Catalytic Innovations for High-Yield Biohydrogen Production in Integrated Dark Fermentation and Microbial Electrolysis Systems
Abstract
1. Introduction
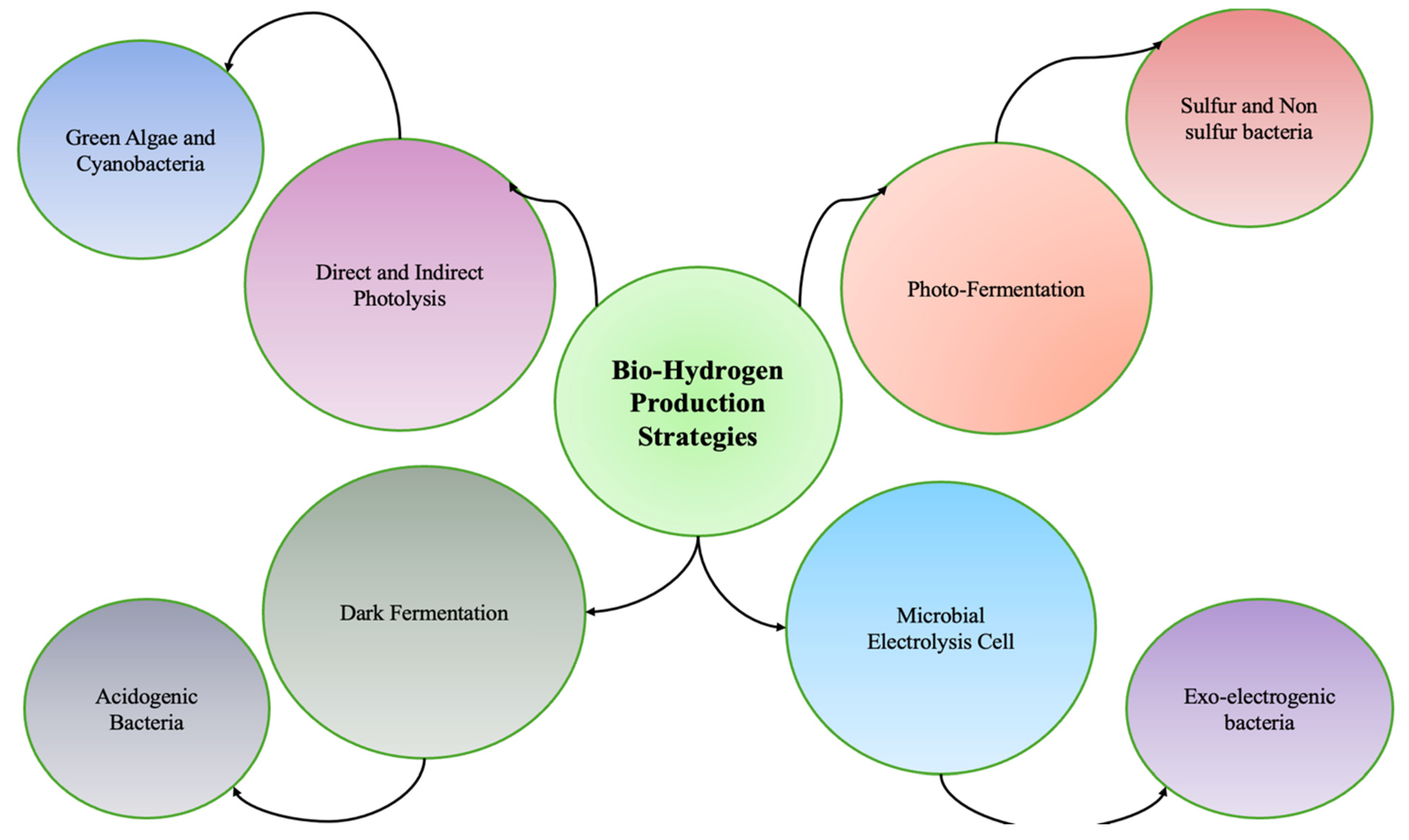
2. Strategies for Producing Biohydrogen
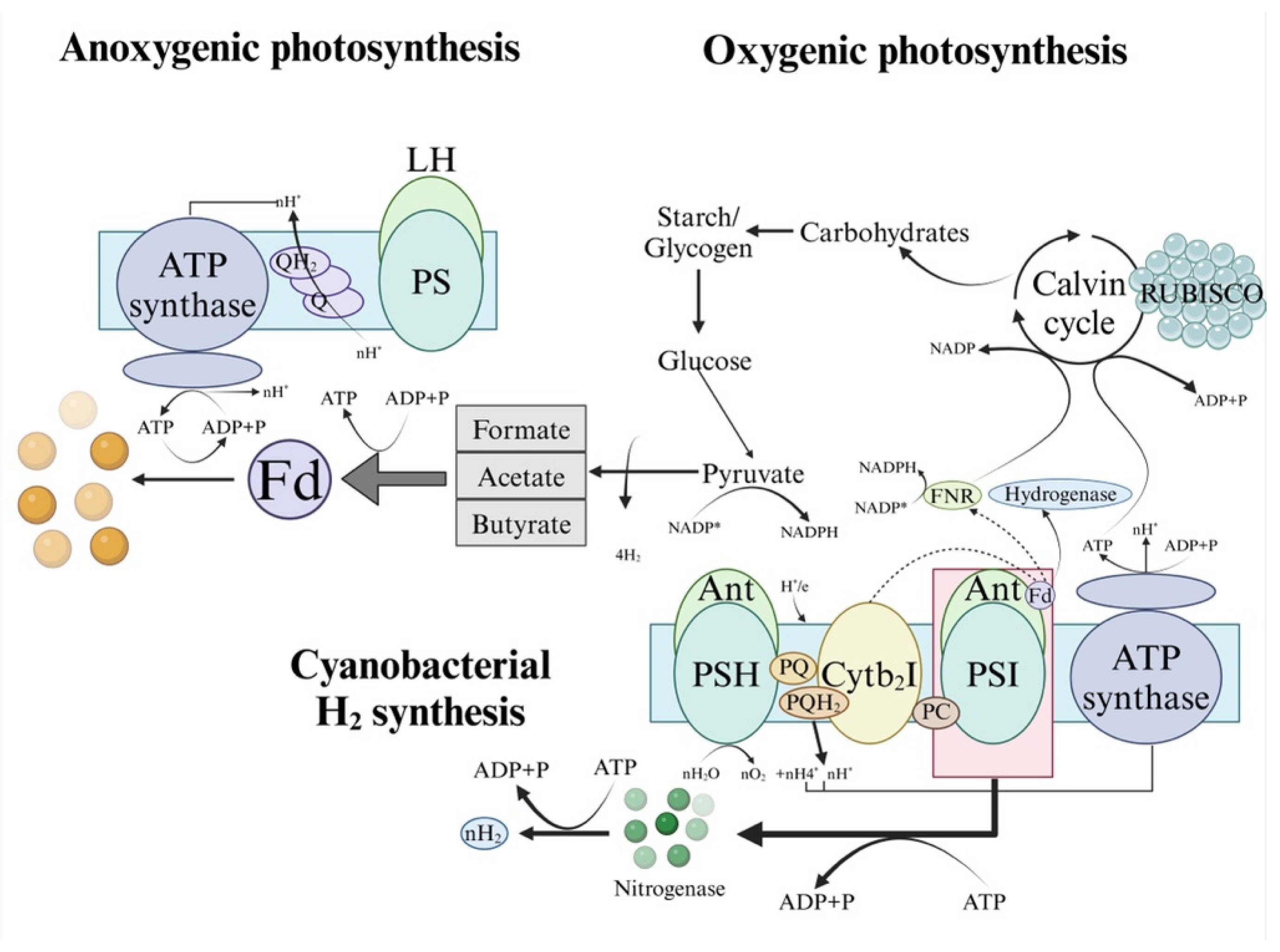
2.1. Direct Photolysis
2.2. Indirect Photolysis
2.3. Photo-Fermentation
2.4. Dark Fermentation
2.5. Microbial Electrolysis Cell (MEC)
3. Catalysts in Dark Fermentation: Enzymatic and Biocatalytic Systems
3.1. Types of Enzymatic Catalysts in Dark Fermentation
3.2. Hydrogenases: The Core of Hydrogen Production
3.3. [Fe-Fe]-Hydrogenases
3.4. [Ni-Fe]-Hydrogenases
3.5. [Fe]-Hydrogenases
3.6. Cellulases
3.7. Amylases
3.8. Proteases
3.9. Oxidorductases: Redox Balance and Electron Transfer
3.10. Pyruvate:Ferredoxin Oxidoreductase
3.11. NADH Ferredoxin Oxidoreductase
3.12. Synthesis and Preparation of Enzymatic Catalysts
3.12.1. Microbial Synthesis of Enzymatic Catalysts
3.12.2. Temperature
3.12.3. Substrate Composition
3.12.4. Nutrient Availability
3.12.5. Genetic Engineering for Enhanced Enzyme Production
3.12.6. Pretreatment of Inocula
3.12.7. Catalytic Activity and Kinetics
3.12.8. Catalytic Mechanisms in Dark Fermentation
4. DF–MEC Combination
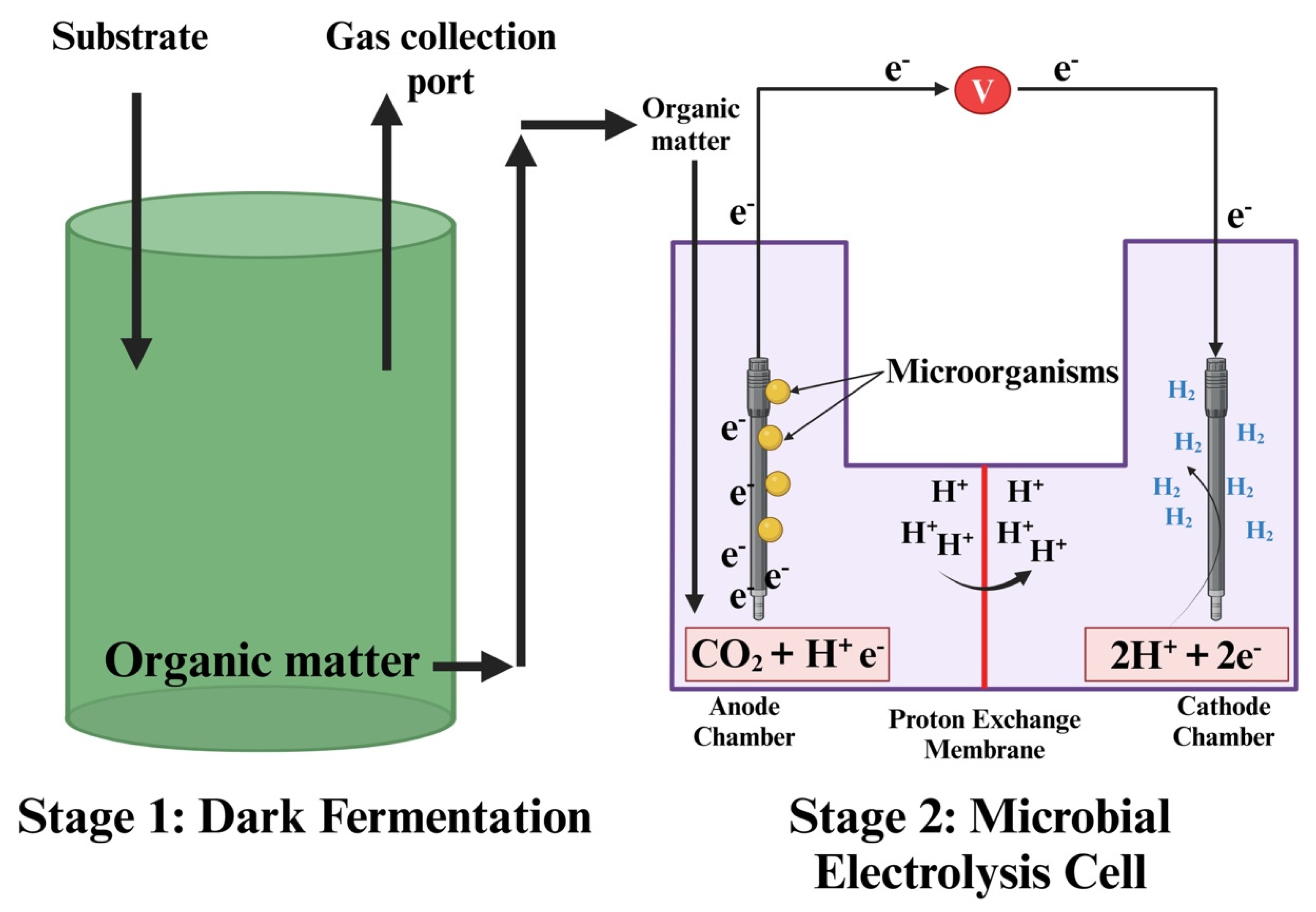
4.1. Stage I: Dark Fermentation Description
4.2. Stage II: Microbial Electrolysis Cell (MEC)
| S.No | Inoculum Used | Anode | Cathode | H2 Production Rate | Reference |
|---|---|---|---|---|---|
| 1 | Waste water MEC effluent | Graphite felt | Ni-containing electrode | 0.54 L H2/L−d | [79] |
| 2 | Wastewater | Graphite brush | Carbon cloth with Pt content | 1.7 L H2/L−d | [90] |
| 3 | Anaerobic Sludge | Graphite felt | Ni-containing electrode | 1.46 L H2/L−d | [91] |
| 4 | Municipal Wastewater | Graphite cloth | Carbon paper with Pt content | 0.07 L H2/L−d | [92] |
| 5 | Anaerobic Sludge | Graphite felt | SS Mesh | 0.05 L H2/L−d | [93] |
4.3. DF–MEC System Coupled for H2 Production
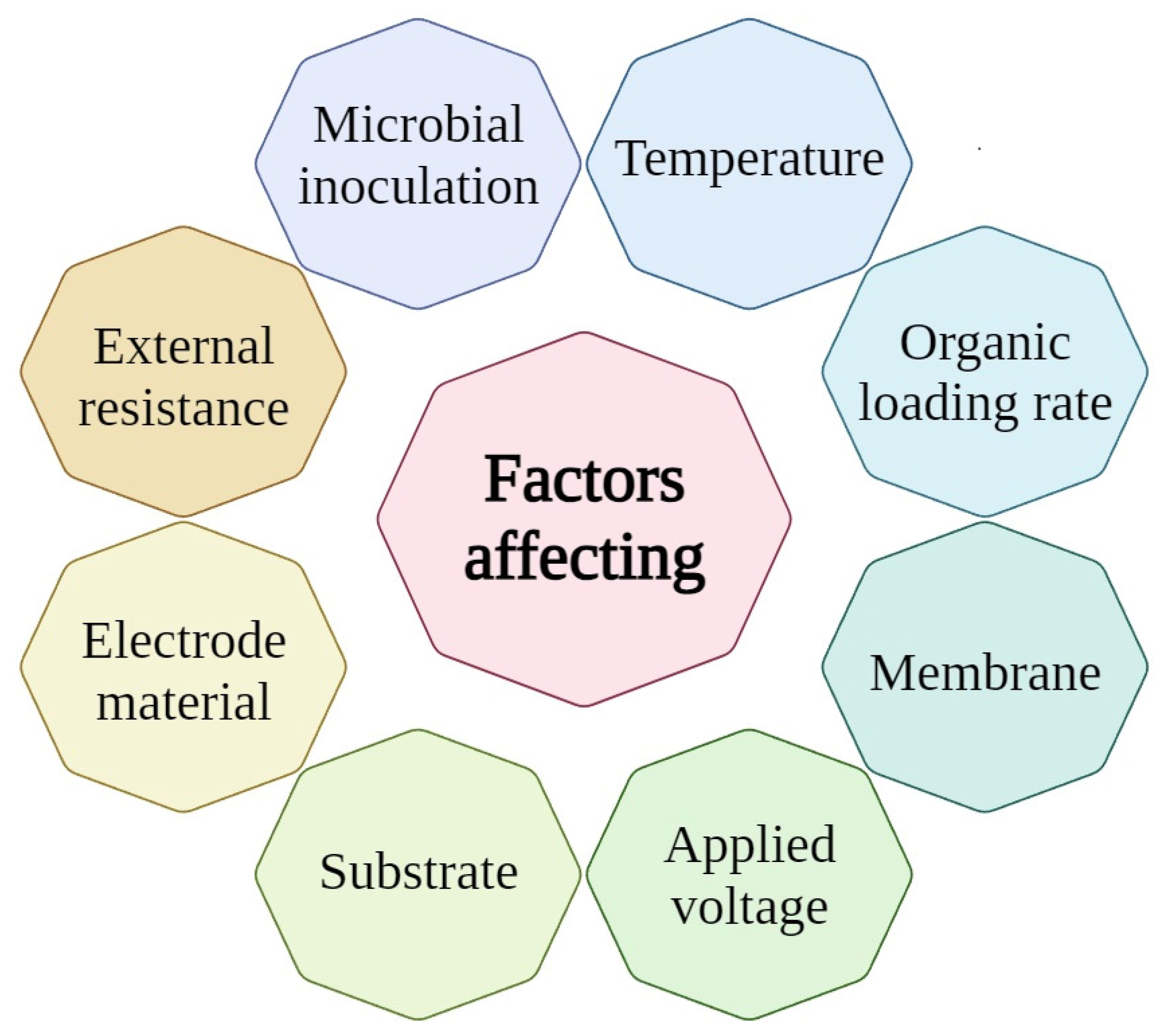
5. Factors Affecting Coupled DF–MEC System Performance
5.1. Substrates
5.2. Increased Potential at the Electrodes
5.3. pH
5.4. Electrode Difficulties
5.5. Unwanted Electron Sinks
6. Current Major Research in the Field of Coupled DF–MEC
7. Challenges of MEC–DF Integration
8. Techno-Economic Analysis of DF–MEC
8.1. TEA–LCA
8.2. Cost Analysis
9. Circular Economy
10. Integrating Dark Fermentation with Other Processes
10.1. Anaerobic Digestion
10.2. Photo-Fermentation
10.3. Microbial Fuel Cell
11. Future Perspective
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Meers, Erik and Roussel, Jimmy and Browne, Jack and Kirchmeyr, Franz and Volta, Marialuisa and Esteves, Sandra and Decorte, Mieke. Available online: https://www.researchgate.net/publication/388677876_BIP_Task-Force-52_RI-Digestate-Valorisation_Sept2024 (accessed on 21 May 2025).
- Energies|An Open Access Journal from MDPI. Available online: https://www.mdpi.com/journal/energies (accessed on 21 May 2025).
- Tapia-Venegas, E.; Ramirez-Morales, J.E.; Silva-Illanes, F.; Toledo-Alarcón, J.; Paillet, F.; Escudie, R.; Lay, C.-H.; Chu, C.-Y.; Leu, H.-J.; Marone, A.; et al. Biohydrogen Production by Dark Fermentation: Scaling-up and Technologies Integration for a Sustainable System. Rev. Environ. Sci. Biotechnol. 2015, 14, 761–785. [Google Scholar] [CrossRef]
- Srivastava, P.; García-Quismondo, E.; Palma, J.; González-Fernández, C. Coupling Dark Fermentation and Microbial Electrolysis Cells for Higher Hydrogen Yield: Technological Competitiveness and Challenges. Int. J. Hydrogen Energy 2024, 52, 223–239. [Google Scholar] [CrossRef]
- Malićanin, M.V.; Švarc-Gajić, J.; Lević, S.M.; Rac, V.A.; Salević-Jelić, A.S.; Pešić, M.B.; Milinčić, D.D.; Pasarin, D.; Rakić, V.M. Valorization of Grape Seed Cake by Subcritical Water Extraction. Processes 2025, 13, 1597. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Integrated Approach to Aerodynamic Optimization of Darrieus Wind Turbine Based on the Taguchi Method and Computational Fluid Dynamics (CFD). Available online: https://www.mdpi.com/2076-3417/15/10/5739 (accessed on 21 May 2025).
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Bakonyi, P.; Buitrón, G.; Valdez-Vazquez, I.; Nemestóthy, N.; Bélafi-Bakó, K. A Novel Gas Separation Integrated Membrane Bioreactor to Evaluate the Impact of Self-Generated Biogas Recycling on Continuous Hydrogen Fermentation. Appl. Energy 2017, 190, 813–823. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Periyasamy, S.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K. Lignocellulose Biohydrogen: Practical Challenges and Recent Progress. Renew. Sustain. Energy Rev. 2015, 44, 728–737. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Saakes, M.; Buisman, C.J.N. Ni Foam Cathode Enables High Volumetric H2 Production in a Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2010, 35, 12716–12723. [Google Scholar] [CrossRef]
- Lee, H.-S.; Vermaas, W.F.J.; Rittmann, B.E. Biological Hydrogen Production: Prospects and Challenges. Trends Biotechnol. 2010, 28, 262–271. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, D.; Zhang, H.; Jing, Y.; Tahir, N.; Zhang, Y.; Zhang, Q. Comparative Study on Bio-Hydrogen Production from Corn Stover: Photo-Fermentation, Dark-Fermentation and Dark-Photo Co-Fermentation. Int. J. Hydrogen Energy 2020, 45, 3807–3814. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial Electrohydrogenesis Cell and Dark Fermentation Integrated System Enhances Biohydrogen Production from Lignocellulosic Agricultural Wastes: Substrate Pretreatment towards Optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Park, S.-G.; Rajesh, P.P.; Sim, Y.-U.; Jadhav, D.A.; Noori, M.T.; Kim, D.-H.; Al-Qaradawi, S.Y.; Yang, E.; Jang, J.-K.; Chae, K.-J. Addressing Scale-up Challenges and Enhancement in Performance of Hydrogen-Producing Microbial Electrolysis Cell through Electrode Modifications. Energy Rep. 2022, 8, 2726–2746. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in Biological Hydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Mahata, C.; Das, D. Current Status and Prospects of Biohydrogen Production Process. In Microbial Biotechnology for Renewable and Sustainable Energy; Saini, J.K., Sani, R.K., Eds.; Springer Nature: Singapore, 2022; pp. 99–133. ISBN 9789811638527. [Google Scholar]
- Abdul-Ganiyu, S.; Quansah, D.A.; Ramde, E.W.; Seidu, R.; Adaramola, M.S. Techno-Economic Analysis of Solar Photovoltaic (PV) and Solar Photovoltaic Thermal (PVT) Systems Using Exergy Analysis. Sustain. Energy Technol. Assess. 2021, 47, 101520. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative Hydrogen Production: Principles, Progress, and Prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen Production: Current Perspectives and the Way Forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Biohydrogen Produced via Dark Fermentation: A Review. Available online: https://www.mdpi.com/2674-0389/3/3/29 (accessed on 18 May 2025).
- Morra, S.; Valetti, F.; Gilardi, G. [FeFe]-Hydrogenases as Biocatalysts in Bio-Hydrogen Production. Rend. Fis. Acc. Lincei 2017, 28, 183–194. [Google Scholar] [CrossRef]
- He, S.H.; Teixeira, M.; LeGall, J.; Patil, D.S.; Moura, I.; Moura, J.J.; DerVartanian, D.V.; Huynh, B.H.; Peck, H.D. EPR Studies with 77Se-Enriched (NiFeSe) Hydrogenase of Desulfovibrio baculatus. Evidence for a Selenium Ligand to the Active Site Nickel. J. Biol. Chem. 1989, 264, 2678–2682. [Google Scholar] [CrossRef]
- Furdui, C.; Ragsdale, S.W. The Role of Pyruvate Ferredoxin Oxidoreductase in Pyruvate Synthesis during Autotrophic Growth by the Wood-Ljungdahl Pathway. J. Biol. Chem. 2000, 275, 28494–28499. [Google Scholar] [CrossRef]
- Petitdemange, H.; Cherrier, C.; Raval, R.; Gay, R. Regulation of the NADH and NADPH-Ferredoxin Oxidoreductases in Clostridia of the Butyric Group. Biochim Biophys Acta 1976, 421, 334–337. [Google Scholar] [CrossRef]
- Cáceres, J.C.; Michellys, N.G.; Greene, B.L. Nitric Oxide Inhibition of Glycyl Radical Enzymes and Their Acti-vases. J. Am. Chem. Soc. 2025, 147, 11777–11788. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Diffusion Network of CO in FeFe-Hydrogenase|The Journal of Chemical Physics|AIP Publishing. Available online: https://pubs.aip.org/aip/jcp/article/149/20/204108/197086/Diffusion-network-of-CO-in-FeFe-Hydrogenase (accessed on 18 May 2025).
- Mishra, P.; Pradhan, N. A Synergistic Association between Iron Reduction and Enhanced Hydrogen Production in Clostridium pasteurianum. Biochem. Eng. J. 2024, 203, 109216. [Google Scholar] [CrossRef]
- Scienze Fisiche e Naturali. [FeFe]-Hydrogenases as Biocatalysts in Bio-Hydrogen Production|Rendiconti Lincei. Available online: https://link.springer.com/article/10.1007/s12210-016-0584-9 (accessed on 18 May 2025).
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Cellulose Degradation: A Therapeutic Strategy in the Improved Treatment of Acanthamoeba Infections. Parasit. Vectors 2015, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Kuligowski, K.; Świerczek, L.; Cenian, A. Review of Lignocellulosic Biomass Pretreatment Using Physical, Thermal and Chemical Methods for Higher Yields in Bioethanol Production. Sustainability 2025, 17, 287. [Google Scholar] [CrossRef]
- Amylase—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/materials-science/amylase (accessed on 18 May 2025).
- Dark Hydrogen Fermentation from Hydrolyzed Starch Treated with Recombinant Amylase Originating from Caldimonas Taiwanensis On1|Request PDF. Available online: https://www.researchgate.net/publication/5919748_Dark_Hydrogen_Fermentation_from_Hydrolyzed_Starch_Treated_with_Recombinant_Amylase_Originating_from_Caldimonas_taiwanensis_On1 (accessed on 18 May 2025).
- (PDF) Pyruvate:Ferredoxin Oxidoreductase Is Coupled to Light-Independent Hydrogen Production in Chlamydomonas Reinhardtii. Available online: https://www.researchgate.net/publication/233964670_PyruvateFerredoxin_Oxidoreductase_Is_Coupled_to_Light-independent_Hydrogen_Production_in_Chlamydomonas_reinhardtii (accessed on 18 May 2025).
- Recent Trends in Lignocellulosic Biofuels and Bioenergy: Advancements and Sustainability Assessment|SpringerLink. Available online: https://link.springer.com/book/10.1007/978-981-96-4636-4 (accessed on 18 May 2025).
- Hess, V.; Schuchmann, K.; Müller, V. The Ferredoxin:NAD+ Oxidoreductase (Rnf) from the Acetogen Acetobacterium Woodii Requires Na+ and Is Reversibly Coupled to the Membrane Potential. J. Biol. Chem. 2013, 288, 31496–31502. [Google Scholar] [CrossRef]
- Maeda, T.; Sanchez-Torres, V.; Wood, T.K. Metabolic Engineering to Enhance Bacterial Hydrogen Production. Microb. Biotechnol. 2008, 1, 30–39. [Google Scholar] [CrossRef]
- Xuan, J.; He, L.; Wen, W.; Feng, Y. Hydrogenase and Nitrogenase: Key Catalysts in Biohydrogen Production. Molecules 2023, 28, 1392. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Bogaert, G.V.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical Systems (BES) for Sustainable Energy Production and Product Recovery from Organic Wastes and Industrial Wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Baba, R.; Morita, M.; Asakawa, S.; Watanabe, T. Transcription of [FeFe]-Hydrogenase Genes during H2 Production in Clostridium and Desulfovibrio spp. Isolated from a Paddy Field Soil. Microbes Environ. 2017, 32, 125–132. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; da Cruz Tarelho, L.A.; Capela, M.I. Biohythane Production via Anaerobic Digestion Process: Fundamentals, Scale-up Challenges, and Techno-Economic and Environmental Aspects. Environ. Sci. Pollut. Res. 2024, 31, 49935–49984. [Google Scholar] [CrossRef]
- Mesophilic Bacterium—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/mesophilic-bacterium (accessed on 18 May 2025).
- de Medeiros, T.D.M.; Dufossé, L.; Bicas, J.L. Lignocellulosic Substrates as Starting Materials for the Production of Bioactive Biopigments. Food Chem. X 2022, 13, 100223. [Google Scholar] [CrossRef] [PubMed]
- Hydrogenase—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/hydrogenase (accessed on 18 May 2025).
- Homologous Overexpression of [FeFe] Hydrogenase in Enterobacter cloacae IIT-BT 08 to Enhance Hydrogen Gas Production from Cheese Whey|Request PDF. Available online: https://www.researchgate.net/publication/251581449_Homologous_overexpression_of_FeFe_hydrogenase_in_Enterobacter_cloacae_IIT-BT_08_to_enhance_hydrogen_gas_production_from_cheese_whey (accessed on 18 May 2025).
- Effects of Heat Shocks on Microbial Community Structure and Microbial Activity of a Methanogenic Enrichment Degrading Benzoate|Request PDF. Available online: https://www.researchgate.net/publication/305890123_Effects_of_heat_shocks_on_microbial_community_structure_and_microbial_activity_of_a_methanogenic_enrichment_degrading_benzoate (accessed on 18 May 2025).
- Acclimation of Acid-Tolerant Methanogenic Culture for Bioaugmentation: Strategy Comparison and Microbiome Succession|ACS Omega. Available online: https://pubs.acs.org/doi/10.1021/acsomega.9b03783 (accessed on 18 May 2025).
- Impacts of 2-Bromoethanesulfonic Sodium on Methanogenesis: Methanogen Metabolism and Community Structure|Request PDF. Available online: https://www.researchgate.net/publication/366533899_Impacts_of_2-bromoethanesulfonic_sodium_on_methanogenesis_Methanogen_metabolism_and_community_structure (accessed on 18 May 2025).
- A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Available online: https://www.mdpi.com/2073-4344/9/4/353 (accessed on 18 May 2025).
- Catalytic Turnover of [FeFe]-Hydrogenase Based on Single-Molecule Imaging|Request PDF. Available online: https://www.researchgate.net/publication/51642470_Catalytic_Turnover_of_FeFe-Hydrogenase_Based_on_Single-Molecule_Imaging (accessed on 18 May 2025).
- Environments|An Open Access Journal from MDPI. Available online: https://www.mdpi.com/journal/environments (accessed on 21 May 2025).
- Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis. Available online: https://www.mdpi.com/2071-1050/16/23/10755 (accessed on 18 May 2025).
- Dark Fermentation—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/dark-fermentation (accessed on 18 May 2025).
- Valorization of Algal Biomass to Biofuel: A Review. Available online: https://www.mdpi.com/2673-8783/5/2/26 (accessed on 21 May 2025).
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial Waste as a Resource for Volatile Fatty Acids Production via Anaerobic Fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef] [PubMed]
- Ergal, İ.; Fuchs, W.; Hasibar, B.; Thallinger, B.; Bochmann, G.; Rittmann, S.K.-M.R. The Physiology and Biotechnology of Dark Fermentative Biohydrogen Production. Biotechnol. Adv. 2018, 36, 2165–2186. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.-B.; Tan, G.-Y.A.; Wang, J.-Y. Caproate Formation in Mixed-Culture Fermentative Hydrogen Production. Bioresour. Technol. 2010, 101, 9550–9559. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Pérez-Bernal, M.F.; Bernet, N.; Trably, E. Sequential Dark Fermentation and Microbial Electrolysis Cells for Hydrogen Production: Volatile Fatty Acids Influence and Energy Considerations. Bioresour. Technol. 2023, 374, 128803. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Corrigendum to “Hydrogen Production from Biomass Using Dark Fermentation” [Renew Sustain Energy Rev 91 (2018) 665–94]. Renew. Sustain. Energy Rev. 2018, 95, 354. [Google Scholar] [CrossRef]
- Khongkliang, P.; Jehlee, A.; Kongjan, P.; Reungsang, A.; O-Thong, S. High Efficient Biohydrogen Production from Palm Oil Mill Effluent by Two-Stage Dark Fermentation and Microbial Electrolysis under Thermophilic Condition. Int. J. Hydrogen Energy 2019, 44, 31841–31852. [Google Scholar] [CrossRef]
- Dessì, P.; Porca, E.; Lakaniemi, A.-M.; Collins, G.; Lens, P.N.L. Temperature Control as Key Factor for Optimal Biohydrogen Production from Thermomechanical Pulping Wastewater. Biochem. Eng. J. 2018, 137, 214–221. [Google Scholar] [CrossRef]
- Bayard, R.; Liu, X.; Benbelkacem, H.; Buffiere, P.; Gourdon, R. Can Biomethane Potential (BMP) Be Predicted from Other Variables Such As Biochemical Composition in Lignocellulosic Biomass and Related Organic Residues? Bioenerg. Res. 2016, 9, 610–623. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark Fermentative Biohydrogen Production from Lignocellulosic Biomass: Technological Challenges and Future Prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A Review of Biological Delignification and Detoxification Methods for Lignocellulosic Bioethanol Production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Elefsiniotis, P.; Li, D. The Effect of Temperature and Carbon Source on Denitrification Using Volatile Fatty Acids. Biochem. Eng. J. 2006, 28, 148–155. [Google Scholar] [CrossRef]
- Grootscholten, T.I.M.; Steinbusch, K.J.J.; Hamelers, H.V.M.; Buisman, C.J.N. High Rate Heptanoate Production from Propionate and Ethanol Using Chain Elongation. Bioresour. Technol. 2013, 136, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-R.; Jeon, J.-M.; Yi, D.-H.; Song, H.-S.; Yang, S.-Y.; Choi, T.-R.; Bhatia, S.K.; Yoon, J.-J.; Kim, Y.-G.; Brigham, C.J.; et al. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate-Co-3-Hydroxyhexanoate) Terpolymer Production from Volatile Fatty Acids Using Engineered Ralstonia Eutropha. Int. J. Biol. Macromol. 2019, 138, 370–378. [Google Scholar] [CrossRef]
- Lavagnolo, M.C.; Girotto, F.; Rafieenia, R.; Danieli, L.; Alibardi, L. Two-Stage Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste—Effects of Process Conditions during Batch Tests. Renew. Energy 2018, 126, 14–20. [Google Scholar] [CrossRef]
- Ali, M.; Elreedy, A.; Ibrahim, M.G.; Fujii, M.; Tawfik, A. Hydrogen and Methane Bio-Production and Microbial Community Dynamics in a Multi-Phase Anaerobic Reactor Treating Saline Industrial Wastewater. Energy Convers. Manag. 2019, 186, 1–14. [Google Scholar] [CrossRef]
- Rafieenia, R.; Lavagnolo, M.C.; Pivato, A. Pre-Treatment Technologies for Dark Fermentative Hydrogen Production: Current Advances and Future Directions. Waste Manag. 2018, 71, 734–748. [Google Scholar] [CrossRef]
- Ta, D.T.; Lin, C.-Y.; Ta, T.M.N.; Chu, C.-Y. Biohythane Production via Single-Stage Anaerobic Fermentation Using Entrapped Hydrogenic and Methanogenic Bacteria. Bioresour. Technol. 2020, 300, 122702. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. ‘Renewable’ Hydrogen: Prospects and Challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Kossalbayev, B.D.; Zayadan, B.K.; Tomo, T.; Veziroglu, T.N.; Allakhverdiev, S.I. Hydrogen Production from Phototrophic Microorganisms: Reality and Perspectives. Int. J. Hydrogen Energy 2019, 44, 5799–5811. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Lee, D.-J.; Show, K.-Y.; Su, A. Dark Fermentation on Biohydrogen Production: Pure Culture. Bioresour. Technol. 2011, 102, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, T.; Liu, H. Microbial Diversity of a Mesophilic Hydrogen-Producing Sludge. Appl. Microbiol. Biotechnol. 2002, 58, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Liang, D.-W.; Bai, Y.-X.; Fan, Y.-T.; Hou, H.-W. Enhanced H2 Production from Corn Stalk by Integrating Dark Fermentation and Single Chamber Microbial Electrolysis Cells with Double Anode Arrangement. Int. J. Hydrogen Energy 2014, 39, 8977–8982. [Google Scholar] [CrossRef]
- Moreno, R.; Escapa, A.; Cara, J.; Carracedo, B.; Gómez, X. A Two-Stage Process for Hydrogen Production from Cheese Whey: Integration of Dark Fermentation and Biocatalyzed Electrolysis. Int. J. Hydrogen Energy 2015, 40, 168–175. [Google Scholar] [CrossRef]
- Tommasi, T.; Ruggeri, B.; Sanfilippo, S. Energy Valorisation of Residues of Dark Anaerobic Production of Hydrogen. J. Clean. Prod. 2012, 34, 91–97. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Qian, Y.; Angelidaki, I.; Zhang, Y. The Impact of Anode Acclimation Strategy on Microbial Electrolysis Cell Treating Hydrogen Fermentation Effluent. Bioresour. Technol. 2017, 236, 37–43. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Hafez, H.; Lee, H.-S. Hydrogen Production from Sugar Beet Juice Using an Integrated Biohydrogen Process of Dark Fermentation and Microbial Electrolysis Cell. Bioresour. Technol. 2015, 198, 223–230. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Guo, K.; Prévoteau, A.; Rabaey, K. A Novel Tubular Microbial Electrolysis Cell for High Rate Hydrogen Production. J. Power Sources 2017, 356, 484–490. [Google Scholar] [CrossRef]
- Badia-Fabregat, M.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen Production from Crude Glycerol in an Alkaline Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2019, 44, 17204–17213. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Kalil, M.S.; Abdeshahian, P.; Hamid, A.A. A Review of the Substrates Used in Microbial Electrolysis Cells (MECs) for Producing Sustainable and Clean Hydrogen Gas. Renew. Energy 2014, 71, 466–472. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.J. Microbial Electrolysis Cells for Waste Biorefinery: A State of the Art Review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Fan, Y.; Hou, H. Improved Bio-Hydrogen Production from Glucose by Adding a Specific Methane Inhibitor to Microbial Electrolysis Cells with a Double Anode Arrangement. J. Biosci. Bioeng. 2016, 122, 488–493. [Google Scholar] [CrossRef]
- Chabert, N.; Amin Ali, O.; Achouak, W. All Ecosystems Potentially Host Electrogenic Bacteria. Bioelectrochemistry 2015, 106, 88–96. [Google Scholar] [CrossRef]
- Liu, W.; Huang, S.; Zhou, A.; Zhou, G.; Ren, N.; Wang, A.; Zhuang, G. Hydrogen Generation in Microbial Electrolysis Cell Feeding with Fermentation Liquid of Waste Activated Sludge. Int. J. Hydrogen Energy 2012, 37, 13859–13864. [Google Scholar] [CrossRef]
- Escapa, A.; Lobato, A.; García, D.M.; Morán, A. Hydrogen Production and COD Elimination Rate in a Continuous Microbial Electrolysis Cell: The Influence of Hydraulic Retention Time and Applied Voltage. Environ. Prog. Sustain. Energy 2013, 32, 263–268. [Google Scholar] [CrossRef]
- Rivera, I.; Buitrón, G.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K. Hydrogen Production in a Microbial Electrolysis Cell Fed with a Dark Fermentation Effluent. J. Appl. Electrochem. 2015, 45, 1223–1229. [Google Scholar] [CrossRef]
- Rivera, I.; Bakonyi, P.; Cuautle-Marín, M.A.; Buitrón, G. Evaluation of Various Cheese Whey Treatment Scenarios in Single-Chamber Microbial Electrolysis Cells for Improved Biohydrogen Production. Chemosphere 2017, 174, 253–259. [Google Scholar] [CrossRef]
- Bakonyi, P.; Kumar, G.; Koók, L.; Tóth, G.; Rózsenberszki, T.; Bélafi-Bakó, K.; Nemestóthy, N. Microbial Electrohydrogenesis Linked to Dark Fermentation as Integrated Application for Enhanced Biohydrogen Production: A Review on Process Characteristics, Experiences and Lessons. Bioresour. Technol. 2018, 251, 381–389. [Google Scholar] [CrossRef]
- Biofilm Inhibition Against Staphylococcus aureus and Alizarin Red Dye-Removing Capability of Plant-Based Green Synthesis of Lanthanum Oxide (La2O3NPs) Nanoparticles. Available online: https://www.mdpi.com/2624-781X/6/2/32 (accessed on 21 May 2025).
- Wrana, N.; Sparling, R.; Cicek, N.; Levin, D.B. Hydrogen Gas Production in a Microbial Electrolysis Cell by Electrohydrogenesis. J. Clean. Prod. 2010, 18, S105–S111. [Google Scholar] [CrossRef]
- Yang, E.; Omar Mohamed, H.; Park, S.-G.; Obaid, M.; Al-Qaradawi, S.Y.; Castaño, P.; Chon, K.; Chae, K.-J. A Review on Self-Sustainable Microbial Electrolysis Cells for Electro-Biohydrogen Production via Coupling with Carbon-Neutral Renewable Energy Technologies. Bioresour. Technol. 2021, 320, 124363. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Abbassi, R.; Yadav, A.K.; Garaniya, V.; Asadnia, M. A Review on the Contribution of Electron Flow in Electroactive Wetlands: Electricity Generation and Enhanced Wastewater Treatment. Chemosphere 2020, 254, 126926. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Abbassi, R.; Yadav, A.; Garaniya, V.; Asadnia, M.; Lewis, T.; Khan, S.J. Influence of Applied Potential on Treatment Performance and Clogging Behaviour of Hybrid Constructed Wetland-Microbial Electrochemical Technologies. Chemosphere 2021, 284, 131296. [Google Scholar] [CrossRef]
- An, J.; Li, N.; Wan, L.; Zhou, L.; Du, Q.; Li, T.; Wang, X. Electric Field Induced Salt Precipitation into Activated Carbon Air-Cathode Causes Power Decay in Microbial Fuel Cells. Water Res. 2017, 123, 369–377. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Popat, S.C.; Torres, C.I. Critical Transport Rates That Limit the Performance of Microbial Electrochemistry Technologies. Bioresour. Technol. 2016, 215, 265–273. [Google Scholar] [CrossRef]
- Srikanth, S.; Pant, D.; Dominguez-Benetton, X.; Genné, I.; Vanbroekhoven, K.; Vermeiren, P.; Alvarez-Gallego, Y. Gas Diffusion Electrodes Manufactured by Casting Evaluation as Air Cathodes for Microbial Fuel Cells (MFC). Materials 2016, 9, 601. [Google Scholar] [CrossRef]
- Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J.G.; Yan, Y. Correlating Hydrogen Oxidation and Evolution Activity on Platinum at Different pH with Measured Hydrogen Binding Energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent Advances and Emerging Challenges in Microbial Electrolysis Cells (MECs) for Microbial Production of Hydrogen and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless Steel Is a Promising Electrode Material for Anodes of Microbial Fuel Cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef]
- Satinover, S.J.; Schell, D.; Borole, A.P. Achieving High Hydrogen Productivities of 20 L/L-Day via Microbial Electrolysis of Corn Stover Fermentation Products. Appl. Energy 2020, 259, 114126. [Google Scholar] [CrossRef]
- Aulenta, F.; Catapano, L.; Snip, L.; Villano, M.; Majone, M. Linking Bacterial Metabolism to Graphite Cathodes: Electrochemical Insights into the H2-Producing Capability of Desulfovibrio sp. ChemSusChem 2012, 5, 1080–1085. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical Hydrogen Production from Urban Wastewater on a Pilot Scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Croese, E.; Jeremiasse, A.W.; Marshall, I.P.G.; Spormann, A.M.; Euverink, G.-J.W.; Geelhoed, J.S.; Stams, A.J.M.; Plugge, C.M. Influence of Setup and Carbon Source on the Bacterial Community of Biocathodes in Microbial Electrolysis Cells. Enzym. Microb. Technol. 2014, 61–62, 67–75. [Google Scholar] [CrossRef]
- Xie, X.; Criddle, C.; Cui, Y. Design and Fabrication of Bioelectrodes for Microbial Bioelectrochemical Systems. Energy Environ. Sci. 2015, 8, 3418–3441. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Chendake, A.D.; Schievano, A.; Pant, D. Suppressing Methanogens and Enriching Electrogens in Bioelectrochemical Systems. Bioresour. Technol. 2019, 277, 148–156. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhang, L.; Xu, T.; Ding, K. Influence of Initial Anolyte pH and Temperature on Hydrogen Production through Simultaneous Saccharification and Fermentation of Lignocellulose in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2017, 42, 22663–22670. [Google Scholar] [CrossRef]
- Optimizing Solar–Biomass Pyrolysis: Innovations in Reactor Design and Thermal–Solar System Efficiency. Available online: https://www.mdpi.com/1996-1073/18/10/2640 (accessed on 21 May 2025).
- Yang, G.; Yin, Y.; Wang, J. Microbial Community Diversity during Fermentative Hydrogen Production Inoculating Various Pretreated Cultures. Int. J. Hydrogen Energy 2019, 44, 13147–13156. [Google Scholar] [CrossRef]
- Favaro, L.; Alibardi, L.; Lavagnolo, M.C.; Casella, S.; Basaglia, M. Effects of Inoculum and Indigenous Microflora on Hydrogen Production from the Organic Fraction of Municipal Solid Waste. Int. J. Hydrogen Energy 2013, 38, 11774–11779. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Ren, N. Bioreactor Performance and Quantitative Analysis of Methanogenic and Bacterial Community Dynamics in Microbial Electrolysis Cells during Large Temperature Fluctuations. Environ. Sci. Technol. 2012, 46, 6874–6881. [Google Scholar] [CrossRef]
- Marone, A.; Ayala-Campos, O.R.; Trably, E.; Carmona-Martínez, A.A.; Moscoviz, R.; Latrille, E.; Steyer, J.-P.; Alcaraz-Gonzalez, V.; Bernet, N. Coupling Dark Fermentation and Microbial Electrolysis to Enhance Bio-Hydrogen Production from Agro-Industrial Wastewaters and by-Products in a Bio-Refinery Framework. Int. J. Hydrogen Energy 2017, 42, 1609–1621. [Google Scholar] [CrossRef]
- Das, D.; Khanna, N.; Dasgupta, C.N. Biohydrogen Production: Fundamentals and Technology Advances; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-08656-4. [Google Scholar]
- Sara, H.R.; Enrico, B.; Mauro, V.; Andrea, D.C.; Vincenzo, N. Techno-Economic Analysis of Hydrogen Production Using Biomass Gasification -A Small Scale Power Plant Study. Energy Procedia 2016, 101, 806–813. [Google Scholar] [CrossRef]
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Jahim, J.M. Biohydrogen Production through Photo Fermentation or Dark Fermentation Using Waste as a Substrate: Overview, Economics, and Future Prospects of Hydrogen Usage. Biofuels Bioprod. Biorefining 2013, 7, 334–352. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-Economic and Environmental Impact Assessment of Hydrogen Production Processes Using Bio-Waste as Renewable Energy Resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Lim, S.S.; Fontmorin, J.-M.; Ikhmal Salehmin, M.N.; Feng, Y.; Scott, K.; Yu, E.H. Enhancing Hydrogen Production through Anode Fed-Batch Mode and Controlled Cell Voltage in a Microbial Electrolysis Cell Fully Catalysed by Microorganisms. Chemosphere 2022, 288, 132548. [Google Scholar] [CrossRef]
- Li, Y.-C.; Liu, Y.-F.; Chu, C.-Y.; Chang, P.-L.; Hsu, C.-W.; Lin, P.-J.; Wu, S.-Y. Techno-Economic Evaluation of Biohydrogen Production from Wastewater and Agricultural Waste. Int. J. Hydrogen Energy 2012, 37, 15704–15710. [Google Scholar] [CrossRef]
- Segundo-Aguilar, A.; González-Gutiérrez, L.V.; Payá, V.C.; Feliu, J.; Buitrón, G.; Cercado, B. Energy and Economic Advantages of Simultaneous Hydrogen and Biogas Production in Microbial Electrolysis Cells as a Function of the Applied Voltage and Biomass Content. Sustain. Energy Fuels 2021, 5, 2003–2017. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Zheng, Y.; Ji, M.-K.; Li, X.; Salama, E.-S. Metabolic Pathways for Microalgal Biohydrogen Production: Current Progress and Future Prospectives. Bioresour. Technol. 2020, 318, 124253. [Google Scholar] [CrossRef]
- James, B.D.; Baum, G.N.; Perez, J.; Baum, K.N. Technoeconomic Boundary Analysis of Biological Pathways to Hydrogen Production; National Renewable Energy Lab (NREL): Golden, CO, USA, 2009. [Google Scholar]
- Show, K.-Y.; Yan, Y.-G.; Lee, D.-J. Chapter 13—Biohydrogen Production from Algae: Perspectives, Challenges, and Prospects. In Biofuels from Algae, 2nd. ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 325–343. ISBN 978-0-444-64192-2. [Google Scholar]
- Bundhoo, Z.M.A. Coupling Dark Fermentation with Biochemical or Bioelectrochemical Systems for Enhanced Bio-Energy Production: A Review. Int. J. Hydrogen Energy 2017, 42, 26667–26686. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.; Mohammadi, P.; Mirghorayshi, M.; Ibrahim, S.; Younesi, H.; Mohamed, A.R. An Anaerobic Hybrid Bioreactor of Granular and Immobilized Biomass for Anaerobic Digestion (AD) and Dark Fermentation (DF) of Palm Oil Mill Effluent: Mass Transfer Evaluation in Granular Sludge and Role of Internal Packing. Biomass Bioenergy 2017, 103, 1–10. [Google Scholar] [CrossRef]
- Magdalena, J.A.; González-Fernández, C. Microalgae Biomass as a Potential Feedstock for the Carboxylate Platform. Molecules 2019, 24, 4404. [Google Scholar] [CrossRef]

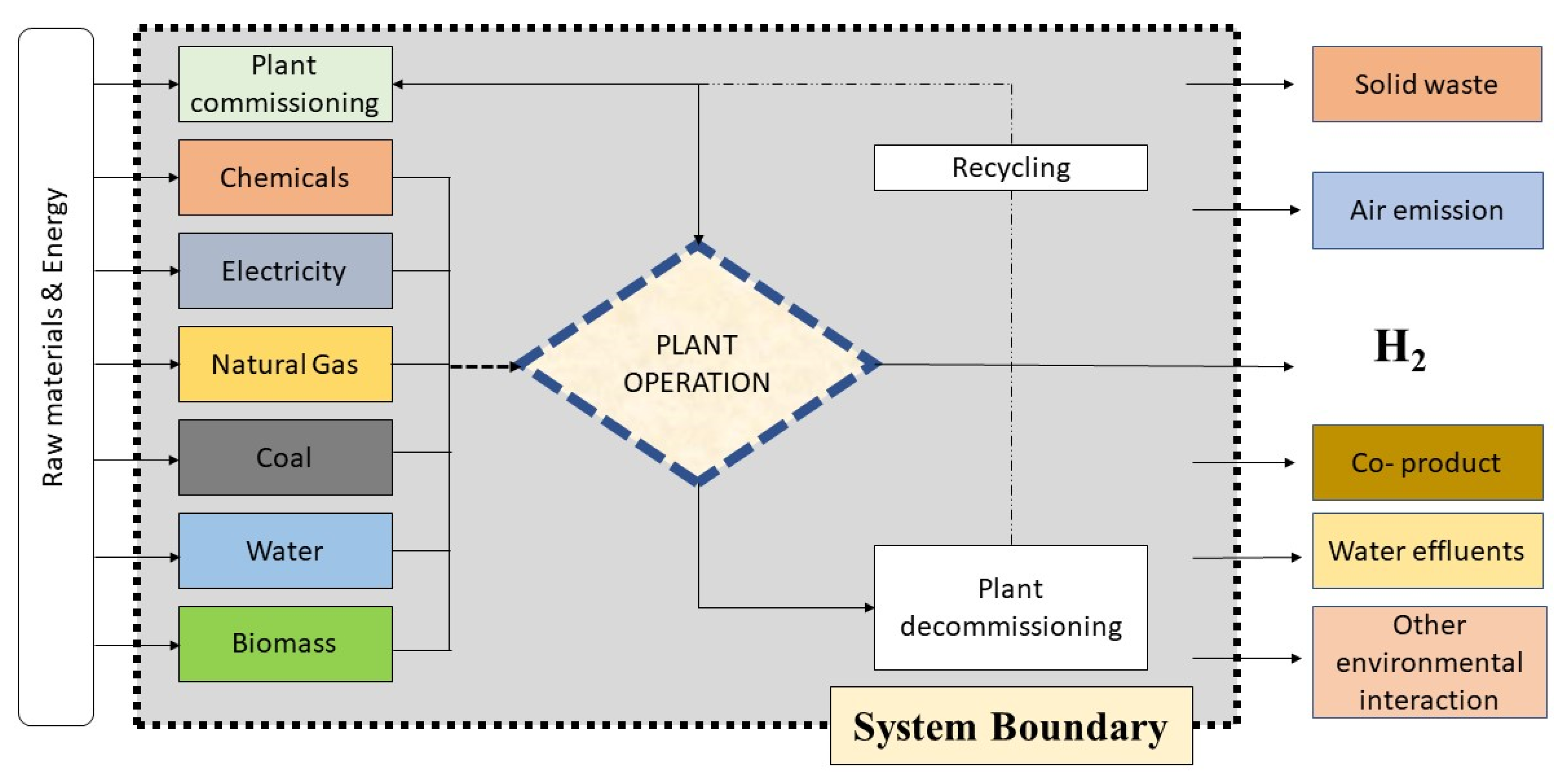
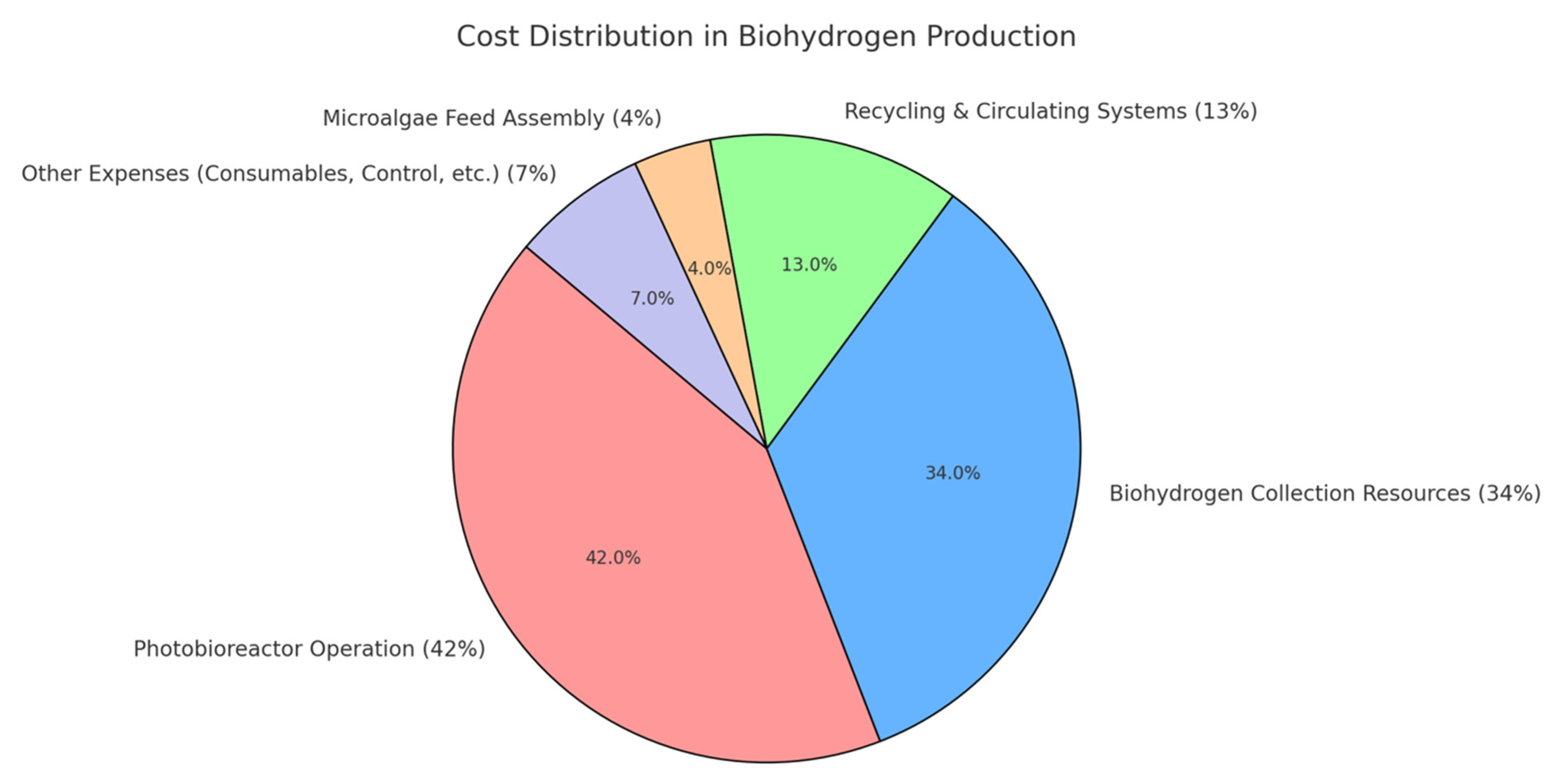
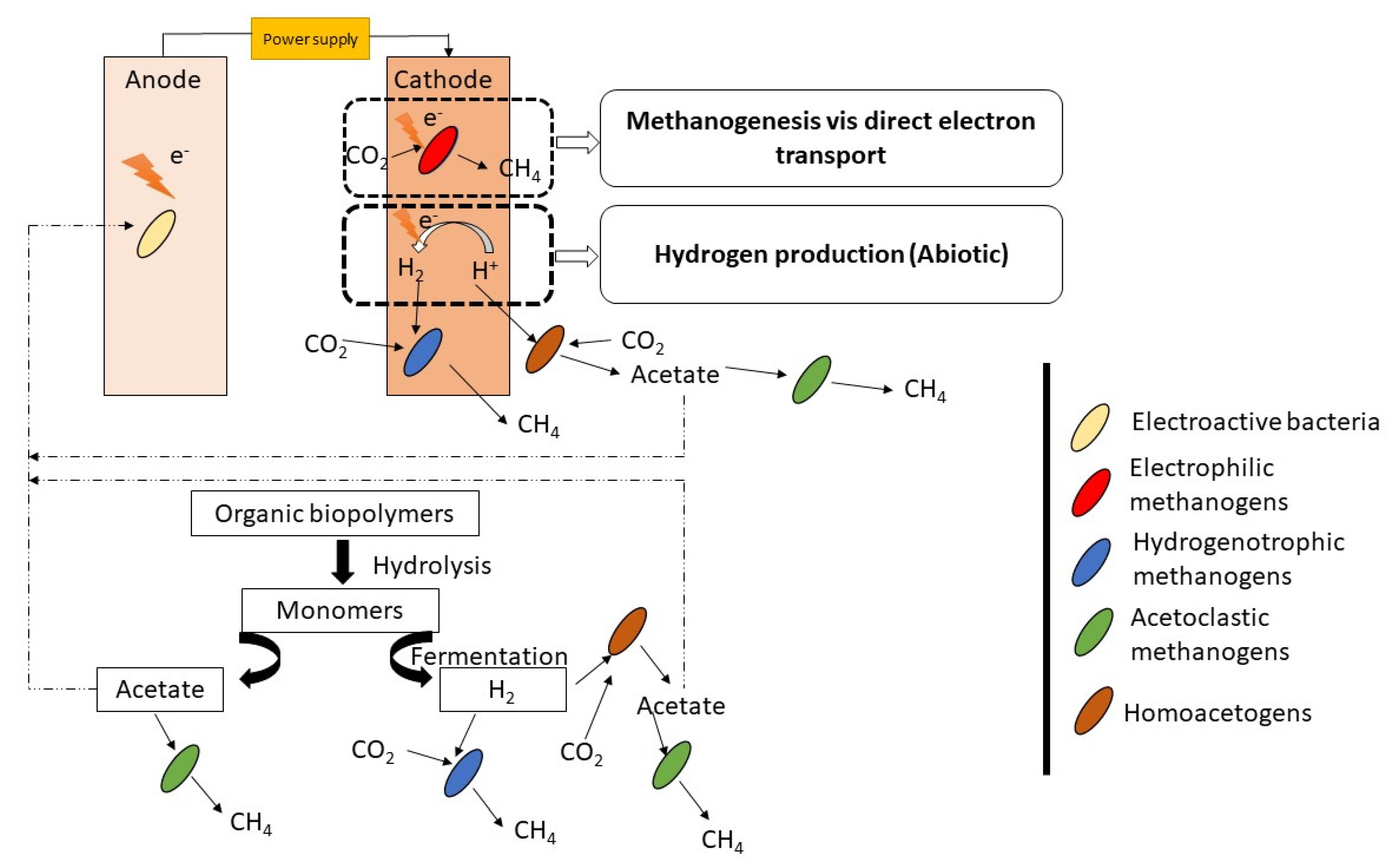
| Techniques | Merits of the Technique | Demerits of the Technique |
|---|---|---|
| Photo-fermentation | 1. A broad spectrum of light energy can be used by the bacteria in use. 2. Fit for a range of organic waste kinds. | 1. The produced O2 inhibits the nitrogenase. 2. Slow hydrogen production 3. High implementation cost. |
| Thermochemical gasification | Maximize the substrate’s conversion to biohydrogen. | The specifications for the unique circumstances of gas maintenance |
| Supercritical conversion. | 1. An ongoing procedure that is nighttime operational. 2. Elevated hydrogen concentration 3. The substrate does not need to be dried 4. High H2 selectivity and gasification efficiency. | Conditions for choosing a supercritical media. |
| Indirect bio-photolysis | 1. The blue–green algae use water to create hydrogen. 2. The atmospheric fixation of nitrogen gas. | 1. Certain hydrogenase enzymes must be eliminated in order to halt the H2 breakdown process. 2. Oxygen makes up 30% of the gas produced. |
| Dark fermentation | 1. The procedure is easy to use and economical. 2. Attained a high rate of H2 production 3. Ability to produce H2 in the absence of light | 1. O2 has the ability to inhibit hydrogenase 2. Reduce the output of biohydrogen. 3. An increase in H2 pressure makes the process less favorable thermodynamically. |
| Direct bio-photolysis | 1. Uses only water and sunlight to generate H2 2. Cost-effective process 3. Energy discussion significantly increased when compared to other biomass (crops or forests). | 1. The requirement for strong lighting. 2. The simultaneous production of H2 and O2, with the latter having a detrimental effect on the entire system. 3. Reduced efficiency of photochemistry |
| Microbial electrolysis | 1. Direct production of biohydrogen from waste streams 2. A viable and efficient method for producing hydrogen from wastewater in the future | 1. We still have a good understanding of the metabolic pathways involved. 2. Reduced H2 generation at low electrode power densities |
| Enzyme | Microorganism | Role in Dark Fermentation | Active Site | Turnover Frequency (k_cat) | Key Characteristics | Reference |
|---|---|---|---|---|---|---|
| [Fe-Fe] Hydrogenase | Clostridium spp. (e.g., C. acetobutylicum, C. pasteurianum) | Catalyzes proton reduction to H2 using electrons from reduced ferredoxin | Di-iron H-cluster with CN− and CO ligands | ~104 s−1 | High efficiency, low redox potential, sensitive to O2 and high H2 partial pressure | [22] |
| [Ni-Fe] Hydrogenase | Enterobacter spp., Desulfovibrio spp. | Facilitates H2 production via proton reduction with electrons from ferredoxin or NADH | Nickel-iron with cysteine ligands | 102–103 s−1 | Moderate efficiency, higher redox potential, less sensitive to O2 than [Fe-Fe] | [23] |
| Pyruvate-Ferredoxin Oxidoreductase (PFOR) | Clostridium spp., Thermotoga maritima | Converts pyruvate to acetyl-CoA, reducing ferredoxin for hydrogenase activity | Iron-sulfur clusters | ~102 s−1 | Essential for electron transfer to hydrogenases, operates in acetate pathway | [24] |
| NADH-Ferredoxin Oxidoreductase (NFOR) | Clostridium thermocellum, C. butyricum | Regenerates reduced ferredoxin from NADH, supporting [Fe-Fe] hydrogenase activity | Iron-sulfur clusters | ~101–102 s−1 | Enhances electron flow, critical for sustained H2 production | [25] |
| Pyruvate-Formate Lyase (PFL) | Enterobacter aerogenes, Escherichia coli | Converts pyruvate to formate, providing an alternative electron donor for H2 production | Glycyl radical | ~102 s−1 | Active in mixed acid fermentation, less common in strict anaerobes | [26] |
| Catalyst/Enzyme System | Substrate | VmaxV (mmol H2·h−1·cm−2 or μmol·min−1·mg−1) | KmK_m (mM) | Temperature (°C) | pH | Notes on Performance |
|---|---|---|---|---|---|---|
| Ni–Mo alloy cathode | Acetate | 0.145 | 2.5 | 30 | 7.0 | High activity under neutral conditions |
| Pt/C catalyst | Glucose | 0.160 | 3.0 | 25 | 7.0 | Low overpotential, high hydrogen yield |
| MoS2 nanosheets | Lactate | 0.125 | 1.8 | 35 | 7.0 | Good stability, suitable for long-term operation |
| [FeFe]-hydrogenase immobilized on CNTs | Reduced ferredoxin | 0.210 | 0.05 | 25 | 8.0 | Very low KmK_m, high specificity for electron donor |
| S.No | Inoculum Used | Substrate Used | H2 Production Rate | Reference |
|---|---|---|---|---|
| 1 | Cow Dung Compost | Corn Stalk | 1.71 L H2/L−d | [78] |
| 2 | Digested sludge from wastewater treatment | Cheese Whey | 1.54 L H2/L−d | [79] |
| 3 | Anaerobic digester sludge | Glucose | 1.1 L H2/L−d | [80] |
| 4 | Cow dung compost | Pretreated corn stalk | 1.7 L H2/L−d | [81] |
| 5 | Anaerobic digested sludge | Sugar beet juice | 1.91 L H2/L−d | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandit, C.; Srivastava, S.; Chang, C.-T. Catalytic Innovations for High-Yield Biohydrogen Production in Integrated Dark Fermentation and Microbial Electrolysis Systems. Catalysts 2025, 15, 848. https://doi.org/10.3390/catal15090848
Pandit C, Srivastava S, Chang C-T. Catalytic Innovations for High-Yield Biohydrogen Production in Integrated Dark Fermentation and Microbial Electrolysis Systems. Catalysts. 2025; 15(9):848. https://doi.org/10.3390/catal15090848
Chicago/Turabian StylePandit, Chetan, Siddhant Srivastava, and Chang-Tang Chang. 2025. "Catalytic Innovations for High-Yield Biohydrogen Production in Integrated Dark Fermentation and Microbial Electrolysis Systems" Catalysts 15, no. 9: 848. https://doi.org/10.3390/catal15090848
APA StylePandit, C., Srivastava, S., & Chang, C.-T. (2025). Catalytic Innovations for High-Yield Biohydrogen Production in Integrated Dark Fermentation and Microbial Electrolysis Systems. Catalysts, 15(9), 848. https://doi.org/10.3390/catal15090848






