Abstract
The ubiquitous presence of tetracycline in water sources due to its prevalent usage in antibiotics poses a potential risk to ecosystems and to human health. There is therefore a dire need for efficient methods of tetracycline removal. To this end, we constructed a series of super-small-sized BiVO4 composites with different loading amounts of 5 wt%, 10 wt%, 15 wt%, 20 wt%, and 25 wt% on SiO2 support by a hydrothermal–calcination method for efficient photocatalytic degradation of tetracycline. XRD and SEM results revealed that these composites all exhibited good crystallinity and well-dispersed morphologies. Particularly, the 20 wt% BiVO4@SiO2 of this series of composites showed superior photocatalytic performance, with 84.1% efficiency in tetracycline degradation, higher than any of its counterparts. This can be explained by its wide light-absorption range and high charge-separation efficiency, as confirmed by UV-Vis absorption spectra and quenched fluorescence spectra. This work offers a new method for the design and construction of tetracycline degradation photocatalysts.
1. Introduction
As a broad-spectrum antibiotic, tetracycline (TC) has been widely used in aquaculture and clinical medicine for effective treatment of bacterial infection and for prevention of disease [1,2,3]. Due to overuse, and the incomplete absorption of tetracycline in organisms, tetracycline residues have been continuously released into water environments; such residues have been detected in various water sources, including surface water, rivers, and domestic water in a growing number of cities [4,5]. The presence of TC poses a serious risk to the ecological environment and to human health as a result of bio-accumulation in living organisms and the growth of antibiotic-resistant bacteria [6,7,8,9]. To address this issue, various methods have been employed; these include traditional water treatment methods such as physical and chemical adsorption [10], biodegradation [5], and chemical oxidation [4]. However, due to the low efficiency of these methods in removing tetracycline from water, new approaches involving advanced oxidation processes (AOPs) have been adopted, including Fenton or Fenton-like reactions, ozonation or catalytic ozonation, photocatalytic oxidation, electrochemical oxidation, and ionizing radiation [11]. Among these, the photocatalytic oxidation method has received particular attention due to its economic and green characteristics, including use of sunlight, mild reaction conditions, fast reaction rates, etc.
As a matter of fact, the choice of photocatalysts is rather critical in achieving high catalytic performance and efficiency. Bismuth-based materials exhibit the advantages of low cost, non-toxicity, high efficiency in visible-light utilization, good photocatalytic activity, and stability; they have therefore attracted much attention as promising photocatalyst candidates [12]. BiVO4, one of the most important bismuth-based materials, has drawn increasing interest in recent years because of its superior properties; these include a wide range of light absorption, strong catalytic ability, and high stability associated with its n-type semiconducting nature [13,14]. There are three main crystal types of BiVO4, i.e., monoclinic scheelite (Eg = 2.40 eV), tetragonal scheelite (Eg = 2.34 eV), and tetragonal zircon (Eg = 2.90 eV). The monoclinic crystalline phase of BiVO4 exhibits superior ability in organic pollutant degradation and water decomposition under visible-light conditions owing to the lone-pair distortion of the Bi 6s orbital in the BiVO4 semiconductor. However, the narrow band gap of BiVO4 leads to a high photogenerated carrier recombination rate, which reduces catalytic efficiency. It is worth noting that particle size is rather fundamental to the photocatalytic activity of a certain material. Particles of small and uniform size are key to decreasing the charge recombination rate due to the easy migration of photo-induced charge carriers to the particle surface in small-sized particles [15,16]. Moreover, small-sized particles possess a higher surface area, which is conducive to improving adsorption capacity [17]. Therefore, preparation of small-sized BiVO4 particles should facilitate the separation efficiency of photogenerated charge carriers, and improve photocatalytic performance.
In situ synthesis of photocatalysts using inert supports is a practical method for preparing small-sized BiVO4. Common inert carriers include activated carbon, silica, aluminum oxide, and other minerals. Among these, activated carbon has a large specific surface area, but it may mask the active sites, and it may also participate in side reactions. The specific surface area of Al2O3 is usually lower than that of silicon oxide, and it is difficult to prepare. SiO2 is one of the most important and popular carriers in the field of photocatalysis. With the advantages of large surface area, uniform pore topology, and high stability in harsh reaction environments (such as acid and high-temperature), silica serves as a rather good supporting and dispersing material for constructing high-photocatalytic-performance composites with small-sized photoactive structures [18]. Diana et al. [19] used SiO2 as a supporting material for TiO2 to build composites which reduced the agglomeration of TiO2 and facilitated the photogenerated charge separation of TiO2, significantly improving the photocatalytic efficiency of Cr(VI) and Pb(II) removal. Ferreira-Neto et al. [20] found that preparation of TiO2 nanoparticles using tetrabutyl titanate resulted in particle agglomeration due to rapid hydrolysis and condensation during synthesis, and this greatly reduced the specific surface area of the catalyst as well as that of exposed active sites. The dispersion of TiO2 and the corresponding catalytic activity could be effectively improved by introducing SiO2 particles into the TiO2 precursor solution. Therefore, SiO2 has great potential to be a supporting and dispersing photocatalyst with enhanced photocatalytic performance.
Hence, in this work, we employed SiO2 as a support and sodium oleate (surfactants) as a dispersant to construct a series of super-small-sized BiVO4@SiO2 composites using a hydrothermal–calcination method for photocatalytic degradation of tetracycline. Synthetic conditions were optimized by varying the calcination temperature, with 500 °C as the optimal temperature. BiVO4@SiO2 composites with give different BiVO4 loading amounts, i.e., 5 wt%, 10 wt%, 15 wt%, 20 wt%, and 25 wt%, were fabricated via this synthetic method. XRD and SEM results revealed that these composites exhibited good crystallinity and well-dispersed morphologies of small-sized particles. Particularly, the 20 wt% BiVO4@SiO2 of this series of composites showed superior photocatalytic performance, with the highest recorded efficiency of 84.1% in tetracycline degradation, due to its wide light-absorption range and efficient charge separation, as confirmed by solid-state UV-Vis absorption spectra and quenched fluorescence spectra.
2. Results and Discussion
A series of small-sized BiVO4@SiO2 composites with different BiVO4 loading amounts were synthesized via a hydrothermal–calcination method. First, BiVO4 was obtained by a hydrothermal method. This was then loaded onto SiO2 support by calcination, producing BiVO4@SiO2 composites. These composites were then characterized by a range of spectroscopies, including polycrystalline X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), UV-vis absorption spectroscopy, and fluorescence spectroscopy.
2.1. Synthesis Conditions
2.1.1. Calcination Temperature
To obtain the optimum BiVO4@SiO2 composite, a range of temperatures from 400 °C to 600 °C were employed for the calcination process, and the adopted mass ratio of BiVO4 and SiO2 was 1/5. Figure 1 shows XRD patterns for composites prepared under different calcination temperatures. All the composites displayed intense XRD patterns, and the corresponding peaks were assigned to BiVO4 due to their good matching with the monoclinic scheelite phase of BiVO4 (JCPDS card No. 14-0688). This indicated the monoclinic crystal phase nature of these composites, exactly the structure which was sought. No other signals could be observed, due to the amorphous nature of the SiO2 support. In addition, with continuous increase in calcination temperature, diffraction peaks gradually intensified, revealing the continuously enhanced crystallinity of the corresponding composites.
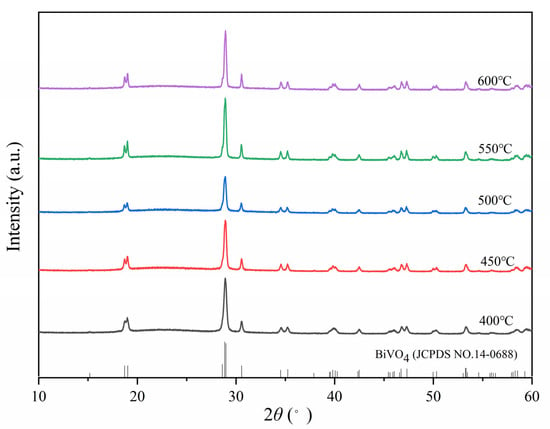
Figure 1.
XRD patterns of BiVO4@SiO2 composites prepared at different calcination temperatures.
The morphologies of these composites are shown in the SEM images in Figure 2. The composite calcinated at 400 °C exhibited small-sized particles in a range of 50–60 nm which were agglomerated with a morphology of elongated strips. With increase in the calcination temperature from 400 °C to 450 and then 500 °C, the particles became more uniform and elliptical, and their surfaces became smoother. Particle size also increased slightly due to the sintering effect of the increased calcination temperature. However, a further increase in temperature to 550 °C led to sintering between particles and, thus, the formation of cylinder-shaped particles with length of ca. 200 nm. When the temperature was finally raised to 600 °C, the sintering situation became aggravated, and the particles were closely connected with each other, forming larger agglomerations. On the basis of the above results, 500 °C was considered to be the optimum temperature for the calcination process to obtain the corresponding small-sized and uniform particles. Although slight particle agglomeration due to thermal sintering can be found in the SEM images (Figure 2e,f) for the optimal calcination temperature of 500 °C, its potential drawback is outweighed by the concurrent significant improvement in crystallinity as evidenced by the XRD analysis detailed above, leading to the superior photocatalytic performance as discussed below.
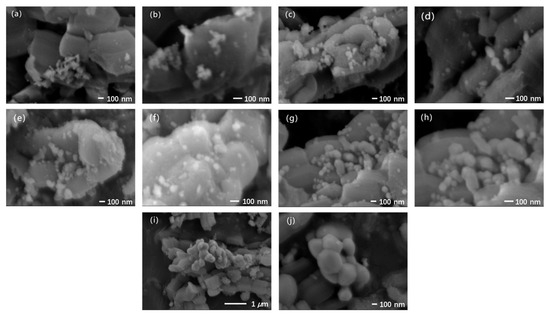
Figure 2.
SEM images of 20% BiVO4@SiO2 composites with different calcination temperatures: 400 °C (a,b); 450 °C (c,d); 500 °C (e,f); 550 °C (g,h); 600 °C (i,j).
2.1.2. Loading Amount
The mass ratio of BiVO4 and SiO2 is also an important factor in the photocatalytic performance of the composites. Therefore, five different mass ratios of BiVO4 and SiO2, 1/20, 1/10, 1/6.6, 1/5, and 1/4, were employed for the calcination process of the composites, leading to five different BiVO4 loading amounts of BiVO4@SiO2 composites, i.e., 5 wt%, 10 wt%, 15 wt%, 20 wt%, and 25 wt%, respectively.
As shown in Figure 3, the XRD patterns of all the prepared composites showed characteristic peaks corresponding to the monoclinic scheelite phase of BiVO4, and no other peaks were observed, indicating that there were no other impurities in the as-prepared composites. Moreover, the intensity of signals increased gradually with the increase in BiVO4 loading amount, indicating the better crystallinity of the higher BiVO4 loading amount for BiVO4@SiO2 composites.
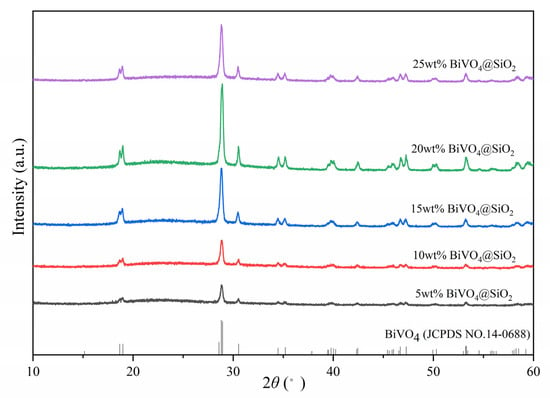
Figure 3.
XRD spectra of BiVO4@SiO2 composites with different BiVO4 loading amounts.
Figure 4 shows the morphologies of this series of BiVO4@SiO2 composites. As can be seen in the figure, the particle sizes of small BiVO4 were in a range of 60 to 100 nm, and the number of BiVO4 nanoparticles increased with increases in the loading ratio of BiVO4/SiO2. As illustrated in Figure 4, the amount of BiVO4 nanoparticles in both 5 wt% and 10 wt% BiVO4@SiO2 composites was rather sparse, suggesting inadequate loading of BiVO4 on SiO2 in the two samples. In the case of both 15 wt% and 20 wt% BiVO4@SiO2, the quantity of BiVO4 nanoparticles significantly increased, due to the higher loading percentage of BiVO4 on SiO2. It is noteworthy that the BiVO4 nanoparticles were closely connected with each other and evenly dispersed on SiO2 in the 20 wt% BiVO4@SiO2 composites. Compared to BiVO4 nanoparticles in samples prepared with lower loadings, the BiVO4 particles in the 25 wt% BiVO4@SiO2 sample were larger, with average size as high as ca. 150 nm, a result which can be attributed to the agglomeration of BiVO4 nanoparticles associated with the large loading amount.
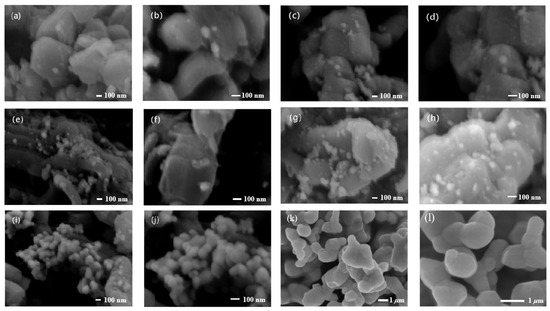
Figure 4.
SEM images of BiVO4@SiO2 composites calcined at 500 °C with different BiVO4 loading amounts: (a,b) 5 wt% BiVO4@SiO2; (c,d) 10 wt% BiVO4@SiO2; (e,f) 15 wt% BiVO4@SiO2; (g,h) 20 wt% BiVO4@SiO2; (i,j) 25 wt% BiVO4@SiO2. (k,l) Pure BiVO4 without SiO2 support.
Further EDS analysis of 20 wt% BiVO4@SiO2 revealed an even distribution of Bi, V, Si, and O elements in the composite. As can be observed in Figure 5, the elements of Si and V were abundant and evenly distributed throughout the composite, while the even presence of the Bi element indicated that the nanoparticles of BiVO4 were uniformly dispersed on SiO2, confirming that BiVO4 nanostructured composites were successfully constructed.
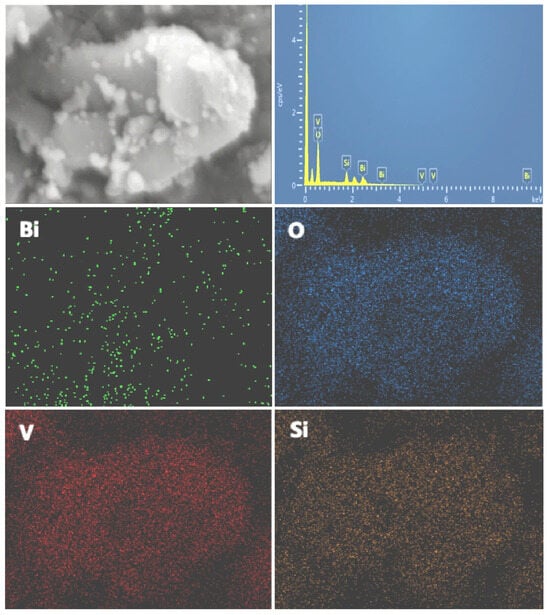
Figure 5.
Distribution of elements in EDS analysis of 20% BiVO4@SiO2 composite.
2.2. Optical Properties
To investigate the optical properties of the as-prepared composites, UV-vis diffuse reflectance spectra were recorded for all the different loading BiVO4@SiO2 samples. As shown in Figure 6, all the composites exhibited intense absorption in the range of 400 nm to 600 nm, suggesting their good potential for light absorption in photocatalysis. Moreover, absorption was intensified in the order of 10 wt%, 5 wt%, 15 wt%, 25 wt%, 20 wt% BiVO4 loading amounts for these composites, suggesting the superior light-utilization capacity of 20 wt% BiVO4@SiO2. The 20 wt% BiVO4@SiO2 exhibited the most visible absorption boundary, compared to other BiVO4@SiO2 composites, suggesting its superior light-utilization capacity for photocatalysis.
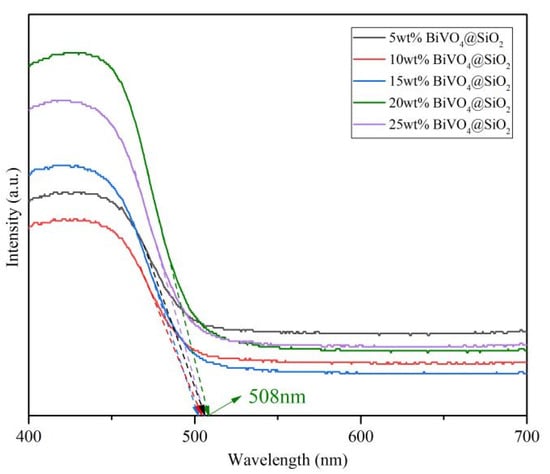
Figure 6.
UV-vis diffuse reflectance spectra for BiVO4@SiO2 composites with different BiVO4 loading amounts. The arrows exhibited the tangent of the spectra curves which were intersected with the wavelength axis.
According to the following equations, the positions of the valence band and conduction band of the semiconductor can be further estimated [21]:
EVB = X − Ee + 0.5Eg
ECB = EVB − Eg
In the equations, EVB and ECB are the valence band potential and conduction band potential, respectively, of semiconductors; and X is the electronegativity of semiconductors, which can be obtained by calculating the geometric average of the absolute electronegativity of the constituent atoms. This is the mathematical average of the electronic affinity and the first dissociation energy of atoms. For BiVO4, the X value is 6.04 eV. Ee is the hydrogen standard potential of free electrons (~4.5 eV). The EVB and ECB values for 20 wt% BiVO4@SiO2 composites were found to be 2.76 and 0.32 eV, respectively, by calculation.
To shed light on the potential of these composites with regard to photocatalytic performance, fluorescence spectroscopy was carried out for all the composites. Figure 7 shows the fluorescence spectra of all the BiVO4@SiO2 composites. With an excitation wavelength of 360 nm, an emission peak appearing at 570 nm can be observed for all the composites. With increases in the loading percentage of BiVO4 from 5% to 10%, 15%, and finally, 20%, the fluorescence intensity of the corresponding catalysts gradually decreased. However, the fluorescence intensity exhibited a dramatic rise when the loading percentage of BiVO4 was further increased, to 25%. This in turn resulted in the lowest fluorescence intensity for the 20 wt% BiVO4@SiO2 composites, indicating its superior photoinduced charge-separation efficiency and excellent photocatalytic performance potential, compared to its counterparts.
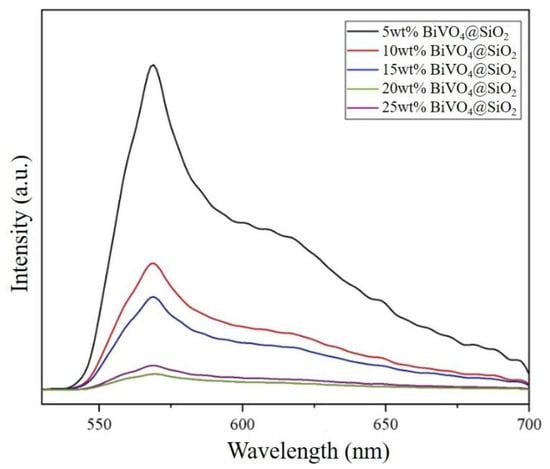
Figure 7.
Fluorescence spectra for BiVO4@SiO2 composites with different BiVO4 loading amounts.
2.3. Photocatalytic Performance in Tetracycline Degradation
Due to their excellent optical properties, these calcinated composites were employed for photocatalytic degradation of tetracycline. Figure 8 displays the efficiency in tetracycline degradation achieved by these photocatalysts. As can be seen, the degradation efficiency for all the catalysts exhibited a pattern of decreasing with degradation time, finally reaching a plateau at about 3 h. After 3 h of irradiation, the degradation efficiency was calculated to be 62.7% for 5 wt% BiVO4@SiO2, 67.3% for 10 wt% BiVO4@SiO2, 74% for 15 wt% BiVO4@SiO2, 84.1% for 20 wt% BiVO4@SiO2, and 79.8% for 25 wt% BiVO4@SiO2, respectively. As a result, the photocatalytic degradation efficiency of these composites increased gradually with increases in the BiVO4 loading percentage from 5 wt% to 10 wt%, 15 wt%, and 20 wt%, with a maximum value of 84.1% being obtained. A further increase in the BiVO4 loading percentage, to 25 wt%, led to a decrease in the corresponding degradation efficiency, to 79.8%. This revealed that the 20 wt% BiVO4@SiO2 was the best candidate of this series of composites for photocatalytic tetracycline degradation. This can be explained as follows: by increasing the super small-sized BiVO4 nanoparticles loading on the SiO2, the specific surface area was thereby reduced, resulting in a decrease in degradation efficiency. This result can also be illuminated by the superior light-absorption property and photoinduced charge-separation efficiency exhibited by the 20 wt% BiVO4@SiO2 composites, compared to all other composites, as confirmed by the UV-vis absorption and fluorescence spectra detailed above. In addition, these small-sized BiVO4@SiO2 composites exhibited superior photocatalytic performance compared to normal-sized BiVO4 particles (about 500 nm), confirming that the improved charge-separation efficiency of small-sized particles was the result of their small size. Moreover, the degradation reaction kinetics of these synthesized composites were also studied, demonstrating the first-order kinetics of their photodegradation reactions, with their rate constants being obtained by calculation with the following first-order kinetic equation:
ln(C0/C) = kappt
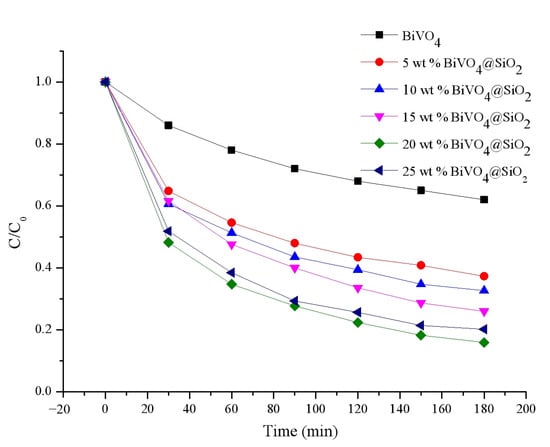
Figure 8.
Photocatalytic tetracycline degradation efficiency of BiVO4@SiO2 composites with different BiVO4 loading amounts.
The apparent rate constants for the pure BiVO4, 5 wt%, 10 wt%, 15 wt%, 20 wt%, and 25 wt% BiVO4@SiO2 composites were calculated to be 0.0025, 0.0049, 0.0056, 0.0071, 0.0094, and 0.0083 min−1, respectively. These results were in line with the photocatalytic efficiency detailed above, confirming the optimal photocatalytic kinetics of the 20 wt% BiVO4@SiO2 composites.
Further, the photocatalytic degradation performance of various bismuth-based catalysts toward TC was compared. The results are presented in Table 1, and show the promising degradation potential of these newly-fabricated small-sized BiVO4@SiO2 composites. As can be seen from Table 1, 20 wt% BiVO4@SiO2 exhibited excellent photocatalytic degradation of TC, with superior efficiency of 84.1% in 180 min, compared to pure bismuth vanadate without heterojunction or other decorated structures [22,23,24,25]. This demonstrates the effectiveness of the small-sized construction strategy in enhancing photocatalytic performance.

Table 1.
Comparison of TC degradation performance of different BiVO4-based catalysts.
As shown in Scheme 1, in the photocatalytic process, electrons (e−) and holes (h+) are generated under light irradiation and migrate to the surface of the photocatalyst, inducing redox reactions and forming reactive oxygen species such as hydrogen peroxide (H2O2), hydroxyl radical (·OH) etc. From the perspective of potential analysis, it can be seen that the EVB and ECB values for the 20 wt% BiVO4@SiO2 composites are 2.76 eV and 0.32 eV, and that holes in the valence band directly drive the oxidation-mediated generation of ·OH radicals (), while electrons in the BiVO4 conduction band efficiently reduce O2 to produce H2O2 (). As has been reported by other researchers [22,23,24,25,27,30], the photocatalytic degradation of tetracycline is primarily driven by reactive oxygen species generated from photoexcited charge carriers, as follows:
BiVO4 + hv → BiVO4 (e− + h+)
e− (BiVO4) +O2 → H2O2
h+ +H2O →·OH
(h+, H2O2, ·OH) + tetracycline → degradation products
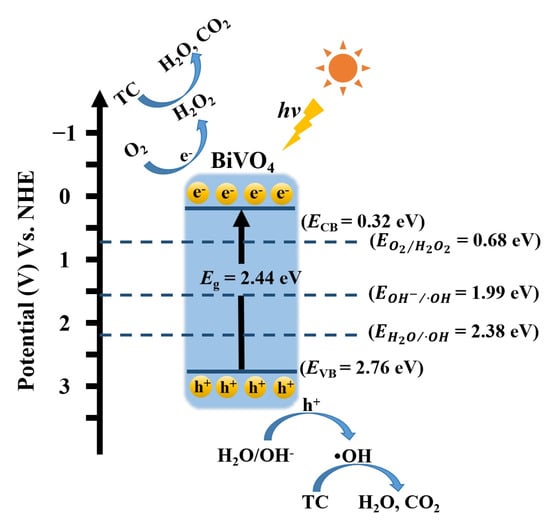
Scheme 1.
The mechanism of small-sized BiVO4 photocatalytic degradation towards tetracycline.
The process typically begins with initial attacks on vulnerable functional groups, such as N-demethylation at the C4 position or hydroxylation of the aromatic ring. These reactions lead to ring-opening and to formation of various smaller intermediate products [31]. The preserved electrons and holes of the 20 wt% BiVO4@SiO2 composite, with their strong redox capability, facilitate the generation of ·OH radicals and H2O2, thereby enabling the highly efficient degradation of tetracycline.
3. Materials and Experimental Methods
3.1. Materials
Bismuth (III) nitrate pentahydrate (Bi(NO3)3·5H2O, 98%) was purchased from Alfa Aesar (Haverhill, MA, USA). Ammonium vanadate (NH4VO3, 99.0%), silicon dioxide (SiO2, 99%), cyclohexane (C6H12, 99.7%), and sodium oleate (C18H33NaO2, 98%) were purchased from Macklin (Shanghai, China). Alcohol (C2H5OH, 99.9%) was purchased from XIHUA (Shantou, China). All chemicals were analytical-grade and were used without further purification.
Deionized water was obtained from a Persee (Beijing, China) water purification system.
3.2. Preparation of BiVO4 Photocatalysts
The BiVO4 photocatalysts were prepared by a hydrothermal synthetic method using deionized water as solvent, Bi(NO3)3·5H2O as a Bi source, and NH4VO3 as a V source. Sodium oleate (surfactants) was used as the dispersant to prepare BiVO4 nanoparticles. The BiVO4 photocatalysts with different loadings were prepared with SiO2 (commercial silica) as the base.
Firstly, 1.6 mmol sodium oleate was added to 40 mL deionized water and stirred for 30 min to form a uniform solution. Then, 0.8 mmol Bi(NO3)3·5H2O was added to this solution. Meanwhile, 0.8 mmol of NH4VO3 was dissolved in 40 mL deionized water; this was then added to the above solution and stirred for 30 min. The resulting solution was transferred to a 100 mL Teflon-lined stainless-steel autoclave and heated at 100 °C for 10 h. After cooling down to room temperature, the BiVO4 product was obtained by filtration, then washed several times with cyclohexane and alcohol, and dried at 60 °C.
To obtain different loading composites, the as-prepared BiVO4 and SiO2 were added to 60 mL amounts of cyclohexane with different mass ratios (1:20, 1:10, 1:6.6, 1:5, 1:4), then sonicated for 30 min and stirred vigorously for 2 h. The mixture was then transferred to an oil bath at 80 °C and stirred to vaporize cyclohexane. The obtained solids were calcined at certain temperature (400 °C, 450 °C, 500 °C, 550 °C, 600 °C) for 1 h to obtain (5 wt%, 10 wt%, 15 wt%, 20 wt%, 25 wt%) BiVO4@SiO2 composites.
3.3. Characterization
The UV–visible absorption spectra of the samples were recorded using UV–Vis diffuse reflectance spectroscopy (UV-vis DRS, UV-2501PC, Shimadzu, Kyoto, Japan) in the spectral range of 400–700 nm. The crystal structure and composition of the as-prepared samples were characterized by X-ray diffraction (XRD, Rigaku, D/max2500, Akishima, Japan), and the patterns were recorded over the angular range 10–70°, using a scan rate of 8°/min, Cu-Kα radiation and a Ni filter, and working voltage and current of 40 kV and 20 mA, respectively. The surface morphology of each sample was obtained using scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan). Analysis of photoluminescence spectra (PL, Renishaw in Via, 325 nm He-Gd) was carried out using the 380 nm line of a Xe lamp as an excitation source at room temperature.
3.4. Photocatalytic Activity Measurements
A 0.2 g amount of calcined samples and 200 mL of 10 mg/L tetracycline solution were placed into in a glass reactor, then stirred uniformly and sonicated for 10 min. The reactor was placed in the dark for 1 h to reach the adsorption–desorption equilibrium of tetracycline on the catalysts. After that, the reactor was irradiated for 3 h under a 350 W xenon lamp (Shenzhen Stone-lighting Opto Device Co., Ltd., Shenzhen, China) with the UV part of the light removed by a cut-off filter (λ > 420 nm). During the irradiation, 2 mL of the solution was drawn every 30 min. The drawn solution was filtered using a 0.22 μm needle filter, and concentrations of tetracycline were measured by high-performance liquid chromatography (LC2000, Shanghai Techcomp Instrument Co., Ltd., Shanghai, China) to evaluate the photocatalytic activity of BiVO4@SiO2 composites.
4. Conclusions
This study successfully fabricated a series of super-small-sized BiVO4@SiO2 composites with different BiVO4 loadings by a surfactant-assisted hydrothermal–calcination method for efficient photocatalytic degradation of tetracycline. XRD and SEM results revealed that these composites exhibited good crystallinity along with well-dispersed morphologies of small-sized particles. Their optical properties were revealed by UV-vis absorption and fluorescence spectroscopy, indicating their potential use as a high-performance photocatalyst. In particular, the 20 wt% BiVO4@SiO2 composite exhibited photocatalytic tetracycline degradation performance which was superior to that of other BiVO4@SiO2 composites and of pure BiVO4 obtained by a SiO2-matrix-free synthesis method, due to its uniformly dispersed morphology of small-sized BiVO4 particles, wide light-absorption range, and high charge-separation efficiency. These findings lay a solid foundation for the design and fabrication of small-sized particle composites with efficient photocatalytic performance.
Author Contributions
L.X. and Z.Z. conducted all the experiments and wrote the full text, while L.J. was responsible for the overall experimental design, and Y.Z. handled the manuscript review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the GuangDong Basic and Applied Basic Research Foundation (grant number 2023A1515010721) and by Science and Technology Projects in Guangzhou (grant number 2023A04J0061).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Guangdong University of Technology and the public service platform of Rongjiang Laboratory are gratefully acknowledged.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Xie, Z.J.; Feng, Y.P.; Wang, F.L.; Chen, D.N.; Zhang, Q.X.; Zeng, Y.Q.; Lv, W.Y.; Liu, G.G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chen, Q.W.; Zhang, J.Y.; Dong, J.W.; Yan, H.L.; Chen, C.; Feng, R.R. Characterization and source identification of tetracycline antibiotics in the drinking water sources of the lower Yangtze River. J. Environ. Manag. 2019, 244, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Couch, M.; Agga, G.E.; Kasumba, J.; Parekh, R.R.; Loughrin, J.H.; Conte, E.D. Abundances of Tetracycline Resistance Genes and Tetracycline Antibiotics during Anaerobic Digestion of Swine Waste. J. Environ. Qual. 2019, 48, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.H.; Stemig, A.M.; Wammer, K.H.; Strathmann, T.J. Oxidation of Antibiotics during Water Treatment with Potassium Permanganate: Reaction Pathways and Deactivation. Environ. Sci. Technol. 2011, 45, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Ochoa, J.; Amrane, A. Biodegradation and biosorption of tetracycline and tylosin antibiotics in activated sludge system. Process Biochem. 2009, 44, 1302–1306. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Y.; Fei, L.; Cheng, X.; Zhu, X.; Deng, L.; Ma, X. Z-scheme CuO/Fe3O4/GO heterojunction photocatalyst: Enhanced photocatalytic performance for elimination of tetracycline. Chemosphere 2022, 309, 136721. [Google Scholar] [CrossRef]
- Li, Y.H.; Cao, H.; Liu, W.L.; Liu, P.T. Effective degradation of tetracycline via recyclable cellulose nanofibrils/polyvinyl alcohol/Fe3O4 hybrid hydrogel as a photo-Fenton catalyst. Chemosphere 2022, 307, 135665. [Google Scholar] [CrossRef]
- Xiao, C.B.; Yuan, J.L.; Li, L.Y.; Zhong, N.B.; Zhong, D.J.; Xie, Q.H.; Chang, H.X.; Xu, Y.L.; He, X.F.; Li, M. Photocatalytic synergistic biofilms enhance tetracycline degradation and conversion. Environ. Sci. Ecotechnol. 2023, 14, 100234. [Google Scholar] [CrossRef]
- Ben, Y.J.; Fu, C.X.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C.M. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Nguyen, T.K.T.; Chen, W.H.; Chen, C.W.; Bui, X.T.; Patel, A.K.; Dong, C.D. Hydrothermal and pyrolytic conversion of sunflower seed husk into novel porous biochar for efficient adsorption of tetracycline. Bioresour. Technol. 2023, 373, 128711. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qin, C.; Zhong, J.; Li, J.; Huang, S. In-situ fabrication of Bi0/BiVO4 photocatalysts with boosted photocatalytic activity. Mater. Lett. 2022, 306, 130802. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Li, M.K.; Zhu, N.L.; Cheng, Y.; Lam, S.S.; Chen, J.; Gao, Y.X.; Zhao, J.T. Bi-based visible light-driven nano-photocatalyst: The design, synthesis, and its application in pollutant governance and energy development. Nano-Day 2022, 43, 101432. [Google Scholar] [CrossRef]
- Dong, M.; Yang, S.; Zhou, P.; Gao, Y.; Wang, A.; Tu, S. Construction of MWCNT-Bridged ternary S-scheme BiVO4/NH2-UiO-66 (Zr) heterojunction for enhanced visible-light photocatalytic degradation of tetracycline. Mater. Sci. Semicond. Process. 2026, 202, 110188. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar]
- Xiao, L.; Wang, Y. Research progress on monoclinic crystalline bismuth vanadate photocatalytic material. Mod. Chem. Ind. 2023, 43, 41–44+49. [Google Scholar]
- Tong, Y.B.; Zhao, S.X.; Kang, J.; Shen, J.M.; Chen, Z.L.; Wang, B.Y.; Bi, L.B.; Deng, J.J. Preparation of small-sized BiVO4 particles with improved photocatalytic performance and its photocatalytic degradation of doxycycline in water. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 620, 126412. [Google Scholar] [CrossRef]
- Bahari, M.B.; Jalil, A.A.; Mamat, C.R.; Hassan, N.S.; Setiabudi, H.D.; Vo, D.-V.N. Insight into the development of silica-based materials as photocatalysts for CO2 photoconversion towards CH3OH: A review and recent progress. Surf. Interfaces 2022, 31, 102049. [Google Scholar] [CrossRef]
- Eddy, D.R.; Puri, F.N.; Noviyanti, A.R. Synthesis and photocatalytic activity of silica-based sand quartz as the supporting TiO2 photocatalyst. Procedia Chem. 2015, 17, 55–58. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; Simoes, M.B.; Perissinotto, A.P.; de Vicente, F.S.; Noeske, P.L.M.; Ribeirob, S.J.L.; Ro-drigues, U.P. Solvent-controlled deposition of titania on silica spheres for the preparation of SiO2@TiO2 core@shell nanoparticles with enhanced photocatalytic activity. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 570, 293–305. [Google Scholar] [CrossRef]
- Nethercot, A.H., Jr. Prediction of Fermi Energies and Photoelectric Thresholds Based on Electronegativity Concepts. Phys. Rev. Lett. 1974, 33, 1088–1091. [Google Scholar] [CrossRef]
- Piccirillo, G.; Aroso, R.T.; Rodrigues, F.M.S.; Carrilho, R.M.B.; Pinto, S.M.A.; Calvete, M.J.F.; Pereira, M.M. Oxidative Degradation of Pharmaceuticals: The Role of Tetrapyrrole-Based Catalysts. Catalysts 2021, 11, 1335. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Dawi, E.A.; Sharma, G. Rare earth doped ZnO nanoparticles as spintronics and photocatalyst for degradation of pollutants. Molecules 2023, 28, 2838. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yu, T.; Zheng, C.; Lei, X.; Li, J.; Liu, X.; Guo, R. Bi nanoclusters construction and oxygen vacancy engineering for enhanced piezo-photocatalytic degradation performance of BiVO4. Chem. Eng. J. 2025, 522, 167714. [Google Scholar] [CrossRef]
- Cheng, C.; Tan, H.; Zhu, W.; Liu, L.; Chen, K. The transition of tetragonal to monoclinic phase in BiVO4 coupled with peroxymonosulfate for photocatalytic degradation of tetracycline hydrochloride. Environ. Res. 2025, 267, 120631. [Google Scholar] [CrossRef]
- Yang, G.; Lang, Y. Photodegradation of Antibiotic Drugs by BiVO4 Nanocomposites. Mater. Sci. (Medžiagotyra) 2023, 29, 507–514. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Xu, Y.; Lu, C.; Ma, H.; Guo, J.; Wang, L. Visible light-driven photocatalytic degradation of BiOBr/BiVO4 S-scheme: Performances, DFT calculations and mechanism. Mater. Res. Bull. 2026, 196, 113892. [Google Scholar] [CrossRef]
- Wang, J.; Mao, L.J.; Duan, Y.J.; Lei, K.; Zeng, X.H.; Sun, Y. Efficient photocatalytic degradation of tetracycline hydrochloride by Cu2O/BiVO4 p-n heterostructure. Dig. J. Nanomater. Biostructures 2024, 19, 539–548. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhang, H.; Zhang, J.; Li, Z.; Wang, X.; Xu, J.; Cao, D.; Li, J.; Lu, C. Photothermal-assisted photocatalytic degradation of tetracycline in simulated natural water by BiVO4/CuBi2O4 Z-scheme heterojunction: Mechanisms insight, degradation pathways and toxicity assessment. Process Saf. Environ. Prot. 2024, 188, 1292–1305. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci Eur 2021, 33, 64. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.