[(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2/CuI as Cocatalyst for Coupling-Cyclization of 2-Iodophenol with Terminal Alkynes in Water
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Machine
3.2. General Experimental Procedure for the Coupling-Cyclization Reaction of 2-Iodophenol with Various Aromatic Acetylenes
3.3. Analytical Data of Products (Supplementary Materials)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takasugi, M.; Nagao, S.; Masamune, T.; Shirata, A.; Takahashi, K. Structures of moracins E, F, G, and H, new phytoalexins from diseased mulberry. Tetrahedron Lett. 1979, 20, 4675–4678. [Google Scholar] [CrossRef]
- Pacher, T.; Seger, C.; Engelmeier, D.; Vajrodaya, S.; Hofer, O.; Greger, H. Antifungal stilbenoids from Stemonacollinsae. J. Nat. Prod. 2002, 65, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Wine-Show, S.; lan-Lih, T.; Teng, C.M.; Chen, I.S. Nor-neolignan and phenyl propanoid from Zanthoxylumailanthoides. Phytochemistry 1994, 36, 213–215. [Google Scholar] [CrossRef]
- Jenab, M.; Thompson, L.U. The influence of flaxseed and lignans on colon carcinogenesis and β-glucuronidase activity. Carcinogenesis 1996, 17, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Kondo, K.; Kuroda, T.; Moritani, Y.; Yamagata, S.; Sugiura, M.; Kikkawa, H.; Kaminuma, O.; Ikezawa, K. Novel Selective PDE IV Inhibitors as Antiasthmatic Agents. Synthesis and Biological Activities of a Series of 1-Aryl-2,3-bis(hydroxymethyl)naphthalene Lignans. J. Med. Chem. 1996, 39, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.; Castro, M.; Corral, J.M.; Lopez-Vazquez, M.; Feliciano, A.S.; Faircloth, G.T. In vivo immunosuppressive activity of some cyclolignans. Bioorg. Med. Chem. Lett. 1997, 7, 2781–2786. [Google Scholar] [CrossRef]
- Zacchino, S.; Rodriguez, G.; Pezzenati, G.; Orellana, G. In vitro evaluation of antifungal properties of 8.O.4′-neolignans. J. Nat. Prod. 1997, 60, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Dupont, R.; Cotelle, P. An expeditious synthesis of polyhydroxylated 2-arylbenzo[b]furans. Tetrahedron 2001, 57, 5585–5589. [Google Scholar] [CrossRef]
- Johnson, F.; Subramanian, R. Cheminform Abstract: Halocarbon Chemistry. Part 3. Ortho Metallation-Induced Cyclization of Acetylenes: One-Flask Synthesis of 2,3-Disubstituted Benzofurans, Thianaphthenes, and Indoles. Cheminform 1987, 18, 393–399. [Google Scholar] [CrossRef]
- Moure, M.J.; Sanmartin, R.; Dominguez, E. Copper Pincer Complexes as Advantageous Catalysts for the Heteroannulation of ortho-Halophenols and Alkynes. Adv. Synth. Catal. 2014, 356, 2070–2080. [Google Scholar] [CrossRef]
- Zanardi, A.; Mata, J.A.; Peris, E. Domino Approach to Benzofurans by the Sequential Sonogashira/Hydroalkoxylation Couplings Catalyzed by New N-Heterocyclic-Carbene-Palladium Complexes. Organometallics 2009, 28, 4335–4339. [Google Scholar] [CrossRef]
- Sun, S.X.; Wang, J.J.; Xu, Z.J.; Cao, L.Y.; Shi, Z.F.; Zhang, H.L. Highly efficient heterogeneous synthesis of benzofurans under aqueous condition. Tetrahedron 2014, 70, 3798–3806. [Google Scholar] [CrossRef]
- Hudson, R.; Bizier, N.P.; Esdale, K.N.; Katz, J.L. Synthesis of indoles, benzofurans, and related heterocycles via an acetylene-activated SNAr/intramolecular cyclization cascade sequence in water or DMSO. Org. Biomol. Chem. 2015, 13, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Urban, S.; Beiring, B.; Glorius, F. Ruthenium NHC Catalyzed Highly Asymmetric Hydrogenation of Benzofurans. Angew. Chem. Int. Ed. 2012, 51, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.G.; Saejueng, P.; Murphy, J.M.; Venkataraman, D. Synthesis of 2-Arylbenzo[b]furans via Copper(I)-Catalyzed Coupling of o-Iodophenols and Aryl Acetylenes. Org. Lett. 2002, 4, 4727–4729. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Mo, S.; Lu, Y.; Shen, Z. Domino Sonogashira Coupling/Cyclization Reaction Catalyzed by Copper and ppb Levels of Palladium: A Concise Route to Indoles and Benzo[b]furans. Adv. Synth. Catal. 2011, 353, 713–718. [Google Scholar] [CrossRef]
- Arcadi, A.; Blesi, F.; Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Marinelli, F. Multisubstitutedbenzo[b]furans through a copper- and/or palladium-catalyzed assembly and functionalization process. Tetrahedron 2013, 69, 1857–1871. [Google Scholar] [CrossRef]
- Arcadi, A.; Marinelli, F.; Cacchi, S. Palladium-Catalyzed Reaction of 2-Hydroxyaryl and Hydroxyheteroaryl Halides with 1-Alkynes: An Improved Route to the Benzo[b]furan Ring System. Synthesis 1986, 9, 749–751. [Google Scholar] [CrossRef]
- Pal, M.; Subramanian, V.; Yeleswarapu, K.R. Pd/C mediated synthesis of 2-substituted benzo[b]furans/nitrobenzo[b]furans in water. Tetrahedron 2003, 44, 8221–8225. [Google Scholar] [CrossRef]
- Bochicchio, A.; Chiummiento, L.; Funicello, M.; Lopardo, M.T.; Lupattelli, P. Efficient synthesis of 5-nitro-benzo[b]furans via 2-bromo-4-nitro-phenyl acetates. Tetrahedron Lett. 2010, 51, 2824–2827. [Google Scholar] [CrossRef]
- Gil-Molto, J.; Najera, C. Palladium(II) Chloride and a (Dipyridin-2-ylmethyl)amine-Derived Palladium(II) Chloride Complex as Highly Efficient Catalysts for the Synthesis of Alkynes in Water or in NMP and of Diynes in the Absence of Reoxidant. Eur. J. Org. Chem. 2005, 4073–4081. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, J.; Saikh, F. A new synthesis of 2-aryl/alkylbenzofurans by visible light stimulated intermolecular Sonogashira coupling and cyclization reaction in water. Tetrahedron Lett. 2012, 53, 5883–5886. [Google Scholar] [CrossRef]
- Guo, M.P.; Zhang, Q.C. An inexpensive and highly stable palladium(II) complex for room temperature Suzuki coupling reactions under ambient atmosphere. Tetrahedron Lett. 2009, 50, 1965–1968. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Xu, L.; Wang, Q.; Chen, J.; Ding, J.; Wu, H. Palladium-Catalyzed Addition of Potassium Aryltrifluoroborates to Aliphatic Nitriles: Synthesis of Alkyl Aryl Ketones, Diketone Compounds, and 2-Arylbenzo[b]furans. J. Org. Chem. 2013, 78, 5273–5281. [Google Scholar] [CrossRef] [PubMed]
- Mandali, P.K.; Chand, D.K. Palladium Nanoparticles Catalyzed Synthesis of Benzofurans by a Domino Approach. Synthesis 2015, 47, 1661–1668. [Google Scholar]
- Pauli, L.; Tannert, R.; Scheil, R.; Pfaltz, A. Asymmetric Hydrogenation of Furans and Benzofurans with Iridium-Pyridine-Phosphinite Catalysts. Chem. Eur. J. 2015, 21, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
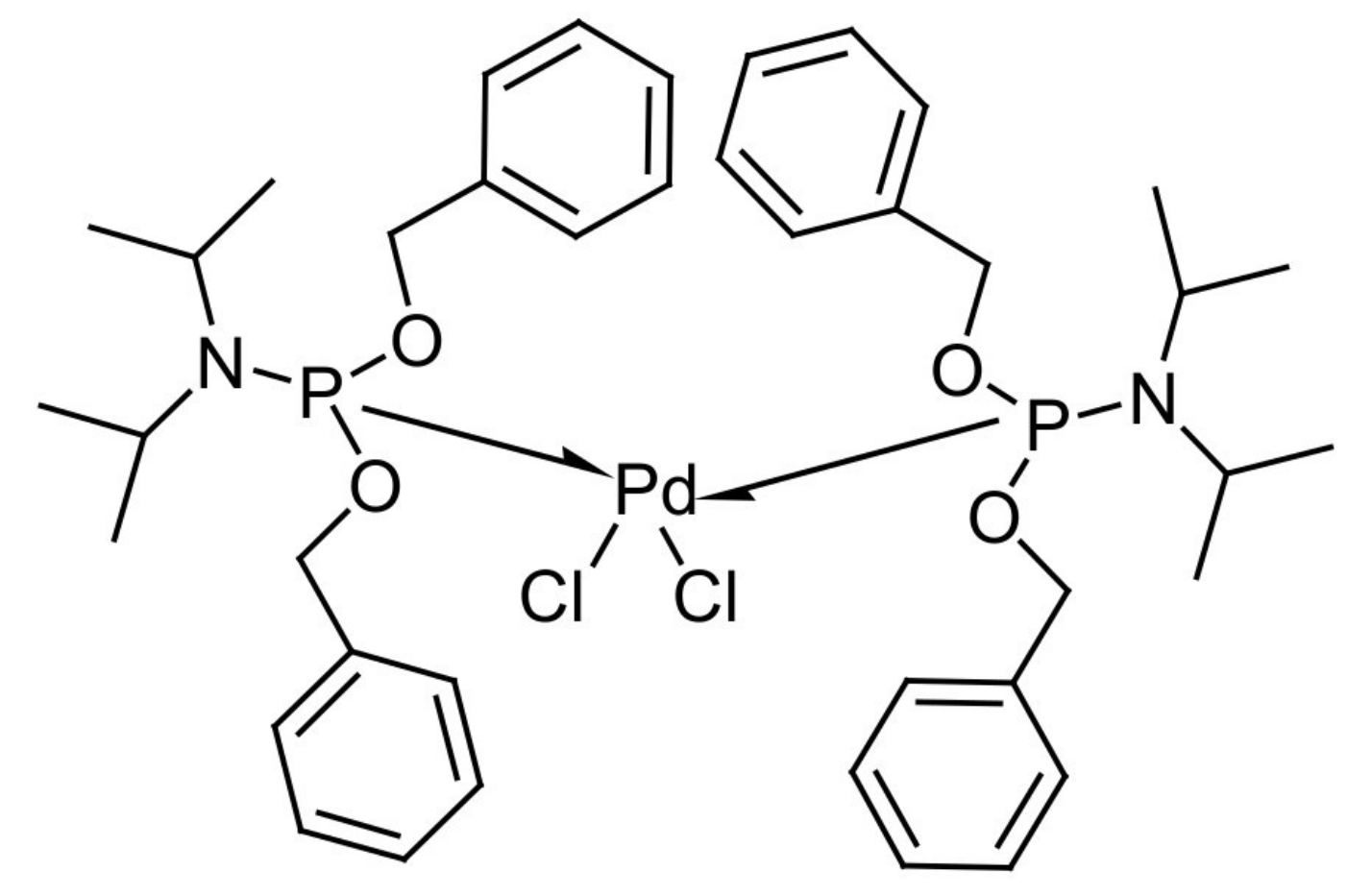
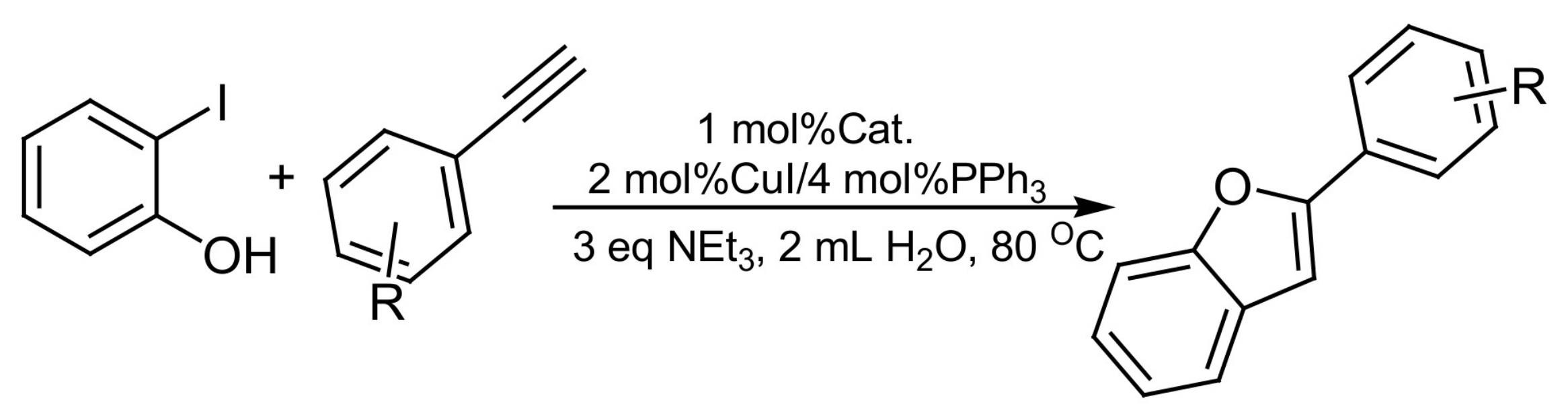

| Entry | Base | CuI (mol %) | PPh3 (mol %) | Time (h) | Temperature (°C) | Yield b (%) |
|---|---|---|---|---|---|---|
| 1 | NEt3 | 2 | 0 | 4 | 80 | 51 |
| 2 | NEt3 | 2 | 2 | 4 | 80 | 60 |
| 3 | NEt3 | 2 | 4 | 4 | 80 | 97 |
| 4 | NEt3 | 2 | 6 | 4 | 80 | 67 |
| 5 | NEt3 | 2 | 8 | 4 | 80 | 70 |
| 6 c | NEt3 | 0 | 4 | 4 | 80 | N.D. |
| 7 | NEt3 | 1 | 4 | 4 | 80 | 66 |
| 8 | NEt3 | 3 | 4 | 4 | 80 | 64 |
| 9 | NEt3 | 4 | 4 | 4 | 80 | 83 |
| 10 | Cs2CO3 | 2 | 4 | 4 | 80 | 28 |
| 11 | KOH | 2 | 4 | 4 | 80 | 19 |
| 12 | NaOH | 2 | 4 | 4 | 80 | 21 |
| 13 | Pyridine | 2 | 4 | 4 | 80 | trace |
| 14 | K2CO3 | 2 | 4 | 4 | 80 | trace |
| 15 | t-BuOH | 2 | 4 | 4 | 80 | 31 |
| 16 | NEt3 | 2 | 4 | 2 | 80 | 90 |
| 17 | NEt3 | 2 | 4 | 6 | 80 | 87 |
| 18 | NEt3 | 2 | 4 | 8 | 80 | 79 |
| 19 | NEt3 | 2 | 4 | 4 | 60 | 70 |
| 20 | NEt3 | 2 | 4 | 4 | 100 | 96 |
| 21 | NEt3 | 2 | 4 | 4 | 120 | 96 |
| 22 | NEt3 | 2 | 4 | 4 | 140 | 75 |
| 23 d | NEt3 | 2 | 4 | 4 | 80 | 53 |
| 24 e | NEt3 | 2 | 4 | 4 | 80 | 51 |
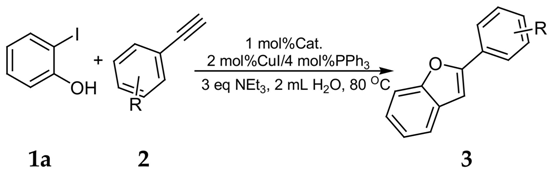
| Entry | Alkyne | Product | Yield b (%) |
|---|---|---|---|
| 1 |  | 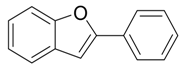 3a 3a | 97 |
| 2 | 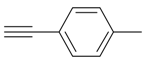 |  3b 3b | 96 |
| 3 |  | 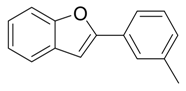 3c 3c | 95 |
| 4 | 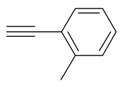 | 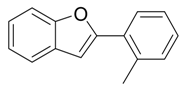 3d 3d | 91 |
| 5 | 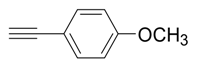 |  3e 3e | 87 |
| 6 | 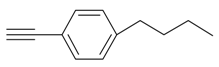 |  3f 3f | 92 |
| 7 | 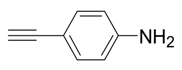 | 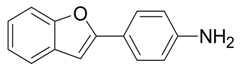 3g 3g | 78 |
| 8 | 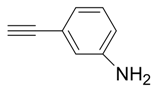 | 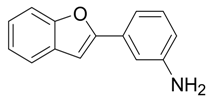 3h 3h | 88 |
| 9 | 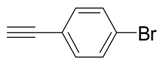 |  3i 3i | 82 |
| 10 |  |  3j 3j | 80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Guo, M.; Fu, L.; Wen, Y.; Shen, X.; Zhou, L. [(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2/CuI as Cocatalyst for Coupling-Cyclization of 2-Iodophenol with Terminal Alkynes in Water. Catalysts 2018, 8, 136. https://doi.org/10.3390/catal8040136
Jiang P, Guo M, Fu L, Wen Y, Shen X, Zhou L. [(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2/CuI as Cocatalyst for Coupling-Cyclization of 2-Iodophenol with Terminal Alkynes in Water. Catalysts. 2018; 8(4):136. https://doi.org/10.3390/catal8040136
Chicago/Turabian StyleJiang, Panli, Mengping Guo, Leiqing Fu, Yongju Wen, Xiuli Shen, and Lanjiang Zhou. 2018. "[(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2/CuI as Cocatalyst for Coupling-Cyclization of 2-Iodophenol with Terminal Alkynes in Water" Catalysts 8, no. 4: 136. https://doi.org/10.3390/catal8040136
APA StyleJiang, P., Guo, M., Fu, L., Wen, Y., Shen, X., & Zhou, L. (2018). [(PhCH2O)2P(CH3)2CHNCH(CH3)2]2PdCl2/CuI as Cocatalyst for Coupling-Cyclization of 2-Iodophenol with Terminal Alkynes in Water. Catalysts, 8(4), 136. https://doi.org/10.3390/catal8040136





