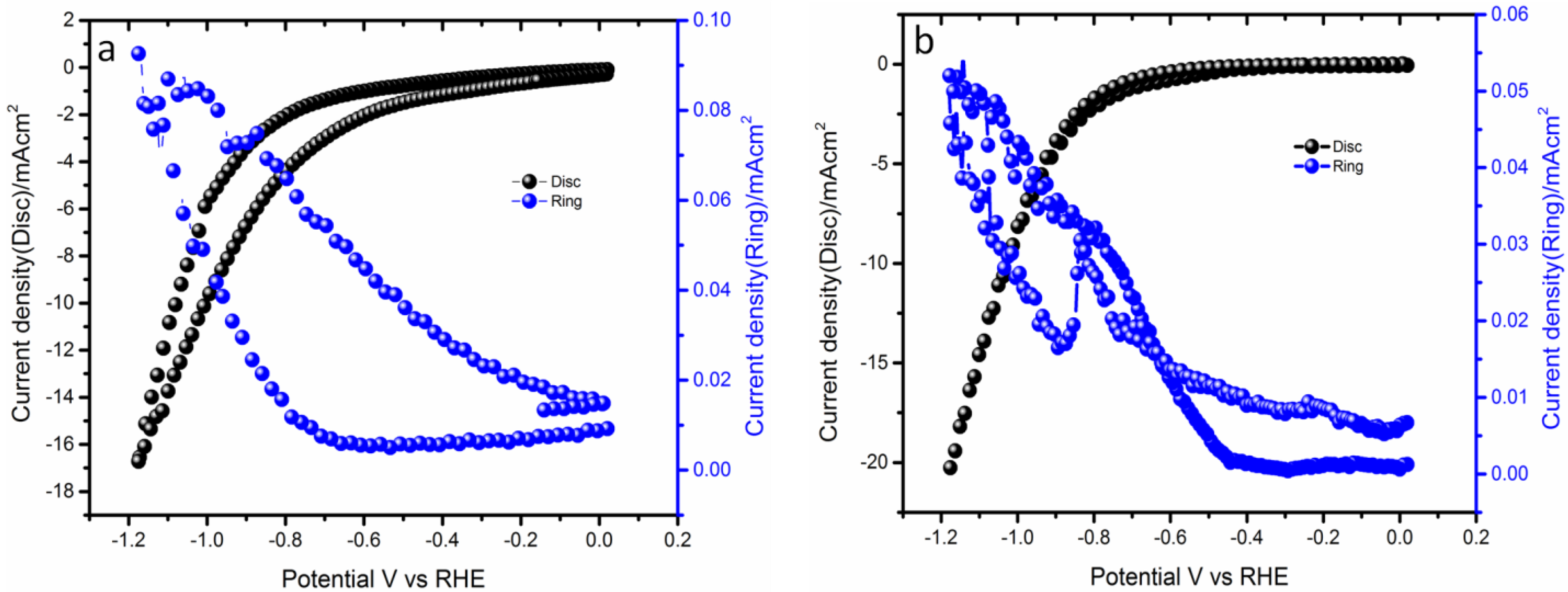
2.1. Application in Oxygen Reduction Reactions
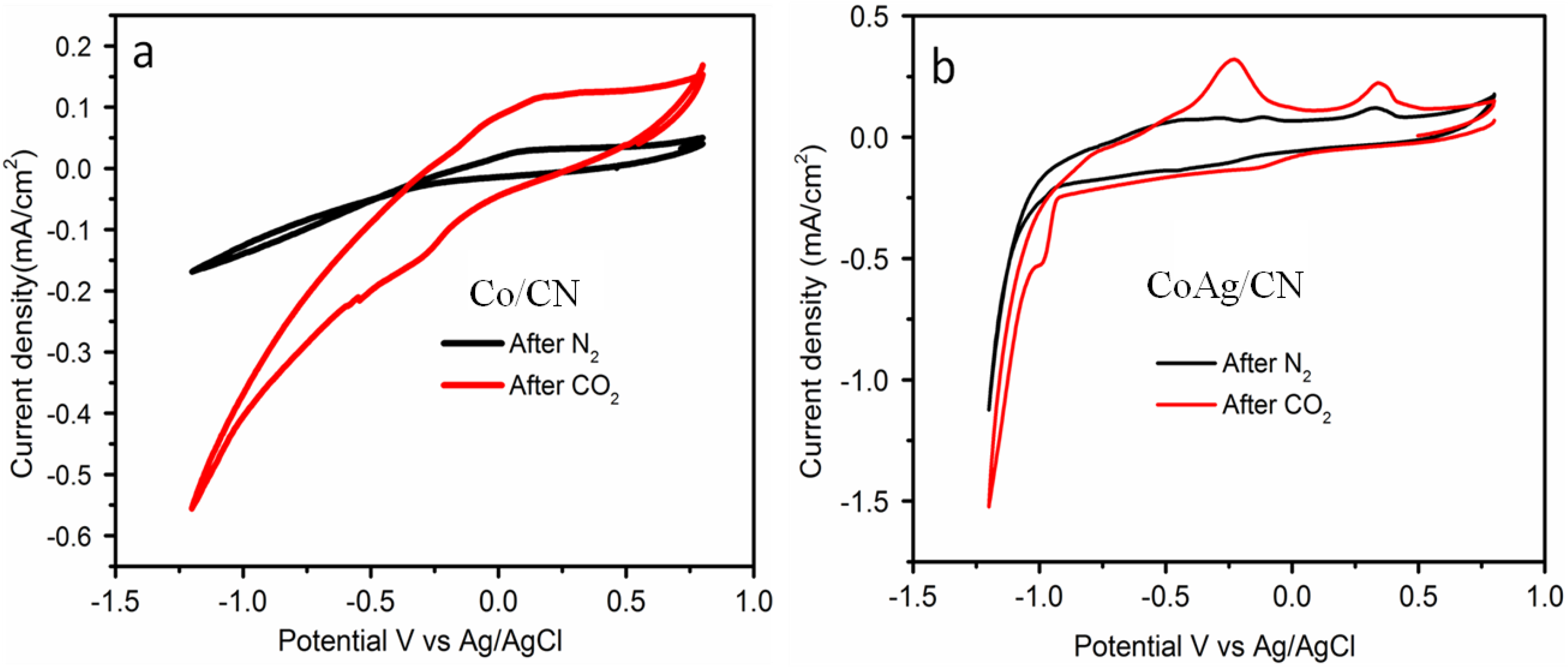
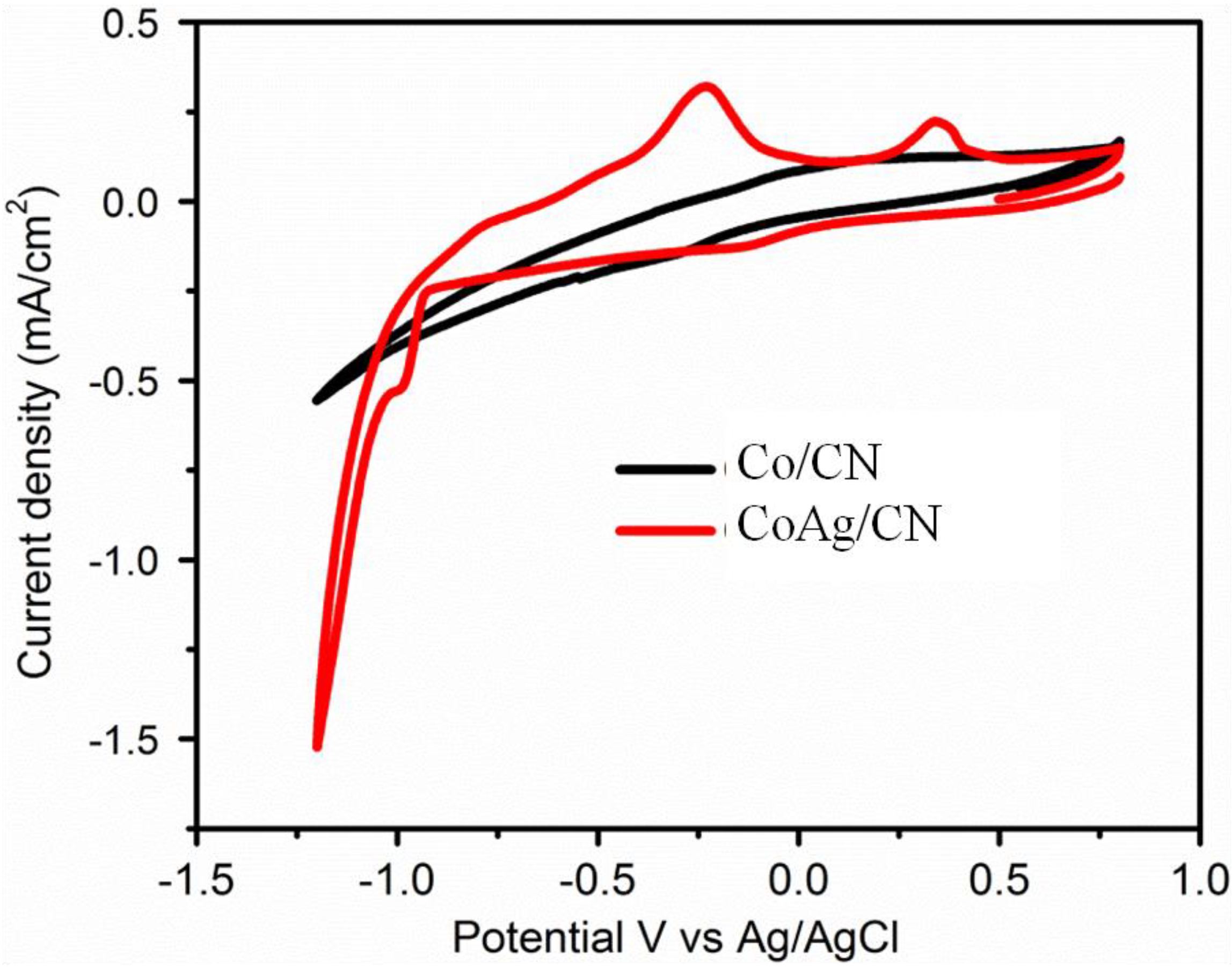
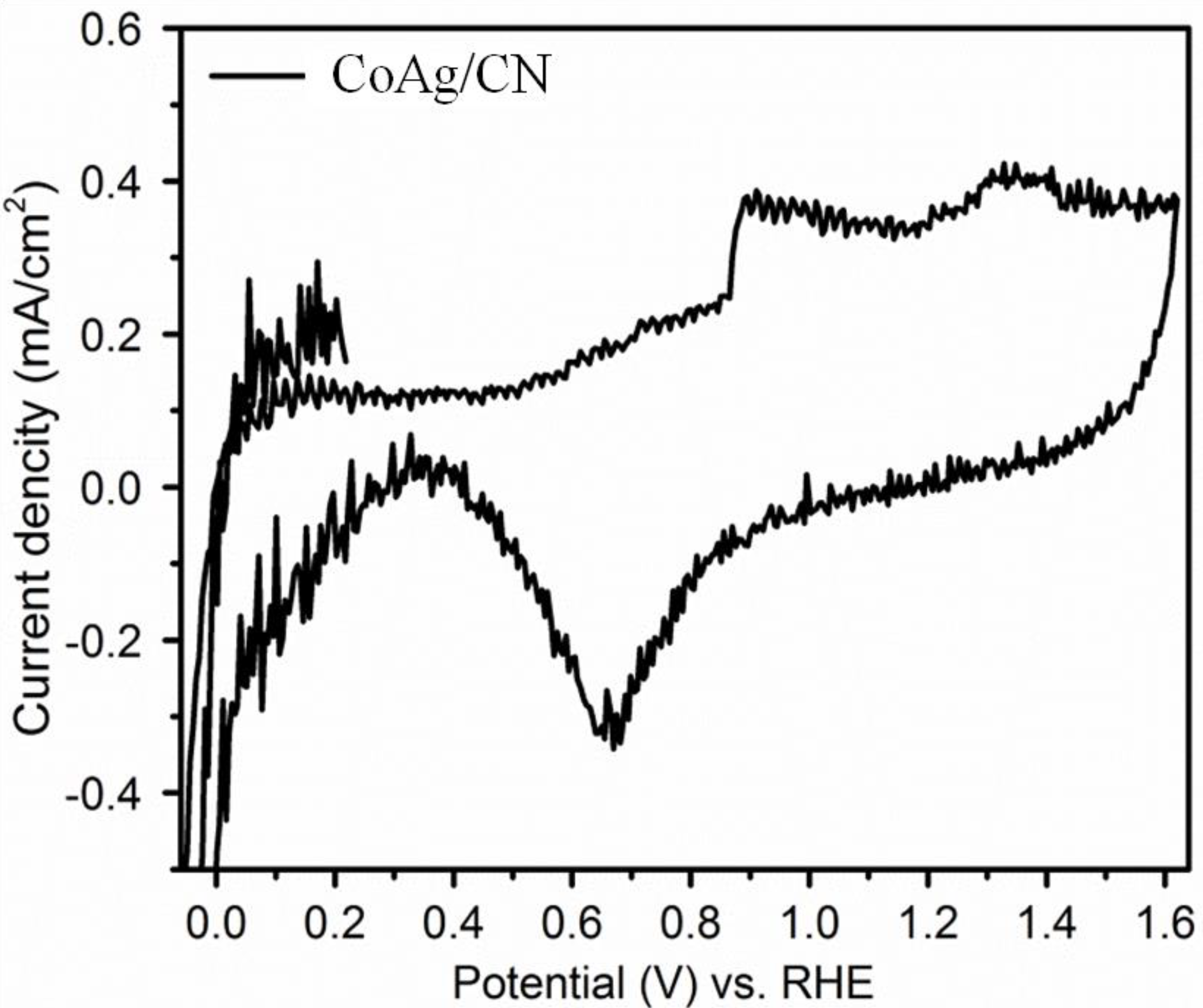
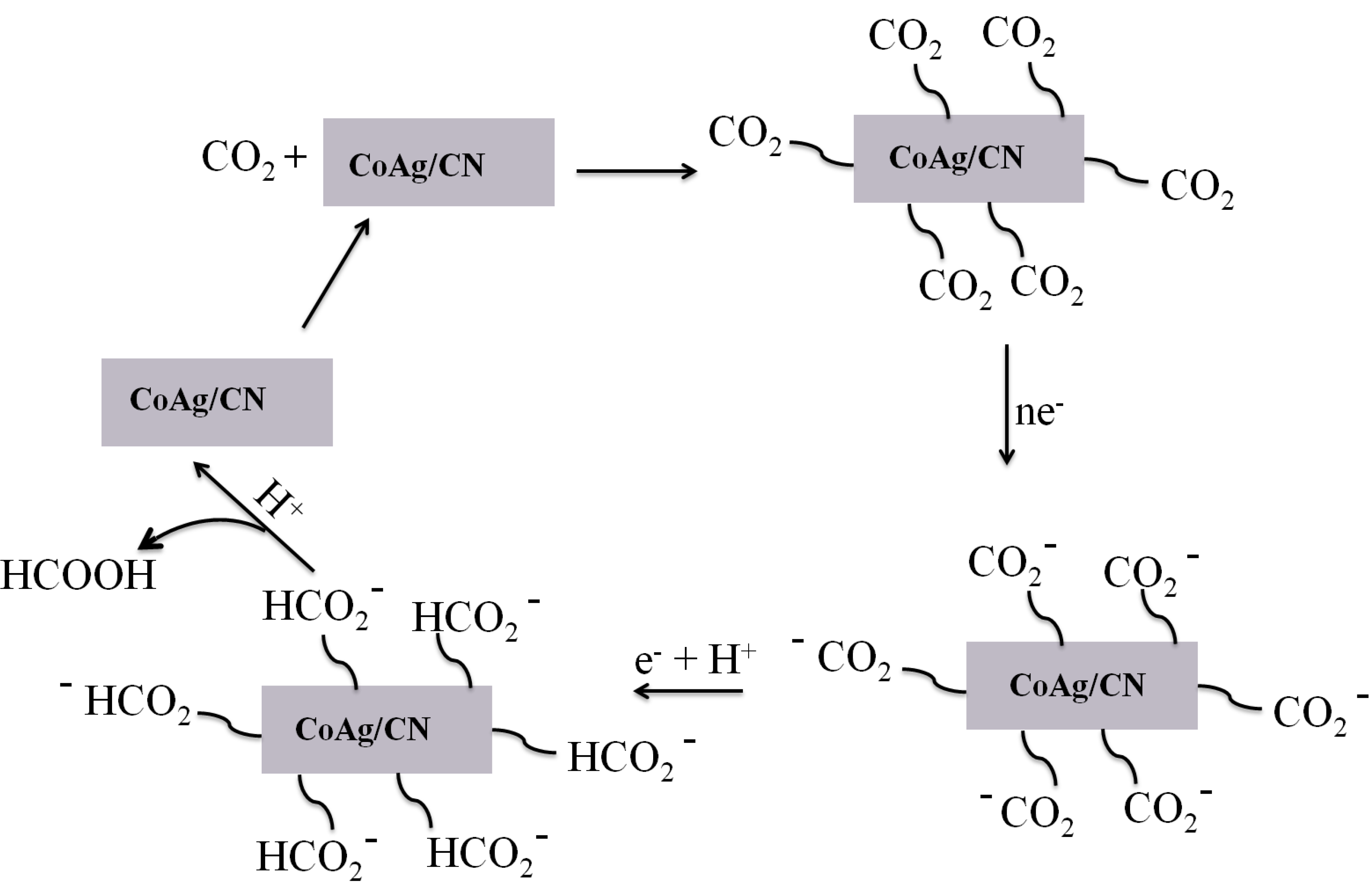
2.2. Application in Carbon Dioxide Conversion
3. Experimental Section
3.1. Synthesis of Carbon Nitride
3.2. Synthesis of Co/CN
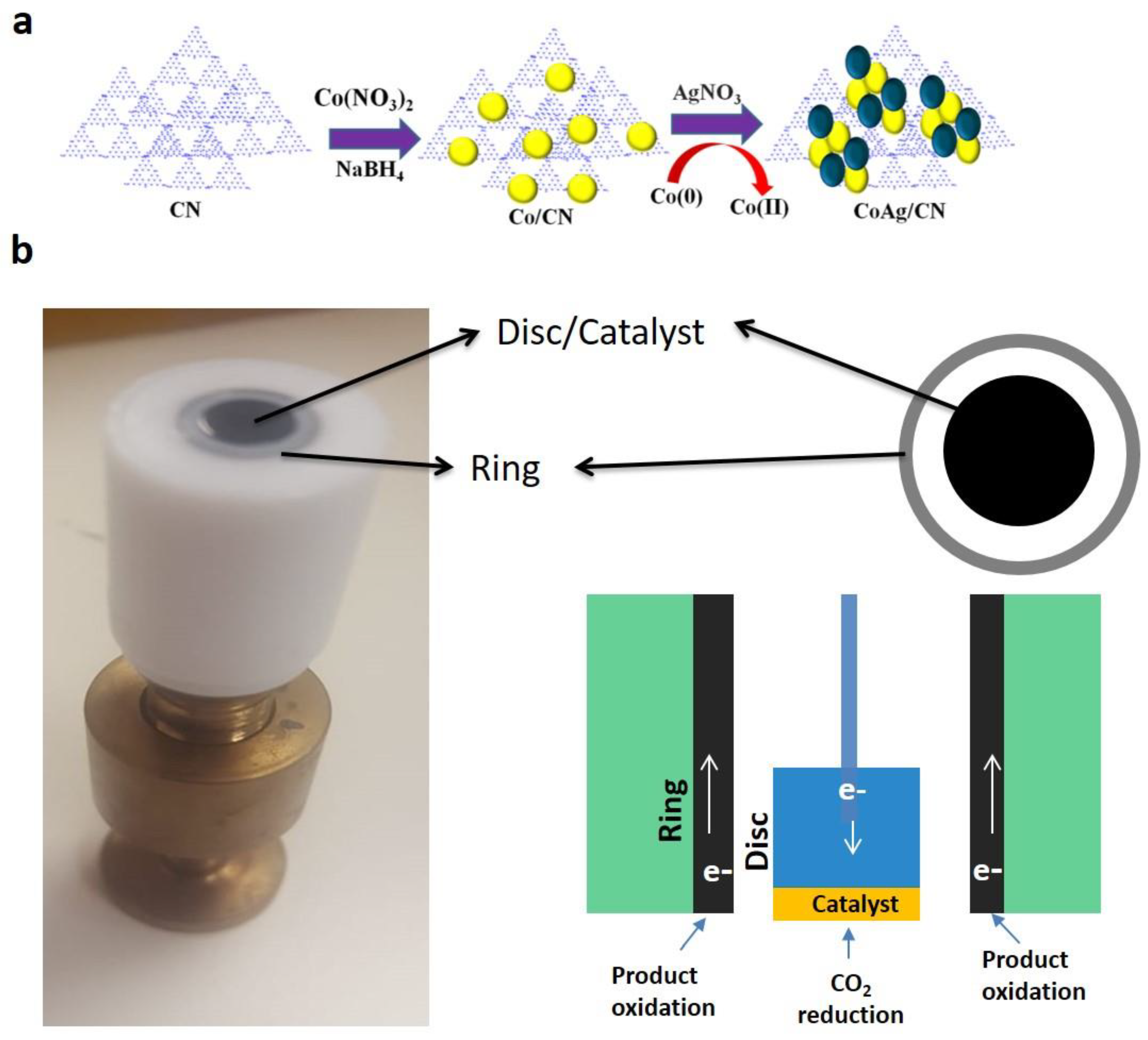
3.3. Synthesis of CoAg/CN Bimetallic NPs
3.4. Characterization Techniques
3.5. Electrode Preparation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayrhofer, K.J.; Arenz, M. Fuel cells: log on for new catalysts. Nat. Chem. 2009, 1, 518. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Hassoun, J.; Park, J.; Sun, Y.; Scrosati, B. An improved high-performance lithium–air battery. Nat. Chem. 2012, 4, 579. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.A.; Kumar, A.; Saad Saleh, M.A.H.; Al-Marri, M.J.; Suslov, S. Zn-enriched PtZn nanoparticle electrocatalysts synthesized by solution combustion for ethanol oxidation reaction in an alkaline medium. MRS Commun. 2018, 8, 411–419. [Google Scholar] [CrossRef]
- Matin, M.; Kumar, A.; Bhosale, R.; Saad, M.S.; Almomani, F.; Al-Marri, M. PdZn nanoparticle electrocatalysts synthesized by solution combustion for methanol oxidation reaction in an alkaline medium. RSC Adv. 2017, 7, 42709–42717. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M=Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.; Almomani, F.; Saad, M.A.H.S.; Suslov, S.; Tarlochan, F. Influence of fuel ratio on the performance of combustion synthesized bifunctional cobalt oxide catalysts for fuel cell application. Int. J. Hydrog. Energy 2019, 44, 436–445. [Google Scholar] [CrossRef]
- Liu, C.; Wei, Y.; Wang, K. Surface condition manipulation and oxygen reduction enhancement of PtAu/C catalysts synergistically modified by CeO2 addition and N2 treatment. J. Phys. Chem. C 2011, 115, 8702–8708. [Google Scholar] [CrossRef]
- Favaro, M.; Agnoli, S.; Perini, L.; Durante, C.; Gennaro, A.; Granozzi, G. Palladium nanoparticles supported on nitrogen-doped HOPG: a surface science and electrochemical study. Phys. Chem. Chem. Phys. 2013, 15, 2923–2931. [Google Scholar] [CrossRef]
- Wolf, E.; Kumar, A.; Mukasyan, A. Combustion synthesis: a novel method of catalyst preparation. Catalysis 2019, 31, 297–346. [Google Scholar]
- Kumar, A. Current trends in cellulose assisted combustion synthesis of catalytically active nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 7681–7689. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Tarlochan, F. Preparation of Nanoparticles via Cellulose-Assisted Combustion Synthesis. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 141–153. [Google Scholar] [CrossRef]
- Perini, L.; Durante, C.; Favaro, M.; Agnoli, S.; Granozzi, G.; Gennaro, A. Electrocatalysis at palladium nanoparticles: Effect of the support nitrogen doping on the catalytic activation of carbonhalogen bond. Appl. Catal. B Environ. 2014, 144, 300–307. [Google Scholar] [CrossRef]
- Leiva, E.; Iwasita, T.; Herrero, E.; Feliu, J.M. Effect of adatoms in the electrocatalysis of HCOOH oxidation. A theoretical model. Langmuir 1997, 13, 6287–6293. [Google Scholar] [CrossRef]
- Smith, S.P.; Abruña, H.D. Structural effects on the oxidation of HCOOH by bismuth modified Pt (111) electrodes with (110) monatomic steps. J. Electroanal. Chem. 1999, 467, 43–49. [Google Scholar] [CrossRef]
- Fu, X.; Hu, X.; Yan, Z.; Lei, K.; Li, F.; Cheng, F.; Chen, J. Template-free synthesis of porous graphitic carbon nitride/carbon composite spheres for electrocatalytic oxygen reduction reaction. Chem. Commun. 2016, 52, 1725–1728. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Z.; Jiang, F.; Yang, L.; Qian, J.; Zhou, Y.; Li, W.; Hong, M. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions. Nanoscale 2014, 6, 6590–6602. [Google Scholar] [CrossRef]
- Lyth, S.M.; Nabae, Y.; Moriya, S.; Kuroki, S.; Kakimoto, M.; Ozaki, J.; Miyata, S. Carbon nitride as a nonprecious catalyst for electrochemical oxygen reduction. Phys. Chem. C 2009, 113, 20148–20151. [Google Scholar] [CrossRef]
- Zhang, Y.; Mori, T.; Ye, J.; Antonietti, M. Phosphorus-doped carbon nitride solid: enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc. 2010, 132, 6294–6295. [Google Scholar] [CrossRef]
- Darabdhara, G.; Amin, M.A.; Mersal, G.A.; Ahmed, E.M.; Das, M.R.; Zakaria, M.B.; Malgras, V.; Alshehri, S.M.; Yamauchi, Y.; Szunerits, S. Reduced graphene oxide nanosheets decorated with Au, Pd and Au–Pd bimetallic nanoparticles as highly efficient catalysts for electrochemical hydrogen generation. J. Mater. Chem. A 2015, 3, 20254–20266. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Kamat, P.V. Galvanic exchange on reduced graphene oxide: designing a multifunctional two-dimensional catalyst assembly. J. Phys. Chem. C 2012, 117, 571–577. [Google Scholar] [CrossRef]
- Bhowmik, T.; Kundu, M.K.; Barman, S. Palladium nanoparticle–graphitic carbon nitride porous synergistic catalyst for hydrogen evolution/oxidation reactions over a broad range of pH and correlation of its catalytic activity with measured hydrogen binding energy. ACS Catal. 2016, 6, 1929–1941. [Google Scholar] [CrossRef]
- Perini, L.; Durante, C.; Favaro, M.; Perazzolo, V.; Agnoli, S.; Schneider, O.; Granozzi, G.; Gennaro, A. Metal–support interaction in platinum and palladium nanoparticles loaded on nitrogen-doped mesoporous carbon for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Nazir, R.; Fageria, P.; Basu, M.; Pande, S. Decoration of carbon nitride surface with bimetallic nanoparticles (Ag/Pt, Ag/Pd, and Ag/Au) via galvanic exchange for hydrogen evolution reaction. J. Phys. Chem. C 2017, 121, 19548–19558. [Google Scholar] [CrossRef]
- Nazir, R.; Fageria, P.; Basu, M.; Gangopadhyay, S.; Pande, S. Decoration of Pd and Pt nanoparticles on a carbon nitride (C 3 N 4) surface for nitro-compounds reduction and hydrogen evolution reaction. New J. Chem. 2017, 41, 9658–9667. [Google Scholar] [CrossRef]
- Taşcı, Z.; Kunduracıoğlu, A.; Kani, İ.; Çetinkaya, B. A New Application Area for Ag-NHCs: CO2 Fixation Catalyst. ChemCatChem 2012, 4, 831–835. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Tarlochan, F. Surface alloying in silver-cobalt through a second wave solution combustion synthesis technique. Nanomaterials 2018, 8, 604. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Matin, M.A.; Tarlochan, F. Synthesis of highly efficient bifunctional Ag/Co3O4 catalyst for oxygen reduction and oxygen evolution reactions in alkaline medium. ACS Omega 2018, 3, 7745–7756. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Jiao, F. Nanostructured metallic electrocatalysts for carbon dioxide reduction. ChemCatChem 2015, 7, 38–47. [Google Scholar] [CrossRef]
- Choudhury, J. New strategies for CO2-to-methanol conversion. ChemCatChem 2012, 4, 609–611. [Google Scholar] [CrossRef]
- Rodemerck, U.; Holeňa, M.; Wagner, E.; Smejkal, Q.; Barkschat, A.; Baerns, M. Catalyst development for CO2 hydrogenation to fuels. ChemCatChem 2013, 5, 1948–1955. [Google Scholar] [CrossRef]
- Meng, A.; Lin, L.; Yuan, X.; Shen, T.; Li, Z.; Li, Q. Ag/ZrO2/MWCNT Nanocomposite as Non-Platinum Electrocatalysts for Enhanced Oxygen Reduction Reaction. ChemCatChem 2019, 11, 2900–2908. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.; An, L. A high-performance supportless silver nanowire catalyst for anion exchange membrane fuel cells. J. Mater. Chem. A 2015, 3, 1410–1416. [Google Scholar] [CrossRef]
- Holewinski, A.; Idrobo, J.; Linic, S. High-performance Ag–Co alloy catalysts for electrochemical oxygen reduction. Nat. Chem. 2014, 6, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Blizanac, B.; Ross, P.N.; Marković, N. Oxygen Reduction on Silver Low-Index Single-Crystal Surfaces in Alkaline Solution: Rotating Ring DiskAg (h kl) Studies. J. Phys. Chem. B 2006, 110, 4735–4741. [Google Scholar] [CrossRef]
- Wiberg, G.K.; Mayrhofer, K.J.; Arenz, M. Investigation of the oxygen reduction activity on silver–a rotating disc electrode study. Fuel Cells 2010, 10, 575–581. [Google Scholar] [CrossRef]
- Singh, P.; Buttry, D.A. Comparison of oxygen reduction reaction at silver nanoparticles and polycrystalline silver electrodes in alkaline solution. J. Phys. Chem. C 2012, 116, 10656–10663. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Liu, Y.; Deng, Y. Silver supported on Co3O4 modified carbon as electrocatalyst for oxygen reduction reaction in alkaline media. Electrochem. Commun. 2013, 31, 108–111. [Google Scholar] [CrossRef]
- Lima, F.H.B.; de Castro, J.; Ticianelli, E.A. Silver-cobalt bimetallic particles for oxygen reduction in alkaline media. J. Power Sources 2006, 161, 806–812. [Google Scholar] [CrossRef]
- Zafferoni, C.; Cioncoloni, G.; Foresti, M.; Dei, L.; Carretti, E.; Vizza, F.; Lavacchi, A.; Innocenti, M. Synergy of cobalt and silver microparticles electrodeposited on glassy carbon for the electrocatalysis of the oxygen reduction reaction: an electrochemical investigation. Molecules 2015, 20, 14386–14401. [Google Scholar] [CrossRef]
- Yi, Q.; Chu, H.; Tang, M.; Yang, Z.; Chen, Q.; Liu, X. Carbon nanotube-supported binary silver-based nanocatalysts for oxygen reduction reaction in alkaline media. J. Electroanal. Chem. 2015, 739, 178–186. [Google Scholar] [CrossRef]
- Yi, Q.; Song, L. Polyaniline-Modified Silver and Binary Silver-Cobalt Catalysts for Oxygen Reduction Reaction. Electroanalysis 2012, 24, 1655–1663. [Google Scholar] [CrossRef]
- Fernández, J.L.; Walsh, D.A.; Bard, A.J. Thermodynamic guidelines for the design of bimetallic catalysts for oxygen electroreduction and rapid screening by scanning electrochemical microscopy. M− Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 2005, 127, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Balbuena, P.B. Design of oxygen reduction bimetallic catalysts: ab-initio-derived thermodynamic guidelines. J. Phys. Chem. B 2005, 109, 18902–18906. [Google Scholar] [CrossRef] [PubMed]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjapanayis, G.C. Borohydride reductions of metal ions. A new understanding of the chemistry leading to nanoscale particles of metals, borides, and metal borates. Langmuir 1992, 8, 771–773. [Google Scholar] [CrossRef]
- Glavee, G.; Klabunde, K.; Sorensen, C.M.; Hadjipanayis, G. Sodium borohydride reduction of cobalt ions in nonaqueous media. Formation of ultrafine particles (nanoscale) of cobalt metal. Inorg. Chem. 1993, 32, 474–477. [Google Scholar] [CrossRef]
- Bala, T.; Arumugam, S.K.; Pasricha, R.; Prasad, B.; Sastry, M. Foam-based synthesis of cobalt nanoparticles and their subsequent conversion to Co core Ag shell nanoparticles by a simple transmetallation reaction. J. Mater. Chem. 2004, 14, 1057–1061. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Jin, L.; Yang, H.; Yang, S. Preparation and magnetic properties of cobalt nanoparticles with dendrimers as templates. Mater. Chem. Phys. 2010, 121, 342–348. [Google Scholar] [CrossRef]
- Lee, D.; Xia, Q.X.; Yun, J.M.; Kim, K.H. High-performance cobalt carbonate hydroxide nano-dot/NiCo (CO3)(OH)2 electrode for asymmetric supercapacitors. Appl. Surf. Sci. 2018, 433, 16–26. [Google Scholar] [CrossRef]
- Gozdziewska, M.; Cichowicz, G.; Markowska, K.; Zawada, K.; Megiel, E. Nitroxide-coated silver nanoparticles: Synthesis, surface physicochemistry and antibacterial activity. RSC Adv. 2015, 5, 58403–58415. [Google Scholar] [CrossRef]
- Chee, S.W.; Tan, S.F.; Baraissov, Z.; Bosman, M.; Mirsaidov, U. Direct observation of the nanoscale Kirkendall effect during galvanic replacement reactions. Nat. Commun. 2017, 8, 1224. [Google Scholar] [CrossRef]
- Chung, H.T.; Won, J.H.; Zelenay, P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat. Commun. 2013, 4, 1922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gupta, K.; Bersani, M.; Darr, J.A.; Shearing, P.R.; Brett, D.J. lectrochemical reduction of carbon dioxide on copper-based nanocatalysts using the rotating ring-disc electrode. Electrochim. Acta 2018, 283, 1037–1044. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Fageria, P.; Nazir, R.; Gangopadhyay, S.; Barshilia, H.C.; Pande, S. Graphitic-carbon nitride support for the synthesis of shape-dependent ZnO and their application in visible light photocatalysts. RSC Adv. 2015, 5, 80397–80409. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, R.; Kumar, A.; Ali, S.; Saad, M.A.S.; Al-Marri, M.J. Galvanic Exchange as a Novel Method for Carbon Nitride Supported CoAg Catalyst Synthesis for Oxygen Reduction and Carbon Dioxide Conversion. Catalysts 2019, 9, 860. https://doi.org/10.3390/catal9100860
Nazir R, Kumar A, Ali S, Saad MAS, Al-Marri MJ. Galvanic Exchange as a Novel Method for Carbon Nitride Supported CoAg Catalyst Synthesis for Oxygen Reduction and Carbon Dioxide Conversion. Catalysts. 2019; 9(10):860. https://doi.org/10.3390/catal9100860
Chicago/Turabian StyleNazir, Roshan, Anand Kumar, Sardar Ali, Mohammed Ali Saleh Saad, and Mohammed J. Al-Marri. 2019. "Galvanic Exchange as a Novel Method for Carbon Nitride Supported CoAg Catalyst Synthesis for Oxygen Reduction and Carbon Dioxide Conversion" Catalysts 9, no. 10: 860. https://doi.org/10.3390/catal9100860
APA StyleNazir, R., Kumar, A., Ali, S., Saad, M. A. S., & Al-Marri, M. J. (2019). Galvanic Exchange as a Novel Method for Carbon Nitride Supported CoAg Catalyst Synthesis for Oxygen Reduction and Carbon Dioxide Conversion. Catalysts, 9(10), 860. https://doi.org/10.3390/catal9100860







